Abstract
Aims
Sphingosine-1-phosphate (S1P) is a signaling lipid, which is involved in several cellular processes including cell growth, proliferation, migration and apoptosis. The associations of serum S1P levels with cardiac geometry and function are still not clear. We investigated the associations of S1P with cardiac structure and systolic function in a population-based sample.
Methods and results
We performed cross-sectional analyses of 858 subjects (467 men; 54.4%), aged 22 to 81 years, from a sub-sample of the population-based Study of Health in Pomerania (SHIP-TREND-0). We analyzed the associations of serum S1P with structural and systolic function left ventricular (LV) and left atrial (LA) parameters as determined by magnetic resonance imaging (MRI) using sex-stratified multivariable-adjusted linear regression models. In men, MRI data showed that a 1 µmol/L lower S1P concentration was associated with an 18.1 mL (95% confidence interval [CI] 3.66–32.6; p = 0.014) larger LV end-diastolic volume (LVEDV), a 0.46 mm (95% CI 0.04–0.89; p = 0.034) greater LV wall thickness (LVWT) and a 16.3 g (95% CI 6.55–26.1; p = 0.001) higher LV mass (LVM). S1P was also associated with a 13.3 mL/beat (95% CI 4.49–22.1; p = 0.003) greater LV stroke volume (LVSV), an 18.7 cJ (95% CI 6.43–30.9; p = 0.003) greater LV stroke work (LVSW) and a 12.6 mL (95% CI 1.03–24.3; p = 0.033) larger LA end-diastolic volume (LAEDV). We did not find any significant associations in women.
Conclusions
In this population-based sample, lower levels of S1P were associated with higher LV wall thickness and mass, larger LV and LA chamber sizes and greater stroke volume and work of the LV in men, but not in women. Our results indicate that lower levels of S1P were associated with parameters related with cardiac geometry and systolic function in men, but not in women.
Graphical abstract
Keywords: Left ventricular geometry and function, Left ventricular mass, Left ventricular hypertrophy, Sphingosine-1-phosphate
Introduction
The last decades brought significant advances in the therapy of cardiovascular diseases (CVDs) and in particular in the field of heart failure. On the other hand, the prevalence of cardiovascular (CV) morbidity and its consequent health loss burden is still increasing, which is attributable in particular to an aging population [1, 2]. CVDs remain the most common reason of morbidity and mortality in Europe, USA and Asia with a significant economic impact on health and social security systems [1, 2].
The ability of the heart muscle to adapt to either pathological or physiological conditions is known as cardiac plasticity [3]. In a pathophysiological perspective, adverse remodeling of the heart is characterized by modification of cardiac shape, size, structure and function [4]. Sphingosine-1-phosphate (S1P) seems to possess physiologic functions that might influence cardiac remodeling.
S1P is a bioactive sphingolipid, transducing its endocrine effects through a group of G-protein coupled cell surface sphingosine-1-phosphate receptors (S1PR 1–5) [5, 6] and is involved in essential cellular processes including cell growth, proliferation, migration and apoptosis [7]. The role of S1P in the field of CVDs is becoming increasingly investigated [8–16]. Previous studies showed, that lower S1P concentrations were associated with deleterious cardiovascular outcomes [12, 14]. Furthermore, S1P mediates protective mechanisms against ischemic and reperfusion injury in cardiomyocytes [8–10, 13, 17, 18], enhances myocardial regeneration after myocardial infarction [11, 19] and is even attributed to be partially responsible for the advantageous effects of ß-blockers in patients with heart failure and cardiac remodeling after myocardial infarction [16, 20]. Altogether, these previous findings support the hypothesis that alterations of serum S1P levels, mainly lower levels, might be related to deleterious cardiovascular outcomes.
To the best of our knowledge, no previous population-based study has investigated the association of S1P concentrations with heart geometry and function. Therefore, the aim of the present study was to investigate the relation between lower S1P and LV and LA parameters of structure and systolic function as assessed by magnetic resonance imaging (MRI) in a large population-based sample.
Materials and methods
Study population
The present cross-sectional analysis is based on data from the population-based Study of Health in Pomerania (SHIP). The study design and recruitment strategy have been described elsewhere in detail [21]. Our analyses were based on data obtained from a sub-sample of the second SHIP cohort (SHIP-TREND-0) established between 2008 and 2012 [22]. In brief, a stratified random sample of 8,826 adults, aged 20–79 years, was selected from the population of West Pomerania, the north-eastern region of Germany. Participation in the first SHIP-START cohort was an exclusion criterion. In total 4,420 subjects participated in SHIP-TREND-0 (response 50.1%). Among them, 957 subjects (427 women, 44.6%), aged 21 to 81 years, who were eligible and willing to undergo whole-body MRI participated in the cardiac MRI substudy (Supplementary Figure I).
We excluded participants with previous self-reported myocardial infarction or stroke (n = 17), pacemaker (n = 1), left bundle block (n = 1) and a LVEF lower than 40% as determined by MRI (n = 9). We also excluded participants with missing values for S1P (n = 61) or any of the covariables used in the regression models (n = 10) (Supplementary Figure I). The final analytical sample comprised 858 subjects (391 women; 45.6%), aged 22–81 years (individuals with good quality images for the LV, n = 838 and for the LA, n = 775) (Supplementary Figure I).
All study participants gave written informed consent. The study was approved by the ethics committee of the University of Greifswald [22] and complies with the Declaration of Helsinki.
Cardiac MRI
Cardiac MRI was performed on a 1.5-T MR system (Magnetom Avanto; Siemens Medical Systems, Erlangen, Germany) [23] with subjects in a supine position.
LV analysis was performed according to the post-processing guidelines of the Society for Cardiovascular Magnetic Resonance [24]. LV concentricity (LVC) was calculated as left ventricular mass (LVM)/left ventricular end-diastolic volume (LVEDV). Left ventricular stroke volume (LVSV), left ventricular stroke work (LVSW) [25], left ventricular cardiac output (LVCO) and left ventricular ejection fraction (LVEF) were calculated following the formulas described in the supplemental material.
For LA analysis, contours of end-diastolic and end-systolic endocardial borders were marked in transversal-axis in all phases. LA stroke volume (LASV), LA cardiac output (LACO) and LA ejection fraction (LAEF) were calculated following the formulas described in the supplemental material.
