Abstract
A 53-year-old woman with a HeartMate III left ventricular assist device (LVAD) was successfully treated under hyperbaric conditions for haemorrhagic cystitis. The HeartMate III LVAD inserted in this patient had not previously been tested or certified for use under hyperbaric conditions. To our knowledge this is the first report of the HeartMate III LVAD being used to support a patient undergoing hyperbaric treatment. The overview detailed here of the safety and technical aspects of managing this patient for hyperbaric treatment was possible due to the collaboration of a multi-disciplinary team. We believe that our experience has demonstrated a pathway to safe hyperbaric treatment of patients dependent upon a HeartMate III LVAD.
Keywords: Bioengineering, Case report, LVAD, Haemorrhagic cystitis
Introduction
There are significant limitations to the safe use of electromedical devices in patients receiving hyperbaric oxygen treatment (HBOT). Few medical devices are manufacturer approved, marketed and licensed for use in the unique environmental conditions that exist in hyperbaric chambers. Devices must be tolerant of the pressure changes involved, and the use of medical devices in the chamber must not present any hazard to the patient or others. In particular, the device must not create any fire ignition hazard, which can be more likely due to the altered operating environment, and which creates the risk of a potentially catastrophic fire given the increased oxygen availability under pressure.[ 1]
To our knowledge, there are only two prior published reports of patients with a left ventricular assist device (LVAD) being treated with HBOT. One group treated their patient by housing the external parts of the LVAD in a pressure resistant housing[ 2] while another performed some modifications to a HeartMate II LVAD that were similar to our approach in order to treat their LVAD-dependent patient.[ 3]
The patient reported here was referred for hyperbaric treatment of haemorrhagic cystitis (HC); a dysfunction of the bladder mucosa leading to bleeding which can be acute or insidious. It can be a complex disease process to manage.
There are a number of potential causative factors and multiple aetiologies can be involved in some cases. Common causative factors are radiation injury,[ 4] chemotherapy,[ 4] haematopoietic stem cell transplantation,[ 5] infection (bacterial/viral/fungal), systemic disease, anticoagulation or idiopathic causes.[ 6]
Conventional management strategies for HC have included options such as clot extraction, continuous bladder irrigation, bladder instillations of haemostatic factors, formalin, arterial embolisation or salvage surgery.[ 6] In recent years, HBOT has been increasingly used in the treatment of HC with growing evidence of efficacy.[ 1 , 7 , 8]
Currently in Australia it is estimated that there are about 110,000 people living with heart failure based upon census data.[ 9] A proportion of these become dependent upon implanted ventricular assist devices which are mechanical pumps that augment the function of the damaged ventricle so as to restore normal haemodynamics and end-organ blood flow. An external power source / controller device is connected to the intra-thoracic pump via an electrical cable through the skin. The assistance provided by an LVAD allows the heart to rest and recover its function and in those who are not expected to recover adequate cardiac function; the LVAD can provide a bridge to transplantation.[ 10]
Case report
CLINICAL FACTORS
The patient consented to the publication of her case details. She is a 53-year-old woman who was referred to the Alfred Hospital’s Hyperbaric Unit (HBU) for management of refractory HC. She presented a complicated past medical history, with localised cervical cancer diagnosed in 2005 and treated with chemotherapy, radiotherapy and a subsequent hysterectomy. She subsequently developed a non-ischaemic dilated cardiomyopathy (NIDCM) that led to severe heart failure, manifested on one occasion by an out-of-hospital cardiac arrest that she survived in 2015. Following this episode, she had an implantable cardiac defibrillator (ICD) inserted. The ICD is a Medtronic Amplia MRI Quad Biventricular ICD implanted in November 2018. Her ICD was approved by Medtronic to be pressurised to 4 atmospheres absolute (atm abs).[ 11] In late 2019 she presented to hospital with decompensated heart failure and this required insertion of a permanent LVAD and a temporary right ventricular assistance device. She was therapeutically anti-coagulated with a heparin infusion for these device insertions and sustained a kidney injury requiring continuous renal replacement therapy post operatively.
