Abstract
Background
Sub-Saharan Africa is one of the regions in the world with the highest numbers of uncontrolled hypertension as well as people living with HIV/AIDS (PLHIV). However, the association between hypertension and antiretroviral therapy is controversial.
Methods
Participant demographics, medical history, laboratory values, WHO clinical stage, current medication, and anthropometric data were recorded at study entry and during study visits at 1, 3, 6 months, and every 6 months thereafter until month 36. Patients who stopped or changed their antiretroviral therapy (tenofovir, lamivudine, efavirenz) were censored on that day. Office blood pressure (BP) was categorized using ≥ 2 measurements on ≥ 2 occasions during the first three visits. Factors associated with systolic and mean BP were analyzed using bivariable and multivariable multilevel linear regression.
Results
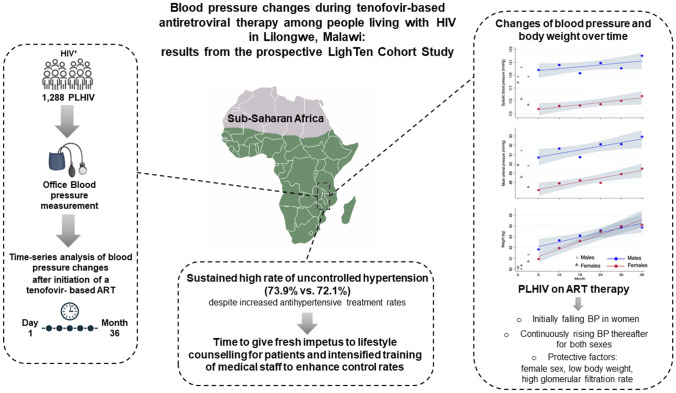
1,288 PLHIV (751 females, 58.3%) could be included and 832 completed the 36 months of observation. Weight gain and a higher BP level at study entry were associated with an increase in BP (p < 0.001), while female sex (p < 0.001), lower body weight at study entry (p < 0.001), and high glomerular filtration rate (p = 0.009) protected against a rise in BP. The rate of uncontrolled BP remained high (73.9% vs. 72.1%) and despite indication treatment, adjustments were realized in a minority of cases (13%).
Conclusion
Adherence to antihypertensive treatment and weight control should be addressed in patient education programs at centers caring for PLHIV in low-resources settings like Malawi. Together with intensified training of medical staff to overcome provider inertia, improved control rates of hypertension might eventually be achieved.
Trial registration
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00392-023-02253-w.
Keywords: HIV infection, Blood pressure, Weight gain, Hypertension prevalence, Hypertension incidence, Uncontrolled hypertension
Introduction
The decades-long rise in cardiovascular disease (CVD) continues in almost all countries outside the high-income world and the CVD burden because of modifiable risk factors increases globally [1]. In 2019, the leading risk factor for attributable deaths was high systolic blood pressure (BP), which accounted for 10.8 million deaths, i.e., 19.2% of all deaths worldwide [2]. Three-quarters of the world population with hypertension are living in low- and middle-income countries [3]. Data on hypertension prevalence in Africa vary widely and depending on the age of the studied population range from 15 to 70% [4, 5]. Especially sub-Saharan Africa is one of the regions in the world with the highest fractions of uncontrolled BP at 91% in men and 87% in women, respectively [6].
Despite the progress made following the introduction of effective and widespread antiretroviral therapy (ART), this region still also has the highest global share of incident cases (64.8%), deaths (74.0%), and people living with HIV/AIDS (PLHIV, 70.7%) [7]. Improved survival on ART and sociodemographic changes have been accompanied by rising numbers of non-communicable diseases (NCD), especially CVD [8–10]. The HIV infection itself is associated with an elevated risk for CVD [11–13] and 20%–40% of PLHIV from the region, corresponding to 5–10 million individuals, are estimated to be hypertensive [14, 15].
Hypertension contributes considerably to the NCD burden, as hypertensive PLHIV are at a higher risk of cardiovascular events and suffer from increased all-cause mortality compared to hypertensive HIV-uninfected individuals or normotensive PLHIV [11–13, 16]. Pathophysiologic mechanisms in hypertensive PLHIV include a state of chronic systemic inflammation, immune dysregulation, viral tropism, microbial translocation [17, 18], and possibly direct ART effects, since the prevalence of hypertension has been found to be higher in PLHIV on ART (14.5%-35.0%) compared to those who were ART naïve (10.5–16.9%) [19–21].
The present analysis intends to contribute data from longitudinal observation to the discussion around the integration of NCD management into existing national HIV care programs in countries with limited resources [22]. To this end, we determined changes in BP and its determinants, prevalence, incidence, and control rate of arterial hypertension in ambulatory PLHIV who had initiated tenofovir-based ART in combination with lamivudine and efavirenz.
Methods
The prospective Lighthouse Tenofovir (LighTen) Cohort Study included adult PLHIV presenting to the main facility of the Lighthouse Clinic located in Malawi’s capital city of Lilongwe for ART initiation. Recruitment started in August 2014 and follow-up ended in October 2019. As per Malawi HIV treatment guidelines at the time of enrollment [23], all patients on newly initiated ART received a fixed-dose combination of 300 mg tenofovir disoproxil fumarate (TDF), 300 mg lamivudine (3TC), and 600 mg efavirenz (EFV). The primary end point of the study was the change in renal function, while the prevalence and incidence of non-communicable co-morbidities including hypertension served as secondary objectives. The original power calculation was based on the expectation to detect a prevalence of 22% for renal impairment. However, due to technical reasons, creatinine and estimated glomerular filtration rate were available in a sufficient number of participants only at baseline. At the Lighthouse Clinic, the majority of routine clinical care is provided by nurses and clinical officers who complete 3–4 years of basic training.
Study population
Adult patients (≥ 18 years) with confirmed HIV infection and no self-reported history of prior ART, willing to participate and able to give written informed consent, were included. Participant demographics and medical history including self-reported hypertension, smoking status, laboratory values, WHO clinical stage, current medication, and anthropometric data (height, body weight, body mass index (BMI)) were recorded at study entry (month 0) as well as during regular study visits at 1, 3, 6 months, and every 6 months thereafter until month 36. Weight was measured with shoes removed and only light clothing. Participants who stopped or changed ART regimen for whatever reason were censored on the date of treatment change as were the participants who withdrew their consent, were transferred to another HIV treatment center, or had missed two appointments or more. Following the guidelines, all participants received cotrimoxazole prophylaxis, while isoniazid preventive therapy was given only to a small minority of participants.
Blood pressure measurements and definitions
Office BP was determined during each visit as the mean of the following measurements: (1) oscillometric BP measurement (Rossmax CF115f and Omron M300) as part of anthropometric measurements at clinical registration, and (2) additional measurement by a qualified nurse if the initial BP was ≥ 140/90 mmHg. All measurements were done in the sitting position 5 min after the cuff had been attached to the upper arm of the dominant hand. As a separate room for unattended BP measurements was not available, office BP was classified according to the current European Society of Cardiology/European Society of Hypertension treatment guidelines [24]. For the presentation of changes in BP categories from study entry to month 36, the classification of the 2020 International Society of Hypertension global hypertension practice guidelines with its lower number of categories was used [25]. Normotension was defined as mean BP < 140/90 mmHg per visit on at least two occasions during the first three visits (month 0, 1, and 3). As a mean BP ≥ 140/90 mmHg on one occasion does not label a patient as “hypertensive”, the individual median or modal BP category during the first three visits was considered as each participant’s baseline (month 0) BP category. Prevalent cases of hypertension were then defined as (i) the number of PLHIV with a median or modal BP category of at least hypertension grade 1 [24] plus (ii) participants on antihypertensive treatment, who were classified as “controlled “ or “uncontrolled” according to the aforementioned limits. Incident cases of hypertension were defined as mean systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg on at least two consecutive visits from month 6 onward. Antihypertensive therapy was supposed to follow the Malawi Standard Treatment Guideline [26] and was initiated with lifestyle counselling (reduced intake of salt and alcohol, increased consumption of vegetables/fruit, combined with regular exercise and preferably normal body weight). Antihypertensive drug treatment was free of charge and prescribed depending on availability in a stepwise fashion (substances most frequently prescribed in square brackets) using a diuretic (hydrochlorothiazide [HCTZ] 25 mg qd, bendrofluazide 2.5 mg qd, furosemide 20 mg qd only in case of unavailability of other diuretics), a dihydropyridine calcium channel blocker (amlodipine [AML] 5-10 mg qd, nifedipine slow release 10-20 mg bid), an angiotensin-converting-enzyme inhibitor (enalapril [ENA] 10-20 mg qd, captopril 12.5-50 mg tid), and finally a betablocker (atenolol [ATEN] 50-100 mg qd, propranolol 40-80 mg tid). Angiotensin-receptor blockers or single pill combinations were not available during the study period.
