Abstract
Introduction
Dual diethylcarbamazine and albendazole (DA) therapy is the standard mass drug administration (MDA) regimen for lymphatic filariasis in Kenya. Following the recent World Health Organization recommendation, Kenya piloted triple therapy with ivermectin, diethylcarbamazine, and albendazole (IDA) in MDA.
Objective
We conducted a community-based, observational, cohort event monitoring study to compare the types, frequency, severity, and predictors of adverse events following dual versus triple therapy in 20,421 eligible residents.
Methods
Residents in Kilifi (n = 10,010) and Mombasa counties (n = 10,411) received DA and IDA through MDA campaigns, respectively. Adverse events were actively monitored through house-to-house visits on days 1, 2, and 7 after MDA. Any clinical events reported before and after MDA were cross-checked and verified to differentiate pre-existing events from MDA-associated adverse events.
Results
Overall, 5807 and 3102 adverse events were reported by 2839 and 1621 individuals in the IDA and DA groups, respectively. The incidence of experiencing one or more adverse events was significantly higher (p < 0.0001) in the IDA group (27.3%; 95% confidence interval [CI] 26.4–28.2) than in the DA group (16.2%; 95% CI 15.5–16.9). Dizziness (15.9% vs 5.9%) and drowsiness (10.1% vs 2.6%) were the most common adverse events and significantly higher in the IDA group compared with the DA group (p < 0.0001). Most adverse events were mild or moderate with a few severe cases (IDA = 0.05%; 95% CI 0.35–0.78, DA = 0.03%; 95% CI 0.14–0.60). Female sex, obesity, taking three or more diethylcarbamazine or ivermectin tablets, and having pre-existing clinical symptoms were significant predictors of adverse events following IDA treatment.
Conclusions
Ivermectin, diethylcarbamazine, and albendazole as a combination is as safe and well tolerated as DA to use in MDA campaigns with no serious life-threatening adverse events. Systemic mild-to-moderate adverse events with a few severe cases and transient adverse events are more common with IDA treatment than with DA treatment. Hence, integrating pharmacovigilance into a MDA program is recommended for the timely detection and management of adverse events.
Key Points
| This comparative safety surveillance study reveals that about one third and one-in-six participants experience mass drug administration-associated adverse events with ivermectin, diethylcarbamazine, and albendazole (IDA) and diethylcarbamazine and albendazole (DA) therapy, respectively (p < 0.0001). Dizziness, drowsiness, diarrhea, headache, stomach pain, and confusion are the most reported adverse events that occurred at a higher frequency in the IDA treatment group compared with the DA treatment group (p < 0.001). |
| Female sex, taking three or more diethylcarbamazine or ivermectin tablets, and having chronic or pre-existing clinical conditions are significantly associated with adverse events following IDA therapy. |
| Both IDA and DA therapy are well tolerated, and the associated adverse events are transient and mild to moderate with a few severe cases. Hence, safety monitoring is recommended for the timely detection and management of adverse events following mass drug administration. |
Introduction
Lymphatic filariasis (LF), commonly known as elephantiasis, is a neglected tropical disease (NTD) of major public health concern affecting people living in tropical and subtropical countries [1]. Lymphatic filariasis, a mosquito-borne disease caused by filarial parasites, is the most debilitating NTD and the second leading cause of chronic disability worldwide, manifested as progressing lymphedema, elephantiasis, and hydroceles [1]. Through the Global Programme to Eliminate Lymphatic Filariasis, the World Health Organization (WHO) recommends mass drug administration (MDA) of antifilarial medicines to the entire at-risk population to interrupt transmission and eliminate LF as a public health problem by the year 2020, but recently the target milestone extended to 2030 [2]. In 2012, Kenya was reported as among the top six high-burden countries with an endemic population of 398 million and accounting for 27% of the global endemic population [3]. Lymphatic filariasis is endemic in six counties of the Kenyan coastal region and approximately 4.1 million people are at a risk of LF infection [4].
The WHO recommends three antifilarial drugs, diethylcarbamazine (DEC), albendazole (ALB), and ivermectin (IVM), for use in MDA programs for the control of LF, as a single, dual, or triple regimen depending on the co-endemicity with either onchocerciasis or loiasis [5, 6]. Sentinel site surveys conducted in the LF-endemic coastal region of Kenya in 2015 and 2016 reported that that some of the areas had filarial antigenemia of up to 6.3% despite MDA with DEC and ALB [7]. Based on reports of better effectiveness from clinical trials [8, 9], the WHO issued updated guidelines endorsing the use of a triple regimen with IVM, DEC, and ALB (IDA) in MDA programs to accelerate the control and elimination of LF in 2017 [10]. Kenya was among the first countries to implement the triple therapy regimen in 2018.
Various factors influence compliance to MDA including but not limited to an increase in the number of tablets to be swallowed and adverse events (AEs) that occur after taking the medicines [11, 12]. Previous studies reported the occurrence of mild and moderate AEs such as fever, headache, dizziness, malaise, myalgia, fatigue, and gastrointestinal upset are common among patients treated with IVM, DEC, and ALB either as a single or dual therapy and that severe AEs occurred more commonly in patients with loiasis [13–15]. However triple therapy may be associated with an increased frequency or more severe AEs as demonstrated by randomized clinical trials that compared the safety of IDA with DA or IA in Papua New Guinea, India, Haiti, Indonesia, and Fiji [9, 16]. Large-scale studies in different LF-endemic settings have been performed using cluster randomized trials in Papua New Guinea, India, Haiti, Indonesia, and Fiji to assess the safety of IDA versus DA [9, 15, 17, 18]. However, no similar community-based comparative safety surveillance has been performed in Africa to test the safety of a triple therapy in MDA campaigns. Treatment-associated AEs can vary between populations partly owing to genetic variations and other factors including co-infections, comorbidities, concomitant medications, nutritional status, and environmental exposure [19, 20].
