Abstract
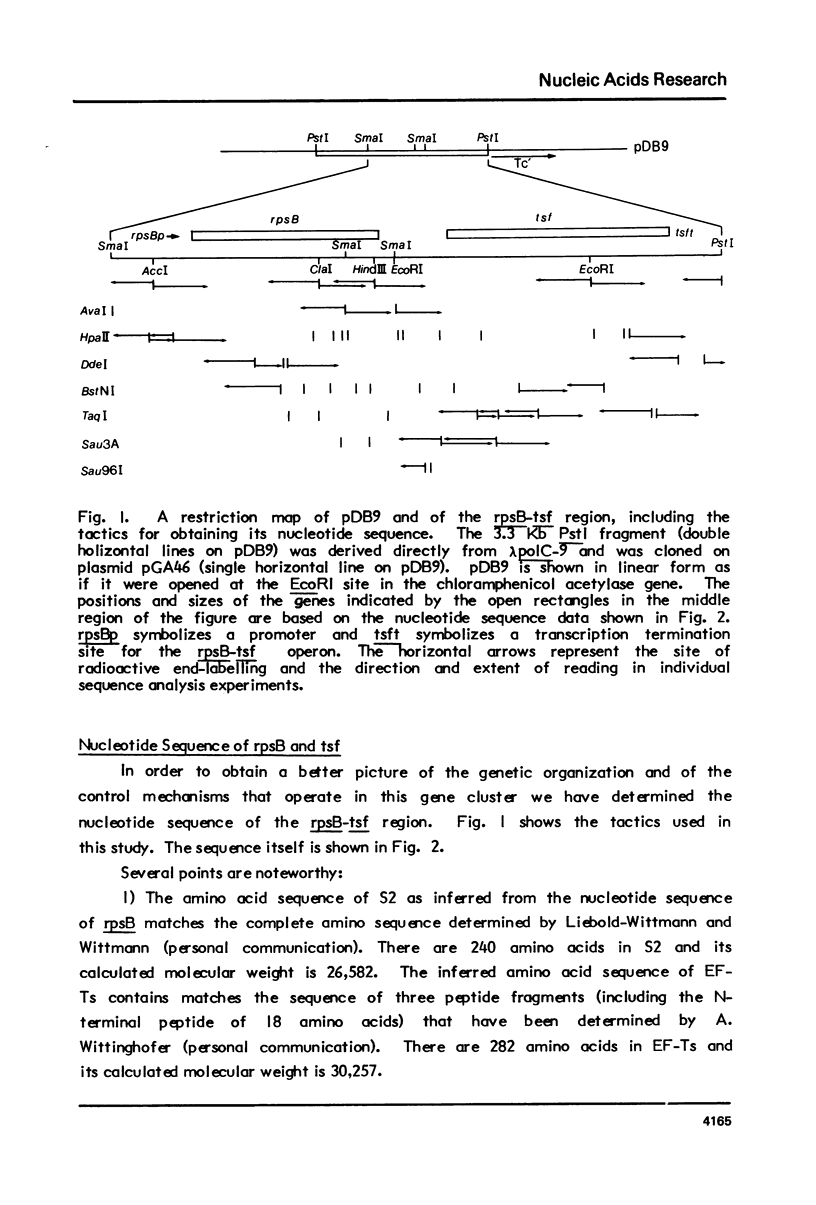
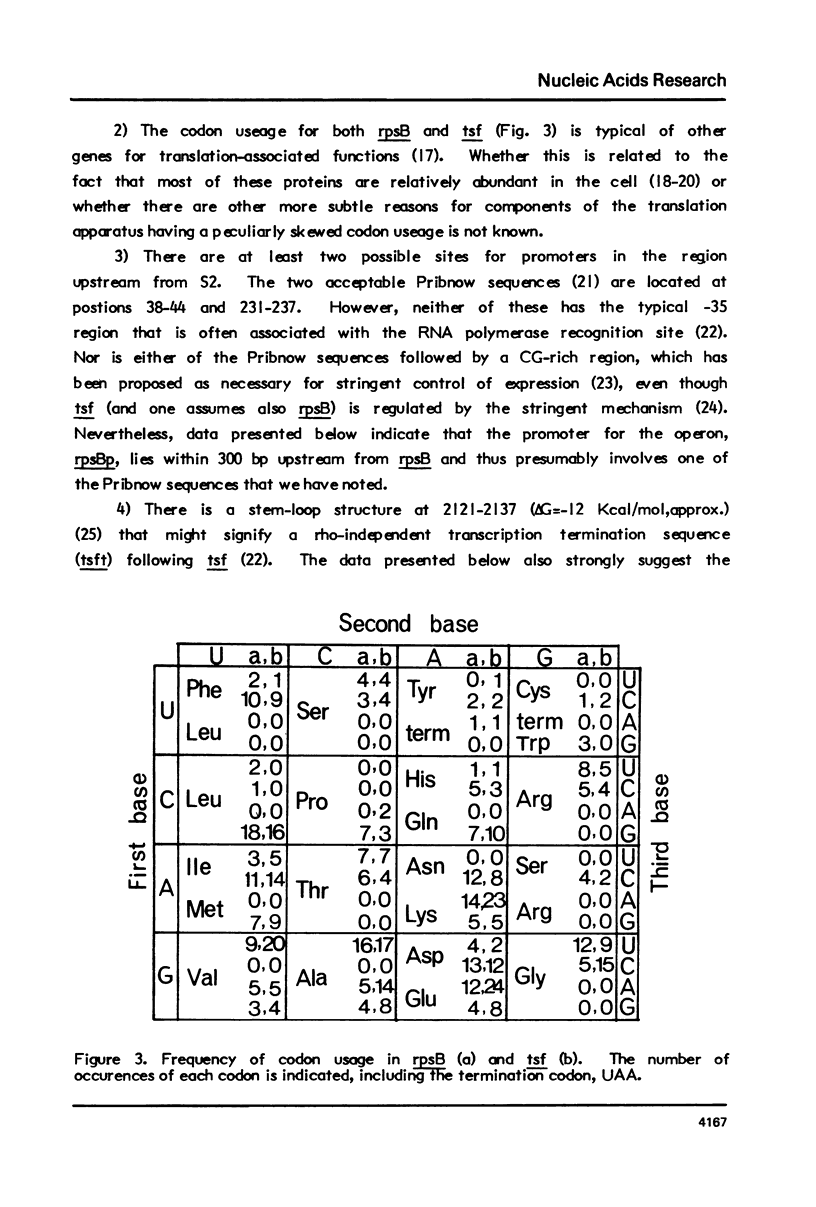
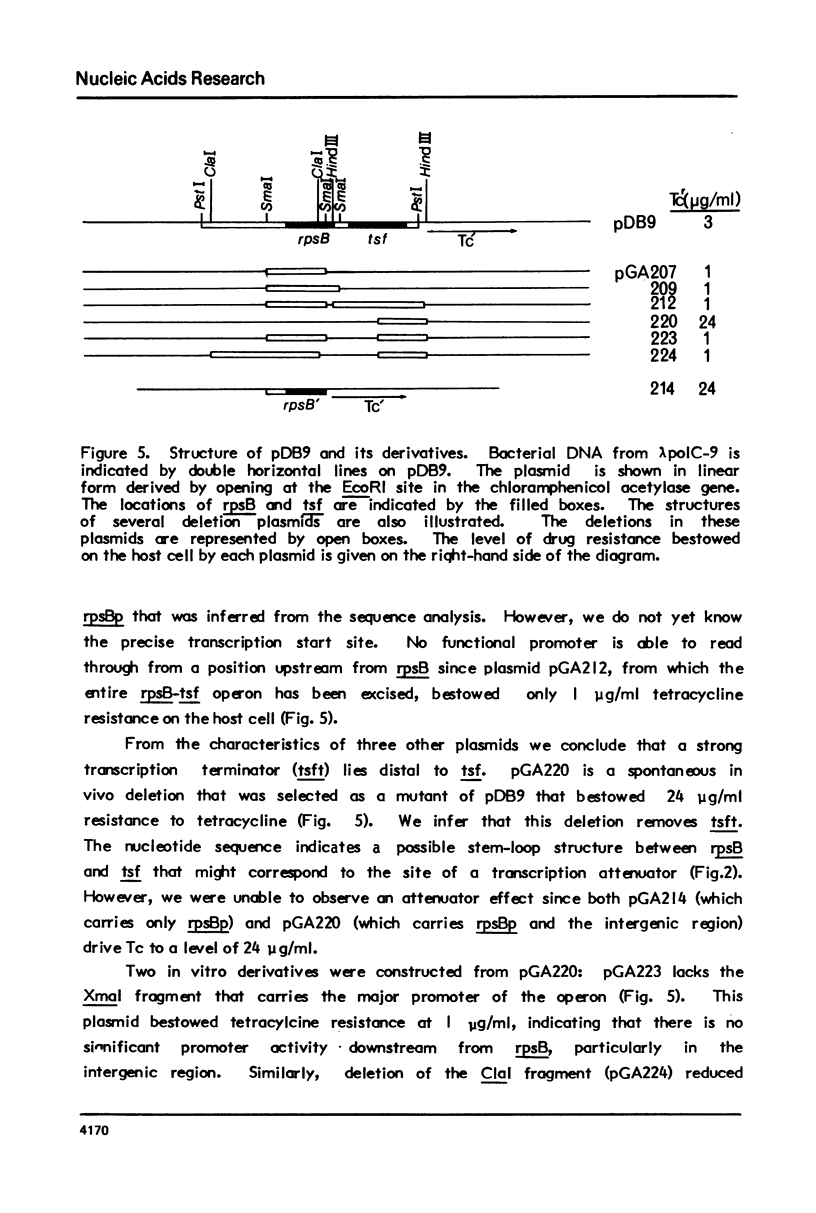
We report the nucleotide sequence of the four min region of the Escherichia coli genetic map that includes the genes for ribosomal protein S2 (rpsB) and translation elongation factor EF-Ts (tsf), and the possible location of regulatory sites within this two gene operon. The data indicate that the gene order is: rpsBp-rpsB-tsf-tsft. One potential regulatory site is a 16 nucleotide sequence in the rpsB leader region encompassing the ribosome binding site and the translation initiation codon. This has a high degree of homology with nucleotides 8 through 23 on the 5' end of 165 ribosomal RNA, and might signify a sequence that is necessary for post-transcriptional control of rpsB expression. The data allow one to infer the amino acid sequences of S2 and EF-Ts.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. Characterization of promoter-cloning plasmids: analysis of operon structure in the rif region of Escherichia coli and isolation of an enhanced internal promoter mutant. J Bacteriol. 1980 Dec;144(3):904–916. doi: 10.1128/jb.144.3.904-916.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G., Friesen J. D. Plasmid vehicles for direct cloning of Escherichia coli promoters. J Bacteriol. 