Serum sphingosine-1-phosphate
Blood samples were taken from the cubital vein and analyzed directly or stored at − 80 °C in the Integrated Research Biobank of the University Medicine Greifswald [26]. Serum S1P was quantified by liquid chromatography tandem mass spectrometry (LC–MS/MS) with minor modifications as previously described [12]. After addition of 20 μL of the internal standard (1 μmol/L S1P-d7 [Avanti Polar Lipids, Alabaster, AL, USA]) to 20 μL serum, proteins were precipitated and passed to centrifugation. The sample isolates were subjected to reverse-phase chromatography and positive electrospray ionization. After elution with a binary gradient S1P was quantified by MS/MS in the multiple reaction mode, monitoring the (M + H) S1P parent ion (m/z = 380) fragmentation to the daughter ion (m/z = 264). The internal standard S1P-d7 with the m/z 387 to 271 transition was used to correct for variations in sample preparation and instrument response. Calibration curves were generated to calculate absolute S1P concentrations in the serum sample and quality controls were included and accepted with a coefficient of variation below 15% [12, 27].
Interview, medical and laboratory examination
Information on age, sex, socio-economic variables and smoking status [28] was collected by trained and certificated medical staff during a standardized computer-assisted interview.
All participants underwent an extensive standardized medical examination, including anthropometric measurements and bioelectrical impedance analysis. Blood pressure was measured after a resting period of at least five minutes. Systolic and diastolic blood pressures as well as heart rate were measured three times on the right arm of seated subjects using an oscillometric digital blood pressure monitor (HEM-705CP, Omron Corporation, Tokyo, Japan) with an interval of three minutes between readings. The mean of the second and third measurements for the systolic and diastolic blood pressures and for the heart rate was calculated and used for the present analyses. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg and/or current self-reported use of any anti-hypertensive medication.
Additionally, fasting and non-fasting blood samples were obtained from all study participants [29], to determine glycated hemoglobin, glucose concentrations, total serum cholesterol, low and high density lipoprotein-cholesterol (LDL-C, HDL-C), serum creatinine and estimated glomerular filtration rate (eGFR).
Statistical analysis
To characterize the study sample, data was reported as the median (25th and 75th percentile) for continuous variables and as percentages for categorical variables stratified by tertiles of S1P and sex.
While the association of S1P concentrations with MRI determined LVM was not modified by age (p-value for interaction = 0.369), it was modified by sex (p-value for interaction = 0.013). Consequently, we decided to evaluate all the associations of S1P with LV and LA parameters stratified by sex and adjusted for age, body fat mass, body fat-free mass, height2.7, systolic blood pressure, use of antihypertensive medication, glycated hemoglobin, use of hypoglycemic medication, smoking status and estimated glomerular filtration rate (eGFR, calculated by the Chronic Kidney Disease-Epidemiology Collaboration [CKD-EPI] equation[30]). In order to evaluate the robustness of our findings in light of individuals that did not take part in the MRI examination, we performed inverse probability weighting[31], assuming a missing at random mechanism [32]. The inverse probability weights were calculated in logistic regression models with participation in the MRI examination as outcome and sociodemographic and health-related variables as predictors. We used fractional polynomials to test potential non-linear relationships between S1P levels and the outcome variables [33].
In sensitivity analyses, we explored the associations of S1P with LVM stratified by hypertension (yes/no) and by smoking status (never, former or current smoker). Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg and/or current self-reported use of any anti-hypertensive medication (Anatomical Therapeutic Chemical code C02, C03, C07, C08 and C09).
A two-sided p-value p < 0.05 was considered as statistically significant. Statistical analyses were performed using Stata 17.0 (Stata Corporation, College Station, TX, USA).
Results
Table 1 shows descriptive data of the study participants stratified by sex-specific tertiles of serum S1P concentrations. The median age was similar between the tertiles for men and women. While the use of hypertensive medication was higher in the first tertile than in the other groups for men, it was similar in all groups in women. The levels of total cholesterol, LDL-cholesterol and HDL-cholesterol were lower in the first tertile than in the other groups for men, but the use of lipid-lowering medication was also higher in this group, when compared to the others. In women the use of lipid-lowering medication was higher in the first tertile group than the others, but there was no significant difference regarding the cholesterol levels. Men also showed a lower eGFR in the first tertile than the others groups. All other characteristics did not differ relevantly between the groups for men and women.
Table 1.
Characteristics of the study sample stratified by tertiles of serum sphingosine-1-phosphate (S1P) levels and sex (n = 858)
| Parameter | First tertile | Second tertile | Third tertile | Total | p-value* | |
|---|---|---|---|---|---|---|
| N (%) | Men | 156 (54.4) | 156 (54.6) | 155 (54.4) | 467 (54.4) | |
| Women | 131 (45.6) | 130 (45.5) | 130 (45.6) | 391 (45.6) | ||
| Sphingosine-1-phosphate (µM) | Men | 0.65 (0.60, 0.69) | 0.81 (0.77, 0.84) | 0.97 (0.93, 1.06) | 0.81 (0.69, 0.93) | |