Laboratory analysis
All participants had a baseline evaluation beyond the standards of the Malawian HIV treatment program, including full blood count (AcT 5diff CP Hematology Analyzer, Beckman Coulter, Atlanta GA, USA), renal function tests (Erba XL200, Erba Mannheim, Germany), CD4 cell counts (Pima CD4-test, Abbott, Cape Town, South Africa), and HIV-RNA plasma levels (Cepheid GeneXpert, HIV viral load, Sunnyvale, CA, USA). The estimated glomerular filtration rate (eGFR) was calculated according to the CKD-EPI equation [27]. CD4 counts and viral load were measured every 6 and 12 months, respectively.
Statistical analysis
Descriptive statistics included mean and standard deviation (for normally distributed variables), median and interquartile range (for non-normally distributed variables), and frequencies (%) for categorical variables.
Information on sex (binary; 0: female, 1: male), age (in years; continuous), months after enrollment (0 to 36; continuous), BP (systolic and diastolic; continuous), body weight and height (continuous), renal function parameter (eGFR according to the CKD-EPI equation, continuous), CD4 count (continuous), viral load (continuous), WHO HIV stage (categorical; 1, 2, 3, 4), and individual status (categorical; 1: alive on ART regimen not changed, 2: changed regimen, 3: lost to follow-up, 4: withdrawn, 5: died) were available for inductive analyses.
Systolic and mean arterial BP values ([2xdiastolic BP + systolic BP]/3) as main outcome variables were confirmed to be normally distributed using Kolmogorov–Smirnov’s test. Afterward, we examined the development of the variables with repeated measurements, i.e., systolic BP, mean arterial BP, and weight using scatter plots. As visual inspection revealed unsteadiness during the initial study period, linear regression was plotted from month 6 onward. Consequently, we assessed the factors associated with systolic and mean arterial BP also from month 6 onward, allowing repeated measurements of weight for individuals using bivariable and multivariable multilevel linear regression. Independent variables were months, sex, weight at each visit, BP at baseline, age at baseline, weight at baseline, log(eGFR) at baseline, and WHO HIV stage at baseline and the personal identifier (second level). Forward variable selection was performed to select the predictors for the multivariable multilevel models.
The BP classification were descriptively analyzed for the available 799 PLHIV being observed for the whole study period using Sankey diagrams comparing baseline and end line.
We compared participants enrolled at baseline vs. still enrolled at month 6 vs. not-enrolled patients seen at the Lighthouse Clinic during the recruitment period to check for selection bias. Missing values were treated as missing at random. We ran Wilcoxon rank-sum test (Mann–Whitney U test) to compare the median of viral load for the groups with controlled vs. uncontrolled hypertension at month 36. For the comparison of weight change for those who could vs. those who could not be followed until 36 months, independent t tests were used. All statistical analyses used 0.05 significance level.
All data analyses were performed using Stata/IC 15.1 for Mac (StataCorp LLC, 4905 Lakeway Drive, College Station, TX 77845, USA).
Results
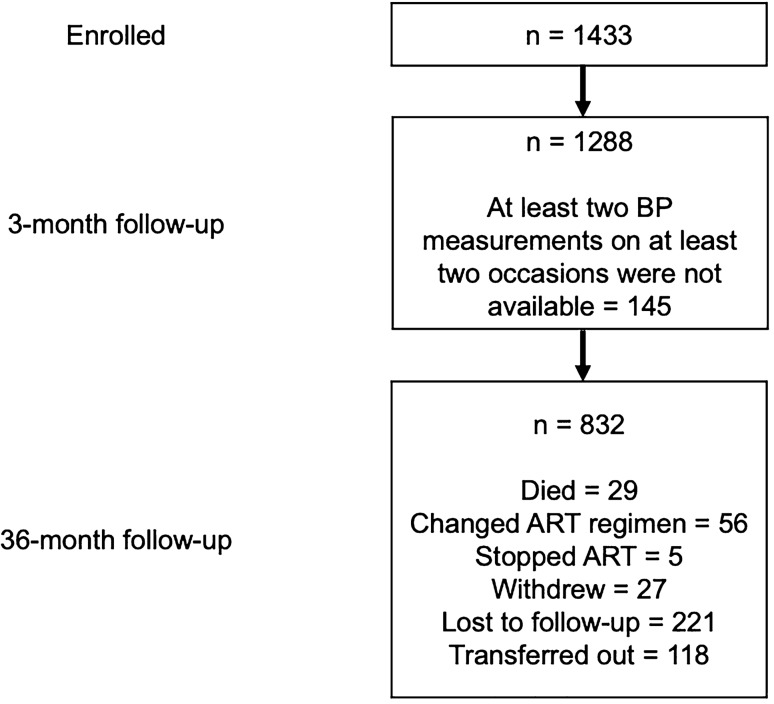
A total of 1,433 participants were enrolled during the 2-year recruitment period. At least two BP measurements on at least two occasions during the first 3 months were available for 1,288 PLHIV (751 females, 58.3%) who constitute the study population and the baseline characteristics are given in Table 1. The comparison of patients enrolled at baseline vs. month 6 vs. not-enrolled PLHIV revealed minor differences with respect to sex, age, BMI, and WHO stage at study entry (see Table 1 in the Supplementary Information).
Table 1.
