Abstract
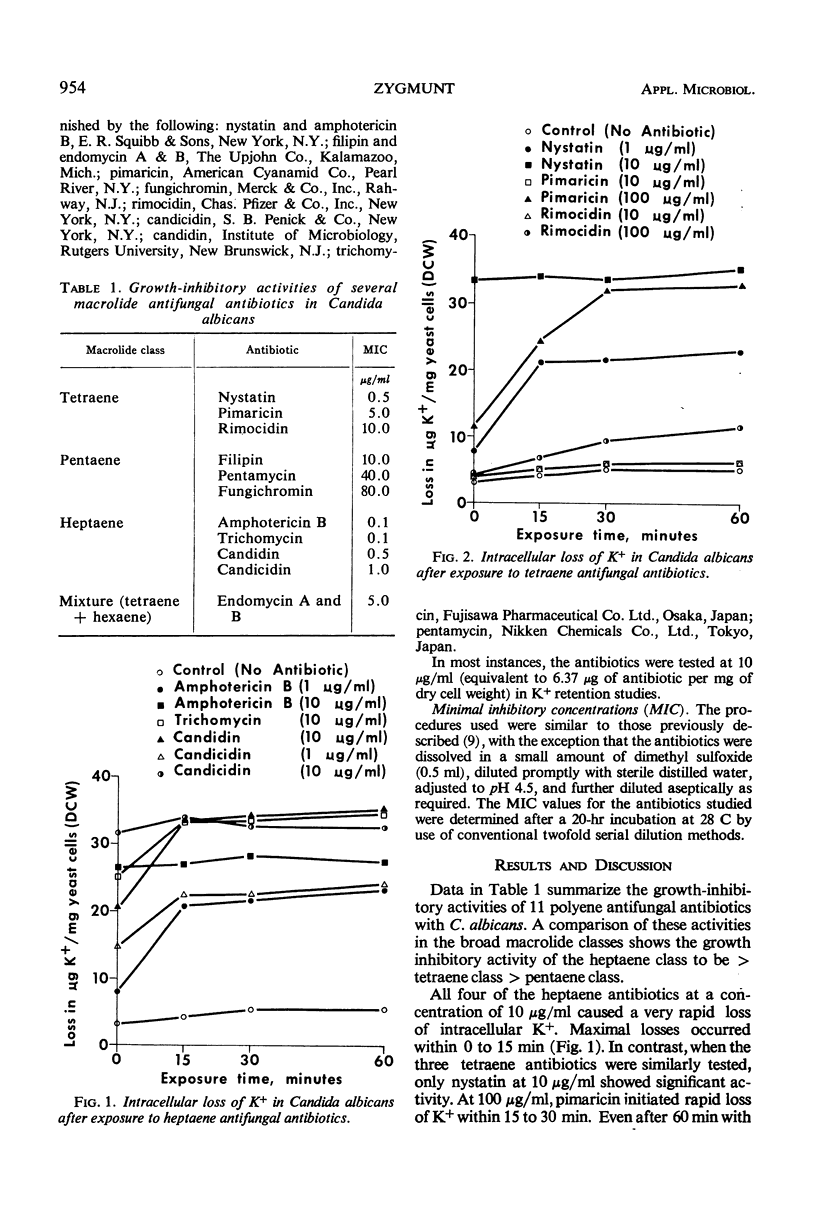
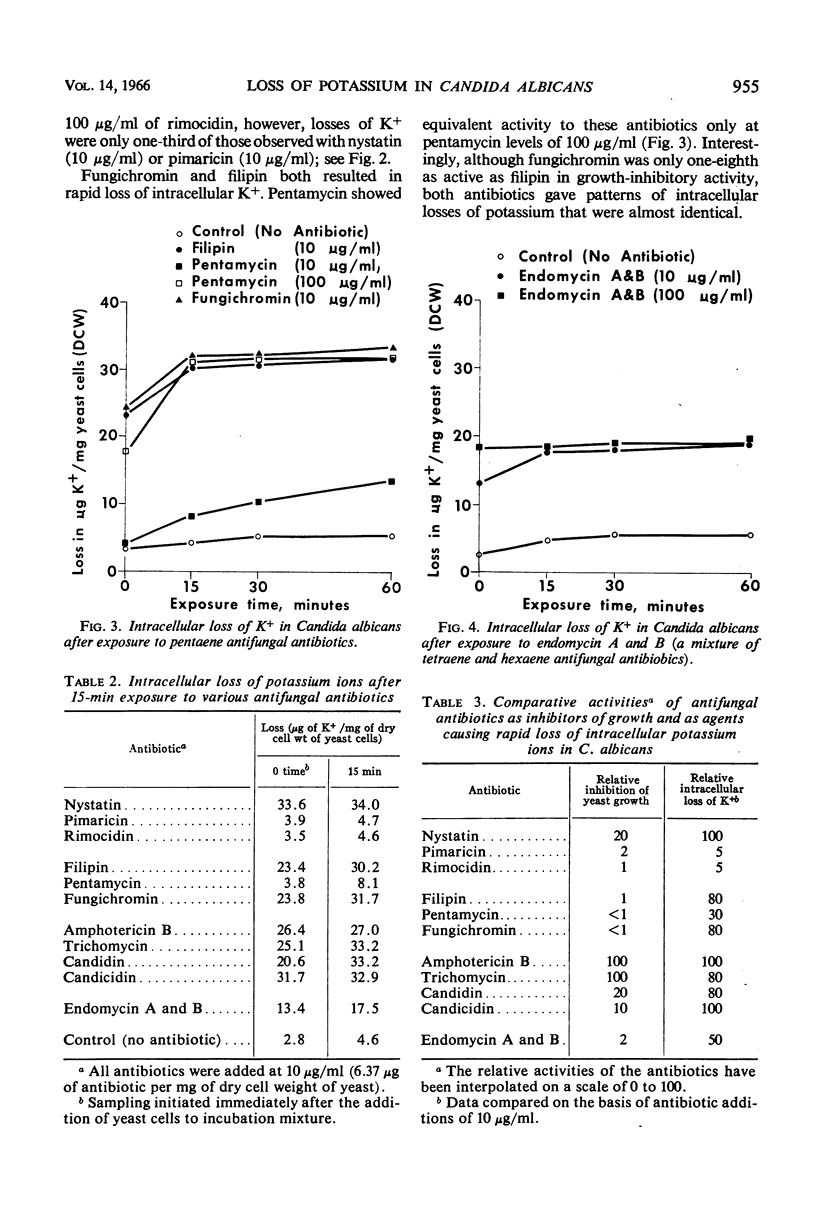
Eleven antifungal antibiotics, representing three broad macrolide classes, were studied in Candida albicans for their effect on growth and on the fate of intracellular K+. Marked differences were observed among these antibiotics between their growth-inhibitory activities and their adverse effects on the integrity of the cellular membrane as evidenced by loss of K+. Antibiotics most active in inhibiting growth of C. albicans were amphotericin B, trichomycin, candidin, candicidin (all heptaenes), and nystatin (a tetraene). In addition to those antibiotics, filipin and fungichromin also caused rapid leakage of K+ from yeast cells. Interestingly, fungichromin was the least active of the 11 antibiotics in inhibiting growth. Concentrations of rimocidin 10 times as great as those required for growth inhibition caused only a slight loss in intracellular K+ after 60 min.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HARSCH M., LAMPEN J. O. MODIFICATION OF K PLUS TRANSPORT IN YEAST BY THE POLYENE ANTIFUNGAL ANTIBIOTIC N-ACETYLCANDIDIN. Biochem Pharmacol. 1963 Aug;12:875–883. doi: 10.1016/0006-2952(63)90118-7. [DOI] [PubMed] [Google Scholar]
