Abstract
Introduction
In the diving community there is a special need to know if asymptomatic or mild COVID-19 disease impacts the cardiopulmonary functioning of individuals with occupational exposure to extreme environments. To date, no controlled studies have been conducted comparing COVID-19-infected hyperbaric employees and non-COVID-19-infected peers in a military setting.
Methods
Between June 2020 and June 2021, healthy, hyperbaric, military personnel aged between 18 and 54 years old, who had recovered from asymptomatic or subclinical COVID-19 disease at least one month earlier, were analysed. Non-COVID-infected peers with medical assessments during the same period were used as the control group. Somatometry, spirometry, VO2 max, and DLCO were measured for each group.
Results
No clinically relevant differences in somatometry, lung function tests, and exercise testing were found between the COVID-19 group and the controls. However, the percentage of individuals with a decrease in estimated VO2-max of 10% or more was significantly greater in the COVID group than in the control group (24 vs. 7.8%, P = 0.004).
Conclusions
After asymptomatic or mild symptomatic COVID-19 infections, military hyperbaric employees are as fit as those who had not encountered COVID-19. As this research was based on a military population, it cannot be extrapolated to a nonmilitary population. Further studies in nonmilitary populations are necessary to determine the medical relevance of the present findings.
Keywords: Fitness to dive, Health status, Health surveillance, Infectious diseases, Lung, Lung function, Pulmonary function
Introduction
Since December 2019, the world has faced the spread of the SARS-CoV-2 virus, which causes the coronavirus disease known as COVID-19.[ 1] Coronaviruses infect epithelial cells throughout the respiratory tract. The majority of patients recover at home with only minor symptoms.[ 2] Histological research indicates that in patients hospitalised with COVID-19, infection can result in different types of pulmonary damage.[ 3] Whether this damage is permanent is still a point of debate. High-resolution computed tomography (HRCT) scans three months after discharge still showed radiological evidence of COVID-19 in 78% of severe cases, declining to 48% after six months.[ 4] Studies have demonstrated decreases in predicted diffusing capacity for carbon monoxide (DLCO), restrictive ventilatory defects, and small airway dysfunction in COVID-19 patients.[ 5]
Little is known about the extent to which these pathophysiological changes and changes in physiological pulmonary parameters also occur in asymptomatic or subclinical COVID-19 patients. One study reports a reduction in diffusion capacity of carbon monoxide (DLCO) as the main effect,[ 5] whereas other studies suggest radiological evidence of COVID-19 in asymptomatic and mild disease.[ 6 - 9] Furthermore, several studies relating to non-severe COVID-19 found a maximal oxygen uptake (VO2-max) lower than expected after illness,[ 10 - 12] although others report no impairment.[ 13]
Multiple guidelines for the medical assessment of post-COVID-19 divers have been developed because of the major concern in the diving industry about the risk of COVID-19-induced pulmonary barotrauma (PBt).[ 14 - 16] Pathological predispositions to PBt include bullae and blebs.[ 17 - 19] Although there have been no published cases of divers with PBt following COVID-19, numerous case reports have linked COVID-19 to bullae-associated pneumothorax,[ 20 - 23] even in mild cases of COVID-19.[ 24] Based upon the latter, and following DMAC 33 regulations,[ 15] a tailormade post-COVID-19 dive medical assessment of divers was implemented at the Royal Netherlands Navy Diving and Submarine Medical Centre (DMC).
Since many previous studies investigating the effect of mild COVID-19 on pulmonary function did not include baseline function data, it was not possible to ascertain whether abnormalities found were pre-existing or due to COVID-19. Consequently, there is still uncertainty regarding the long-term effects of COVID-19 on pulmonary function in both military divers and the general public.
The DMC has a large (historical) database of medical assessments relating to different diving- and hyperbaric-related occupations. This database was used to provide matched, non-COVID-19-infected individuals to act as the control group. This retrospective, cross-sectional study aimed to investigate whether changes in cardiopulmonary function occur in hyperbaric personnel after mild and asymptomatic COVID-19.
Methods
According to national law and legislation, retrospective analyses are not required to be evaluated by a medical ethics committee. Nevertheless, this study has been evaluated and granted permission by the Surgeon General of the Netherlands Armed Forces (reference number DOSCO2020036245). Furthermore, the methods used to handle personal details and privacy were in agreement with the guidelines of the Association of Universities in the Netherlands and the Declaration of Helsinki.
Medical assessments of military hyperbaric personnel, i.e., divers, chamber attendants, and submariners, of the Netherlands Armed Forces are performed annually at the DMC. All fitness-to-dive assessments are performed according to European Diving Technology Committee (EDTC) guidelines,[ 25] except that pulmonary function testing (PFT) results are interpreted relative to the Global Lung Initiative (GLI)-2012 reference set.[ 26] In June 2020, a post-COVID-19 dive medical assessment, adapted from the DMAC-33 guideline,[ 15] was adopted by DMC. In this study, we use the term ‘hyperbaric’ which refers to divers, chamber attendants and submariners.