Characteristics at study entry for the whole study population and the subgroup observed until month 36
| Characteristics | All observations | Visited month 36 | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 1,288 | 100.0 | 832 | 100.0 | |
| Female | 751 | 58.3 | 488 | 58.7 | |
| Male | 537 | 41.7 | 344 | 41.3 | |
| Age [years] (mean ± SD) | 36.1 ± 9.3 | 37.1 ± 9.2 | |||
| Age group [years] | |||||
| 18–24 | 117 | 9.1 | 57 | 6.8 | |
| 25–34 | 503 | 39.1 | 302 | 36.3 | |
| 35–44 | 445 | 34.5 | 307 | 36.9 | |
| 45–54 | 170 | 13.2 | 128 | 15.4 | |
| 55–64 | 43 | 3.3 | 33 | 4.0 | |
| 65 + | 10 | 0.8 | 5 | 0.6 | |
| Diabetes mellitus type 2 | 14 | 1.1 | 9 | 1.1 | |
| History of hypertension at study entry | |||||
| Self-reported, treated, uncontrolled | 27 | 2.1 | 17 | 2.0 | |
| Self-reported, treated, controlled | 10 | 0.8 | 5 | 0.6 | |
| Self-reported, untreated, uncontrolled | 15 | 1.2 | 9 | 1.1 | |
| Self-reported, untreated, controlled | 22 | 1.7 | 14 | 1.7 | |
| Systolic blood pressure [mmHg] (mean ± SD) | 118.3 ± 20.4 | 119.9 ± 20.5 | |||
| Diastolic blood pressure [mmHg] (mean ± SD) | 75.7 ± 14.0 | 76.6 ± 14.1 | |||
| Mean arterial pressure [mmHg] (mean ± SD) | 89.9 ± 15.4 | 90.9 ± 15.5 | |||
| Blood pressure categories (ESC/ESH) | |||||
| Optimal | 653 | 50.7 | 396 | 47.6 | |
| Normal | 261 | 20.3 | 173 | 20.8 | |
| High normal | 157 | 12.2 | 101 | 12.1 | |
| Grade 1 hypertension | 154 | 12.0 | 117 | 14.1 | |
| Grade 2 hypertension | 28 | 2.1 | 18 | 2.2 | |
| Grade 3 hypertension | 35 | 2.7 | 27 | 3.2 | |
| Weight (at baseline) [kg] (mean ± SD) | 60.1 ± 11.8 | 60.7 ± 12.0 | |||
| Missing | 4 | 0.3 | 4 | 0.5 | |
| Body mass index [kg/m2] | |||||
| < 18.5 | 67 | 5.2 | 32 | 3.9 | |
| 18.5–24.9 | 767 | 59.6 | 484 | 58.2 | |
| 25.0–29.9 | 298 | 23.1 | 211 | 25.3 | |
| ≥ 30.0 | 152 | 11.8 | 101 | 12.1 | |
| Missing | 4 | 0.3 | 4 | 0.5 | |
| eGFR [mL/min/1.73m2] (median; IQR) | 102; 84 – 116 | 101; 84 – 114 | |||
| Missing | 12 | 0.9 | 6 | 0.7 | |
| WHO HIV stage | |||||
| 1 | 603 | 46.8 | 403 | 48.4 | |
| 2 | 212 | 16.4 | 147 | 17.7 | |
| 3 | 396 | 30.8 | 237 | 28.5 | |
| 4 | 77 | 6.0 | 45 | 5.4 | |
| CD4 count [cells/mm3] (median; IQR) | 281; 128 – 426 | 296; 151 – 426 | |||
| Missing | 182 | 14.1 | 149 | 17.9 | |
| Viral load [copies/mL] (median; IQR) | 33,992; 6,968 –145,868 | 28,832; 6,012 – 129,634 | |||
| Missing | 40 | 3.1 | 26 | 3.1 | |
| Hemoglobin [g/dL] (median; IQR) | 12.5; 11.0 – 13.8 | 12.6; 11.0 – 13.9 | |||
| Missinga | 236 | 18.3 | 139 | 16.7 | |
| Hematocrit [%] (median; IQR) | 37.7; 33.1 – 41.9 | 38.1; 33.4 – 42.3 | |||
| RBC [106/mm3] (median; IQR) | 4.40; 3.96 – 4.88 | 4.42; 4.00 – 4.92 | |||
| WBC [cells/mm3] (median; IQR) | 4,500; 3,600 –5,600 | 4,450; 3,600 – 5,500 | |||
| Platelet count [103/mm3] (median; IQR) | 226; 174 – 295 | 222; 172 – 286 | |||
| Missing | 363 | 28.2 | 224 | 26.9 | |
SD standard deviation, IQR interquartile range, eGFR estimated glomerular filtration rate, RBC erythrocyte count, WBC leucocyte count, aapplies also to hematocrit, RBC, WBC
During the observation time, a total of 456 participants were lost (see Fig. 1) including 29 participants who died, 56 patients who had their ART regimen changed due to failing viral suppression, and five PLHIV who had decided of their own free will to stop ART completely (for more details see Table 2 in the Supplementary Information). HIV plasma levels were below the detection limit (indicating ART drug adherence) in 96.0%, 98.0%, and 100% of females as well as 94.2%, 93.0%, and 97.1% of males at months 12, 24, and 36, respectively.
Fig. 1.
LighTen Study flowchart
Table 2.
Bivariable multilevel linear regression (≥ 6 months) for systolic and mean arterial BP
| Variable | Systolic BP | Mean arterial BP | ||
|---|---|---|---|---|
| ß | p value | ß | p value | |
| Months | 0.1 | 0.018 | 0.1 | < 0.001 |
| Constant | 116.0 | < 0.001 | 88.3 | < 0.001 |
| Systolic BP at baseline | 0.4 | < 0.001 | – | – |
| Mean arterial BP at baseline | – | – | 0.4 | < 0.001 |
| Constant | 67.6 | < 0.001 | 50.9 | < 0.001 |
| Age at baseline | 0.5 | < 0.001 | 0.4 | < 0.001 |
| Constant | 98.8 | < 0.001 | 76.4 | < 0.001 |
| Sex | ||||
| Females | Ref | < 0.001 | Ref | < 0.001 |
| Males | 5.5 | 3.2 | ||
| Constant | 114.3 | < 0.001 | 88.0 | < 0.001 |
| Weight at baseline | 0.4 | < 0.001 | 0.3 | < 0.001 |
| Constant | 93.2 | < 0.001 | 71.4 | < 0.001 |
| Weight at each visit | 0.5 | < 0.001 | 0.3 | < 0.001 |
| Constant | 86.7 | < 0.001 | 67.7 | < 0.001 |
| log(eGFR) at baseline | − 10.9 | < 0.001 | − 7.8 | < 0.001 |
| Constant | 166.3 | < 0.001 | 125.1 | < 0.001 |
| WHO HIV stage | ||||
| 1 | Ref | 0.604 | Ref | 0.495 |
| 2 | − 1.1 | − 0.8 | ||
| 3 | 0.7 | 0.6 | ||
| 4 | − 0.5 | − 0.6 | ||
| Constant | 116.6 | < 0.001 | 89.3 | < 0.001 |
| CD4 count at baseline | − 0.1 | 0.816 | 0.1 | 0.795 |
| Constant | 117.1 | < 0.001 | 88.8 | < 0.001 |
Months number of months after the baseline visit, BP blood pressure, eGFR estimated glomerular filtration rate
There was a drop in both systolic and mean arterial BP during the first 6 months among female participants. Systolic and mean BP values were higher among males and both main outcomes as well as weight at each visit showed a linear increase with an almost identical slope for both sexes after month 6 (see figure in the Graphical Abstract). The change in weight (weight at last visit – weight at month 6) was correlated with the change in systolic (r = 0.212; p < 0.001) and mean (r = 0.194; p < 0.001) arterial BP (see Fig. 1 and Fig. 2 in the Supplementary Information).
Fig. 2.
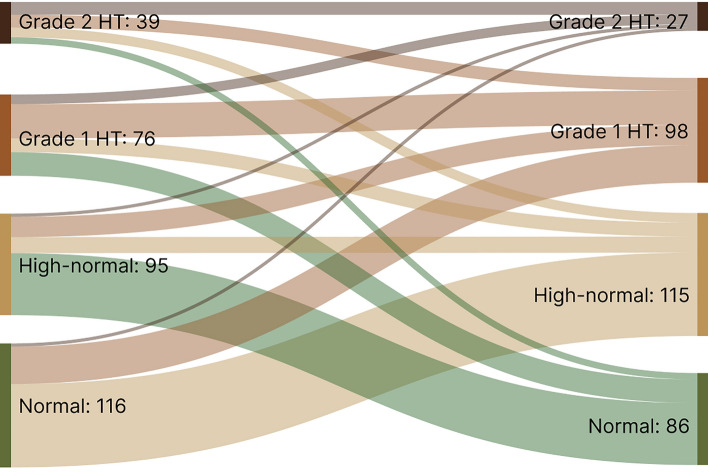
Blood pressure classification development over time (month 0 & 36) according to International Society of Hypertension categories. 473 (80.3%) unchanged observations of normotension are not shown in this figure, (https://sankeymatic.com/build/)
In bivariable analyses, sex, months (i.e., number of months after the baseline visit), and weight at each visit as well as baseline values for BP, age, weight, and log(eGFR) were associated with systolic as well as with mean arterial BP (Table 2). Multivariable analyses yielded similar results for both main outcomes and if stratified by sex (Table 3 and Table 4). In comparison to the bivariable analyses, the adjusted models showed smaller estimates for age at baseline, sex, and log(eGFR) at baseline, while higher weight at baseline changed to a negative effect. Weight at each visit resulted in similar estimates for all models showing an increase over time.
Table 3.
