Abstract
The MICs and MBCs of 15 antibiotics for two strains of Staphylococcus aureus were determined in Mueller-Hinton broth (MHB) and 90% serum–10% MHB. Subsequent experiments established that highly protein-bound antibiotics (≥80%), such as LY333328, demonstrated higher MICs and MBCs, less killing over an 8-h interval, and shorter postantibiotic effects in 90% serum–10% MHB than in MHB alone. Albumin was demonstrated to be almost solely responsible for changes in the aforementioned pharmacodynamic parameters of LY333328.
Currently, in vitro antibiotic susceptibility testing performed in standard microbiological media such as Mueller-Hinton broth (MHB) is used to determine appropriate therapy for patients with infectious diseases (12). The predictive value of antibiotic activity in microbiological media for in vivo drug efficacy has been established in many instances; however, detailed data describing the influence of biological fluids, such as human serum, on antibiotic activity are, at present, limited. Antibiotics vary in their affinity for binding to plasma proteins, and it is believed that only the free, unbound fraction of drug is available for antibacterial action (4). Increases in the MICs in serum of highly-bound cephalosporins such as ceftriaxone for Staphylococcus aureus (7), cefoperazone for Pseudomonas aeruginosa (8), and cefonicid for S. aureus (3) have been noted. The MICs and MBCs of teicoplanin, a highly bound glycopeptide, for S. aureus are increased, and killing over 24 h is decreased, when the bacteria are grown in Mueller-Hinton broth supplemented with human serum (1, 15). At the same time, there is evidence of potential factors in serum which enhance the antibacterial action of some newer cephalosporins against gram-negative bacilli (9, 10) and of fluoroquinolones against S. aureus (6). Our goal was to study the effect of serum on the MICs, MBCs, time-kill curves, and postantibiotic effects (PAEs) of the investigational glycopeptide LY333328 and comparator agents for S. aureus. We want not only to define the influence of human serum on the pharmacodynamics of LY333328 and comparator agents but also to determine the serum component(s) responsible for protein binding and the level of protein binding at which this influence becomes significant.
(This work was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 28 September–October 1997.)
One reference strain (ATCC 29213) and one clinical blood isolate (M709) of S. aureus were used. LY333328 (Lilly Research Laboratories, Indianapolis, Ind.), teicoplanin, vancomycin, amoxicillin/clavulanate, cefazolin, cefotaxime, ceftriaxone, cefuroxime, ciprofloxacin, clindamycin, cloxacillin, erythromycin, gentamicin, imipenem, and rifampin were provided by their manufacturers. Drugs were selected on the basis of their clinical effectiveness against S. aureus infections, representation of different antibiotic classes, and degrees of protein binding. Stock solutions of these antibiotics were prepared from standard powders as described in National Committee for Clinical Laboratory Standards (NCCLS) guidelines (12).
MHB supplemented with cations (25 μg of CaCl2 per ml, 12.5 μg of MgSO4 per ml) was used as the control medium. Pooled human plasma was obtained from the Manitoba Red Cross and stored at −84°C. Serum was prepared daily from plasma by recalcification with 2 ml of 1 M CaCl2 per 100 ml of plasma. A 90% serum–10% MHB (no cations added) (MHBS) medium was prepared, and the pH was adjusted to 7.3 to equal that of MHB. The MHBS was then filter (pore size, 0.45 μm) sterilized. Biochemical analysis showed similar osmolalities for the two media. A solution of MHB containing bovine serum albumin (MHBA) at a concentration equivalent to that of the 90% serum (35 μg/ml) and one containing bovine serum albumin (35 μg/ml) and human α1-acid glycoprotein (mean serum concentration, 0.9 μg/ml) were also prepared (12). Blood agar plates were used for colony counts.