SUBJECTS
The data used in this study were from participants who underwent a post-COVID-19 dive medical assessment for hyperbaric work in the period from June 2020 to June 2021. The data of subjects who tested positive, using either the reverse transcriptase-polymerase chain reaction test (RT-PCR) or serology for SARS-CoV-2, but were neither hypoxic nor hospitalised were included. Thus, only asymptomatic and mildly symptomatic patients were included. Furthermore, active disease or the absence of a previous record of relevant testing were also reasons for exclusion.
The intention was to couple two to three age-, sex-, and occupation-matched controls, who had never tested positive for COVID-19 but who had undergone their last medical in the same period as the COVID-19 group, to every person included in the COVID-19 group. This ensured that the control group had experienced similar disadvantages (e.g., closure of sporting facilities and working at home) as the COVID-19-positive group during the COVID-19 lockdown periods. In terms of age, a one year difference in birth year was accepted (e.g., someone born in 1990 could be coupled to someone born in 1989, 1990, or 1991).
DIVE MEDICAL ASSESSMENT
Regular annual dive medical assessments typically consist of somatometry, spirometry, and exercise testing, along with an interview and physical examination by the dive medical officer, as specified by EDTC guidelines. Post-COVID-19 dive medical assessment additionally included measurements of diffusing capacity and plethysmography. All personnel who came for a COVID-19 dive medical assessment were asked if their results could, anonymously, be used for research purposes. Only the results of those who agreed were included in this study database.
Spirometry, plethysmography, and diffusion capacity
Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and DLCO were measured on MS-PFT PRO (Vyaire, the Netherlands). All measurements were carried out by qualified respiratory technicians according to the European Respiratory Society / American Thoracic Society task force guidelines.[ 27 , 28] For DLCO, the single-breath method was used and is reported as DLCO corrected for haemoglobin. The predicted FEV1 and FVC were calculated using the GLI reference values of 2012.[ 29] Residual volume (RV), vital capacity (VC), and total lung capacity (TLC) plethysmography measurements were carried out in a Vyaire Vyntus Body box (Vyaire, the Netherlands). For plethysmography, the current GLI reference values of 2021 were used.[ 30] For DLCO predicted values, the GLI 2017 reference values were used.[ 27] Calibration was performed following the manufacturer’s instructions.
Exercise testing
Maximal exercise capacity tests were performed with an Excalibur Sport ergometer (Lode, the Netherlands) based on a weight-dependent protocol in which the test starts with a power of 1 watt·kg-1 body weight during the first minute, with incremental increases of 0.5 watt·kg-1 body weight and 0.25 watt·kg-1 body weight in the second and third minutes, respectively, until exhaustion. The load was raised once per minute. All testing was done as advised in the American Thoracic Society protocol.[ 31] In this paper, the term estimated VO2-max (eVO2-max) will be used to express the calculated VO2-max. All electrocardiographs (ECGs) were taken with a Vyaire Vyntus ECG (Vyaire, the Netherlands). Due to COVID-19 regulations, it was not possible to use breath analysis for testing carried out at the beginning of the pandemic. Thus, it was decided to calculate VO2-max using the formula below developed by the DMC where WMAX = maximal load (Watts), A = age (years), W = weight (kg). See formula.
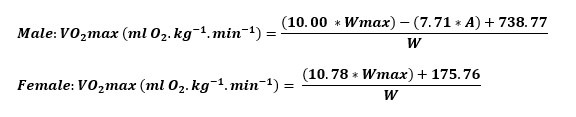
STATISTICAL ANALYSIS
As only a handful of studies have been published with subclinical COVID-19 patients, the sample size estimation was rather difficult. The threshold for the COVID-19 group was set at a minimum of 50 subjects since the sample size of other studies at the time of data gathering was at least 33.[ 5]
Analysis of data was blinded and performed with SPSS Statistics 23 (IBM Corporation), VassarStats (http://vassarstats.net) and SISA (https://www.quantitativeskills.com/sisa/). The latter two were used for their Freeman-Halton extension of Fisher’s exact test. All data were first tested for normality using the Shapiro-Wilk test. Continuous variables were compared using an independent or two-tailed-sample, t-test, or a rank-sum test in the case of nonparametric data. Finally, a linear regression analysis was performed to test whether changes were time-dependent. A probability (P) value of less than 0.05 was considered significant.
Results
Fifty-one post-COVID-19 subjects and 125 healthy controls were included in this study. The baseline characteristics of the post-COVID-19 and control groups were not statistically different (Table 1); however, those in the post-COVID-19 group had their latest dive medical assessment significantly later than those in the control group (1.5 y [IQR 1.75 y] vs 1 y [IQR 1 y], respectively; P = 0.023). On average, the post-COVID-19 group had their post-COVID-19 dive medical assessment 1 month (IQR 2.0 months) after their positive COVID-19 test.