On day seven post-insertion of her cardiac assist devices, she experienced onset of frank haematuria. Imaging of the urinary tract with ultrasound and computed tomography (CT) demonstrated no anatomical cause for the haematuria. Haematuria continued despite conventional therapies including continuous bladder washout and treatment of potential urinary tract infections. She required multiple blood transfusions to combat anaemia resulting from the ongoing haematuria. A rigid cystoscopy demonstrated severe cystitis with multiple dilated and tortuous mucosal capillary vessels that were bleeding. Clot evacuation was performed and visible bleeding vessels were cauterised. She continued to have persistent bleeding for many weeks sufficient to require ongoing blood product transfusions. She was transitioned to anticoagulation with warfarin during this time in view of her LVAD. Further rigid cystoscopies were performed with injection of intra-vesical alum and further diathermy. Intra-vesical prostaglandin was ruled out as a viable option due to her brittle asthma. Viral causes such as cytomegalovirus and adenovirus infections were investigated and ruled out as potential contributors. Therapeutic anticoagulation was eventually ceased to moderate HC; requiring acceptance that this carried an increased risk of stroke. Her multiple comorbidities made her medically unsuitable to undergo a radical cystectomy or an urgent heart transplant and she was referred to the HBU for consideration of HBOT three months after onset of her HC.
She was considered a likely responder to HBOT given that the features of her HC were consistent with being a late radiation side effect.[ 5] Significant considerations arose during her assessment and consent for HBOT however.
These included the tolerance and reliability of her ICD in the chamber at pressure, the need for her LVAD to work at pressure without risk of catastrophic cardiac failure if it failed and the safety risks these devices might pose in chamber. Her brittle asthma also needed consideration. These issues led to a significant delay of eight weeks whilst the LVAD technology issue was addressed. She had temporising bilateral nephrostomies performed during this period to minimise her bleeding risk by urinary diversion. She commenced HBOT in late December 2019 and successfully received 38 treatments in total, each at involving oxygen breathing at 243 kPa (2.4 atmospheres absolute) for a duration of approximately 90 minutes.
ADAPTION OF THE HEARTMATE III DEVICE
The HeartMate III LVAD is a widely used LVAD which is manufactured by Abbott Cardiovascular. Its components are:
The implanted pump unit, consisting of a fully sealed electronically controlled motor driving a blood flow impeller via magnetic coupling;
a power cable that connects the implanted pump with its external controller (termed 'the driveline' by Abbott); this cable can be separated external to the patient’s body should the controller need replacing;
a pump controller which incorporates a liquid crystal display (LCD) screen and which has two power input cables;
a mains power supply that can be used to power the LVAD when the patient will remain close to an electrical power outlet;
and battery units to allow mobility.
Normally, one of the two power supply inputs should remain connected whenever the other is changed so that the LVAD is never without power. The controller incorporates a small battery which can power the LVAD should both of the power inputs be disconnected simultaneously or fail for any reason however the duration of operation enabled by this controller battery is a few minutes only.
The following summarises the evaluation and modification process for enabling use of the HeartMate III within the hyperbaric chamber at The Alfred hospital, an institution that has a strong history of assessing, developing and modifying medical devices for hyperbaric use. Several Alfred HBU hyperbaric technical officers hold qualifications in biomedical engineering.
The process commenced with an overview evaluation of the HeartMate III LVAD, to determine potential suitability for use within the hyperbaric chamber. These controllers were not certified from the manufacturer as hyperbaric compatible, therefore a detailed physical inspection, functional testing and failure modes evaluation was warranted. Abbott cardiovascular do not approve these units for use in hyperbaric chambers; however, due to the urgency of the clinical situation they agreed to supply the Alfred HBU with a new implantable pump system and supporting documentation to facilitate our evaluation and testing processes. The evaluation included a review of all available documentation that included the operating and technical service manuals. The sample pump unit was subject to a detailed evaluation of its internal components. Discussions were undertaken with Abbott’s engineering department and the cardiovascular team at the Alfred, to develop a deeper mutual understandings of the proposed plan for hyperbaric treatment of patients with an LVAD and the options for ensuring continuity of LVAD function. A literature review was also conducted to determine whether previous testing has been reported under hyperbaric conditions.