Safety monitoring of new treatment regimens before a broader use in public health programs is critical for sub-Saharan Africa, where fully functional pharmacovigilance systems included in the NTD programs are lacking [21–23]. Kenya is the first African country to pilot the triple therapy in MDA campaigns in two LF-endemic counties because more safety data were needed before the implementation of IDA at a broader scale to the general target population. Therefore, we conducted a large-scale, community-based, comparative, parallel, active cohort event monitoring study to compare the incidence, type, severity, and risk factors of the newly introduced triple therapy (IDA) versus the standard dual therapy (DA) regimens in MDA campaigns to eliminate LF in Kenya.
Methods
Study Design and Setting
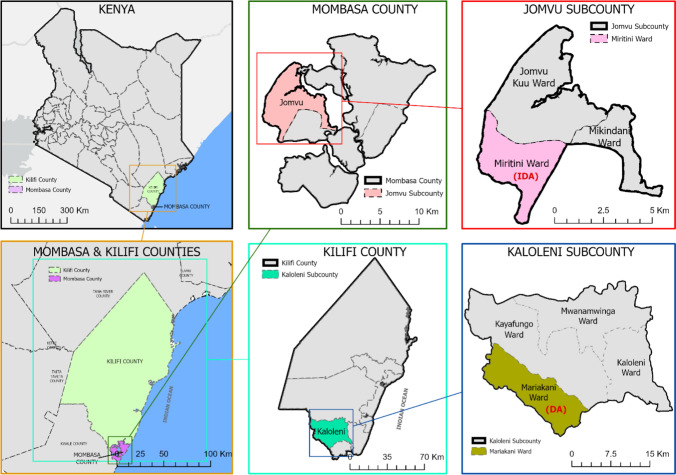
This prospective, observational, comparative, cohort event monitoring study was conducted between November and December 2018 in two LF-endemic coastal regions of Kenya (Kilifi County and Mombasa County). Safety surveillance with dual therapy (DA treatment group) was conducted in the Mariakani Ward, Kaloleni Sub County, Kilifi County, whereas safety surveillance of the triple therapy was conducted in the Miritini Ward (IDA treatment group), Jomvu Sub County, Mombasa County (Fig. 1). Mariakani Ward and Miritini Ward were selected because they are semi-urban areas enabling easy recruitment of large cohort sizes and a follow-up because of the closeness of the households.
Fig. 1.
Map of the study areas. Map of Kenya (top left). Map of Mombasa County showing the Jomvu Sub-County, Miritini Ward (top right) and Kilifi County, Kaloleni Sub-County, Mariakani Ward (bottom left)
Both Kilifi and Mombasa are among the six LF-endemic counties of the coastal region along the Indian Ocean. Mombasa County is in the south-eastern part of the coastal region of Kenya. It covers an area of 229.9 km2 excluding 65 km2 of water mass that is 200 nautical miles inside the Indian Ocean. It borders Kilifi County to the North and Kwale County to the South West, and the Indian Ocean to the East Mombasa County has six sub-counties and at the time of this study the population of Mombasa was projected to be 1,266,358 [24]. The site at the Mariakani Ward in Kilifi County is described previously [25]. In brief, Kilifi County is one of the five counties that border the Kenyan coast to the Indian Ocean, covering 12,246 km2, and has seven sub-counties divided into 35 electoral wards.
Study Population, Enrollment, and Sample Size
Before initiating the study, community sensitization and awareness activities through local public meetings (also called baraza) were conducted to inform the community leaders and the community about the study objectives, methodology, and data collection procedures and the type of data to be collected. The purpose of this sensitization meeting was to obtain community consent to conduct the study.
According to the WHO and Kenyan national MDA guidelines, any person aged ≥ 2 years who is resident in an LF-endemic region and not pregnant is eligible to receive MDA for the prevention and elimination of LF [10]. The study population consisted of all consenting and assenting residents eligible to receive preventive chemotherapy through MDA campaigns to control LF. Written informed consent and/or assent was obtained before study enrollment. The sample size was based on the WHO recommendation that a cohort of 10,000 participants receiving treatment provides a chance of detecting serious AEs at rate of ≤ 0.1%. A sample size of 3000 participants gives a 95% probability of identifying a single rare serious AE with an incidence rate of 1:3000 [26]. At the time of the study, the Kenyan NTD program estimated the target population for MDA at 33,072 and 55,063 for the IDA and DA treatment group, respectively. This provided a high probability of attaining the desired sample size for the two treatment groups that was projected at 10,000 participants in each treatment group. All MDA-eligible residents in the community received treatments sequentially through house-to-house visits. Participants were then given identification numbers according to their order of study entry until the desired number was attained.
Preventive Chemotherapy and Exposure Definition
The study exposure was receiving either dual therapy consisting of diethylcarbamazine citrate plus albendazole (DA) or triple therapy consisting of ivermectin, diethylcarbamazine, and albendazole (IDA) through an MDA campaign to halt the transmission of LF. As onchocerciasis is not endemic in the LF-endemic coastal regions, single-dose DA is the standard MDA regimen for LF in Kenya. Therefore, all eligible residents of the Mariakani Ward at Kaloleni Sub County, Kilifi County received the standard dual therapy of a single-dose diethylcarbamazine (6-mg/kg) plus albendazole (400-mg) combination (DA treatment group) as per the national and WHO MDA guideline [10, 27].
The Kenyan Ministry of Health selected three areas to roll out the pilot of IDA treatment before the scale up to the rest of the country [28]. The Miritini Ward, Jomvu Sub County, Mombasa County was one of the wards selected by the Kenya NTD program for the triple therapy piloting. Accordingly, all consenting eligible residents of the Miritini Ward received triple therapy with a single oral dose of ivermectin (200 μg/kg), diethylcarbamazine (6 mg/kg), and albendazole (400) mg (IDA treatment group). Study participants received the treatment during MDA campaigns led by the Kenyan national NTD program. Community drug distributors working for the NTD program delivered the medications under directly observed therapy. The study team had no role in the MDA planning, selection of the treatment regimen, and provision and administration of the drugs.
Outcome Measures, Assessment, and Validation of MDA-Associated AEs
The primary outcome measure was the incidence of any type of AE (post-MDA-associated AE), defined as experiencing any sign, symptom, or disease that occurred during the 7-day follow-up period after receiving MDA and if the same type of event was not reported at baseline before receiving MDA [29, 30]. Furthermore, any reported symptom between days 3 and 6 was considered as a valid AE if the participant did not experience the same symptom on pre-MDA and preceding days. The secondary outcomes were the type and severity grade of AEs.