1979 Nov;140(2):400–407. doi: 10.1128/jb.140.2.400-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Squires C., Squires C. L. Attenuation and processing of RNA from the rplJL--rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3331–3335. doi: 10.1073/pnas.77.6.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt A., Ebel J. P. The secondary structure of the protein L1 binding region of ribosomal 23S RNA. Homologies with putative secondary structures of the L11 mRNA and of a region of mitochondrial 16S rRNA. Nucleic Acids Res. 1981 Jan 24;9(2):293–307. doi: 10.1093/nar/9.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N., Caldwell P., Weissbach H. Autogenous control of Escherichia coli ribosomal protein L10 synthesis in vitro. Proc Natl Acad Sci U S A. 1980 May;77(5):2592–2595. doi: 10.1073/pnas.77.5.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Dennis P. P. Transcription patterns of adjacent segments on the chromosome of Escherichia coli containing genes coding for four 50S ribosomal proteins and the beta and beta' subunits of RNA polymerase. J Mol Biol. 1977 Oct 5;115(4):603–625. doi: 10.1016/0022-2836(77)90105-x. [DOI] [PubMed] [Google Scholar]
- Fiil N. P., Bendiak D., Collins J., Friesen J. D. Expression of Escherichia coli ribosomal protein and RNA polymerase genes cloned on plasmids. Mol Gen Genet. 1979 May 23;173(1):39–50. doi: 10.1007/BF00267689. [DOI] [PubMed] [Google Scholar]