Table 1. Main baseline characteristics of the two study groups; data are mean (SD) for normally divided parameters and median (IQR, µ = mean) for non-normal parameters; DLCO − diffusing capacity for carbon monoxide; FEV1 – forced expiratory volume in one second; FVC – forced vital capacity; HRmax – maximum heart rate; IQR – interquartile range; SD – standard deviation; TLC – total lung capacity; VC – vital capacity; eVO2max − estimated maximum rate of oxygen consumption .
| Parameter | COVID+ (n = 51) | CONTROL (n = 125) | P |
| Age | 30.00 (IQR 8, μ = 31.78) | 31.00 (IQR 11, μ = 32.66) | 0.497 |
| Male | 49 (96.1%) | 123 (98.4%) | 0.581 |
| Female | 2 (3.9%) | 2 (1.6%) | |
| Height (cm) | 183 (SD 7.6) | 184 (SD 6.8) | 0.726 |
| Weight (kg) | 86.0 (SD 12.5) | 86.6 (SD 11.5) | 0.767 |
| Body mass index (kg·m-2) | 25.4 (SD 2.4) | 25.6 (SD 2.7) | 0.962 |
| Fat percentage | 15.8 (IQR 5.5, μ = 16.4) | 15.8 (IQR 6.2, μ = 16.5) | 0.754 |
| n | 48 | 111 | |
| Occupation | 0.804 | ||
| Diver | 32 (62.7%) | 77 (61.6%) | |
| Submariner | 10 (19.6%) | 27 (21.6%) | |
| Special forces | 7 (13.7%) | 19 (15.2%) | |
| Smoking status | 0.527 | ||
| Never | 32 (62.7%) | 71 (56.8%) | |
| Active | 10 (19.6%) | 22 (17.6%) | |
| Former | 9 (17.6%) | 32 (25.6%) | |
| Smoking pack years | 3.5 (IQR 6.45, μ = 4.19) | 4.05 (IQR 5.63, μ = 5.33) | 0.330 |
| n | 19 | 54 | |
| FEV1 (L) | 4.63 (SD 0.58) | 4.74 (SD 0.61) | 0.316 |
| FEV1 Z-score | -0.14 (SD 0.78) | 0.0 (SD 0.80) | 0.275 |
| n | 51 | 123 | |
| FVC (L) | 5.96 (SD 0.82) | 6.01 (SD 0.76) | 0.679 |
| FVC Z-score | 0.25 (SD 0.82) | 0.27 (SD 0.83) | 0.841 |
| n | 51 | 123 | |
| FEV1/FVC | 77.78 (SD 4.48) | 78.90 (SD 4.55) | 0.141 |
| FEV1/FVC Z-score | 0.65 (SD 0.64) | -0.48 (SD 0.68) | 0.118 |
| n | 51 | 123 | |
| RV (L) | 1.65 (SD 0.48) | 1.53 (SD 0.31) | 0.314 |
| n | 27 | 21 | |
| VCmax (L) | 30 | 21 | 0.138 |
| n | 30 | 21 | |
| TLC (L) | 7.90 (SD 1.09) | 8.03 (SD 1.00) | 0.666 |
| n | 27 | 21 | |
| TLC % of predicted | 105.5 (IQR 14.6, μ = 104.9) | 104.6 (IQR 17.0, μ = 105.0) | 0.856 |
| n | 26 | 21 | |
| DLCO (mmol·min-1·kPa-1) | 11.67 (SD 1.90) | 12.13 (SD 1.68) | 0.306 |
| DLCO % of predicted | 101.7 (SD 14.4) | 102.3 (SD 8.7) | 0.838 |
| n | 32 | 33 | |
| Wmax (watt) | 324 (SD 50) | 324 (SD 45) | 0.920 |
| n | 50 | 122 | |
| eVO2-max (ml·kg-1·min-1) | 43.69 (IQR 6.52, μ = 43.78) | 42.68 (IQR 6.63, μ = 43.10) | 0.284 |
| n | 50 | 122 | |
| HRmax (beats·min-1) | 186 (IQR 20, μ = 184) | 185 (IQR 11, μ = 186) | 0.550 |
| n | 50 | 122 |
SOMATOMETRY
The post-COVID-19 group had a significantly higher body fat percentage (Δfat percentage 2.1% [SD 3.1]) than the control group (0.6% [2.4]; P = 0.002). However, the skinfold thickness measurement method has an error of up to 3.5% for women and up to 5% for men.[ 32]
LUNG FUNCTION TESTING
None of the pre-post parameters for diffusion capacity and plethysmography varied significantly between the COVID-19 and control groups. This was also the case for spirometry except for the change in FEV1 (ΔFEV1), which had a significantly greater decline in the control group than in the post-COVID-19 group (Tables (2 and (3). However, this difference in ΔFEV1 falls within the coefficient of variation.[ 33] Finally, there was no correlation between DLCO % of predicted or change in DLCO % of predicted (ΔDLCO) and either age or months after illness.