Before receiving MDA, all participants were interviewed for any pre-existing clinical symptoms/condition (pre-MDA event). Participants were asked for any of the following clinical symptoms; fever, loss of appetite, dizziness or fainting, confusion, drowsiness, headache, cough, breathing difficulty, nausea, vomiting, diarrhea, stomach pain, itching, rash, and any other symptoms. After drug intake, the study participants were actively followed to monitor for any MDA-associated AEs through house-to-house visits on day 1, day 2, and day 7. Participants were requested to contact the data collectors and report any AEs they may experience between days 3 and 6. Any clinical events reported before and after MDA were cross-checked and verified to differentiate pre-existing clinical symptoms from treatment-associated AEs following MDA.
All reported AEs were graded based on five levels of severity using the Common Terminology Criteria for Adverse Events Version 5.0 [31] as follows:
Grade 1 (mild): asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated.
Grade 2 (moderate): minimal, local, or non-invasive intervention indicated; limiting age-appropriate Instrumental Activities of Daily Living.
Grade 3 (severe): medically significant but not immediately life threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care Activities of Daily Living.
Grade 4: life-threatening consequences; urgent intervention indicated.
Grade 5: death related to an AE.
Data Collection
The study involved 720 data collectors (390 for IDA, and 330 for DA treatment groups) recruited from the study catchment area and trained on how to use the Case report forms (CRFs) and record the reported AEs. For easy access to the community and the households, data collectors were paired with 130 (IDA) and 110 (DA) community drug distributors working for the national NTD program to administer the drugs. Data collection was conducted through house-to-house visits by a total of 130 teams for IDA and 110 teams for DA; each team consisting of one community drug distributor, one data collector, and a village elder (Balozi) to assist with community mobilization in each MDA implementation unit (the entire population in an area where LF transmission occurs). Community health extension workers were assigned as supervisors for the data collection teams.
Sociodemographic, clinical, and medical histories, including any comorbidities, concomitant medications, and nutritional status (using anthropometric data), were collected using a case record format and verified by the supervisor at the study site. Data were collected on paper and double entered into the electronic database, verified, and 10% of the data was used for quality assurance to ensure the accuracy of the data entry. A team of data managers reviewed the entered data to cross-check and rectify for errors, incomplete, and missing data.
Statistical Analysis
All data collected during the study period were cleaned before the statistical analysis. Each type of reported AE was categorized as a dichotomous outcome as “yes” or “no”. An outcome variable of having any type of AE was generated for each participant. Categorical variables were summarized as proportions, while continuous variables were summarized as mean with standard deviation or median and inter-quartile range. Outcomes of interest were (a) the frequency of the AE and (b) the severity of the AE within the 7-day monitoring period. Associations between categorical variables were analyzed using the Chi-square test. Predictors of AEs were analyzed by a univariate analysis followed by a multivariate binomial logistic regression analysis. Predictor variables with p ≤ 0.2 in the univariate analysis were entered into the multivariate model for analysis. A generalized linear model approach (binomial distribution, logit link) was used to assess the risk factors for AEs. Statistical analyses were conducted using STATA Version 15.1 (StataCorp, College Station, TX, USA). A p-value of < 0.05 was considered statistically significant.
Ethical Consideration
This study received ethical approval from the Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (Ref. No. KNH-ERC/A/143). Permission to conduct the study was obtained from relevant County and Sub-County Health Departments, and consent from the community leaders and the communities. The study was coordinated and led by the National Pharmacovigilance Centre based at the Pharmacy and Poison Board, National Medicine Regulatory Authority under the Ministry of Health. Before study enrollment, participants and their parents or legal guardians received information about the study. For participants aged ≤ 12 years, written informed consent was obtained from their parents or guardian. For participants aged > 12 years, written informed consent was obtained from the parent or guardian, and assent was obtained from the study participant.
Results
Characteristics of Study Participants
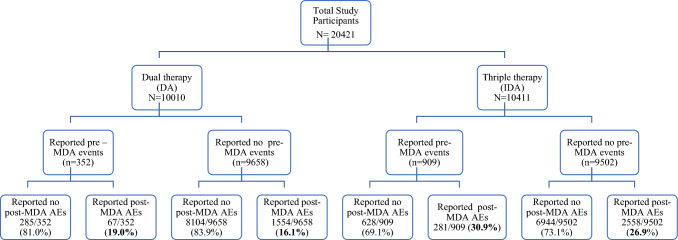
A total of 20,421 participants were enrolled and monitored for MDA-associated AEs between November and December 2018. Among these, 10,010 residents in the Mariakani Ward and 10,411 residents in the Miritini Ward received DA and IDA, respectively. The sociodemographic and baseline characteristics of the study participants are presented in Table 1. About half of the study participants in both treatment groups were female (52% in the DA group, and 53% in the IDA group). There were no significant differences in sociodemographic and baseline characteristics of the study participants between the two treatment groups. The study flow chart indicating the proportion of study participants who reported at least one type of post-MDA AE stratified by the presence or absence of any clinical symptom before receiving MDA (pre-MDA event) in each treatment group is presented in Fig. 2.
Table 1.