| Women | 0.66 (0.60, 0.70) | 0.82 (0.79, 0.85) | 1.00 (0.93, 1.09) | 0.82 (0.70, 0.93) | ||
| Age (years) | Men | 52 (39, 65) | 48 (40, 59) | 47 (40, 49) | 49 (40, 60) | 0.145 |
| Women | 52 (52, 61) | 51 (39, 58) | 49 (40, 59) | 50 (40, 59) | 0.412 | |
| Total body weight (kg) | Men | 87.7 (80.3, 96.6) | 85.3 (77.8, 95.4) | 85.9 (78.0, 96.6) | 86.4 (78.5, 96.3) | 0.375 |
| Women | 68.4 (61.1, 78.9) | 70.2 (63.9, 79.1) | 72.7 (64.1, 82.0) | 70.1 (63.0, 79.8) | 0.164 | |
| Body fat-free mass (kg) | Men | 67.0 (62.7, 72.2) | 66.9 (61.7, 71.0) | 66.0 (60.4, 72.8) | 66.7 (61.6, 72.2) | 0.688 |
| Women | 46.0 (43.8, 50.5) | 47.0 (43.9, 50.5) | 47.4 (44.2, 51.2) | 46.9 (43.9, 50.7) | 0.717 | |
| Body fat mass (kg) | Men | 21.4 (16.4, 25.3) | 19.4 (16.2, 24.5) | 20.0 (16.4, 25.1) | 20.1 (16.3, 25.1) | 0.234 |
| Women | 21.8 (17.1, 28.6) | 23.4 (17.9, 30.8) | 24.8 (18.5, 32.3) | 23.0 (18.0, 30.5) | 0.063 | |
| Height (cm) | Men | 177 (173, 182) | 179 (174, 183) | 177 (173, 181) | 178 (173, 182) | 0.361 |
| Women | 164 (159, 169) | 165 (161, 169) | 164 (160, 168) | 164 (159, 169) | 0.326 | |
| Body mass index (kg/m2) | Men | 28.2 (25.8, 30.5) | 27.3 (24.9, 30.1) | 27.4 (25.0, 30.0) | 27.7 (25.3, 30.2) | 0.097 |
| Women | 25.7 (22.6, 28.9) | 25.8 (22.8, 30.6) | 26.7 (23.8, 30.5) | 26.0 (23.1, 30.1) | 0.219 | |
| Waist circumference (cm) | Men | 95.9 (88.5, 104) | 93.4 (86.6, 103) | 93.6 (87.0, 103) | 94.0 (87.4, 103) | 0.192 |
| Women | 80.0 (73.0, 89.7) | 80.0 (73.5, 92.0) | 81.3 (76.0, 90.2) | 81.0 (73.8, 90.5) | 0.550 | |
| Waist-to-height ratio | Men | 0.55 (0.49, 0.58) | 0.53 (0.48, 0.58) | 0.53 (0.50, 0.58) | 0.54 (0.49, 0.58) | 0.122 |
| Women | 0.50 (0.44, 0.54) | 0.49 (0.44, 0.56) | 0.50 (0.46, 0.56) | 0.50 (0.45, 0.55) | 0.532 | |
| Systolic blood pressure (mmHg) | Men | 132 (125, 142) | 133 (123, 142) | 135 (125, 145) | 133 (124, 143) | 0.583 |
| Women | 116 (106, 127) | 116 (108, 127) | 119 (109, 131) | 117 (108, 128) | 0.333 | |
| Diastolic blood pressure (mmHg) | Men | 78 (73, 85) | 80 (75, 86) | 81 (74, 88) | 80 (74, 86) | 0.243 |
| Women | 74 (67, 79) | 74 (68, 81) | 74 (69, 81) | 74 (68, 81) | 0.236 | |
| Hypertension (%) | Men | 53.9 | 44.2 | 50.3 | 50.5 | 0.228 |
| Women | 38.9 | 30.8 | 33.1 | 34.3 | 0.358 | |
| Antihypertensive medication (%) | Men | 37.2 | 23.7 | 21.9 | 27.6 | 0.004 |
| Women | 32.1 | 22.3 | 26.2 | 29.9 | 0.201 | |
| Glycated hemoglobin (%) | Men | 5.3 (4.9, 5.5) | 5.3 (5.0, 5.7) | 5.3 (5.1, 5.7) | 5.3 (4.9, 5.6) | 0.159 |
| Women | 5.1 (4.7, 5.4) | 5.2 (4.8, 5.5) | 5.3 (4.9, 5.5) | 5.2 (4.8, 5.5) | 0.107 | |
| Type 2 diabetes mellitus (%) | Men | 10.9 | 6.41 | 7.74 | 8.35 | 0.375 |
| Women | 7.63 | 6.15 | 4.62 | 6.14 | 0.629 | |
| Hypoglycemic medication (%) | Men | 6.41 | 3.85 | 1.94 | 4.07 | 0.146 |
| Women | 3.05 | 0.00 | 0.00 | 1.02 | 0.036 | |
| Total cholesterol (mmol/l) | Men | 5.20 (4.50, 6.00) | 5.30 (4.70, 6.10) | 5.50 (4.80, 6.20) | 5.30 (4.70, 6.10) | 0.026 |
| Women | 5.50 (5.00, 6.20) | 5.50 (4.60, 6.30) | 5.40 (4.90, 6.20) | 5.50 (4.90, 6.20) | 0.941 | |
| LDL-cholesterol (mmol/l) | Men | 3.34 (2.73, 3.86) | 3.43 (2.86, 3.96) | 3.59 (2.85, 4.06) | 3.44 (2.83, 3.98) | 0.046 |
| Women | 3.33 (2.84, 3.77) | 3.25 (2.63, 4.14) | 3.42 (2.79, 4.00) | 3.33 (2.76, 3.95) | 0.485 | |
| HDL-cholesterol (mmol/l) | Men | 1.25 (1.08, 1.45) | 1.30 (1.11, 1.51) | 1.33 (1.13, 1.52) | 1.28 (1.11, 1.49) | 0.053 |
| Women | 1.59 (1.29, 1.87) | 1.60 (1.38, 1.83) | 1.61 (1.36, 1.87) | 1.60 (1.35, 1.86) | 0.802 | |
| Total cholesterol/HDL-C ratio | Men | 4.18 (3.53, 4.83) | 4.17 (3.37, 5.05) | 4.29 (3.37, 4.96) | 4.19 (3.41, 4.93) | 0.957 |
| Women | 3.37 (2.89, 4.29) | 3.32 (2.73, 4.15) | 3.51 (2.86, 4.17) | 3.38 (2.86, 4.17) | 0.569 | |
| Hypercholesterolemic (%) | Men | 43.6 | 45.5 | 44.5 | 44.5 | 0.943 |
| Women | 38.2 | 35.4 | 32.3 | 35.3 | 0.612 | |
| Lipid-lowering medication (%) | Men | 14.1 | 6.41 | 5.16 | 8.57 | 0.013 |
| Women | 9.16 | 1.54 | 3.08 | 4.60 | 0.011 | |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | Men | 90.2 (79.3, 103) | 94.9 (81.9, 105) | 94.6 (86.1, 107) | 93.9 (81.9, 105) | 0.039 |
| Women | 93.5 (82.6, 104) | 92.6 (79.5, 102) | 92.4 (84.8, 105) | 91.7 (82.6, 104) | 0.650 | |
| Smoking (%) | Men | |||||
| Never | 33.3 | 32.7 | 32.9 | 33.0 | ||
| Current | 16.7 | 22.4 | 28.4 | 22.5 | ||
| Former | 50.0 | 44.9 | 38.7 | 44.5 | 0.136 | |
| Women | ||||||
| Never | 51.9 | 51.5 | 40.8 | 48.1 | ||
| Current | 21.4 | 20.0 | 26.9 | 22.8 | ||
| Former | 26.7 | 28.5 | 32.3 | 29.2 | 0.350 |
Data are expressed as median 25th and 75th percentile (continuous data) or percentage (categorical data)
A p-value p < 0.05 was considered as statistically significant and therefore highlighted in bold
*p-values are based on the chi-squared test (for cells with less than 10 individuals the p-values are based on the Fisher´s exact test) for categorical variables and the Kruskal–Wallis tests for continuous variables
Reversion of the x-axis scale for serum S1P levels
While a previous study[27] of our research group, designed to define reference values with a sub-sample of 1339 healthy participants from the SHIP-TREND-0 cohort, showed that S1P concentrations were not associated with aging for men and women, the analyses of the whole population sample with all subjects with S1P measurements (n = 4194) showed that older age was related to lower values of S1P in both men and women (Supplementary Figure II), probably as a result of the presence of unhealthy participants. In line with that, the core objective of our study were the associations of lower values of S1P concentrations with parameters of cardiac geometry and systolic function, after adjustment for age and other covariates. Accordingly, all relations between S1P and heart variables were plotted with a reverse x-axis to permit a more intuitive analysis of the results.