Multivariable multilevel linear regression (≥ 6 months) for systolic BP stratified by sex
| Variable | Both sexes | Females | Males | |||
|---|---|---|---|---|---|---|
| ß | p-value | ß | p-value | ß | p-value | |
| Months | − 0.1 | < 0.001 | − 0.1 | 0.081 | − 0.1 | 0.001 |
| Systolic BP at baseline | 0.4 | < 0.001 | 0.4 | < 0.001 | 0.3 | < 0.001 |
| Age at baseline | 0.1 | 0.004 | 0.1 | 0.007 | 0.1 | 0.201 |
| Sex | ||||||
| Females | Ref | < 0.001 | – | – | – | – |
| Males | 4.6 | – | – | – | – | |
| Weight at baseline | − 0.4 | < 0.001 | − 0.4 | < 0.001 | − 0.5 | < 0.001 |
| Weight at each visit | 0.6 | < 0.001 | 0.5 | < 0.001 | 0.8 | < 0.001 |
| log(eGFR) at baseline | − 6.2 | < 0.001 | − 7.3 | < 0.001 | − 4.8 | 0.008 |
| Constant | 81.4 | < 0.001 | 83.6 | < 0.001 | 83.1 | < 0.001 |
Months number of months after the baseline visit, BP blood pressure, eGFR estimated glomerular filtration rate
Table 4.
Multivariable multilevel linear regression (≥ 6 months) for mean arterial BP stratified by sex
| Variable | Both sexes | Females | Males | |||
|---|---|---|---|---|---|---|
| ß | p-value | ß | p-value | ß | p-value | |
| Months | 0.1 | 0.866 | 0.1 | 0.318 | − 0.1 | 0.468 |
| Mean arterial BP at baseline | 0.4 | < 0.001 | 0.4 | < 0.001 | 0.4 | < 0.001 |
| Age at baseline | 0.1 | 0.009 | 0.1 | 0.023 | 0.1 | 0.126 |
| Sex | ||||||
| Females | Ref | < 0.001 | – | – | – | – |
| Males | 2.8 | – | – | – | – | |
| Weight at baseline | − 0.3 | < 0.001 | − 0.2 | < 0.001 | − 0.3 | < 0.001 |
| Weight at each visit | 0.4 | < 0.001 | 0.3 | < 0.001 | 0.5 | < 0.001 |
| log(eGFR) at baseline | − 4.5 | < 0.001 | − 4.5 | < 0.001 | − 3.9 | 0.004 |
| Constant | 60.5 | < 0.001 | 59.9 | < 0.001 | 60.8 | < 0.001 |
Months number of months after the baseline visit, BP blood pressure, eGFR estimated glomerular filtration rate
At study entry (month 0), a BP ≥ 140/90 mmHg was found in 217 participants (16.8%) and 74 PLHIV reported a history of hypertension, of whom 37 were not taking antihypertensive drugs. BP values were always < 140/90 mmHg during the first three visits in 22 of these 37 PLHIV with self-reported, but untreated hypertension, hence they were defined as normotensive. The remaining 52 participants were considered as truly hypertensive and together with newly detected cases (n = 121) the prevalence of PLHIV with confirmed hypertension was 13.4% for the whole group at study entry (11.5% for females and 16.2% for males). During the observation time, 50 PLHIV normotensive at study entry were diagnosed with hypertension for the first time, resulting in an incidence of 4.5% over up to 36 months. The number of prevalent and incident cases of hypertension was higher among male participants, but there were also more males in the older age groups (Table 5).
Table 5.
Prevalent and incident cases of hypertension in female and male participants
| Age group [years] | Females | Males | Both sexes | ||||
|---|---|---|---|---|---|---|---|
| Prevalent cases [n] | Incident cases [n] | Total female [n] | Prevalent cases [n] | Incident cases [n] | Total male [n] | ||
| 18–24 | 4 | 1 | 96 | 1 | 0 | 21 | 117 |
| 25–34 | 13 | 7 | 322 | 11 | 8 | 181 | 503 |
| 35–44 | 30 | 9 | 225 | 40 | 12 | 220 | 445 |
| 45–54 | 29 | 7 | 89 | 21 | 4 | 81 | 170 |
| 55–64 | 8 | 0 | 16 | 12 | 1 | 27 | 43 |
| 65 + | 2 | 0 | 3 | 2 | 1 | 7 | 10 |
| Total | 86 | 24 | 751 | 87 | 26 | 537 | 1288 |
The change in the distribution of BP categories from baseline to month 36 is shown in Fig. 2 except for those whose BP remained normal (n = 473). Among these participants, the total number of PLHIV with a confirmed diagnosis of hypertension increased from 121 (15.1%) to 142 (17.8%). The number of patients on antihypertensive drug treatment nearly tripled (from 23 to 61); however, the rate of uncontrolled treated hypertension remained essentially unchanged: 17/23 PLHIV (73.9%) at study entry vs. 44/61 patients (72.1%) at month 36. Viral load at baseline did not differ between participants who finally had controlled vs. uncontrolled hypertension (28,832; 6,775–137,957 vs. 41,426; 7,794–149,943; p = 0.113). HIV replication was unsuppressed in one patient with controlled hypertension (on antihypertensive drug treatment) compared to three patients with uncontrolled hypertension (following lifestyle advice, but not on drug treatment). During the study, most hypertensive patients received antihypertensive monotherapy (n = 46, 85% on HCTZ), followed by free combinations of two (n = 24, 63% on ENA plus HCTZ), three (n = 10, 70% on ENA plus HCTZ plus AML), and four drugs, respectively (n = 3, ENA, HCTZ, AML plus ATEN). During 1,462 visits, modifications of antihypertensive drug regimens would have been indicated in 576 cases according to current guideline recommendations; however, the treating clinical officers and nurses adjusted the antihypertensive treatment during only 75 visits (13%).
Discussion
The principal findings of the present prospective cohort study are sex-related differences in BP change during the first 6 months, followed by continuously rising BP thereafter in PLHIV who had started tenofovir-based ART. Weight gain during follow-up and a higher BP level at study entry favored a rising BP, while a negative association was found with female sex, lower body weight, and high glomerular filtration rate at baseline. Finally, despite an increase in antihypertensive drug prescriptions, the rate of uncontrolled hypertension remained essentially unchanged.
Pathophysiologic mechanisms in hypertensive PLHIV include a state of chronic systemic inflammation, immune dysregulation, viral tropism, and microbial translocation [17, 18] as well as HIV-mediated activation of the sympathetic nervous system [28, 29]. Neuroendocrine hyperactivity and autonomic dysfunction may be partially explained by psychosocial stressors [30], especially among PLHIV from low- and-middle income countries who often live at the minimum level of subsistence. Direct ART effects may also play a role, since the prevalence of hypertension has been found to be higher in PLHIV on ART (14.5%-35.0%) compared to those who were ART naïve (10.5–16.9%) [19–21]. The latter is comparable to the 16.8% in the present study, when only the office BP measurements from the first visit (month 0) were analyzed. In a large cross-sectional, population-based study in rural and urban Malawi using BP data from BP measurements of only one visit [31], the national prevalence was 15.8%. However, it is well known that the prevalence of hypertension is overestimated when office BP data from only a single visit are used [32]. In the present study, the number of hypertensive cases was lower (13.4%), once several BP measurements over several weeks were considered as requested by international guidelines to confirm a diagnosis of hypertension [24, 25, 33]. As could be expected, the percentage of hypertensive postmenopausal women was higher than men from the same age groups [34]. The observed significant sex difference in the overall hypertension prevalence (11.5% in females vs. 16.2% in males; p < 0.001) in the LighTen Study might be explained by the fact that more than 85% of female PLHIV were premenopausal.
There are already several investigations addressing weight gain on ART [35–41], and existing studies estimate the weight gain on EFV- and TDF-containing regimens to be moderate, in the range of 2–3 kg over 96 weeks [35, 42]. In a prospective study from South Africa ART with TDF, FTC, and efavirenz, a weight gain of 1 kg had been observed after 48 weeks [42]. The increase in body weight among LighTen participants is comparable to these findings.