Sociodemographic and baseline characteristics of study participants stratified by treatment group
| Variable | Treatment group | p-value | |
|---|---|---|---|
| Triple therapy (IDA) | Dual therapy (DA) | ||
| n (%) | n (%) | ||
| Sex | |||
| Female | 5369 (51.6) | 5343 (53.4) | 0.01 |
| Male | 5042 (48.4) | 4667 (46.6) | |
| Age, years | |||
| 2–15 | 3224 (31.0) | 4456 (44.5) | < 0.001 |
| 16–20 | 1173 (11.3) | 1315 (13.1) | |
| 21–64 | 5892 (56.6) | 3949 (39.5) | |
| 65–99 | 122 (1.2) | 290 (2.9) | |
| Body mass index | |||
| Underweight | 1331 (12.8) | 1898 (19.0) | < 0.001 |
| Normal | 3521 (33.9) | 3145 (31.5) | |
| Overweight | 2603 (25.1) | 2239 (22.5) | |
| Obese | 2918 (28.1) | 2693 (27.0) | |
| HAZ (age 10–19 years) | |||
| Stunted | 1461 (35.9) | 2056 (37.4) | 0.15 |
| Normal | 2607 (64.1) | 3448 (62.7) | |
| BAZ (age 10–19 years) | |||
| Wasted | 680 (16.7) | 1305 (23.8) | < 0.001 |
| Normal | 3385 (83.3) | 4187 (76.2) | |
| WAZ (age 2–9 years) | |||
| Underweight | 346 (16.7) | 662 (24.1) | < 0.001 |
| Normal | 1722 (83.3) | 2085 (75.9) | |
| Concomitant medication | |||
| Yes | 236 (2.3) | 436 (4.4) | < 0.001 |
| No | 10,175 (97.7) | 9574 (95.6) | |
| Received MDA for in the previous year (2017) | |||
| Yes | 3427 (37.2) | 5875 (62.2) | < 0.001 |
| No | 5788 (62.8) | 3572 (37.8) | |
| Slept under a bed net the previous night | |||
| Yes | 9278 (89.9) | 9296 (93.5) | < 0.001 |
| No | 1048 (10.2) | 642 (6.5) | |
| House has mosquito mesh screen on windows | |||
| Yes | 5805 (56.2) | 4800 (48.4) | < 0.001 |
| No | 4522 (43.8) | 5127 (51.7) | |
| Indoor spraying to prevent mosquitoes | |||
| No | 7178 (69.5) | 6703 (67.8) | < 0.001 |
| Yes | 3145 (30.5) | 3184 (32.2) | |
| Number of DEC tablets taken during MDA | |||
| 1 | 1260 (12.1) | 1721 (17.2) | < 0.001 |
| 2 | 1328 (12.8) | 2383 (23.8) | |
| 3 | 4200 (40.4) | 5880 (58.7) | |
| 4 | 3606 (34.7) | 26 (0.3) | |
| Number of ivermectin tablets taken during MDA | |||
| 1 | 1232 (11.8) | - | |
| 2 | 1317 (12.7) | - | |
| 3 | 4220 (40.5) | - | |
| 4 | 3642 (35.0) | - | |
| Chronic illness | |||
| No | 10,147 (97.5) | 9928 (99.2) | < 0.001 |
| Yes | 264 (2.5) | 82 (0.8) |
BAZ body mass index-for-age z-score, DA diethylcarbamnzine + albendazole, DEC diethylcarbamazine citrate, HAZ height-for-age z-score, IDA ivermectin + diethylcarbazine + albendazole, MDA mass drug admnistrtaion, WAZ weight-for-age z-score
Fig. 2.
Study flow chart of participants enrollment, follow-up, and incidence of mass drug administration (MDA)-associated adverse events (AEs) in each treatment group. The incidence of experiencing one or more MDA-associated AEs is indicated in bold. DA diethylcarbamnzine + albendazole, DEC diethylcarbamazine citrate, IDA ivermectin + dethylcarbazine + albendazole
Safety of IDA Versus DA
In the IDA treatment group, 2839 out of the 10,411 participants experienced one or more MDA-associated AEs. The proportion of participants who experienced one, two, or three or more types of MDA-associated AEs were 14.9% (n = 1549), 7.4% (n = 772), 2.7% (n = 220), and 2.2% (n = 223), respectively. In the DA treatment group, 1621 individuals out of 10,010 participants reported at least one type of post-MDA AE. The proportions of participants who experienced one, two, or three or more types of AEs were 9.2% (n = 917), 4.6% (n = 464), and 2.4% (n = 240), respectively.
The cumulative incidence of experiencing one or more type of AEs over a 7-day follow-up period was significantly higher (p < 0.0001) in the IDA treatment group (27.3%; 95% CI 26.4– 28.2) compared with the DA treatment group (16.2%; 95% CI 5.5–16.9). The proportion of people who experienced MDA-associated AEs and the total number of AEs reported were higher in the IDA treatment group than in the DA treatment group.
In the IDA treatment group, the cumulative incidence of experiencing at least one type of MDA-associated AE among those who reported pre-existing clinical symptoms (30.9%; 95% CI 27.9–33.9) was significantly higher (p = 0.01) compared with those who did not report any pre-MDA clinical events (26.9%; 95% CI 26.0–27.8). Likewise, in the DA treatment group, the cumulative incidence of experiencing at least one type of MDA-associated AE was higher among those who reported pre-MDA clinical events (19.0%; 95% CI 14.9–23.15) than those with no pre-MDA event (16.1%; 95% CI 5.4–16.8), but this difference was not statistically significant (p = 0.14) (Fig. 2).
Proportion of Various Types of AEs
In the IDA treatment group, a total of 5807 AEs was reported by 2839 participants over the 7-day follow-up. Of the total AEs reported, 88.3% (n = 5126) occurred on day 1, 7.2% (n = 420) occurred on day 2, while 4.5% (n = 261) occurred between days 3 and 7 of MDA. A total of 3102 AEs was reported by the 1621 participants in the DA treatment group, of which 26.9% (n = 2695), 3.3% (n = 332), and 0.7% (n = 73) of AEs occurred on day 1, day 2, and between days 3 and 7, respectively.
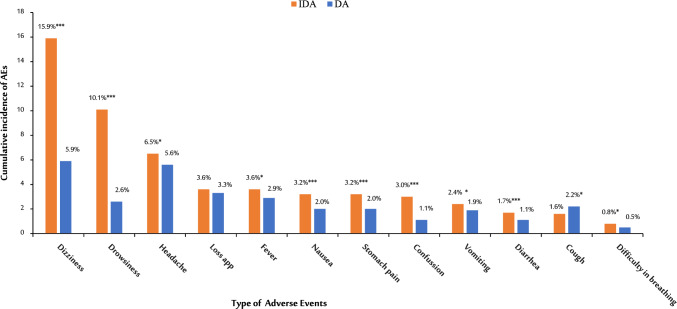
Dizziness (15.9%, n = 1657), drowsiness (10.1%, n = 1053), and headache (6.5%, n = 678) were the three most reported AEs among those treated with IDA. In contrast, dizziness (5.9%, n = 586), headache (5.6%), and loss of appetite (3.3%, n = 332) were the most reported AEs in the DA treatment group. Comparison of the proportion of each type of AE between the two treatment groups is presented in Fig. 3. Of the most reported AEs, dizziness, drowsiness, diarrhea, headache, stomach pain, and confusion were significantly higher in the IDA group than the DA group (p < 0.001) (see Fig. 3).