MICs and MBCs were determined by using NCCLS broth macrodilution guidelines (12, 13). Antibiotic killing of bacteria over time was determined as previously described (13, 18), with viable colony counts (CFU per milliliter) occurring at 0, 1, 2, 3, 4, 6, 8, 12, and 24 h. S. aureus cultures were incubated at 37°C in a shaking water bath and exposed to antibiotic at a concentration equal to the previously determined MIC in MHB for that strain. Growth controls were run to ensure equivalent rates of growth in all media. PAEs were determined by the standard method (5, 18). After 2 h of exposure to an antibiotic at a concentration equal to the MIC in MHB, cultures were diluted 1:1,000 to remove the drug, and the time for the culture to replicate 1 log10 unit was determined. Cultures were incubated at 37°C in a shaking water bath, and colony counts were performed when bacteria were first introduced to the antibiotic, 2 h later (both before and after the 1:1,000 dilution), and every 0.5 h thereafter for a minimum of 4 h after the dilution. The PAE was calculated as previously described (18). All MIC, MBC, time-kill curve, and PAE determinations were performed a minimum of three times per strain on separate occasions.
MICs and MBCs were interpreted as described in NCCLS guidelines (1, 13), which state that a fourfold or greater difference in values is significant. Time-kill curves were analyzed at 0, 8, and 24 h by using repeated-measure analysis of variance, while PAEs were analyzed by factorial analysis of variance. Post hoc comparisons were then made by use of Scheffe’s method.
MICs and MBCs for the reference strain of S. aureus, ATCC 29213, in MHB and MHBS are shown in Table 1 along with literature values for protein binding of each antibiotic in serum (2). The MICs and MBCs for the clinical strain M709 differed from those for strain ATCC 29213 by no more than a factor of 2, and repeat experiments with both strains showed no significant variation from the median values shown.
TABLE 1.
Antibiotic MIC and MBC determinations for S. aureus ATCC 29213
| Antibiotic | % Protein bound | MIC (μg/ml) in:
|
MBC (μg/ml) in:
|
||
|---|---|---|---|---|---|
| MHB | MHBS | MHB | MHBS | ||
| Gentamicin | 5–10 | 0.5 | 0.5 | 1 | 1 |
| Amoxicillin/clavulanateb | 17/20 | 0.5/0.25 | 0.5/0.25 | 2/1 | 2/1 |
| Imipenem | 20 | 0.015 | 0.03 | 0.03 | 0.06 |
| Vancomycin | 30 | 1 | 1 | 4 | 4 |
| Cefotaxime | 35 | 2 | 4 | 4 | 4 |
| Cefuroxime | 35 | 2 | 2 | 4 | 4 |
| Ciprofloxacin | 40 | 0.5 | 0.5 | 1 | 1 |
| Erythromycin | 60 | 0.5 | 0.25 | 8 | 8 |
| Rifampin | 75 | 0.015 | 0.03 | 0.125 | 0.25 |
| Cefazolin | 80 | 0.5 | 2a | 1 | 4a |
| Ceftriaxone | 83–96 | 4 | 16a | 8 | 32a |
| LY333328 | >80 (rat) | 0.5 | 4a | 0.5 | 4a |
| Teicoplanin | 90 | 0.5 | 2a | 0.5 | 2a |
| Clindamycin | 94 | 0.125 | 1a | 2 | 16a |
| Cloxacillin | 94 | 0.25 | 2a | 0.5 | 4a |
This is a value that is significantly (P ≤ 0.05) larger in MHBS.
Values for each drug in this combination are given.
Antibiotics showing significant differences in MICs determined in MHB and MHBS were LY333328, cefazolin, ceftriaxone, teicoplanin, clindamycin, and cloxacillin (Table 1). Susceptibilities to these six antibiotics were then retested a minimum of three times in MHBA. The antibiotic MIC and MBC values, respectively, determined in MHBA were as follows: LY333328, 4 and 4 μg/ml; cefazolin, 1 and 2 μg/ml;ceftriaxone, 8 and 16 μg/ml; teicoplanin, 2 and 2 μg/ml; clindamycin, 0.25 and 2 μg/ml; and cloxacillin, 2 and 4 μg/ml. Clindamycin MIC and MBC values were also determined in MHBA supplemented with α1-acid glycoprotein (0.9 μg/ml); they were 1 and 16 μg/ml, respectively. The MICs and MBCs for the clinical strain M709 differed from those for strain ATCC 29313 by no more than a factor of 2, and repeat experiments with both strains showed no significant variation from the median values presented.