Associations of S1P values with structural parameters of LV
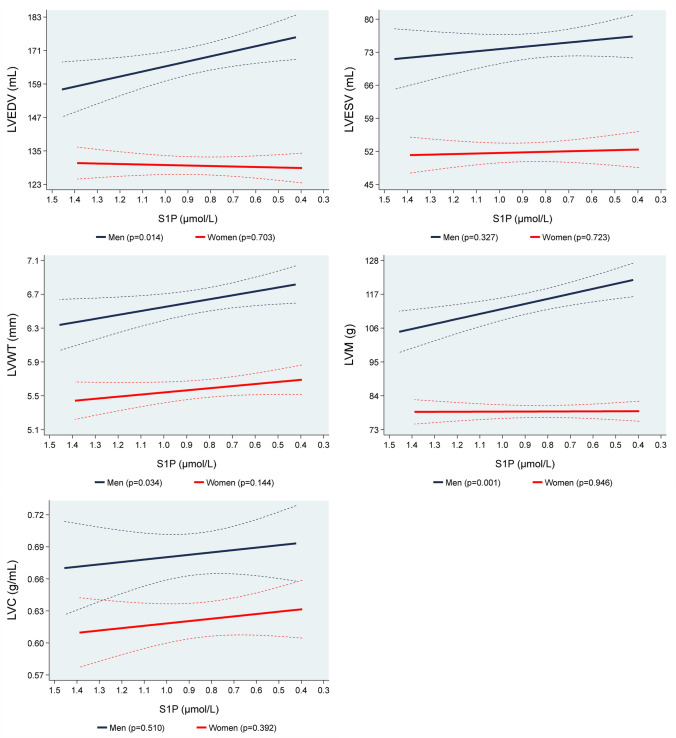
Figure 1 shows the associations of S1P concentrations with MRI determined LVEDV, LVWT, LVM and LVC. In multivariable adjusted regression analyses we observed statistically significant inverse associations of S1P concentrations with LVEDV, LVWT and LVM for men, while none of these associations was found for women. In detail, in men, a 1 µmol/L lower S1P concentration was associated with an 18.1 mL (95% confidence interval [CI] 3.66–32.6; p = 0.014) bigger LVEDV, a 0.46 mm (95% CI 0.04–0.89; p = 0.034) higher LVWT and a 16.3 g (95% CI 6.55–26.1; p = 0.001) greater LVM (Table 2, Supplemental Figure III). We could not show significant associations of S1P concentrations with LVESV and LVC for both sexes.
Fig. 1.
Adjusted* line (95% CI) showing the associations between sphingosine-1-phosphate (S1P) with mean magnetic resonance imaging determined left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), left ventricular wall-thickness (LVWT), left ventricular mass (LVM) and left ventricular concentricity (LVC) stratified by sex (men = 457; women = 381). *Linear regression adjusted for age, body fat mass, body fat-free mass, height2.7, systolic blood pressure, use of antihypertensive medication, glycated hemoglobin, use of hypoglycemic medication, smoking status and eGFR. Data was weighted according to subjects that did not take part in the MRI examination
Table 2.
Adjusted* ß-coefficient (95%-CI) of the associations of S1P with magnetic resonance imaging determined leftventricular and leftatrial cardiac geometry and function parameters stratified by sex (LV: men = 457; women = 381, LA: men = 421; women = 354)
| Parameter | Men ß-coefficient (95% CI), p-value |
Women ß-coefficient (95% CI), p-value |
|---|---|---|
| Left ventricular end-diastolic volume (mL) | − 18.1 (− 32.6 to − 3.66), p = 0.014 | 1.78 (− 7.34 to 10.9), p = 0.703 |
| Left ventricular end-systolic volume (mL) | − 4.63 (− 13.9 to 4.63), p = 0.327 | − 1.19 (− 7.77 to 5.40), p = 0.723 |
| Left ventricular wall-thickness (mm) | − 0.46 (− 0.89 to − 0.04), p = 0.034 | − 0.25 (− 0.58 to 0.09), p = 0.144 |
| Left ventricular mass (g) | − 16.3 (− 26.1 to − 6.55), p = 0.001 | − 0.21 (− 6.37 to 5.94), p = 0.946 |
| Left ventricular concentricity (g/mL) | − 0.02 (− 0.09 to 0.04), p = 0.510 | − 0.02 (− 0.07 to 0.03), p = 0.392 |
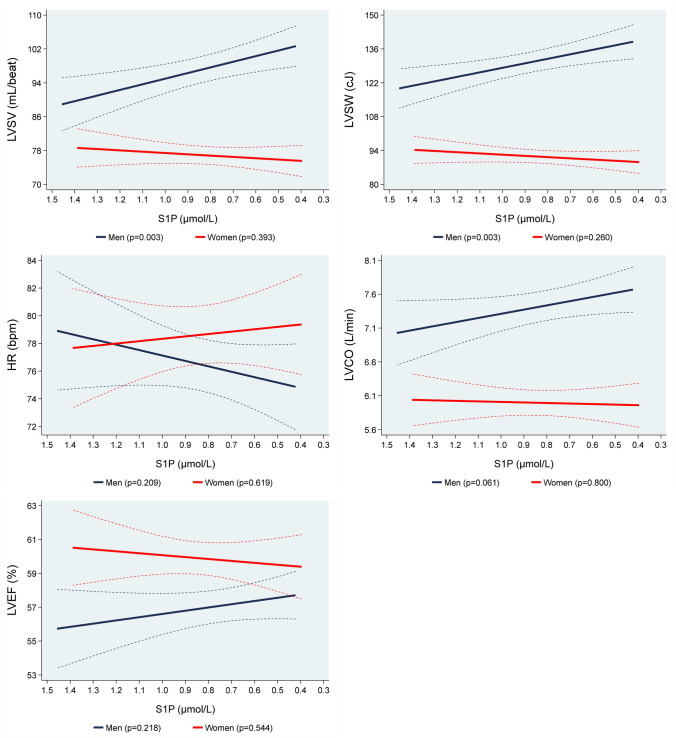
| Left ventricular stroke volume (mL/beat) | − 13.3 (− 22.1 to − 4.49), p = 0.003 | 3.10 (− 4.03 to 10.2), p = 0.393 |
| Left ventricular stroke work (cJ) | − 18.7 (− 30.9 to − 6.43), p = 0.003 | 5.10 (− 3.78 to 13.9), p = 0.260 |
| Heart rate (bpm) | 3.91 (− 2.20 to 10.0), p = 0.209 | − 1.72 (− 8.52 to 5.10), p = 0.619 |
| Left ventricular cardiac output (L/min) | − 0.62 (− 1.27 to 0.03), p = 0.061 | 0.08 (− 0.53 to 0.70), p = 0.800 |
| Left ventricular ejection fraction (%) | − 1.91 (− 5.00 to 1.13), p = 0.218 | 1.14 (− 2.55 to 4.82), p = 0.544 |
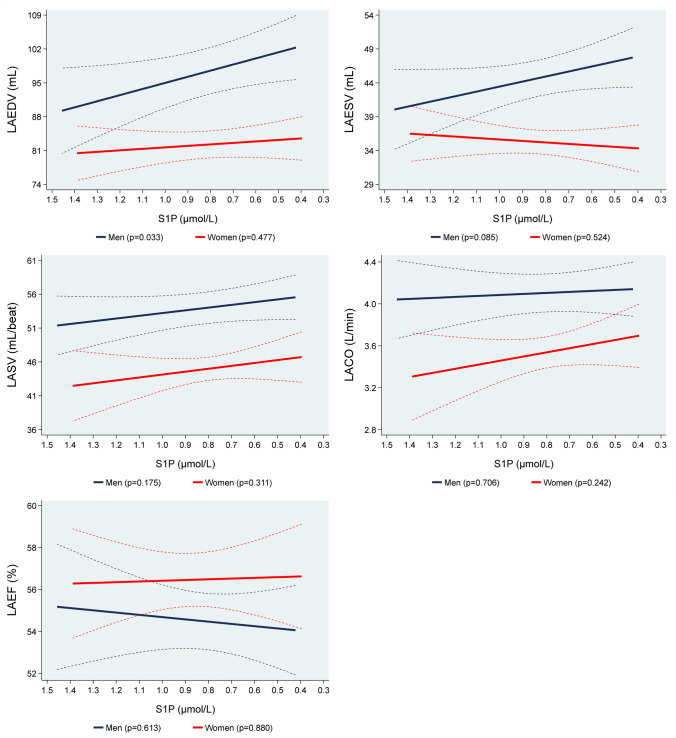
| Left atrial end-diastolic volume (mL) | − 12.6 (− 24.3 to − 1.03), p = 0.033 | − 3.07 (− 11.6 to 5.41), p = 0.477 |
| Left atrial end-systolic volume (mL) | − 7.42 (− 15.9 to 1.03), p = 0.085 | 2.19 (− 4.60 to 8.95), p = 0.524 |
| Left atrial stroke volume (mL/beat) | − 4.03 (− 9.85 to 1.80), p = 0.175 | − 4.29 (− 12.6 to 4.02), p = 0.311 |