According to a meta-analysis including more than 2.3 million participants, the risk of hypertension increased continuously with increasing BMI or weight gain [43]. Cross-sectional studies have confirmed overweight or obesity as determinants of arterial hypertension in countries from sub-Saharan Africa including Malawi, in PLHIV [44–47]. The hypertension incidence per 100 patient-years in prospective studies of PLHIV from sub-Saharan Africa ranges from 5.4 to 16.0, respectively [48–52]. This large variation may be explained by methodological differences related to the technique of BP measurement. Thus, the lower rate in the study from South Africa [50] was based upon three BP measurements on at least two occasions, while the highest rate was obtained in Ethiopia from two measurements on only one occasion [48], emphasizing the need to follow the international guidelines for an accurate diagnosis of hypertension [24, 25, 33]. In contrast to the LighTen Study, patients received a wide range of different combinations of NNRTIs and NRTIs [49–52] in these studies, but also DTG-based ART regimen [48]. Kidney disease with impaired eGFR is strongly associated with hypertension in PLHIV [50, 51, 53], which in turn may explain why high eGFR at study entry was found to have the strongest protective impact in LighTen participants.
According to a systematic review with meta-analysis, less than 40% of hypertensive individuals in the general population of sub-Saharan Africa were diagnosed as such, less than 20% of those diagnosed received antihypertensive medication and in less than 10% of treated individuals BP was controlled, i.e., < 140/90 mmHg [4]. A similar pattern can be observed among hypertensive PLHIV from sub-Saharan Africa, where the rate of uncontrolled hypertension is very high, ranging from 61 to 85%, and the rates of hypertension awareness are typically < 30% [54–57]. According to previous findings from an approach integrating hypertension management into HIV care facilities in Malawi, the BP control rates after 6 months were 38% and 30% among PLHIV with hypertension grade 1 or 2, respectively [57], which is comparable to a control rate of 37% for those on antihypertensive drug treatment in the general population [28]. Studies using an intensified model of care with visits every 6 weeks until BP was controlled led to higher control rates of 47.4% [58]. However, a recently published study of Malawian PLHIV with a median age of 51.0 years who were on ART for a median duration of 5.9 years (78.5% on efavirenz/lamivudine/tenofovir) revealed a control rate of only 19% after 1 year of follow-up for those on antihypertensive drug treatment [59]. The authors identified self-reported non-adherence to BP lowering medications as the only factor significantly associated with uncontrolled hypertension in this patient cohort with > 95% viral suppression [59]. Besides patient-related factors such as non-adherence, the reasons for uncontrolled BP are manifold, including irregular supply of antihypertensive drugs with frequent changes in prescriptions (and hence reduced adherence), substandard quality of drugs, shortage of health workers and centers, and poverty in general [60, 61]. In addition, physician inertia is a well-known phenomenon in the case of treated, but uncontrolled hypertension [62]. The same phenomenon has been observed for treating clinical officers and nurses in the present LighTen Study with its low rate (13%) of treatment adjustments in patients with BP ≥ 140/90 mmHg during patient visits.
In sub-Saharan Africa, girls and young women are twice as likely to be living with HIV than men [63]. The age distribution in the current study from Malawi mirrors this observation from epidemiological surveys of the region. Systolic and mean arterial BP were nearly identical for male and female participants when entering the study, but a stepwise decrease in BP over the next 6 months was observed in female PLHIV. Most patients (> 90%) were recruited on the same day as the diagnosis of HIV infection was confirmed. For women, the confirmed diagnosis might be more stressful with increased anxiety, as the fear of stigma, discrimination, abandonment, partner violence, and economic pressure in raising their children are more prevalent among women in Africa [64–68]. Also, a white coat effect, which is associated with anxiety [69] and more common among women [70, 71], may have been attenuated with repeated visits [72], hence explaining the observed sex-related differences during the first 6 months.
Systolic and diastolic BP independently predict adverse cardiovascular outcomes, although systolic BP has a greater effect [73], especially in older patients [74], while MAP is probably a better predictor in people aged < 60 years [75]. Also, MAP had been used to analyze the decline in renal function in patients with chronic kidney disease [76] and was recently found to have the highest predictability in detecting hypertension-related cerebrovascular alterations compared to systolic or diastolic BP separately [77].
According to current European and American guidelines BP should be measured in both arms at the first visit and to use the arm with the higher value as reference to avoid misclassification of BP [24, 33]. Difficulties to measure BP in both arms in a busy outpatient department may arise due to lack of time and workforce; thus, the ISH deemed bilateral measurements not essential for low- and middle-income countries ISH [25]. In a large cross-sectional, population-based study in rural and urban Malawi, measurements always in the right arm were used [31] in contrast to the WHO Steps survey, where BP measurements had to be taken always in the left arm [78]. Inter-arm pressure differences are inconsistent [79] and often not reproducible [80]. The BP is typically higher in the right arm [79, 81] and this has been explained by the handedness of people: right arm systolic BP ≥ 5 mmHg higher than left arm in 64% of right-handed participants (1,730 of 2,692 healthy volunteers) and left arm systolic BP ≥ 5 mmHg higher than right arm in 62% of left-handed participants (211 of 338 healthy volunteers) with differences < 5 mmHg in 30% and 35%, respectively [82]. To avoid shifting measurement sites, BP measurements were always taken in the upper arm of the dominant hand of the LighTen participants, thereby reducing variability in the individual BP change over time. In view of the above-mentioned inter-arm differences, the number of cases whose BP category would differ due to this type of measurement seems negligible.
Strengths and limitations
The results of the LighTen Study are based on findings from a well-defined population with regular follow-up visits. Patients were included in the analysis as long as they received the same antiretroviral drug combination with TDF, FTC, and efavirenz they had initiated at study entry. No major differences were identified compared to non-enrolled PLHIV seen at the Lighthouse through the recruitment period. 65% of the study cohort completed the 36 months visit; thus, a third of patients has been lost to follow-up, mainly because they had defaulted two or more scheduled visits or had been transferred out to other HIV treatment centers. Again, compared to the whole cohort, no major differences in demographic data were identified. The initial weight gain for those who did not complete the study was not different from those who could be followed until 36 months (see Table 3 in the Supplementary Information). However, less increase after month 6 and finally a weight loss were observed for the former group. Patients who had died or needed a change in ART due to viral resistance had been included in this group. Thus, it cannot be excluded that the study findings are biased toward a “healthier” group of PLHIV, but once identified, they probably represent the target patient population for interventions aiming at cardiovascular risk reduction.
Only BP values ≥ 140/90 mmHg were rechecked by a qualified nurse or clinical officer in their offices. Since even attended automated BP measurements as used here are lower than conventional office BP values [83], this probably had not introduced a major rate of misclassified truly hypertensive BP categories. 24-h ambulatory BP monitoring (24-h-ABPM) was not available when recruitment for the LighTen Study started. However, when 24-h-ABPM was performed in a subgroup of 117 LighTen participants, a median of 26 months (6–8 visits) after enrollment, white coat hypertension or white coat effect was identified in 18.8% of those with office hypertension [84]. Self-reported hypertension could have been another important source of bias and is known to be considerably flawed [85], but patients without antihypertensive drug treatment and BP values < 140/90 mmHg were not included in the calculation of the prevalence of hypertension. Data on creatinine plasma levels were available only at study entry with no information on albuminuria; thus, the exact KDIGO classification of kidney function [86] was not possible. Also, since the changes in BP and the prevalence and incidence of hypertension were defined as secondary outcomes, the findings of the present study may only be considered as the results of a descriptive data analysis.
We did not address adherence to antihypertensive drug treatment directly, e.g., by pill counting and the inference from viral load suppression as a marker of good adherence may be equivocal. However, PLHIV on ART with undetectable HIV viral load had lower systolic BP among those diagnosed with hypertension [10]. HIV viral replication was suppressed already at study entry in 51 participants (4%); hence, they must have been pretreated, but the type of ART for these patients is unknown. This low number probably has no major impact on the results. Diabetes mellitus type 2 was known in 14 PLHIV with metformin given in 3 cases. Due to the low numbers, these participants were not separately analyzed. Patients were not screened for diabetes, thus, its true prevalence in this cohort is unknown. However, newly diagnosed diabetes needing glucose-lowering drugs was recently reported in only 1 of 100 overweight or obese PLHIV taking ART at the Lighthouse Clinic [87].