The MICs and MBCs of cefazolin in MHBS for both strains of S. aureus tested were fourfold higher than those in MHB, while both values determined in MHBA were twofold higher than those in MHB. Ceftriaxone MICs and MBCs were increased fourfold in MHBS relative to those in MHB, and when tested in MHBA, changes in ceftriaxone MICs and MBCs were both limited to twofold increases over those in MHB. Clindamycin and cloxacillin MICs and MBCs in MHBS were both increased eightfold over those in MHB. When MHBA was used, cloxacillin again showed results eightfold higher than those in MHB. Clindamycin, however, showed no significant changes in its MICs and MBCs in MHBA compared to those in MHB until α1-acid glycoprotein (0.9 μg/ml) was added, at which point an eightfold increase such as that demonstrated with MHBS occurred.
Time-kill curve experiments were performed in MHB, MHBA, and MHBS with the eight antibiotics listed in Table 2. Bacterial killing following 8 h of incubation in MHBS and MHBA was negligible for LY333328, teicoplanin, cefazolin, ceftriaxone, clindamycin, cloxacillin, and vancomycin, while cultures incubated with ciprofloxacin demonstrated 1 log10 kill at 8 h (Table 2). At 24 h of incubation, bacterial killing had been significantly reduced with all antibiotics in all three media tested except for cloxacillin in MHB (data not shown). The results for growth controls in all media showed no significant differences between each other.
TABLE 2.
Killing of S. aureus ATCC 29213 by antibiotics at 1× MIC following 8 h of incubation in three mediaa
| Antibiotic | Killing (%) at 8 h in:
|
||
|---|---|---|---|
| MHB | MHBS | MHBA | |
| LY333328 | 99.9 | 0 | 5 |
| Teicoplanin | 10 | 0 | 0 |
| Cefazolin | 95 | 0 | 0 |
| Ceftriaxone | 95 | 0 | 0 |
| Clindamycin | 50 | 0 | 0b |
| Cloxacillin | 99.9 | 0 | 0 |
| Vancomycin | 20 | 20 | 15 |
| Ciprofloxacin | 99 | 90 | 90 |
Killing represented by subtracting inoculum at 8 h from the initial inoculum; 0 represents no kill or net growth at 8 h.
Result for MHBA plus α1-acid glycoprotein (0.9 μg/ml).
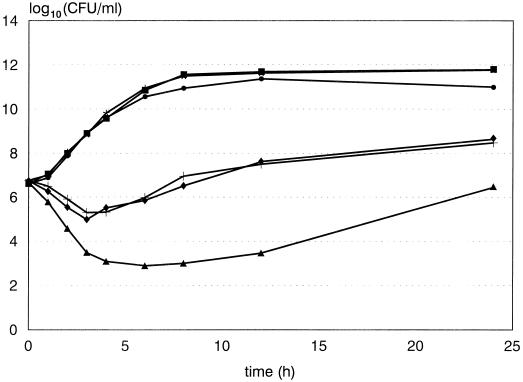
Less killing of the two strains of S. aureus occurred (P < 0.001) in MHBA and MHBS than in MHB, but there was no significant difference between killing in MHBA and that in MHBS by LY333328 (Fig. 1) and teicoplanin (data not shown). Similarly, no significant difference was noted between cloxacillin killing in MHBA and that in MHBS, but there was significantly less (P < 0.001) killing in both of these media than in MHB. Ceftriaxone and cefazolin showed decreased killing (P < 0.001) in MHBA and MHBS compared to that in MHB, but in addition a small but significant (P < 0.01) decrease in killing was demonstrated by both antibiotics in MHBS compared to that in MHBA (data not shown). Clindamycin showed less killing (P < 0.001) in MHBS than in MHBA and MHB, but there was no significant difference in killing by clindamycin between that in MHBA and that in MHB. However, when α1-acid glycoprotein (0.9 μg/ml) was added to MHBA, a decrease in killing (P < 0.001) compared to that in MHB was noted, and the difference in killing by clindamycin between that in MHBA and that in MHBS disappeared. No significant differences in killing over 24 h between media were noted for either ciprofloxacin or vancomycin.