Table 2. Main post-measurement characteristics of the two study groups; data are mean (SD) for normally divided parameters and median (IQR, µ = mean) for non-normal parameters; DLCO − diffusing capacity for carbon monoxide; FEV1 – forced expiratory volume in one second; FVC – forced vital capacity; HRmax – maximum heart rate; IQR – interquartile range; SD – standard deviation; TLC – total lung capacity; VC – vital capacity; eVO2max − estimated maximum rate of oxygen consumption .
| Parameter | COVID+ (n = 51) | CONTROL (n = 125) | P |
| Age | 31.0 (IQR 9, μ = 33.9) | 32.0 (IQR 10, μ = 34.5) | 0.609 |
| Years since previous medical | 1.50 (IQR 1.75, μ = 2.17) | 1.00 (IQR 1.00, μ = 1.69) | 0.023 |
| n | 51 | 124 | |
| Months since infection | 1.0 (IQR 2.0, μ = 2.5) | N/A | N/A |
| Weight (kg) | 87.4 (SD 12.3) | 86.6 (SD 11.6) | 0.773 |
| Body mass index (kg·m-2) | 26.3 (IQR 3.4, μ = 25.9) | 25.4 (IQR 3.4, μ = 25.6) | 0.340 |
| Fat percentage | 18.4 (IQR 4.4, μ = 18.0) | 16.8 (IQR 6.2, μ = 17.5) | 0.165 |
| n | 47 | 117 | |
| Δ Fat percentage | 2.1 (SD 3.1) | 0.6 (SD 2.4) | 0.002 |
| n | 46 | 105 | |
| FEV1 (L) | 4.62 (SD 0.57) | 4.62 (SD 0.57) | 0.953 |
| n | 50 | 123 | |
| FEV1 Z-score | -0.04 (SD 0.81) | -0.13 (SD 0.74) | 0.476 |
| n | 50 | 123 | |
| ΔFEV1 (L) | -0.01 (SD 0.31 | -0.12 (SD 0.21) | 0.023 |
| n | 50 | 121 | |
| FVC (L) | 5.94 (SD 0.75) | 5.92 (SD 0.72) | 0.898 |
| n | 50 | 123 | |
| FVC Z-score | 0.31 (SD 0.78) | 0.17 (SD 0.80) | 0.320 |
| n | 50 | 123 | |
| FEV1/FVC | 77.76 (SD 5.67) | 78.25 (SD 5.17) | 0.583 |
| n | 50 | 123 | |
| FEV1/FVC Z-score | -0.58 (SD 0.82) | -0.51 (SD 0.76) | 0.619 |
| n | 50 | 123 | |
| RV (L) | 1.59 (SD 0.39) | 1.56 (SD 0.45) | 0.815 |
| n | 50 | 13 | |
| VCmax (L) | 6.04 (SD 0.77) | 6.08 (SD 1.00) | 0.891 |
| n | 50 | 13 | |
| TLC (L) | 7.62 (SD 0.95) | 7.63 (SD 1.24) | 0.959 |
| n | 50 | 13 | |
| DLCO (mmol·min-1·kPa-1) | 11.26 (SD 1.69) | 11.70 (SD 2.66) | 0.519 |
| n | 50 | 18 | |
| Wmax (Watt) | 320 (SD 53) | 328 (SD 44) | 0.325 |
| n | 51 | 119 | |
| eVO2-max (ml·kg-1·min-1) | 42.41 (IQR 4.88, μ = 42.23) | 42.20 (IQR 6.74, μ = 43.35) | 0.435 |
| n | 46 | 118 | |
| Δ eVO2-max (ml·kg-1·min-1) | -0.99 (IQR 5.93, μ = -1.57) | 0.13 (IQR 3.12, μ = 0.22) | 0.020 |
| n | 50 | 115 | |
| % change eVO2-max | -2.4% (IQR 13.9, μ = -3.0) | 0.3% (IQR 7.3, μ = 0.59) | 0.028 |
| Categorial % change eVO2-max | |||
| >-10% | 12 (24%) | 9 (7.8%) | 0.004 |
| -10 to 0% | 18 (36%) | 39 (33.9%) | 0.795 |
| 0 to 10% | 17 (34%) | 58 (50.4%) | 0.053 |
| >10% | 3 (6%) | 9 (7.8%) | 0.683 |
| HRmax (beats·min-1) | 185 (IQR 16, μ = 183) | 184 (IQR 11, μ = 183) | 0.742 |
| n | 51 | 118 |
Table 3. Before and after spirometric parameters for COVID-19+ subjects; data are mean (SD); FEV1 – forced expiratory volume in one second; FVC – forced vital capacity; TLC – total lung capacity .