- Fiil N. P., Friesen J. D., Downing W. L., Dennis P. P. Post-transcriptional regulatory mutants in a ribosomal protein-RNA polymerase operon of E. coli. Cell. 1980 Apr;19(4):837–844. doi: 10.1016/0092-8674(80)90074-4. [DOI] [PubMed] [Google Scholar]
- Friesen J. D., Parker J., Watson R. J., Bendiak D., Reeh S. V., Pedersen S., Fiil N. P. A transducing bacteriophage lambda carrying the structural gene for elongation factor Ts. Mol Gen Genet. 1976 Oct 18;148(1):93–98. doi: 10.1007/BF00268549. [DOI] [PubMed] [Google Scholar]
- Lindahl S., Yamamoto M., Nomura M. Mapping of a cluster of genes for components of the transcriptional and translational machineries of Escherichia coli. J Mol Biol. 1977 Jan 5;109(1):23–47. doi: 10.1016/s0022-2836(77)80044-2. [DOI] [PubMed] [Google Scholar]
- Linn T., Scaife J. Identification of a single promoter in E. coli for rplJ, rplL and rpoBC. Nature. 1978 Nov 2;276(5683):33–37. doi: 10.1038/276033a0. [DOI] [PubMed] [Google Scholar]
- Littlechild J., Dijk J., Garrett R. A. The identification of new RNA-binding proteins in the Escherichia coli ribosome. FEBS Lett. 1977 Mar 1;74(2):292–294. doi: 10.1016/0014-5793(77)80867-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McConnell D. J. Control sites in the sequence at the beginning of T7 gene 1. Nucleic Acids Res. 1979 Aug 10;6(11):3491–3503. doi: 10.1093/nar/6.11.