FIG. 1.
Representative time-kill curve of ATCC 29213 S. aureus exposed to LY333328 at a concentration equal to the MIC in MHB (0.5 μg/ml). Symbols: ■, MHB control; ▴, MHB plus LY333328; •, MHBA control; ⧫, MHBA plus LY333328; ★, MHBS control; ✚, MHBS plus LY333328.
Mean results of PAE determinations of antibiotics for both strains of S. aureus in MHB, MHBA, and MHBS are shown in Table 3. In each case, PAE values of antibiotics for the reference and clinical strains showed no significant difference when compared to each other. Significantly shorter PAEs (P < 0.001) were observed in both MHBA and MHBS than in MHB for LY333328, teicoplanin, cefazolin, ceftriaxone, cloxacillin, and clindamycin (but only when α1-acid glycoprotein [0.9 μg/ml] was added to the MHBA). In addition, no significant difference between PAEs in MHBA and MHBS was noted for these antibiotics. Clindamycin showed no significant decrease in PAE in MHBA without α1-acid glycoprotein as compared to that in MHB. Vancomycin had similar PAEs in all three media. The PAE of ciprofloxacin was significantly (P < 0.05) longer in MHBS than in MHB and MHBA.
TABLE 3.
Mean PAE determinations for S. aureus ATCC 29213 and M709
| Antibiotic | PAE (min [mean ± SD]) in:
|
||
|---|---|---|---|
| MHB | MHBS | MHBA | |
| LY333328 | 65 ± 7 | 5 ± 3 | 5 ± 3 |
| Teicoplanin | 83 ± 5 | 7 ± 5 | 9 ± 4 |
| Cefazolin | 48 ± 3 | 2 ± 2 | 7 ± 4 |
| Ceftriaxone | 56 ± 4 | 1 ± 3 | 15 ± 7 |
| Clindamycin | 88 ± 4 | 11 ± 4 | 73 ± 6 (15 ± 4)a |
| Cloxacillin | 37 ± 3 | 1 ± 3 | 3 ± 3 |
| Vancomycin | 50 ± 4 | 50 ± 4 | 48 ± 5 |
| Ciprofloxacin | 60 ± 5 | 99 ± 4 | 58 ± 5 |
Result for MHBA plus α1-acid glycoprotein (0.9 μg/ml) is shown in parentheses.
We observed that highly protein-bound antibiotics such as LY333328 possess decreased activity in human serum, decreased killing over time, and shorter PAEs. From our experiments, it appears that this reduction in activity is significant when the bound protein fraction of antibiotic is 80% or higher, as was the case with LY333328 (80% bound in rat), cefazoline (80%), ceftriaxone (83 to 96%), teicoplanin (90%), clindamycin (94%), and cloxacillin (94%). It should also be noted that further experiments showed that when antibiotic concentrations in MHBS were raised to their MICs in that medium (data not shown), the differences in killing over time and PAEs between MHB and MHBS were no longer significant (i.e., the higher total drug concentration in MHBS is believed to have resulted in a free drug concentration equivalent to that in MHB).