Fig. 3.
Comparison of the cumulative incidence of mass drug administration-associated adverse events (AEs) between participants who received triple versus dual therapy for the control of lymphatic filariasis. DA diethylcarbamnzine + albendazole, IDA ivermectin + diethylcarbazine + albendazole, *p ≤ 0.01; **p ≤ 0.001; ***p ≤ 0.001
Severity Grading of the AEs
The proportion of AEs graded for severity were 84% (IDA, n = 4868) and 92% (DA, n = 2865) respectively. The grading scale did not vary significantly between the two groups and most of the AEs reported were mild (IDA = 89.4%, DA = 87.3%), followed by moderate (IDA = 10.4 %, DA = 12.4%) and severe (IDA = 0.6%, DA = 0.3%) (see Table 2). Of the severe AEs, headache was the most reported in both treatment groups and none of the participants required medical intervention. The rates of severe AEs in the IDA and DA treatment groups were 0.05% (95% CI 0.35–0.78) and 0.03% (95% CI 0.14–0.60), respectively.
Table 2.
Severity grading of adverse events following mass IDA versus DA therapy for the control of lymphatic filariasis
| Type of event | IDA (n = 4868) | DA (n = 2865) | ||||
|---|---|---|---|---|---|---|
| Grade 1 (mild) | Grade 2 (moderate) | Grade 3 (severe) | Grade 1 (mild) | Grade 2 (moderate) | Grade 3 (severe) | |
| Fever | 298 (92.3%) | 23 (7.1%) | 2 (0.6%) | 218 (91.2%) | 21 (8.8%) | |
| Loss of appetite | 327 (95.3%) | 15 (4.4%) | 1 (0.3%) | 288 (92.3%) | 24 (7.7%) | |
| Dizziness | 1341 (85.2%) | 33 (13.5%) | 1 (0.4%) | 489 (88.9% | 60 (10.9%) | 1 (0.2%) |
| Confusion | 251 (90.9%) | 22 (8.0%) | 3 (1.1%) | 93 (94.9 %) | 5 (5.1%) | |
| Drowsiness | 892 (91.2%) | 83 (7.8%) | 3 (0.3%) | 222 (90.6%) | 23 (9.4%) | |
| Headache | 435 (91.4%) | 37 (7.8%) | 4 (0.8%) | 426 (80.4%) | 97 (18.3%) | 7 (1.3%) |
| Cough | 79 (81.4%) | 16 (16.5%) | 2 (2.1%) | 187 (89.9%) | 21 (10.1%) | |
| Difficulty breathing | 483 (88.9%) | 5 (9.3%) | 1 (1.9%) | 30 (81.1 %) | 7 (18.9%) | |
| Nausea | 222 (91%) | 21 (8.6%) | 1 (0.4%) | 163 (88.1%) | 21 (11.4%) | 1 (0.5%) |
| Vomiting | 183 (96.3%) | 7 (3.7%) | 144 (82.3 %) | 31 (17.7%) | ||
| Diarrhea | 93 (92.1%) | 8 (7.9%) | 86 (83.5 %) | 17 (16.5%) | ||
| Stomach pain | 184 (86.8%) | 26 (12.3%) | 2 (0.9%) | 156 (85.2 %) | 27 (14.8%) | |
| Total | 4353 (89.4%) | 489 (10.0%) | 26 (0.5%) | 2502 (87.3%) | 354 (12.4%) | 9 (0.3%) |
DA diethylcarbamnzine + albendazole, IDA ivermectin + diethylcarbazine + albendazole
Factors Associated with AEs Following Triple (IDA) Versus Dual (DA) Therapy
The frequency of experiencing one or more MDA-associated AEs was significantly higher among adults in both the IDA and DA treatment groups (p < 0.0001). In the IDA group, those aged 21–64 years (29.4%) reported more AEs, whereas in the DA group, those aged 16–20 years (19.7%) reported the most AEs (Table 3). Female participants reported more AEs than male participants, which was statistically significant in the IDA group (p = 0.037). An increase in the number of DEC or IVM tablets taken was significantly associated with the occurrence of AEs (p < 0.0001). Having a chronic illness, taking concomitant medications, or high-protein or high-fat meals before MDA were significantly associated with the occurrence of AEs.
Table 3.