| Left atrial cardiac output (L/min) | − 0.10 (0.59 to 0.40), p = 0.706 | − 0.39 (− 1.05 to 0.27), p = 0.242 |
| Left atrial ejection fraction (%) | 1.08 (− 3.12 to 5.28), p = 0.613 | − 0.34 (− 4.82 to 4.13), p = 0.880 |
A p-value p < 0.05 was considered as statistically significant and therefore highlighted in bold
*Linear regression adjusted for age, body fat mass, body fat-free mass, height2.7, systolic blood pressure, use of antihypertensive medication, glycated hemoglobin, use of hypoglycemic medication, smoking status and eGFR. Data was weighted according to subjects that did not take part in the MRI examination
In sensitivity analyses, in men, we found that a 1 µmol/L lower S1P concentration was associated with a 21.6 g (95% CI 6.46–36.7; p = 0.005) higher LVM in hypertensive individuals, but there was no association in normotensive subjects (p = 0.306). There were no associations of S1P levels with LVM for both hypertensive and normotensive women. Likewise, in men, we observed that a 1 µmol/L lower S1P concentration was associated with a 27.9 g (95% CI 9.76–46.0; p = 0.003) and a 17.3 g (95% CI 1.73–32.8; p = 0.030) greater LVM in current smokers and formers smokers, respectively, but there was no association in never smokers (p = 0.270). There were no associations of S1P levels with LVM regarding smoking status in women. We also performed sensitive analyses to evaluate the associations of S1P with LVM stratified by menopausal status. Both premenopausal and postmenopausal women had no significant associations. Noteworthy, the analyses groups became too small after stratification, which might have influenced the results.
Associations of S1P values with systolic parameters of LV
While we found inverse associations of S1P concentrations with LVSV and LVSW in men, we did not observe associations of these parameters in women. Specifically, in men, a 1 µmol/L lower S1P concentration was associated with a 13.3 mL/beat (95% CI 4.49–22.1; p = 0.003) higher LVSV and an 18.7 cJ (95% CI 6.43–30.9; p = 0.003) higher LVSW (Table 2, Supplemental Figure IV). There were no associations of S1P concentrations with HR, LVCO and LVEF for both sexes (Fig. 2).
Fig. 2.
Adjusted* line (95% CI) showing the associations between sphingosine-1-phosphate (S1P) with mean magnetic resonance imaging determined left ventricular stroke volume (LVSV), left ventricular stroke work (LVSW), heart rate (HR), left ventricular cardiac output (LVCO) and left ventricular ejection fraction (LVEF) stratified by sex (men = 457; women = 381). *Linear regression adjusted for age, body fat mass, body fat-free mass, height2.7, systolic blood pressure, use of antihypertensive medication, glycated hemoglobin, use of hypoglycemic medication, smoking status and eGFR. Data was weighted according to subjects that did not take part in the MRI examination
Associations of S1P values with structural and systolic parameters of LA
After multivariable regression analyses we found a statistically significant inverse association of S1P concentrations with LAEDV in men, but not in women. In detail, in men, a 1 µmol/L lower S1P concentration was associated with a 12.6 mL (1.03–24.3; p = 0.033) bigger LAEDV (Table 2, Supplemental Figure V). There were no associations of S1P concentrations with LAESV, LASV, LACO and LAEF for both men and women (Fig. 3).
Fig. 3.