| Parameter | COVID+ | P | |
| Pre | Post | ||
| FEV1 (n = 50) | 4.63 (0.59) | 4.62 (0.57) | 0.852 |
| FVC (n = 50) | 5.94 (0.81) | 5.94 (0.75) | 0.298 |
| TLC (n = 26) | 7.80 (0.96) | 7.85 (0.83) | 0.358 |
EXERCISE TESTING
The absolute value of eVO2-max did not differ significantly between the two groups. By contrast, compared with baseline, there was a significant decline in eVO2-max (ΔeVO2-max) in the post-COVID-19 group (-0.99 mL·kg-1·min-1, IQR 5.93) when compared to the control group (+0.13 mL·kg-1·min-1, IQR 3.12; P = 0.020). This was also the case for the percentage change (post-COVID-19: -2.4%, IQR 13.9; control: +0.3%, IQR 3.12; P = 0.028). Although these differences are statistically significant, they are nevertheless small in absolute terms. Interestingly, the proportion of subjects with a decrease of more than 10% in eVO2-max was significantly higher in the post-COVID-19 group than in the control group (24% vs 7.8%, P = 0.004). It is important to mention that although 24% of our COVID-19 positives had a decrement of more than 10% in eVO2-max, all were still fit enough to pass their assessment for hyperbaric work. All other categories and parameters were statistically equivalent between the groups. Finally, there was no correlation between ΔeVO2-max and the time elapsed since COVID-19 infection (Figure 1).
Figure 1.
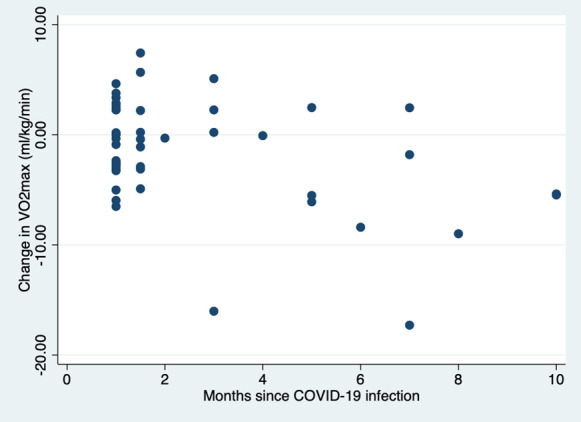
Correlation between ∆VO2-max (ml O2·kg-1·min-1) and months since infection with COVID-19 in the COVID+ group
Discussion
The present study showed no clinically relevant differences in somatometry, lung function, or exercise testing between a post-COVID-19 group and a previously uninfected control group. However, the number of subjects with more than a 10% reduction in their eVO2-max relative to their baseline data was significantly higher in the post-COVID-19-group than in the control group.
To the best of our knowledge, this is the first study to compare the pre-post effect of COVID-19 on lung function and exercise testing in hyperbaric workers previously infected with COVID-19 and in a previously uninfected control group. By contrast, several studies on the effect of COVID-19 on lung function parameters in patients or athletes have been published. Pre- and post-COVID spirometry data showed no significant difference in FEV1 or FVC between COVID-19 and uninfected controls.[ 12 , 13 , 34 , 35] Indeed, in a previous review paper, it was stated that spirometry indices appear to be well preserved.[ 5] Thus, the results of our study align with these previous findings.
Consistent with the spirometry data, we did not find any indication of restriction or alteration in TLC after mild or asymptomatic COVID-19. This is in contrast to recent studies that found restriction and decrease in TLC as the most common abnormalities.[ 5] However, the subjects in those studies were severe, hospitalised patients, whereas our subjects consisted of asymptomatic or only mild symptomatic COVID-19 patients, which suggests that mild COVID-19 does not generate any restriction or changes in TLC. This has been supported by studies that suggest that restriction worsens with increasing severity of COVID-19.[ 5]
In the present study, DLCO was unaffected by COVID-19 infection, which is comparable to those of some previous studies.[ 36 , 37] Although this seems to contradict the common finding that DLCO is decreased in COVID-19 patients one has to keep in mind that the percentage of DLCO predicted (%DLCO pred) was unaffected in patients with mild illness but was lower in patients with severe illness.[ 37] Furthermore, in most studies, the mean age of subjects was higher than that of the subjects in our study, which could have influenced DLCO values since increased age is associated with a graver course of COVID-19.[ 38] In addition, most studies were conducted at the time of patient discharge or within 30 days after discharge. It is possible that DLCO improves most in the months immediately after COVID-19 infection.[ 39 , 40] Our study does not support this as we found no linearity or statistical significance in %DLCO pred or difference between individual pre-COVID-19 and post-COVID-19 values, for any period of time after infection or for any age.