Conclusions
Weight gain during follow-up and initiating ART already at a higher BP level favored a further increase in BP in this cohort of PLHIV. The control rate of hypertension remained low despite increased antihypertensive treatment rates. Our data advocate the inclusion of patient education addressing weight control and antihypertensive treatment adherence as part of NCD management programs at centers caring for PLHIV in low-resources settings like Malawi. Together with intensified training of medical staff to overcome provider inertia in case of indicated treatment adjustments, improved control rates of hypertension might eventually be achieved.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors want to thank the participating patients and all members of the Lighthouse staff for their support throughout the duration of LighTen Study as well as Maik Brune from the Central Laboratory, University Hospital of Heidelberg, Germany. Melani Ratih Mahanani was supported by Deutscher Akademischer Austauschdienst (DAAD)/German Academic Exchange Service Research Grants: This work was supported by the Hector Foundation, Mannheim, Germany, Projekt M72.
Abbreviations
- AML
Amlodipine
- ART
Antiretroviral therapy
- ATEN
Atenolol
- BMI
Body mass index
- BP
Blood pressure
- CVD
Cardiovascular disease
- DTG
Dolutegravir
- EFV
Efavirenz
- eGFR
Estimated glomerular filtration rate
- ENA
Enalapril
- HCTZ
Hydrochlorothiazide
- NCD
Non-communicable diseases
- NNRTI
Non-nucleoside reverse transcriptase inhibitor
- NRTI
Nucleoside reverse transcriptase inhibitors
- PLHIV
People living with HIV
- 3TC
Lamivudine
- TDF
Tenofovir disoproxil fumarate
Author contributions
HMS, FN, HT, and SP conceptualized and designed the study. AN, AdF, TC, TH, and JC were responsible for the acquisition of data and management of patients. MRM and VW performed the statistical analysis and its estimates, MRM and PK generated figures, HMS, MRM, and PK drafted a first version of the manuscript. All authors contributed to the intellectual content, interpretation of data, critical revisions to the drafts of the paper, and approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the National Health Research Commission of the Ministry of Health, Malawi (Appr. No. NHSRC Protocol #1199) and the Ethical Committees of the involved Universities of Cologne (No. 14-251) and Heidelberg (No. S-293/2014).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Hans-Michael Steffen and Melani Ratih Mahanani: These authors share co-first authorship.
Volker Winkler and Sam Phiri: These authors share co-senior authorship.
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. Update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD Risk factors collaborators (2020) global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2019;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control. A systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ataklte F, Erqou S, Kaptoge S, Taye B, Echouffo-Tcheugui JB, Kengne AP. Burden of undiagnosed hypertension in Sub-Saharan Africa. System Rev Meta-Analysis Hyper. 2015;65:291–298. doi: 10.1161/HYPERTENSIONAHA.114.04394. [DOI] [PubMed] [Google Scholar]
- 5.Kaze AD, Schutte AE, Erqou S, Kengne AP, Echouffo-Tcheugui JB. Prevalence of hypertension in older people in Africa: a systematic review and meta-analysis. J Hypertens. 2017;35:1345–1352. doi: 10.1097/HJH.0000000000001345. [DOI] [PubMed] [Google Scholar]
- 6.NCD Risk Factor Collaboration Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD HIV Collaborators (2021) Global, regional, and national sex-specific burden and control of the HIV epidemic, 1990–2019, for 204 countries and territories: the Global Burden of Diseases Study 2019. Lancet HIV. 2019;8:e633–e651. doi: 10.1016/S2352-3018(21)00152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geldsetzer P, Manne-Goehler J, Bärnighausen T, Davies J. What research is needed to address the co-epidemics of HIV and cardiometabolic disease in sub-Saharan Africa? Lancet Diabetes Endocrinol. 2018;6:7–9. doi: 10.1016/S2213-8587(17)30091-8. [DOI] [PubMed] [Google Scholar]
- 9.van Heerden A, Barnabas R, Norris S, Micklesfield L, van Rooyen H, Celum C. High prevalence of HIV and non-communicable disease (NCD) risk factors in rural KwaZulu-Natal. 20. J Int AIDS Soc; 2017. p. e25012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manne-Goehler J, Siedner M, Montana L, et al. Hypertension and diabetes control along the HIV care cascade in rural South Africa. J Int AIDS Soc. 2019;22:e25213. doi: 10.1002/jia2.25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah A, Stelzle D, Lee K, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Systematic review and meta-analysis. Circulation. 2018;138:1100–1112. doi: 10.1161/CIRCULATIONAHA.117.033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsue P, Waters D. Time to recognize HIV infection as a major cardiovascular risk factor. Circulation. 2018;138:1113–1115. doi: 10.1161/CIRCULATIONAHA.118.036211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinstein M, Hsue P, Benjamin L, Bloomfield G, Currier J, Freiberg M, Grinspoon SK, Levin J, Longenecker CT, Post WS. Characteristics, prevention, and management of cardiovascular disease in people living with HIV. A scientific statement from the American Heart Association. Circulation. 2019;140:e98–e124. doi: 10.1161/CIR.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guwatudde D, Nankya-Mutyoba J, Kalyesubula R, et al. The burden of hypertension in sub-Saharan Africa: a four-country cross sectional study. BMC Public Health. 2015;15:1211. doi: 10.1186/s12889-015-2546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todowede O, Mianda S, Sartorius B. Prevalence of metabolic syndrome among HIV-positive and HIV-negative populations in sub-Saharan Africa - a systematic review and meta-analysis. Syst Rev. 2019;8:4. doi: 10.1186/s13643-018-0927-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.So-Armah K, Benjamin LA, Bloomfield GS, Feinstein MJ, Hsue P, Njuguna B, Freiberg MS. HIV and cardiovascular disease. Lancet HIV. 2020;7:e279–e293. doi: 10.1016/S2352-3018(20)30036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahme S, Bloomfield G, Peck R. Hypertension in HIV-infected adults. Novel Pathophysiologic Mechanisms Hypertension. 2018;72:44–55. doi: 10.1161/HYPERTENSIONAHA.118.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masenga S, Hamooya B, Nzala S, Kwenda G, Heimburger D, Mutale W, Munsaka SM, Koethe JR, Kirabo A. Patho-immune mechanisms of hypertension in HIV: a systematic and thematic review. Curr Hypertens Rep. 2019;21:56. doi: 10.1007/s11906-019-0956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nduka C, Stranges S, Sarki A, Kimani P, Uthman O. Evidence of increased blood pressure and hypertension risk among people living with HIV on antiretroviral therapy: a systematic review with meta-analysis. J Hum Hypertens. 2016;30:355–362. doi: 10.1038/jhh.2015.97. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens. 2017;11:530–540. doi: 10.1016/j.jash.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Bigna JJ, Ndoadoumgue AL, Nansseu JR, Tochie JN, Nyaga UF, Nkeck JR, Foka AJ, Kaze AD, Noubiap J. Global burden of hypertension among people with HIV in the era of increased life expectancy: a systematic review and meta-analysis. J Hypertens. 2020;38:1659–1668. doi: 10.1097/HJH.0000000000002446. [DOI] [PubMed] [Google Scholar]
- 22.Ojji D, Aifah A, Dulli L, Iwelunmor J. Early stakeholder engagement lessons from managing hypertension among people living wizh himan-immunodeficiency virus: an integrated model (MAP-IT) Eur Heart J. 2022;43:2347–2349. doi: 10.1093/eurheartj/ehac159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Department of HIV & AIDS (2014) Malawi guidelines for clinical management of HIV in children and adults. 2nd ed. Ministry of Health, Lilongwe.