Power supply continuity is an essential feature of safe LVAD use and this was constrained by the safety related policy of the Alfred HBU that mains electrical power (240 volt AC) is not available in the hyperbaric chamber.
An early conclusion from evaluation of the device was that the controller unit would require modification. The controller’s internal 3 min backup lithium battery was assessed as both at risk of failure and a potential fire hazard if repetitively compressed in the chamber. Rather than attempting to rigorously determine if these concerns were valid, a decision was made to remove this battery (Figure 1), with the consequence that the LVAD would only operate if the controller was continuously connected to external power. We removed the batteries from two LVAD controllers which became dedicated for hyperbaric use only as a primary and backup controller. These were clearly identified with ‘Hyperbaric Modified’ stickers (Figure 2). The controllers were completely stripped down to the bare essentials that would allow us to safely compress them in the chamber (Figure 3).
Figure 1.

Lithium ion battery of the LVAD controller
Figure 2.

Hyperbaric modified LVAD controller
Figure 3.
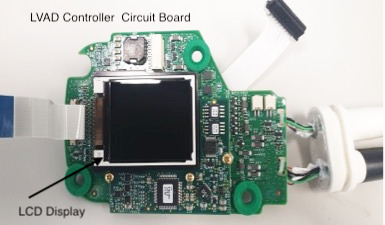
LVAD controller circuit board with minimum components
The department is equipped with highly redundant medical grade, cardiac protected AC supplies, accompanied by an UPS (uninterruptible power supply) to provide power to the LVAD system controller when under pressure from the outside.
A dedicated LVAD chamber penetrator was constructed using an OEM HeartMate III control cable. This custom through-hull penetrator was independently certified to two and a half times the maximum working pressure of the chamber to validate it for pressure and electrical integrity (Figure 4). The cable provided all communication and redundant power to the controller from the outside of the chamber and enabled the Abbott engineers and the VAD coordinators to manipulate controller setting under pressure and download the event logs after each hyperbaric treatment for analysis back at head office. The power provided to the controller via the cable is approximately 14 Volt and 4.8 Amp. In the event of a catastrophic mains power failure and UPS failure, the dedicated VAD console also contained a backup battery system. This provided triple redundancy in the event of a mains power failure.
Figure 4.
Schematic of patient and hyperbaric modified controller in chamber with components present external to chamber
The removal of the internal battery required a modification to a control circuit to inactivate the normal alarm feature that identifies failure of the internal battery. All other alarms and fault logs were assessed as functioning normally during testing.
Pressure testing was undertaken to evaluate the tolerance of the controllers and a sample pump unit to a maximum pressure of 300 kPa gauge (400 kPa absolute, ~4 atm abs) and to pressurisation and depressurisation rates not exceeding 180 kPa·min-1. The controllers were designated as not to be transferred through the medical lock due to the excessive pressurisation rate, thermal and humidity changes that occur during medical lock operation.
A dedicated flow loop was built which enabled comparison of real time sample LVAD flow measurements with flow output indicated on the VAD controller during various hyperbaric pressure profiles. The controller and pump assembly performed according to manufacturer’s specifications during all pressure conditions tested.
Protocols were developed for transfer of the patient to the hyperbaric modified LVAD system in the chamber, with staff training undertaken around both anticipated and emergency operating procedures. For hyperbaric treatments, the patient was transferred from her normal controller to one of the hyperbaric modified controllers prior to her first treatment, and this unit remained in use for the patient for the whole course of her hyperbaric treatment course.
OUTCOME
The patient was successfully treated in our multi-place chamber with no adverse complications or consequences as a result of her HBOT. There were no LVAD functional abnormalities identifies and no alarms were logged throughout the course of 38 hyperbaric treatment sessions.
Hyperbaric treatment reduced, but unfortunately did not eliminate transfusion requirements. Subsequent interventions included discontinuation of warfarin and right iliac artery embolisation. She was eventually discharged to palliative care in May 2020, nine months after her initial admission. At the time of writing this report, she remains well and has only needed intermittent iron and blood transfusions. She has been recommenced on warfarin with manageable HC symptoms.