Adjusted* line (95% CI) showing the associations between sphingosine-1-phosphate (S1P) with mean magnetic resonance imaging left atrial end-diastolic volume (LAEDV), left atrial end-systolic volume (LAESV), left atrial stroke volume (LASV), left atrial cardiac output (LACO) and left atrial ejection fraction (LAEF) stratified by sex (men = 421; women = 354). *Linear regression adjusted for age, body fat mass, body fat-free mass, height2.7, systolic blood pressure, use of antihypertensive medication, glycated hemoglobin, use of hypoglycemic medication, smoking status and eGFR. Data was weighted according to subjects that did not take part in the MRI examination
Discussion
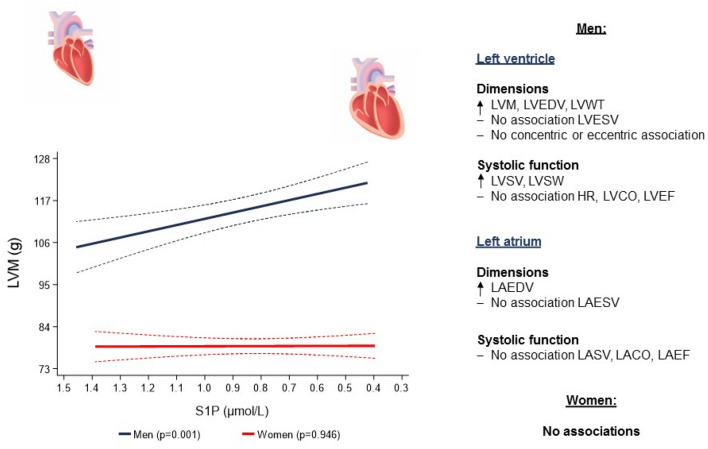
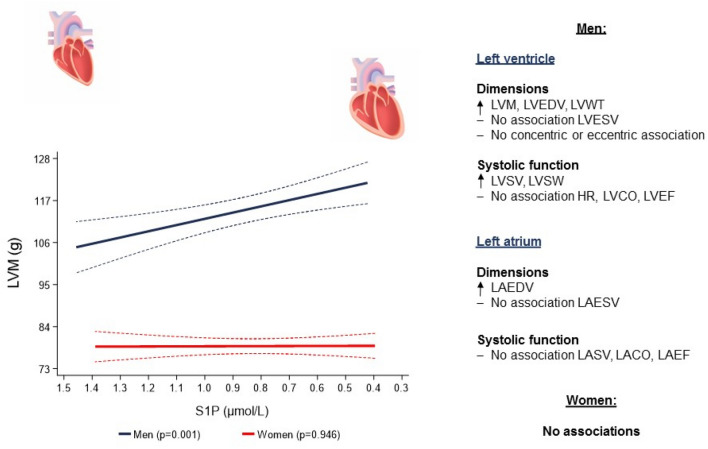
In our community-based sample, we found inverse associations of S1P concentrations with structural and systolic function LV and LA parameters. Importantly, these associations were sex-specific and detectable only in men, but not in women. Specifically, we found that, lower S1P concentrations were associated with larger LV and LA volumes, thicker LVWT and higher LVM, LVSV and LVSW (Fig. 4).
Fig. 4.
Associations of sphingosine-1-phosphate levels with left atrial and left ventricular geometry and systolic function parameters
In the context of the published literature
While there were some studies [34, 35] that described that higher S1P levels were related with hazardous outcomes, several other analyses [11, 12, 14, 15, 36], in agreement with our findings, showed the opposite, i.e. that lower S1P concentrations were associated with pathophysiologic clinical conditions.
A previous animal study [36], with pressure-overloaded cardiomyocytes, showed that the use of S1P could inhibit cardiomyocyte autophagy, thus preventing cardiomyocyte hypertrophy and consequently, protecting the cardiac function, mainly through the activation of the S1PR1.
A clinical study [14] among 74 patients (68% men, mean age of 73 years) with ischemic heart disease showed that lower S1P levels were related to a lower LVEF, as assessed by echocardiography, and a higher severity of heart failure determined by NYHA class. Our findings did not confirm the associations of S1P levels with LVEF or LVCO in both men and women. In this context, however, it must be considered that our sample excluded participants with compromised LV function, which indicate that our results reflect a subclinical stage. Moreover, we used a population-based study with a higher number of participants, a broad age range, sex-specific analysis and the use of MRI to determine the cardiac parameters which is considered to be a more accurate method as compared to echocardiography [37, 38].
Potential mechanisms for the observed associations
The associations of lower S1P levels with cardiac geometry and systolic function parameters might be the result of shared multiple risk factors and comorbidities, such as older age, obesity, hypertension, type 2 diabetes and smoking which can explain these associations as parallel relations rather than direct ones. On the other hand, we have adjusted for many risk factors in our multivariable regression models with no significant modification of our results, which might advocate an independent association of lower S1P levels with these cardiac parameters. Our findings were only significant in men, which suggest that sex hormones might have an important influence, particularly estrogen, which is known for cardioprotective effects. Interestingly, ovariectomized rats experience a decrease of expression of sphingosine kinases 1 and 2, the key enzymes involved in S1P synthesis, alongside with decrease S1P concentrations in aortic tissue [39]. This phenotype was rescued by estradiol valerate treatment. On the other hand, in our sensitive analyses, both premenopausal and postmenopausal women had no significant associations of S1P with LVM.
The role of S1P in heart (patho) physiology is complex. Synthesis of S1P involves endothelial cells, thrombocytes, erythrocytes and other cells, being transported by various carriers including albumin, LDL- and HDL-cholesterol [40]. Activation of the S1P receptor 3 promotes cardiac fibroblast proliferation, but decreases collagen secretion [41, 42]. S1P inhibits proliferation of several muscular cell types via S1P receptor 2 activation and induces cell differentiation, e.g. to cardiomyocytes from of human mesenchymal stem cells [43–45]. Acute administration of the S1PR1, 3–5 agonist fingolimod (FTY720) induces bradycardia [46], but chronic administration in mouse models of heart transplantation or pressure overload protects against cardiac fibrosis, possibly by functional antagonism of S1PR1 in immune cells [47, 48]. Moreover, S1P plays a substantial role in regulation of blood pressure and vascular tone [49, 50]. S1P leads to a strong production of the endothelial nitric oxide (NO) synthase (eNOS)-derived NO in a similar extent like the vascular endothelial growth factor and bradykinin [50]. Mechanistically, S1P-depended activation of S1PR1, the predominant receptor-subtype in endothelial cells, leads through stimulation of the PI3K/Akt/eNOS pathway to endothelial release of NO[51] and a subsequent vasodilatation. Accordingly, lower S1P concentrations might result in decreased NO-liberation and facilitated endothelial dysfunction with a consequent increase in vascular tone. This would result in a raise of the cardiac afterload and subsequent boost of the LVSW. The concomitant increase in the myocardial wall tension would lead to an expand in the LVWT and LVEDV, with an accompanying enlargement of the LVSV and LAEDV, resulting in an enhanced LVM. Interestingly, our findings in sensitivity analyses, that S1P levels were associated with LVM in hypertensive and smoker men might suggest that the associations of low S1P levels could be accentuated in clinical conditions related with endothelial dysfunction.