- 24.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Hear J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 25.Unger T, Borghib C, Charcharc F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38:982–1004. doi: 10.1097/HJH.0000000000002453. [DOI] [PubMed] [Google Scholar]
- 26.MoH (2015) Malawi standard treatment guidelines. 5th ed. Ministry of Health, Lilongwe
- 27.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5:1003–1009. doi: 10.2215/CJN.06870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McIntosh RC. A meta-analysis of HIV and heart rate variability in the era of antiretroviral therapy. Clin Auton Res. 2016;26:287–294. doi: 10.1007/s10286-016-0366-6. [DOI] [PubMed] [Google Scholar]
- 29.Robinson-Papp J, Varuna A, Nmashie A, Sharma SK, Kim-Schulze S, Murray J, George MC, Morgello S, Mueller BR, Lawrence SA, Benn EKT. Sympathetic function and markers of inflammation in well-controlled HIV. Brain Behav Immun Health. 2020;7:100112. doi: 10.1016/j.bbih.2020.100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schutte AE, Botha S, Fourie CMT, Gafane-Matemane LF, Kruger R, Lammertyn L, Malan L, Mels CMC, Schutte R, Smith W, van Rooyen JM, Ware LJ, Huisman HW. Recent advances in understanding hypertension development in sub-Saharan Africa. J Hum Hypertens. 2017;31:491–500. doi: 10.1038/jhh.2017.18. [DOI] [PubMed] [Google Scholar]
- 31.Price AJ, Crampin AC, Amberbir A, et al. Prevalence of obesity, hypertension, and diabetes, and cascade of care in sub-Saharan Africa: a cross-sectional, population-based study in rural and urban Malawi. Lancet Diabetes Endocrinol. 2018;6:208–222. doi: 10.1016/S2213-8587(17)30432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modesti PA, Agostoni P, Agyemang C, et al. Cardiovascular risk assessment in low-resource settings: a consensus document of the European Society of Hypertension Working Group on hypertension and cardiovascular risk in low resource settings. J Hypertens. 2014;32:951–960. doi: 10.1097/HJH.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whelton P, Carey R, Aronow W, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ ASH/ ASPC/ NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 34.Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, Cheng S. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol. 2020;5:19–26. doi: 10.1001/jamacardio.2019.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71:1379–1389. doi: 10.1093/cid/ciz999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feigl AB, Bloom DE, Danaei G, et al. The effect of HIV and the modifying effect of anti-retroviral therapy (ART) on body mass index (BMI) and blood pressure levels in rural South Africa. PLoS ONE. 2016;11:e0158264. doi: 10.1371/journal.pone.0158264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guehi C, Badjé A, Gabillard D, et al. High prevalence of being overweight and obese HIV-infected persons, before and after 24 months on early ART in the ANRS 12136 Temprano Trial. AIDS Res Ther. 2016;13:12. doi: 10.1186/s12981-016-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mave V, Erlandson KM, Gupte N, et al. Inflammation and change in body weight with antiretroviral therapy initiation in a multinational cohort of HIV-infected adults. J Infect Dis. 2016;214:65–72. doi: 10.1093/infdis/jiw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huis in ʼt Veld D, Balestre E, Buyze Jet al (2015) Determinants of weight evolution among HIV-positive patients initiating antiretroviral treatment in low-resource settings. J Acquir Immune Defic Syndr 70:146-154. 10.1097/QAI.0000000000000691 [DOI] [PMC free article] [PubMed]
- 40.Erlandson KM, Kitch D, Tierney C, Sax PE, Daar ES, Tebas P, Melbourne K, Ha B, Jahed NC, McComsey CA. Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. AIDS. 2013;27:2069–2079. doi: 10.1097/QAD.0b013e328361d25d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reda AA, Biadgilign S, DeribewA GB, Deribe K. Predictors of change in CD4 lymphocyte count and weight among HIV infected patients on anti-retroviral treatment in Ethiopia: a retrospective longitudinal study. PLoS ONE. 2013;8:e58595. doi: 10.1371/journal.pone.0058595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381:803–815. doi: 10.1056/NEJMoa1902824. [DOI] [PubMed] [Google Scholar]
- 43.Jayedi A, Rashidy-Pour A, Khorshidi M, Shab-Bidars S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: a systematic review and dose-response meta-analysis of more than 2.3 million participants. Obes Rev. 2018;19:654–667. doi: 10.1111/obr.12656. [DOI] [PubMed] [Google Scholar]
- 44.Lubega G, Mayanja B, Lutaakome J, Abaasa A, Thomson R, Lindan C. Prevalence and factors associated with hypertension among people living with HIV/AIDS on antiretroviral therapy in Uganda. Pan Afr Med J. 2021;38:216. doi: 10.11604/pamj.2021.38.216.28034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dakum P, Avong YK, Okuma J, Sorungbe T, Jatau B, Nedmbi N, Odutola MK, Abimiku A, Mensah CO, Kayode GA. Prevalence and risk factors for obesity among elderly patients living with HIV/AIDS in a low-resource setting. Medicine (Baltimore) 2021;100:e25399. doi: 10.1097/MD.0000000000025399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauer S, Mwanza MW, Chilengi R, Holmes CB, Zyambo Z, Furrer H, Egger M, Wandeler G, Vinikoor MJ. Awareness and management of elevated blood pressure among human immunodeficiency virus-infected adults receiving antiretroviral therapy in urban Zambia: a call to action. Glob Health Action. 2017;10:1359923. doi: 10.1080/16549716.2017.1359923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okeke NL, Davy T, Eron JJ, Napravnik S. Hypertension among HIV-infected patients in clinical care, 1996–2013. Clin Infect Dis. 2016;63:242–248. doi: 10.1093/cid/ciw223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulugeta H, Afenigus AD, Haile D, Amha H, Kassa GM, Wubetu M, Abebaw E, Jara D. Incidence and predictors of hypertension among HIV patients receiving ART at public health facilities, northwest Ethiopia: a one-year multicenter prospective follow-up study. HIV AIDS (Auckl) 2021;13:889–901. doi: 10.2147/HIV.S329838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shamu T, Chimbetete C, Egger M, Mudzviti T. Treatment outcomes in HIV infected patients older than 50 years attending an HIV clinic in Harare, Zimbabwe: a cohort study. PLoS ONE. 2021;16:e0253000. doi: 10.1371/journal.pone.0253000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brennan AT, Jamieson L, Crowther NJ, et al. Prevalence, incidence, predictors, treatment, and control of hypertension among HIV-positive adults on antiretroviral treatment in public sector treatment programs in South Africa. PLoS ONE. 2018;13:e0204020. doi: 10.1371/journal.pone.0204020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodríguez-Arbolí E, Mwamelo K, Kalinjuma AV, Furrer H, Hatz C, Tanner M, Battegay M, Letang E. Incidence and risk factors for hypertension among HIV patients in rural Tanzania – A prospective cohort study. PLoS ONE. 2017;12:e0172089. doi: 10.1371/journal.pone.0172089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okello S, Kanyesigye M, Muyindike WR, Annex BH, Hunt PW, Haneuse S, Siedner MJ. Incidence and predictors of hypertension in adults with HIV-initiating antiretroviral therapy in south-western Uganda. J Hypertens. 2015;33:2039–2045. doi: 10.1097/HJH.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peck RN, Shedafa R, Kalluvya S, Downs JA, Todd J, Suthanthiran M, Fitzgerald DW, Kataraihja JB. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med. 2014;12:125. doi: 10.1186/s12916-014-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohamed SF, Uthman OA, Mutua MK, Asiki G, Abba MS, Gill P. Prevalence of uncontrolled hypertension in people with comorbidities in sub-Saharan Africa: a systematic review and meta-analysis. BMJ Open. 2021;11:e045880. doi: 10.1136/bmjopen-2020-045880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwarisiima D, Atukunda M, Owaraganise A, et al. Hypertension control in integrated HIV and chronic disease clinics in Uganda in the SEARCH study. BMC Public Health. 