Albumin appears to be the primary binding protein for LY333328 and for the β-lactam antibiotics tested, as well as teicoplanin. The effect of MHBA on cloxacillin was virtually identical to that of MHBS in all experiments. With S. aureus, cefazolin and ceftriaxone showed significantly lower activity in MHBA than in MHB, but, unlike cloxacillin, their activities in MHBA and MHBS also showed some differences. The MICs of these antibiotics in MHBA were intermediate between those in MHB and MHBS, and although time-kill curves showed less killing in MHBA than in MHB, there was still more killing in MHBA than in MHBS. Antibiotic PAEs in MHBA were slightly longer than those in MHBS for cefazolin and ceftriaxone but not significantly so. No significant change in any of the results occurred when the albumin in the MHBA was increased to 50 μg/ml, when α1-acid glycoprotein (0.9 μg/ml) was added to the MHBA for antibiotics other than clindamycin, when the calcium and magnesium levels in the MHBA were adjusted to match the concentrations in MHBS, when the MHBS was heated for 30 min at 56°C to inactivate complement, or when human serum albumin was substituted for bovine serum albumin in the MHBA (data not shown). Stratton and Reller found similar discrepancies between human serum and a MHB–5% albumin solution for cefazolin and discovered that the bound fraction of antibiotic was higher in serum than in the albumin solution (16). It is thus possible that there are other minor proteins in serum that bind the cephalosporins cefazolin and ceftriaxone, although the identity of these proteins is uncertain. Sun et al. showed that ceftriaxone is capable of binding to immunoglobulin G in serum and that this binding could be particularly important at low total drug concentrations, such as those used in our experiments (17). The possibility also exists that factors present in MHB are occupying sites on the albumin in MHBA that the two cephalosporins would normally bind to, although it is highly unlikely that such factors would be present in sufficient amounts to have an appreciable effect. Clindamycin, a basic lincosamide, was shown to bind primarily with α1-acid glycoprotein, since when it was added to MHBA, the antibiotic activity was not significantly different from that in MHBS. Further studies showed that α1-acid glycoprotein alone in MHB at physiologic concentrations also showed no significant difference from MHBS when clindamycin activity was being examined, providing further evidence that α1-acid glycoprotein, not albumin, is the main binding protein for this antibiotic.
Previous work by other investigators testing a single strain of methicillin-resistant S. aureus demonstrated results somewhat different from those reported here (11). The addition of albumin (40 μg/ml) to MHB did not significantly alter LY333328 or vancomycin MICs but did result in four- and eightfold increases in MBCs, respectively. A medium containing 50% pooled human serum–50% MHB resulted in inconsistent LY333328 MICs and MBCs and did not significantly alter vancomycin MICs or MBCs. Interestingly, in a concurrent set of experiments performed by the same investigators, the addition of albumin (40 μg/ml) to MHB resulted in an eightfold increase in the LY333328 MIC for a strain of vancomycin-resistant Enterococcus faecium (11). However, no significant changes in MIC and MBC determinations were noted when 50% pooled human serum–50% MHB was used. Investigators in this study were unable to detect the effect of proteins on a strain of methicillin-resistant S. aureus or antibiotic susceptibility (11).
The clinical relevance of antibiotic protein binding is a subject of debate (4). If all else was equal, one would likely select a less-protein-bound antibiotic over a highly bound one for therapy of infectious diseases. In fact, this is rarely the case, since different antibiotics are often quite dissimilar in both intrinsic antibacterial action and in pharmacokinetics and other factors usually take precedence in selection of appropriate antibacterial chemotherapy. Protein binding could be of considerable importance, however, if the antibiotic MIC for an infecting organism was shown to be close to the achievable drug concentration in serum. Although total antibiotic levels may be above the MIC, free drug levels may not, resulting in treatment failure. For infections such as bacterial meningitis or endocarditis, where antibiotic levels above the MBC are desired, protein binding of a potential agent could be even more critical. Indeed, Chambers et al. cite protein binding of cefonicid as the probable reason for its failure in a once-daily regimen for treatment of endocarditis (3).
Acknowledgments
Financial support for the project was generously provided in part by the PMAC Health Research Foundation and the Medical Research Council of Canada. J. A. Karlowsky is a PMAC-HRF/MRC fellow. G. G. Zhanel holds a Merck Frosst Chair in Pharmaceutical Microbiology.
Plasma was provided by the Manitoba Red Cross. L. Sargeant, Department of Clinical Chemistry, Health Sciences Centre, Winnipeg, Canada, performed the biochemical analyses of plasma and MHB.