Factors associated with AEs following mass triple therapy in comparison to dual therapy
| Variables | Triple therapy (IDA) | Dual therapy (DA) | ||
|---|---|---|---|---|
| Incidence of AEs, n (%) | p-value | Incidence of AEs, n (%) | p-value | |
| Sex | ||||
| Female | 1511/5369 (28.1%) | 0.04 | 881/5343 (16.5%) | 0.39 |
| Male | 1328/5042 (26.3%) | 740/4667 (15.9%) | ||
| Age, years | ||||
| 2–15 | 742/3224 (23.0%) | < 0.001 | 661/4456 (14.8%) | < 0.001 |
| 16–20 | 330/1173 (28.1%) | 259/1315 (19.7%) | ||
| 21–64 | 1732/5892 (29.4%) | 638/3949 (16.2%) | ||
| 65–99 | 35/122 (28.7%) | 63/290 (21.7%) | ||
| Took concomitant medication | ||||
| Yes | 175/608 (28.8%) | 0.39 | 113/436 (25.9%) | < 0.001 |
| No | 2664/9803 (27.2%) | 1508/9574 (15.8%) | ||
| Number of DEC tablets taken | ||||
| 1 | 240/1260 (19.0%) | < 0.001 | 233/1721 (13.5%) | < 0.001 |
| 2 | 304/1328 (22.9%) | 357/2383 (15.0%) | ||
| 3 | 1204/4202 (28.7%) | 1026/5880 (17.4%) | ||
| 4 | 1088/3606 (30.2%) | 5/26 (19.2%) | ||
| Ivermectin tablets | ||||
| 1 | 242/1232 (19.6%) | < 0.001 | ||
| 2 | 300/1317 (22.8%) | |||
| 3 | 1209/4220 (28.6%) | |||
| 4 | 1088/3642 (29.9%) | |||
| Chronic illness | ||||
| Yes | 86/264 (32.6%) | 0.05 | 20/82 (24.4%) | 0.04 |
| No | 2753/10147 (27.1%) | 1601/9928 (16.1%) | ||
| Type of meal | ||||
| Carbohydrate | 1635/6598 (24.8%) | < 0.001 | 735/5171 (14.2%) | < 0.001 |
| High fat | 363/1266 (28.7%) | 160/911 (17.6%) | ||
| High protein | 375/1080 (34.7%) | 150/754 (19.9%) | ||
| Pre-MDA clinical events | ||||
| Yes | 281/909 (30.9%) | 0.01 | 67/352 (19.0%) | 0.14 |
| No | 2558/9502 (26.9%) | 1554/9658 (16.1%) | ||
AEs adverse events, DA diethylcarbamnzine + albendazole, DEC diethylcarbamazine citrate, IDA ivermectin + diethylcarbazine + albendazole, MDA mass drug administration
Predictors of AEs Associated with Triple Therapy (IDA)
Risk factors associated with AEs following IDA therapy were analyzed using a univariate analysis followed by a multivariate generalized linear binomial regression (Table 4). In the univariate analysis, age, sex, concomitant medication, number of DEC and IVM tablets, chronic illness, and the type of meal taken before MDA were significant predictors of AEs. A multivariable generalized linear model analysis showed that female sex, obesity, taking three or more tablets of DEC, taking three or more tablets of IVM, and the type of meal taken before MDA were significant factors associated with AEs following IDA therapy. In the DA treatment group, older age, taking concurrent medications, taking three or more tablets of DEC, and the type of meal taken before MDA were significant predictors of AEs [25].
Table 4.
Predictors of adverse events associated with triple therapy with ivermectin, diethylcarbamazine, and albendazole (IDA) for the elimination of lymphatic filariasis
| Variable | Crude risk ratios | 95% CI | p-value | Adjusted risk ratios | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 1.06 | 1.003–1.137 | 0.039 | 1.09 | 1.019–1.975 | 0.01 |
| Male | 1 | 1 | ||||
| Age, years | ||||||
| 2–15 | 0.78 | 0.726–0.843 | 0.0001 | 0.97 | 0.856–1.094 | 0.61 |
| 16–20 | 0.96 | 0.866–1.057 | 0.39 | 1.02 | 0.912–1.145 | 0.71 |
| 21–64 | 1 | |||||
| 65–99 | 0.98 | 0.736–1.295 | 0.87 | 0.87 | 0.620–1.227 | 0.43 |
| BMI | ||||||
| Normal | 1 | 1 | ||||
| Underweight | 0.92 | 0.827–1.021 | 0.12 | 1.01 | 0.899–1.140 | 0.86 |
| Overweight | 0.99 | 0.919–1.079 | 0.93 | 0.99 | 0.904–1.083 | 0.82 |
| Obese | 0.9 | 0.833–0.979 | 0.01 | 0.88 | 0.798–0.961 | 0.005 |
| Concomitant medication | ||||||
| No | 1 | |||||
| Yes | 1.05 | 0.930–1.205 | 0.383 | |||
| Number of DEC tablets taken | ||||||
| 1 | 1 | 1 | ||||
| 2 | 1.2 | 1.033–1.397 | 0.017 | 1.16 | 0.979–1.362 | 0.087 |
| 3 | 1.5 | 1.328–1.699 | < 0.001 | 1.43 | 1.215–1.677 | < 0.001 |
| 4 | 1.58 | 1.399–1.793 | < 0.001 | 1.46 | 1.231–1.74 | < 0.001 |
| Number of ivermectin tablets taken | ||||||
| 1 | 1 | 1 | ||||
| 2 | 1.16 | 0.997–1.348 | 0.054 | 1.102 | 0.935–1.300 | 0.246 |
| 3 | 1.46 | 1.290–1.649 | < 0.001 | 1.364 | 1.159–1.604 | < 0.001 |
| 4 | 1.52 | 1.344–1.721 | < 0.001 | 1.38 | 1.162–1.648 | < 0.001 |
| Chronic illness | ||||||
| No | 1 | |||||
| Yes | 1.2 | 1.006–1.432 | 0.04 | 1.16 | 0.950–1.413 | 0.15 |
| Type of meal | ||||||
| Carbohydrate | 1 | 1 | ||||
| High fat | 1.16 | 1.050–1.274 | 0.003 | 1.14 | 1.038–1.259 | 0.006 |
| High protein | 1.4 | 1.278–1.536 | < 0.001 | 1.37 | 1.248–1.503 | < 0.001 |
| Pre-MDA clinical events | ||||||
| No | 1 | 1 | ||||
| Yes | 1.15 | 1.036–1.272 | 0.008 | 1.08 | 0.961–1.205 | 0.2 |
BMI body mass index, DA diethylcarbamnzine + albendazole, DEC diethylcarbamazine citrate, IDA ivermectin + diethylcarbazine + albendazole, MDA mass drug administration
Significant values are indicated in bold
Discussion
This large-scale, community-based, comparative, parallel, active cohort event monitoring study investigated the safety of the newly introduced triple therapy (IDA) versus the standard dual therapy (DA) regimens in Kenya. Our results indicate a significantly higher rate of MDA-associated AEs among the IDA treatment group than the DA treatment group. About one-third (27.3%) who received IDA reported at least one type of AE, which is almost double the rate in the DA treatment group (16.2%). However, it is worth noting that the AEs reported in both treatment groups were mild to moderate and transient and occurred during the first 2 days after receiving MDA and resolved within a week (Table 3). Of the total AEs reported in the IDA group, 14.9% participants reported one type of AE, 7.4% reported two AEs, and 4.9% three or more AE events, which is similar to DA dual therapy [25]. Other studies reported occurrences of more than one type of AE associated with the use of theses medicines [32, 33]. The most common type of AEs following IDA were dizziness, drowsiness, and headache, in contrast to DA, where dizziness, headache, and loss of appetite were the most reported (Fig. 3). Our findings are consistent with previous studies reporting similar types of common AEs [16, 18, 32, 34]. Female sex, taking three or more DEC or IVM tablets, or having a high-protein or high-fat meal before the drug intake were significant predictors of AEs following IDA therapy (Table 4).