2019;19:511. doi: 10.1186/s12889-019-6838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hyle E, Bekker L, Martey E, Huang M, Xu A, Parker R, Walensky RP, Middelkoop K. Cardiovascular risk factors among ART-experienced people with HIV in South Africa. J Int AIDS Soc. 2019;22:e25274. doi: 10.1002/jia2.25274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel P, Speight C, Maida A, Loustalot F, Giles D, Phiri S, Gupta S, Raghunathan P. Integrating HIV and hypertension management in low-resource settings: lessons from Malawi. PLoS Med. 2018;15:e1002523. doi: 10.1371/journal.pmed.1002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heller DJ, Balzer LB, Kazi D, et al. Hypertension testing and treatment in Uganda and Kenya through the SEARCH study: an implementation fidelity and outcome evaluation. PLoS ONE. 2020;15:e0222801. doi: 10.1371/journal.pone.0222801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffman RM, Chibwana F, Kahn D, et al. High rates of uncontrolled blood pressure in Malawian adults living with HIV and hypertension. Glob Heart. 2021;16:81. doi: 10.5334/gh.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seedat Y. Why is control of hypertension in sub-Saharan Africa poor? Cardiovasc J Afr. 2015;26:193–195. doi: 10.5830/CVJA-2015-065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macquart de Terline D, Diop B, Bernard M, et al. Substandard drugs among five common antihypertensive generic medications: an analysis From 10 African countries. J Hypertens. 2018;36:395–401. doi: 10.1097/HJH.0000000000001560. [DOI] [PubMed] [Google Scholar]
- 62.Kollias A, Foukarakis E, Karakousis K, HYPEDIA Study Group Implementation of the 2018 ESC/ESH Guidelines for the management of hypertension in primary care: the HYPEDIA study. J Hum Hypertens. 2023;37:449–454. doi: 10.1038/s41371-022-00713-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.http://www.unaids.org/en/resources/fact-sheet. last Accessed Mar 26th, 2023
- 64.Parcesepe A, Tymejczyk O, Remien R, Gadisa T, Kulkarni SG, Hoffmann S, Melaku Z, Elul B, Nash D. HIV-related stigma, social support, and psychological distress among individuals initiating ART in Ethiopia. AIDS Behav. 2018;22:3815–3825. doi: 10.1007/s10461-018-2059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peltzer K, Pengpid S. Prevalence and associated factors of enacted, internalized and anticipated stigma among people living with HIV in South Africa: results of the first national survey. HIV AIDS (Auckl) 2019;11:275–285. doi: 10.2147/HIV.S229285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaynes B, Pence B, Eron J, Miller W. Prevalence and comorbidity of psychiatric diagnoses based on reference standard in an HIV+ patient population. Psychosom Med. 2008;70:505–511. doi: 10.1097/PSY.0b013e31816aa0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saadat M, Behboodi Z, Saadat E. Comparison of depression, anxiety, stress, and related factors among women and men with human immunodeficiency virus infection. J Hum Reprod Sci. 2015;8:48–51. doi: 10.4103/0974-1208.153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ivanova E, Hart T, Wagner A, Aljassem K, Loutfy M. Correlates of anxiety in women living with HIV of reproductive age. AIDS Behav. 2012;16:2181–2191. doi: 10.1007/s10461-011-0133-6. [DOI] [PubMed] [Google Scholar]
- 69.Jhalani J, Goyal T, Clemow L, Schwartz JE, Pickering TG, Gerin W. Anxiety and outcome expectations predict the white-coat effect. Blood Press Monit. 2005;10:317–319. doi: 10.1097/00126097-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 70.Tocci G, Presta V, Figliuzzi I, El Halabieh NA, Battistoni A, Coluccia R, D’Agostino M, Ferrucci A, Volpe M. Prevalence and clinical outcomes of white-coat and masked hypertension: analysis of a large ambulatory blood pressure database. J Clin Hypertens (Greenwich) 2018;20:297–305. doi: 10.1111/jch.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas O, Shipman KE, Day K, Thomas M, Martin U, Dasgupta I. Prevalence and determinants of white coat effect in a large UK hypertension clinic population. J Hum Hypertens. 2016;30:386–391. doi: 10.1038/jhh.2015.95. [DOI] [PubMed] [Google Scholar]
- 72.Stenehjem AE, Os I. Reproducibility of blood pressure variability, white-coat effect and dipping pattern in untreated, uncomplicated and newly diagnosed essential hypertension. Blood Press. 2004;13:214–224. doi: 10.1080/08037050410021432. [DOI] [PubMed] [Google Scholar]
- 73.Flint AC, Conell C, Ren X, Banki NM, Chan SL, Ra VA, Melles RB, Bhatt DL. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. New Engl J Med. 2019;381:243–251. doi: 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
- 74.Franklin SS, Lopez VA, Wong NA, Mitchell GF, Larson MG, Vasan RS, Levy D. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:243–250. doi: 10.1161/CIRCULATIONAHA.108.797936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sesso HD, Stampfer MJ, Rosner B, Hennekens CH, Gaziano JM, Manson JE. Glynn RJ (2000) Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension. 2000;36:801–807. doi: 10.1161/01.HYP.36.5.801. [DOI] [PubMed] [Google Scholar]
- 76.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G for the Modification of Diet in Renal Disease Study Group The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 77.Kandil H, Soliman A, Alghamdi NS, Jennings JR, El-Baz A. Using mean arterial pressure in hypertension diagnosis versus using either systolic or diastolic blood pressure measurements. Biomedicines. 2023;11:849. doi: 10.3390/biomedicines11030849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ministry of Health. Malawi National STEPwise Survey for non-communicable disease risk factors 2017 Report. Lilongwe: Ministry of Health; 2017
- 79.Eguchi K, Yacoub M, Jhalani J, Gerin W, Schwartz JE, Pickering TG. Consistency of blood pressure differences between the left and right arms. Arch Intern Med. 2007;167:388–393. doi: 10.1001/archinte.167.4.388. [DOI] [PubMed] [Google Scholar]
- 80.Duprez DA, Jacobs DR, Jr, Andrews LIB, Brumback LC, Denenberg JO, Mcclelland RL, Thomas IC, Criqui MH, Allison MA. Inter-arm systolic blood pressure difference: non-persistence and association with incident cardiovascular disease in the Multi-ethnic Study of Atherosclerosis. J Hum Hypertens. 2023;37:197–204. doi: 10.1038/s41371-022-00669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cassidy P, Jones K. A study of inter-arm blood pressure differences in primary care. J Hum Hypertens. 2001;15:519–522. doi: 10.1038/sj.jhh.1001224. [DOI] [PubMed] [Google Scholar]
- 82.Essa RA, Ahmed SK. Prevalence of inter-arm blood pressure difference among young healthy adults: results from a large cross-sectional study on 3235 participants. Ann Med Surg (Lond) 2022;70:103631. doi: 10.1016/j.amsu.2022.103631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paiva AMG, Mota-Gomes MA, Feitosa ADM, Azevedo TCP, Amorim NW, Mion D, Jr, Sposito AC, Nadruz W., Jr Differences in the diagnosis of high blood pressure using unattended and attended automated office blood pressure. J Hum Hypertens. 2022;36:370–372. doi: 10.1038/s41371-021-00593-6. [DOI] [PubMed] [Google Scholar]
- 84.Kasper P, Nhlema A, de Forest A, Tweya H, Chaweza T, Matanje Mwagomba B, Mula AM, Chiwoko J, Neuhann F, Phiri S, Steffen HM. 24-h-ambulatory blood pressure monitoring in sub-Saharan Africa: hypertension phenotypes and dipping patterns in Malawian HIV+ patients on antiretroviral therapy. Glob Heart. 2021;16:67. doi: 10.5334/gh.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gonçalves V, Andrade K, Carvalho K, Silva M, Pereira M, Galvao T. Accuracy of self-reported hypertension: a systematic review and meta-analysis. J Hypertens. 2018;36:970–978. doi: 10.1097/HJH.000000000000164. [DOI] [PubMed] [Google Scholar]
- 86.Levey AS, Eckardt KU, Dorman NM, Christiansen SL, Cheung M, Jadoul M, Winkelmayer WC. Nomenclature for kidney function and disease - executive summary and glossary from a Kidney disease: improving global outcomes (KDIGO) consensus conference. Eur Heart J. 2020;41:4592–4598. doi: 10.1093/eurheartj/ehaa650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumwenda T, Buleya S, Matanje B, Heller T, Phiri S, Neuhann F, Steffen HM. Cardiovascular risk assessment for primary prevention in Malawian HIV patients on ART: are measurements of glycated hemoglobin and lipids worth the effort? AIDS. 2021;35:997–999. doi: 10.1097/QAD.0000000000002822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.