In the present study, we did not find a significant difference in eVO2-max between the COVID-19 population and the control group. This is in line with a recent study that found that mild or asymptomatic COVID-19 does not influence average VO2-max.[ 13] One can hypothesize that the mild course of the disease in asymptomatic or mild symptomatic patients may have enabled them to restart training faster and recuperate more quickly from the detraining effect of COVID-19 illness than severe symptomatic patients. Indeed, a previous study reported that post-COVID-19 subjects who received six weeks of rehabilitation improved their six-minute walking distance (6MWD) by 50 metres, while no improvement was observed for the group who did not undergo rehabilitation, which supports the detraining hypothesis.[ 40]
Recently, it was reported that the percentage of recruits who had a decline of more than 10% in VO2-max was significantly higher in a COVID-19 symptomatic group than in either COVID-19 asymptomatic or noninfected groups (18.8% vs 1.9% and 0%).[ 11] The reverse applied to recruits with an increase of more than 10% in their VO2-max; while none of the COVID-19 symptomatic recruits had an increase of more than 10%, the noninfected and asymptomatic groups had increases of 13.9% and 7.6%, respectively. Similar results were obtained in the present study; while 24% of the COVID-19-positive group had a decreased eVO2-max of more than 10%, only 7.8% of the COVID-19-negative group had a decreased eVO2-max of 10% or more. By contrast, the proportion of individuals with an eVO2-max increase of more than 10% was not statistically significantly different between the two groups. We suggest that the non-significant increase in VO2-max could be because subjects did not engage in sports on a daily basis, which is in contrast to military recruits who perform basic military training regularly.[ 41]
Based on the above, we hypothesize that the significant increase in the proportion of hyperbaric personnel who showed a > 10% decrease in eVO2-max in the COVID-19 group in this study was not due to COVID-19 directly but to a detraining effect. Dive medical assessments in the future are needed to test this hypothesis. Yet, more importantly, the present results underline the importance to use exercise capacity as a parameter for returning to hyperbaric work as has been suggested by others.[ 42]
The strengths of this study include its study design, sample size, the inclusion of only asymptomatic or mild symptomatic hyperbaric personnel, the use of a control group, and practical relevance for professional hyperbaric work. Nevertheless, the study has some limitations.
First, our subjects were stratified based on the result of their PCR test. Asymptomatic subjects were only included in the COVID-19 group if they had undergone a PCR test for other purposes (e.g., travel or part of contact tracing research) and had tested positive. Asymptomatically infected individuals who had not undergone a PCR test could have been erroneously assigned to the control group. As we do not know to what extent this occurred, it is possible that this oversight could have biased the results of the control group. For the purpose of this study, we had to rely on the honesty of divers to report any illnesses and the results of their PCR tests.
Second, for some outcome measures, data were unavailable for subjects in the control group because not all measurements are standardly performed at every regular dive medical assessment. For example, data for only a small group of control subjects were available for determining the pre-post values of both DLCO and plethysmography.
Third, it should be considered that our data gathering was performed during the period when the Alpha and Delta SARS-Cov-2 variants were most prevalent, and most people were not vaccinated. Vaccination status and virus variants could influence clinical outcomes and thus lung function outcomes. A follow-up study with enhanced knowledge of patient infection and vaccination status may shed light on this limitation.
Finally, the vast majority of our study population was male, with only two females in the COVID-19 group and two female matched-controls. We do not think this biased our results as it was reported that age, but not gender, had an impact on the recovery of mild-to-moderate COVID-19 patients.[ 43] However, to investigate any gender influence, a more balanced male-female population would be needed in future studies.
Conclusion
The present study is the first to measure pre-post changes, relating to lung function and exercise testing, in asymptomatic or mild symptomatic COVID-19 hyperbaric personnel and a matched control group. Based on the results of this study, we concluded that there are no negative effects on either lung function or exercise testing due to asymptomatic or mild symptomatic COVID-19 infection. Since our study population primarily comprised male, hyperbaric employees who were in good physical condition before being infected by COVID-19, this conclusion cannot be generalised to the whole hyperbaric population. Further studies with different populations will be necessary to determine the dive medical relevance of the present findings.
Footnotes
Conflict of interest and funding: nil
Contributor Information
Jan-Peter Schaap, Royal Netherlands Navy Diving and Submarine Medical Center, 1780 CA Den Helder, The Netherlands.
Margy E Zuluaga Fernandez, Center for Man in Aviation, Royal Netherlands Air Force, 3769 DE Soesterberg, The Netherlands.
Antoinette Houtkooper, Royal Netherlands Navy Diving and Submarine Medical Center, 1780 CA Den Helder, The Netherlands.
Edwin L Endert, Royal Netherlands Navy Diving and Submarine Medical Center, 1780 CA Den Helder, The Netherlands.