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Post L. E., Nomura M. DNA sequences from the str operon of Escherichia coli. J Biol Chem. 1980 May 25;255(10):4660–4666. [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Promoter sequence for stringent control of bacterial ribonucleic acid synthesis. J Bacteriol. 1980 Feb;141(2):973–976. doi: 10.1128/jb.141.2.973-976.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Nomura M. Contranscription of genes for RNA polymerase subunits beta and beta' with genes for ribosomal proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3891–3895. doi: 10.1073/pnas.75.8.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Strycharz W. A., Nomura M. Identification of genes for elongation factor Ts and ribosomal protein S2 in E. coli. Cell. 1976 May;8(1):129–138. doi: 10.1016/0092-8674(76)90194-x. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Arfsten A. E., Nomura M. In vitro expression of Escherichia coli ribosomal protein genes: autogenous inhibition of translation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1837–1841. doi: 10.1073/pnas.77.4.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Nomura M. E. coli ribosomal protein L4 is a feedback regulatory protein. Cell. 1980 Sep;21(2):517–522. doi: 10.1016/0092-8674(80)90489-4. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Mueckl D., Lindahl L. Protein L4 of the E. coli ribosome regulates an eleven gene r protein operon. Cell. 1980 Sep;21(2):523–535. doi: 10.1016/0092-8674(80)90490-0. [DOI] [PubMed] [Google Scholar]