Study limitations
Our study has some limitations that are needed to be mentioned. Our study only consisted of Caucasians, therefore, extrapolation to other ethnicities is not appropriate. Since we have a non-random subsample, we cannot exclude a selection bias. Although our cardiac MRI dataset provided detailed information on structural parameters and on parameters of systolic function, we have no data regarding diastolic function. Additionally, while we have adjusted as best as possible for confounding factors, causal assumptions have still to be done with caution due to the cross-sectional study design and the possibility of residual confounding. Future longitudinal studies and replication might help to elucidate these associations.
Notwithstanding, our study has also some important strengths including the large sample size, the standardized assessment of MRI with detailed measurement of cardiac geometry and function and the possibility to adjust for multiple metabolic risk factors like body fat mass, body fat-free mass, glycated hemoglobin, that were available in our study.
Conclusions
Our results indicate that lower levels of S1P were associated with parameters reflecting cardiac geometry and systolic function in men. Specifically, in this population-based sample, lower levels of S1P were associated with higher LV wall thickness and mass, larger LV and LA chamber sizes and greater stroke volume and work of the LV in men, but not in women. Further experimental and clinical studies investigating the S1P signaling pathway in cardiovascular pathology should in particular be vigilant on sex differences and impact of sex hormones.
Perspectives
Clinical competencies
In our study we demonstrated that lower S1P concentrations were associated with larger chamber sizes and higher wall-thickness, stroke volume, stroke work and mass of the left heart in men. We believe these results indicate that S1P might be associated with cardiac geometry and systolic function parameters in men.
Translational outlook
Future longitudinal studies are necessary to substantiate the associations of S1P and heart geometry, as well as its sexual dimorphism that we found. Since most experimental studies investigating S1P are only performed with male animals [7], future studies should include male and female individuals, that would be exposed to conditions leading to modifications of cardiac shape, size, structure and function. A more mechanistic design, such as genetic receptor knockout or use of S1PR- agonist/antagonist substances might clarify causal pathways and possible pharmacological interventions.
Acknowledgements
The authors wish to thank Francisco Couto, who drew the hearts’ graphic figures used in Fig. 4 and in the Graphical abstract.
Abbreviations
- CKD-EPI
Chronic kidney disease epidemiology collaboration
- CV
Cardiovascular
- CVDs
Cardiovascular diseases
- eGFR
Estimated glomerular filtration rate
- HR
Heart rate
- LA
Left atrial
- LACO
Left atrial cardiac output
- LAEDV
Left atrial end-diastolic volume
- LAEF
Left atrial ejection fraction
- LAESV
Left atrial end-systolic volume
- LASV
Left atrial stroke volume
- LV
Left ventricular
- LVC
Left ventricular concentricity
- LVCO
Left ventricular cardiac output
- LVEDV
Left ventricular end-diastolic volume
- LVEF
Left ventricular ejection fraction
- LVESV
Left ventricular end-systolic volume
- LVM
Left ventricular mass
- LVSV
Left ventricular stroke volume
- LVSW
Left ventricular stroke work
- LVWT
Left ventricular wall thickness
- MRI
Magnetic resonance imaging
- SHIP
Study of Health in Pomerania
- S1P
Sphingosine-1-phosphate
- S1PR
Sphingosine-1-phosphate-receptor
Funding
Open Access funding enabled and organized by Projekt DEAL. The Study of Health in Pomerania (SHIP) is part of the Community Medicine Research net (CMR) (http://www.medizin.uni-greifswald.de/icm) of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg-West Pomerania. MRI scans in SHIP-2 and SHIP-TREND-0 have been supported by a joint grant from Siemens Healthineers, Erlangen, Germany and the Federal State of Mecklenburg-West Pomerania. This study was carried out in collaboration with the German Centre for Cardiovascular Research (DZHK) and the German Center for Diabetes Research (DZD), which are supported by the German Federal Ministry of Education and Research (BMBF).
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to data protection aspects but are available in an anonymized form from the corresponding author on reasonable request.
Declarations
Conflict of interest
None declared.
References
- 1.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358(13):1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 4.Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21(5):365–371. doi: 10.1016/j.carpath.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Hodun K, Chabowski A, Baranowski M. Sphingosine-1-phosphate in acute exercise and training. Scand J Med Sci Sports. 2021;31(5):945–955. doi: 10.1111/sms.13907. [DOI] [PubMed] [Google Scholar]
- 6.Means CK, Brown JH. Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc Res. 2009;82(2):193–200. doi: 10.1093/cvr/cvp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jozefczuk E, Guzik TJ, Siedlinski M. Significance of sphingosine-1-phosphate in cardiovascular physiology and pathology. Pharmacol Res. 2020;156:104793. doi: 10.1016/j.phrs.2020.104793. [DOI] [PubMed] [Google Scholar]
- 8.Karliner JS, et al. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33(9):1713–1717. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- 9.Egom EE, et al. Activation of Pak1/Akt/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am J Physiol Heart Circ Physiol. 2011;301(4):H1487–H1495. doi: 10.1152/ajpheart.01003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in the heart: a decade of progress. Biochim Biophys Acta. 2013;1831(1):203–212. doi: 10.1016/j.bbalip.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klyachkin YM, et al. Pharmacological elevation of circulating bioactive phosphosphingolipids enhances myocardial recovery after acute infarction. Stem Cells Transl Med. 2015;4(11):1333–1343. doi: 10.5966/sctm.2014-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soltau I, et al. Serum-sphingosine-1-phosphate concentrations are inversely associated with atherosclerotic diseases in humans. PLoS One. 2016;11(12):e0168302. doi: 10.1371/journal.pone.0168302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yung BS, et al. Selective coupling of the S1P(3) receptor subtype to S1P-mediated RhoA activation and cardioprotection. J Mol Cell Cardiol. 2017;103:1–10. doi: 10.1016/j.yjmcc.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polzin A, et al. Plasma sphingosine-1-phosphate concentrations are associated with systolic heart failure in patients with ischemic heart disease. J Mol Cell Cardiol. 2017;110:35–37. doi: 10.1016/j.yjmcc.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Feuerborn R, et al. Elevating endogenous sphingosine-1-phosphate (s1p) levels improves endothelial function and ameliorates atherosclerosis in low density lipoprotein receptor-deficient (LDL-R-/-) mice. Thromb Haemost. 2018;118(8):1470–1480. doi: 10.1055/s-0038-1666870. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang J, et al. The role of sphingosine 1-phosphate and its receptors in cardiovascular diseases. J Cell Mol Med. 2020;24(18):10290–10301. doi: 10.1111/jcmm.15744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in cardioprotection. J Cardiovasc Pharmacol. 2009;53(3):189–197. doi: 10.1097/FJC.0b013e3181926706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theilmeier G, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114(13):1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 19.Kuang Y, et al. Vascular endothelial S1pr1 ameliorates adverse cardiac remodelling via stimulating reparative macrophage proliferation after myocardial infarction. Cardiovasc Res. 2021;117(2):585–599. doi: 10.1093/cvr/cvaa046. [DOI] [PubMed] [Google Scholar]