- KINSKY S. C. Alterations in the permeability of Neurospora crassa due to polyene antibiotics. J Bacteriol. 1961 Dec;82:889–897. doi: 10.1128/jb.82.6.889-897.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C. Nystatin binding by protoplasts and a particulate fraction of Neurospora crassa, and a basis for the selective toxicity of polyene antifungal antibiotics. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1049–1056. doi: 10.1073/pnas.48.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPEN J. O., ARNOW P. M., BOROWSKA Z., LASKIN A. I. Location and role of sterol at nystatin-binding sites. J Bacteriol. 1962 Dec;84:1152–1160. doi: 10.1128/jb.84.6.1152-1160.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINI F., ARNOW P., LAMPEN J. O. The effect of monovalent cations on the inhibition of yeast metabolism by nystatin. J Gen Microbiol. 1961 Jan;24:51–62. doi: 10.1099/00221287-24-1-51. [DOI] [PubMed] [Google Scholar]
- SUTTON D. D., ARNOW P. M., LAMPEN J. O. Effect of high concentrations of nystatin upon glycolysis and cellular permeability in yeast. Proc Soc Exp Biol Med. 1961 Oct;108:170–175. doi: 10.3181/00379727-108-26882. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT W. A., MARTIN T. A. 3-BROMOPYRUVIC ACID, A HIGHLY SELECTIVE ANTIMICROBIAL AGENT AGAINST CERTAIN YEASTS. J Pharm Sci. 1964 Nov;53:1420–1421. doi: 10.1002/jps.2600531136. [DOI] [PubMed] [Google Scholar]