Reports from the literature indicate that AEs associated with various antifilarial regimens range between 12.6% and 61.1%. Large studies in different LF-endemic settings, most outside of Africa, is performed. A cluster of randomized trials conducted in Papua New Guinea, India, Haiti, Indonesia, and Fiji reported similar rates of AEs in the IDA and DA treatment groups in the general target population (12.1% vs 12%), but persons with microfilaremia experienced more AEs [17, 35]. In our study, the cumulative incidence of AEs among those treated with IDA was 27.3% compared with those treated with DA at 16.2%. Our finding is in line with a recent, open-label, cluster randomized trial in MDA-eligible residents from Papua New Guinea that reported significantly higher rates of AEs in IDA versus DA (20% vs 18%) [18]. An open-label randomized controlled trial among LF-infected participants from Côte d’Ivoire reported higher rates of AEs amongst those treated with IDA than those treated with IA (47% vs 40%) [32]. A recent study reported a higher frequency of AEs in microfilariae-positive participants treated with IDA compared with those treated with DA (39% vs 24%) [18]. Although we did not pre-screen participants for LF infection, presumably the higher rates of AEs in the IDA group could be due to higher rates of immunological reaction from the dying parasite in LF-infected individuals as a result of the higher efficacy of the triple therapy compared with the dual therapy.
Interestingly, we found an association of sex with AEs in the IDA group, but not in the DA treatment group. In the IDA group, the rate of experiencing one or more MDA-associated AEs was significantly higher among female individuals than male individuals. Though not statistically significant, a similar finding was observed in an open-label, block-randomized community study that compared the safety of IDA versus DA in India, where more AEs were reported among female individuals than male individuals in each treatment arm [16, 25]. This is also consistent with results from other studies [14, 36]. Higher rates of MDA-associated AEs in female individuals than in male individuals who received IDA for LF in Tanzania [29], or praziquantel and albendazole in Ethiopia and Rwanda have been reported recently [30, 37]. Significant variation in the rate of treatment-related AEs between male and female individuals could be due to sex-dependent biological and physiological differences influencing drug metabolism and disposition [38, 39]. Nevertheless, sex-dependent variation in the pharmacokinetics of DEC, IVM, or ALB and its association with susceptibility to treatment-associated AEs remains to be investigated. It is also notable that women are likely to use more medication than men, tend to pay more attention to their well-being, and are more likely to report AEs than men. Furthermore, men are less likely to report mild and moderate reactions [40]. Therefore, it is essential to sensitize the communities, more so with men, on the importance of reporting all suspected AEs even when mild.
In both treatment groups, most AEs were mild to moderate and occurred during the first 2 days of MDA treatment and resolved within a week. The observed higher rate of treatment-associated AEs in adults could be due to a higher prevalence of LF infection in adults compared with children [16, 18]. Dizziness, drowsiness, headache, loss of appetite, and fever were the most commonly reported types of AEs in both treatment groups, which is consistent with previous studies [16, 18, 32, 34]. The top three common AEs in the IDA group were dizziness, drowsiness, and headache, whereas dizziness, headache, and loss of appetite were seen in the DA group.
We found a significant association of AEs with increasing tablets of DEC or IVM taken, indicating the possibility of a dose-dependent effect on the occurrence of AEs. A previous study reported that the combination of IVM and DEC did not show any synergistic interaction on the clearance of microfilaria but rather an additive reaction to each other [41]. This could explain the increased prevalence of systemic AEs in the IDA versus the DA group, which could be because of an immunological reaction triggered by death of the parasites. A food–drug interaction as a predictor of AEs is also found in this study [42]. In both treatment groups, participants who had eaten either a high-fat or high-protein meal were more likely to report AEs in comparison to those who had eaten carbohydrates.
Our study provides a relevant evidence-based recommendation for the local and regional MDA program managers and NTD public health programs on the safety of drugs used in MDA campaigns to halt the transmission of LF. Although the rate of mild-to-moderate AEs associated with IDA is significantly higher than the standard dual DA therapy, the tolerability and transient nature of observed AEs are encouraging to scale up the use of IDA to all LF-endemic communities in Kenya and Sub-Saharan Africa. This is particularly important because the superior effectiveness of IDA in clearing microfilariae [15, 34] will accelerate and contribute towards achieving Ending the Neglect to Attain Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030 [43].
To our knowledge, this is the first large-scale active safety surveillance study to compare the safety of MDA with triple versus dual therapy in Sub-Saharan Africa, where millions of people annually receive medications to control the disease. The main strength of our active safety surveillance is the large sample size to detect rare AEs as recommended by the WHO. Second, AEs was assessed using active surveillance for up to 7 days post-MDA with high rates of participant follow-up. Hence, the results from this study can be extrapolated to other LF-endemic zones in Kenya and Sub-Saharan Africa. Our study has some limitations. As MDA is given to all of the at-risk population without a prior diagnosis for the disease, we did not pre-screen our study participants for LF infection before study enrollment. Consequently, the incidence and type of AEs between LF-infected and non-infected people could not be explored. Furthermore, pre-existing clinical conditions (pre-MDA events) were recorded with the intention to cross-check and validate whether the reported events occurred newly after receiving MDA or pre-existed. Only newly occurred MDA-associated AEs were recorded as valid MDA-associated AEs. Worsening of a pre-existing clinical condition after receiving MDA was not explored mainly because the worsening could be due to the MDA received or due ot the untreated pre-existing illness/symptom getting worse over time.