- 20.Cannavo A, et al. β(1)-blockade prevents post-ischemic myocardial decompensation via β(3)AR-dependent protective sphingosine-1 phosphate signaling. J Am Coll Cardiol. 2017;70(2):182–192. doi: 10.1016/j.jacc.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Völzke H, et al. (2022) Cohort profile update: the study of health in pomerania (SHIP). Int J Epidemiol. [DOI] [PubMed]
- 22.Volzke H, et al. Cohort profile: the study of health in Pomerania. Int J Epidemiol. 2011;40(2):294–307. doi: 10.1093/ije/dyp394. [DOI] [PubMed] [Google Scholar]
- 23.Bulow R, et al. Reference ranges of left ventricular structure and function assessed by contrast-enhanced cardiac MR and changes related to ageing and hypertension in a population-based study. Eur Radiol. 2018;28(9):3996–4005. doi: 10.1007/s00330-018-5345-y. [DOI] [PubMed] [Google Scholar]
- 24.Schulz-Menger J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: society for cardiovascular magnetic resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schramm W. The units of measurement of the ventricular stroke work: a review study. J Clin Monit Comput. 2010;24(3):213–217. doi: 10.1007/s10877-010-9234-4. [DOI] [PubMed] [Google Scholar]
- 26.Winter T, et al. (2020) The integrated research biobank of the university medicine greifswald. Open J Bioresour 7
- 27.Moritz E, et al. Reference intervals for serum sphingosine-1-phosphate in the population-based Study of Health in Pomerania. Clin Chim Acta. 2017;468:25–31. doi: 10.1016/j.cca.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Maziak W, et al. Ten-year trends in smoking behaviour among adults in southern Germany. Int J Tuberc Lung Dis. 2002;6(9):824–830. [PubMed] [Google Scholar]
- 29.Baumeister SE, et al. Impact of fatty liver disease on health care utilization and costs in a general population: a 5-year observation. Gastroenterology. 2008;134(1):85–94. doi: 10.1053/j.gastro.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hogan JW, Roy J, Korkontzelou C. Handling drop-out in longitudinal studies. Stat Med. 2004;23(9):1455–1497. doi: 10.1002/sim.1728. [DOI] [PubMed] [Google Scholar]
- 32.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 33.Sauerbrei W, et al. Multivariable regression model building by using fractional polynomials: description of SAS, STATA and R programs. Comput Stat Data Anal. 2006;50(12):3464–3485. doi: 10.1016/j.csda.2005.07.015. [DOI] [Google Scholar]
- 34.Kowalski GM, et al. Plasma sphingosine-1-phosphate is elevated in obesity. PLoS One. 2013;8(9):e72449. doi: 10.1371/journal.pone.0072449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deutschman DH, et al. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146(1):62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 36.Chen YZ, et al. Sphingosine 1 phosphate receptor-1 (S1PR1) signaling protects cardiac function by inhibiting cardiomyocyte autophagy. J Geriatr Cardiol. 2018;15(5):334–345. doi: 10.11909/j.issn.1671-5411.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellenger NG, et al. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2(4):271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 38.Bellenger NG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21(16):1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, et al. The imbalance in the aortic ceramide/sphingosine-1-phosphate rheostat in ovariectomized rats and the preventive effect of estrogen. Lipids Health Dis. 2020;19(1):95. doi: 10.1186/s12944-020-01279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daum G, et al. Determinants of serum- and plasma sphingosine-1-phosphate concentrations in a healthy study group. TH Open. 2020;4(1):e12–e19. doi: 10.1055/s-0040-1701205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takuwa N, et al. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc Res. 2010;85(3):484–493. doi: 10.1093/cvr/cvp312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benamer N, et al. Electrophysiological and functional effects of sphingosine-1-phosphate in mouse ventricular fibroblasts. Biochem Biophys Res Commun. 2011;408(1):6–11. doi: 10.1016/j.bbrc.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 43.Grabski AD, et al. Sphingosine-1-phosphate receptor-2 regulates expression of smooth muscle alpha-actin after arterial injury. Arterioscler Thromb Vasc Biol. 2009;29(10):1644–1650. doi: 10.1161/ATVBAHA.109.191965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medlin MD, et al. Sphingosine 1-phosphate receptor 2 signals through leukemia-associated RhoGEF (LARG), to promote smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2010;30(9):1779–1786. doi: 10.1161/ATVBAHA.110.209395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang L, et al. Synergistic effect of bioactive lipid and condition medium on cardiac differentiation of human mesenchymal stem cells from different tissues. Cell Biochem Funct. 2016;34(3):163–172. doi: 10.1002/cbf.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmouder R, et al. FTY720: placebo-controlled study of the effect on cardiac rate and rhythm in healthy subjects. J Clin Pharmacol. 2006;46(8):895–904. doi: 10.1177/0091270006289853. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed N, et al. Sphingosine 1-phosphate receptor modulator fingolimod (FTY720) attenuates myocardial fibrosis in post-heterotopic heart transplantation. Front Pharmacol. 2017;8:645. doi: 10.3389/fphar.2017.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W, et al. A novel immunomodulator, FTY-720 reverses existing cardiac hypertrophy and fibrosis from pressure overload by targeting NFAT (nuclear factor of activated T-cells) signaling and periostin. Circ Heart Fail. 2013;6(4):833–844. doi: 10.1161/CIRCHEARTFAILURE.112.000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cantalupo A, et al. Nogo-B regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure. Nat Med. 2015;21(9):1028–1037. doi: 10.1038/nm.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cantalupo A, et al. S1PR1 (sphingosine-1-phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension. 2017;70(2):426–434. doi: 10.1161/HYPERTENSIONAHA.117.09088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, et al. Endothelial S1pr1 regulates pressure overload-induced cardiac remodelling through AKT-eNOS pathway. J Cell Mol Med. 2020;24(2):2013–2026. doi: 10.1111/jcmm.14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to data protection aspects but are available in an anonymized form from the corresponding author on reasonable request.