Pieter-Jan AM van Ooij, Royal Netherlands Navy Diving and Submarine Medical Center, 1780 CA Den Helder, The Netherlands; Department of Pulmonary Medicine, Amsterdam University Medical Center, Amsterdam, The Netherlands.
References
- World Health Organisation [internet]. WHO Timeline - COVID-19. [cited 2022 Jul 22]. Available from: https://www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19M
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel Coronavirus diseases (COVID-19) – China, 2020 China CDC Wkly. 2020; 2: 113–122. [PMC free article] [PubMed] [Google Scholar]
- Polak SB, Van Gool IC, Cohen D, von der Thüsen JH, van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33:2128–38. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–54. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Price OJ, Hull JH. Pulmonary function and COVID-19. Curr Opin Physiol. 2021;21:29–35. doi: 10.1016/j.cophys.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–34. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui S, Fujikawa A, Jitsu M, Kunishima N, Watanabe S, Suzuki Y, et al. Chest CT findings in cases from the cruise ship Diamond Princess with coronavirus disease (COVID-19). Radiol Cardiothorac Imaging. 2020;2:e200110. doi: 10.1148/ryct.2020200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui S, Fujikawa A, Jitsu M, Kunishima N, Watanabe S, Suzuki Y, et al. . Erratum: Chest CT findings in cases from the cruise ship "Diamond Princess" with coronavirus disease 2019 (COVID-19). Radiol Cardiothorac Imaging. 2020; 2: e204002. 10.1148/ryct.2020204002 Erratum for: Radiol Cardiothorac Imaging. 2020;2(2):e200110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Xiong R, He R, Lin W, Hao B, Zhang L, et al. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J Infect. 2020;81:e33–e39. doi: 10.1016/j.jinf.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavario P, De Marzo V, Lotti R, Barbara C, Porcile A, Russo C, et al. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int J Cardiol. 2021;340:113–8. doi: 10.1016/j.ijcard.2021.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri GAG, Bielecki M, Züst R, Buehrer TW, Stanga Z, Deuel JW. Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020. Euro Surveill. 2020;25:2001542. doi: 10.2807/1560-7917.ES.2020.25.36.2001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavario P, De Marzo V, Lotti R, Barbara C, Porcile A, Russo C, et al. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int J Cardiol. 2021;340:113–8. doi: 10.1016/j.ijcard.2021.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovancev A, Avakumovic J, Lakicevic N, Stajer V, Korovljev D, Todorovic N, et al. Cardiorespiratory fitness in volleyball athletes following a COVID-19 infection: a cross-sectional study. Int J Environ Res Public Health. 2021;18:4059. doi: 10.3390/ijerph18084059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duikgeneeskunde.nl [internet]. Hartig F Target organ lung – diving after Covid 19 disease? [cited 2022 Jul 23]. Available from: https://www.duikgeneeskunde.nl/wp-content/uploads/2020/04/diving-after-Covid-19.pdf.
- The Diving Medical Advisory Committee [Internet]. Return to diving after COVID-19. [cited 2022 May 15]. Available from: https://www.dmac-diving.org/guidance/DMAC33.pdf.
- Aerospacemedicine.ca [Internet]. Return to diving fitness after respiratory infection with COVID-19 illness – RCN surgeon communication 04/20. [cited 2020 Jul 5]. Available from: https://www.aerospacemedicine.ca/04-20_EN.pdf.