Conclusion
The HeartMate III LVAD had not previously been tested or certified for use under hyperbaric conditions. To our knowledge this is the first report of the HeartMate III LVAD being used to support a patient undergoing hyperbaric treatment. The safety and management of the patient and her device would not have been possible without the collaboration of the Alfred Hospital’s heart failure team, intensive care and key representatives from Abbot Cardiovascular. We believe that our experience has demonstrated a pathway to safe hyperbaric treatment of patients dependent upon a HeartMate III LVAD.
Footnotes
Conflict of interest and funding: nil
Contributor Information
Arun Ilancheran, Hyperbaric Service, Alfred Health, Melbourne, Australia; Monash University, Melbourne, Australia.
Ian Millar, Hyperbaric Service, Alfred Health, Melbourne, Australia; Monash University, Melbourne, Australia.
Theo Tsouras, Hyperbaric Service, Alfred Health, Melbourne, Australia.
References
- Hughes AJ, Schwarer AP, Millar IL. Hyperbaric oxygen in the treatment of refractory haemorrhagic cystitis. Bone Marrow Transplant. 1998;22:585–6. doi: 10.1038/sj.bmt.1701376. [DOI] [PubMed] [Google Scholar]
- Kot J, Siodalski P, Lenkiewicz E. The hyperbaric protective tube: a housing for a left ventricular assist device (LVAD) in a multiplace hyperbaric chamber. Diving Hyperb Med. 2019;49:137–40. doi: 10.28920/dhm49.2.137-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwig D, Logue C, Hendriksen S, Westgard B, Walter J, Pullis M, et al. An approach to treating a patient with a HeartMate II™ left ventricular assist device in a multiplace hyperbaric chamber: a case report. Undersea Hyperb Med. 2018;45:89–93. [PubMed] [Google Scholar]
- Degener S, Pohle A, Strelow H, Mathers MJ, Zumbé J, Roth S, et al. Long-term experience of hyperbaric oxygen therapy for refractory radio- or chemotherapy-induced haemorrhagic cystitis. BMC Urol. 2015;15:38. doi: 10.1186/s12894-015-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintini C, Venturini M, Palese A. Haemorrhagic cystitis, preventive and treatment interventions in patients undergoing haematopoietic stem cell transplantation: a scoping review. Eur J Oncol Nurse. 2019;42:50–62. doi: 10.1016/j.ejon.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Traxer O, Desgrandchamps F, Sebe P, Haab F, Le Duc A, Gattegno B, et al. Cystite hémorragique: étiologie et traitement [Hemorrhagic cystitis: etiology and treatment] Prog Urol. 2001;11:591–601. (Fre). [PubMed] [Google Scholar]
- Dellis A, Deliveliotis C, Kalentzos V, Vavasis P, Skolarikos A. Is there a role for hyperbaric oxygen as primary treatment for grade IV radiation-induced haemorrhagic cystitis? A prospective pilot-feasibility study and review of literature. Int Braz J Urol. 2014;40:296–305. doi: 10.1590/S1677-5538.IBJU.2014.03.02. [DOI] [PubMed] [Google Scholar]
- Oscarsson N, Müller B, Rosén A, Lodding P, Mölne J, Giglio D, et al. Radiation-induced cystitis treated with hyperbaric oxygen therapy (RICH-ART): a randomised, controlled, phase 2-3 trial. Lancet Oncol. 2019; 20: 1602-14. 10.1016/S1470-2045(19)30494-2. Erratum in: Lancet Oncol. 2019 Sep 23. [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics 2018 . National Health Survey 2017–18. Data customised using Table Builder.
- Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. New Eng J Med. 1998;339:1522–33. doi: 10.1056/NEJM199811193392107. [DOI] [PubMed] [Google Scholar]
- https://wwwp.medtronic.com/crs-upload/letters/50/50_CQES-Standard_Letter_Pressure_HBOT-Rev3-2017Apr.pdf.