REFERENCES
- 1.Bailey E M, Rybak M J, Kaatz G W. Comparative effect of protein binding on the killing activities of teicoplanin and vancomycin. Antimicrob Agents Chemother. 1991;35:1089–1092. doi: 10.1128/aac.35.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benet L Z, Oie S, Swartz J B. Appendix II: design and optimization of dosage regimens; pharmacokinetic data. In: Hardman J G, Limbird L E, Molinoff P B, Ruddon R W, Gilman A G, editors. The pharmacological basis of therapeutics. 9th ed. New York, N.Y: McGraw-Hill; 1996. pp. 1707–1792. [Google Scholar]
- 3.Chambers, H. F., J. Mills, T. A. Drake, and M. A. Sande. 1984. Failure of a once-daily regimen of cefonicid for treatment of endocarditis due to Staphylococcus aureus. Rev. Infect. Dis. 6(Suppl. 4):S870–S874. [DOI] [PubMed]
- 4.Craig W A, Ebert S C. Protein binding and its significance in antibacterial therapy. Infect Dis Clin N Am. 1989;3:407–421. [PubMed] [Google Scholar]
- 5.Craig W A, Gudmundsson S. The postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 403–431. [Google Scholar]
- 6.Davidson R J, Zhanel G G, Phillips R, Hoban D J. Human serum enhances the postantibiotic effect of fluoroquinolones against Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:1261–1263. doi: 10.1128/aac.35.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones R N, Barry A L. Antimicrobial activity of ceftriaxone, cefotaxime, desacetylcefotaxime, and cefotaxime-desacetylcefotaxime in the presence of human serum. Antimicrob Agents Chemother. 1987;31:818–820. doi: 10.1128/aac.31.5.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam Y W F, Duroux M H, Gambertoglio J G, Barriere S L, Guglielmo B J. Effect of protein binding on serum bactericidal activities of ceftazidime and cefoperazone in healthy volunteers. Antimicrob Agents Chemother. 1988;32:298–302. doi: 10.1128/aac.32.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang, S. D. R., G. L. Cameron, and P. R. Mullins. 1981. Anomalous effect of serum on the antimicrobial activity of cefoperazone. Drugs 22(Suppl. 1):52–59. [DOI] [PubMed]
- 10.Leggett J E, Craig W A. Enhancing effect of serum ultrafiltrate on the activity of cephalosporins against gram-negative bacilli. Antimicrob Agents Chemother. 1989;33:35–40. doi: 10.1128/aac.33.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercier R-C, Houlihan H H, Rybak M J. Pharmacodynamic evaluation of a new glycopeptide, LY333328, and in vitro activity against Staphylococcus aureus and Enterococcus faecium. Antimicrob Agents Chemother. 1997;41:1307–1312. doi: 10.1128/aac.41.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Methods for dilution in antimicrobial susceptibility tests for bacteria that grow aerobically. M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents. M25-T. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 14.Schmid K. α1-Acid glycoprotein. In: Putnam F W, editor. The plasma proteins, vol. 1. Structure, function, and genetic control. 2nd ed. New York, N.Y: Academic Press; 1975. pp. 183–228. [Google Scholar]
- 15.Stanley D, McGrath B J, Lamp K C, Rybak M J. Effect of human serum on killing activity of vancomycin and teicoplanin against Staphylococcus aureus. Pharmacotherapy. 1994;14:35–39. doi: 10.1002/j.1875-9114.1994.tb02786.x. [DOI] [PubMed] [Google Scholar]
- 16.Stratton C W, Reller L B. Serum dilution test for bactericidal activity. I. Selection of a physiologic diluent. J Infect Dis. 1977;136:187–195. doi: 10.1093/infdis/136.2.187. [DOI] [PubMed] [Google Scholar]
- 17.Sun H E, Chow M S S, Maderaso E G. Characteristics of ceftriaxone binding to immunoglobulin G and potential clinical significance. Antimicrob Agents Chemother. 1991;35:2232–2237. doi: 10.1128/aac.35.11.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhanel G G, Karlowsky J A, Davidson R J, Hoban D J. Effect of pooled human cerebrospinal fluid on the postantibiotic effects of cefotaxime, ciprofloxacin, and gentamicin against Escherichia coli. Antimicrob Agents Chemother. 1992;36:1136–1139. doi: 10.1128/aac.36.5.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]