- Edmonds C, Lowry C, Pennefather J, Walker R, editors . Diving and subaquatic medicine, 4th ed. CRC Press; 2001. doi: 10.1201/b15307 [DOI] [Google Scholar]
- Tetzlaff K, Reuter M, Leplow B, Heller M, Bettinghausen E. Risk factors for pulmonary barotrauma in divers. Chest. 1997;112:654–9. doi: 10.1378/chest.112.3.654. [DOI] [PubMed] [Google Scholar]
- Wingelaar TT, Bakker L, Nap FJ, van Ooij PAM, Endert EL, van Hulst RA. Routine chest X-rays are inaccurate in detecting relevant intrapulmonary anomalies during medical assessments of fitness to dive. Front Physiol. 2021;11:613398. doi: 10.3389/fphys.2020.613398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli AW, Ingle T, Newman J, Nadeem I, Jackson K, Lane ND, et al. COVID-19 and pneumothorax: a multicentre retrospective case series. Eur Respir J. 2020;56:2002697. doi: 10.1183/13993003.02697-2020.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen ML, van Manen MJG, Cretier SE, Braunstahl GJ. Pneumothorax in patients with prior or current COVID-19 pneumonia. Respir Med Case Rep. 2020;31:101187. doi: 10.1016/j.rmcr.2020.101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower L, Carter JL, Rosales Lopez J, Henry AM. Tension pneumothorax in a patient with COVID-19. BMJ Case Rep. 2020;13:e235861. doi: 10.1136/bcr-2020-235861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Lago VC, Cezare TJ, Fortaleza CMCB, Okoshi MP, Baldi BG, Tanni SE. Does COVID-19 increase the risk for spontaneous pneumothorax? Am J Med Sci. 2020; 360: 735–7. 10.1016/j.amjms.2020.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunna K, Braun AB. Development of a large spontaneous pneumothorax after recovery from mild COVID-19 infection. BMJ Case Rep. 2021;14:e238863. doi: 10.1136/bcr-2020-238863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling JED, Nome T. Medical assessment of working divers. Fitness to dive standards of European Diving Technology Committee, 1st ed. Biele-Biene: Hyperbaric Editions; 2004. [cited 2021 Dec 21 Available from: http://www.edtc.org/EDTC-Fitnesstodivestandard-2003.pdf. [Google Scholar]
- Wingelaar TT, Clarijs P, van Ooij PA, Koch DA, van Hulst RA. Modern assessment of pulmonary function in divers cannot rely on old reference values. Diving Hyperb Med. 2018;48:17–22. doi: 10.28920/dhm48.1.17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BL, Brusasco V, Burgos F, Cooper BG, Jensen R, Kendrick A, et al. . 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017; 49: 1600016. 10.1183/13993003.00016-2016. Erratum in: Eur Respir J. 2018;52(5). [DOI] [PubMed] [Google Scholar]
- Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- Quanjer PH, Hall GL, Stanojevic S, Cole TJ, Stocks J. Global lungs initiative. Age- and height-based prediction bias in spirometry reference equations. Eur Respir J. 2012;40:190–7. doi: 10.1183/09031936.00161011. [DOI] [PubMed] [Google Scholar]
- Hall GL, Filipow N, Ruppel G, Okitika T, Thompson B, Kirkby J, et al. Official ERS technical standard: global lung function initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J. 2021;57(3):2000289. doi: 10.1183/13993003.00289-2020. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society; American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003; 167: 211- 77. doi: 10.1164/rccm.167.2.211. Erratum in: Am J Respir Crit Care Med. 2003;1451–2. [DOI] [PubMed] [Google Scholar]
- Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- Enright PL, Beck KC, Sherrill DL. Repeatability of spirometry in 18,000 adult patients. Am J Respir Crit Care Med. 2004;169:234–8. doi: 10.1164/rccm.200204-347OC. [DOI] [PubMed] [Google Scholar]
- Gervasi SF, Pengue L, Damato L, Monti R Pradella S, Pirronti T, et al. . Is extensive cardiopulmonary screening useful in athletes with previous asymptomatic or mild SARS-CoV-2 infection? Br J Sports Med. 2021; 55: 54–61. 10.1136/bjsports-2020-102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Borst B, Peters JB, Brink M, Schoon Y, Bleeker-Rovers CP, Schers H, et al. Comprehensive health assessment 3 months after recovery from acute Coronavirus Disease 2019 (COVID-19). Clin Infect Dis. 2021;73:e1089–e1098. doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazovic B, Zlatkovic-Svenda M, Grbovic J, Milenković B, Sipetic-Grujicic S, Kopitovic I, et al. Comparison of lung diffusing capacity in young elite athletes and their counterparts. Rev Port Pneumol (2006). 2017;S2173-5115(17)30150-1. doi: 10.1016/j.rppnen.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Cortés-Telles A, López-Romero S, Figueroa-Hurtado E, Pou-Aguilar YN, Wong AW, Milne KM, et al. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir Physiol Neurobiol. 2021;288:103644. doi: 10.1016/j.resp.2021.103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Jung SI. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020;52:154–64. doi: 10.3947/ic.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnweber T, Sahanic S, Pizzini A, Luger A, Schwabl C, Sonnweber B, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57:2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement Ther Clin Pract. 2020;39:101166. doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo K, Piirainen JM, Tanskanen-Tervo MM, Kyröläinen H, Huovinen J, Linnamo V. Effects of military basic training on VO2-max, body composition, muscle strength and neural responses in conscripts of different aerobic conditions. Biomed Hum Kinet. 2019;11:167–74. doi: 10.2478/bhk-2019-0023. [DOI] [Google Scholar]
- Sadler C, Alvarez Villela M, Van Hoesen K, Grover I, Lang M, Neuman T, et al. Diving after SARS-CoV-2 (COVID-19) infection: Fitness to dive assessment and medical guidance. Diving Hyperb Med. 2020;50:278–87. doi: 10.28920/dhm50.3.278-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinsky I, Baristaite G, Gurwitz D. Effects of age and sex on recovery from COVID-19: analysis of 5,769 Israeli patients. J Infect. 2020;81:e102–e103. doi: 10.1016/j.jinf.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]