Conclusions
Like dual therapy with DA, triple therapy with IDA is generally as safe and well tolerated as MDA regimens for controlling and eliminating LF. Associated AEs with both treatment regimens are primarily systemic, transient, and mild to moderate with a few severe cases and no significant differences in severity grading between IDA and DA. However, the incidence of experiencing one or more type of AE is about two-fold higher with IDA than with DA. Female sex, taking three or more tablets of DEC or IVM, or having pre-existing clinical conditions are risk factors for experiencing AEs following IDA. Because of its better effectiveness and tolerability, scaling up the use of triple therapy in MDA campaigns is recommended. However, the integration of pharmacovigilance into the NTD programs and safety monitoring during MDA in collaboration with the National Pharmacovigilance Center is recommended for the timely detection and management of AEs and to ensure rare AEs are documented and properly managed.
Acknowledgments
Open access funding is provided by the Karolinska Institutet. The authors thank the Kenyan National Neglected Tropical Disease Programme Team members and the Jomvu and Kaloleni Sub County Health Management Team Sub Counties, particularly the NTD coordinators and their teams, for their support during community sensitization, mobilization, and coordination of the work with the data collectors. They also thank the staff of the Product Safety Department for their support during the data collection and community sensitization and a special thanks to all data collectors, community drug distributors, the community leaders (village Balozis), and the study participants for their cooperation during the data collection and follow-up processes.
Funding
Open access funding provided by Karolinska Institute.
Declarations
Funding
This study was conducted as part of the pharmacovigilance infrastructure and post-marketing surveillance system capacity building for regional medicine regulatory harmonization in East Africa (PROFORMA) project funded by the European and Developing Countries Clinical Trials Partnership (EDCTP-2) program supported by the European Union (grant number CSA2016S-1618) and the Swedish International Development Cooperation Agency (Sida).
Conflicts of Interest/Competing Interests
Christabel Khaemba, Abbie Barry, Wyckliff P. Omondi, Elvis Kirui, Margaret Oluka, Gurumurthy Parthasarathi, Sammy M. Njenga, Anastacia Guantai, and Eleni Aklillu have no conflicts of interest that are directly relevant to the content of the article.
Ethics Approval
This study was approved by the Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (Ref. No. KNH-ERC/A/413).
Consent to Participate
Participant consent was documented on a written informed consent form, which was accompanied by written information for parents/guardians and the informed consent forms were approved by the same ethics committees that approved this protocol.
Consent for Publication
Not applicable.
Availability of Data and Material
All data generated or analyzed during this study are included in this article.
Code Availability
Not applicable.
Authors’ Contributions
CK, AB, PG, AG, MO, and EA participated in the conception and design of the study. CK, AB, AG, MO, WPO, MM, SMN, and EA participated in material preparation and investigation. AG, SMN, MO, and EA supervised the implementation of the project. CK and EK performed the data curation. CK, AB, EK, and EA performed the data analysis and interpretation. CK prepared the original manuscript and AB, PG, AG, SMN, MO, WPO, MM, and EA edited and reviewed the manuscript. PG, SMN, and WPO provided technical advice. All authors read and approved the final version.
Contributor Information
Christabel Khaemba, Email: cnkhaemba@gmail.com.
Abbie Barry, Email: abbiebarry9@gmail.com.
Wyckliff P. Omondi, Email: wyckliff.omondi@gmail.com
Elvis Kirui, Email: elvokip@gmail.com.
Margaret Oluka, Email: olukamarga@yahoo.com.
Gurumurthy Parthasarathi, Email: partha18@gmail.com.
Sammy M. Njenga, Email: sammynjenga@gmail.com
Anastacia Guantai, Email: anguantai@yahoo.com.
Eleni Aklillu, Email: eleni.aklillu@ki.se.
References
- 1.World Health Organization. Global programme to eliminate lymphatic filariasis: progress report, 2017. 2018. https://apps.who.int/iris/bitstream/handle/10665/275719/WER9344.pdf?ua=1. Accessed 20 Feb 2023.
- 2.Casulli A. New global targets for NTDs in the WHO roadmap 2021–2030. PLoS Negl Trop Dis. 2021;15(5):e0009373. doi: 10.1371/journal.pntd.0009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramaiah KD, Ottesen EA. Progress and impact of 13 years of the global programme to eliminate lymphatic filariasis on reducing the burden of filarial disease. PLoS Negl Trop Dis. 2014;8(11):e3319. doi: 10.1371/journal.pntd.0003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Weekly epidemiological record record No. 43, 23 October 2020. 2020. https://apps.who.int/iris/bitstream/handle/10665/336185/WER9543-eng-fre.pdf. Accessed 26 Feb 2023.
- 5.Tisch DJ, Michael E, Kazura JW. Mass chemotherapy options to control lymphatic filariasis: a systematic review. Lancet Infect Dis. 2005;5(8):514–523. doi: 10.1016/S1473-3099(05)70192-4. [DOI] [PubMed] [Google Scholar]
- 6.Gyapong JO, Kumaraswami V, Biswas G, Ottesen EA. Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opin Pharmacother. 2005;6(2):179–200. doi: 10.1517/14656566.6.2.179. [DOI] [PubMed] [Google Scholar]
- 7.Njenga SM, Kanyi HM, Mutungi FM, Okoyo C, Matendechero HS, Pullan RL, et al. Assessment of lymphatic filariasis prior to re-starting mass drug administration campaigns in coastal Kenya. Parasit Vectors. 2017;10(1):99. doi: 10.1186/s13071-017-2044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer PU, King CL, Jacobson JA, Weil GJ. Potential value of triple drug therapy with ivermectin, diethylcarbamazine, and albendazole (IDA) to accelerate elimination of lymphatic filariasis and onchocerciasis in Africa. PLoS Negl Trop Dis. 2017;11(1):e0005163. doi: 10.1371/journal.pntd.0005163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubray CL, Sircar AD, Beau de Rochars VM, Bogus J, Direny AN, Ernest JR, et al. Safety and efficacy of co-administered diethylcarbamazine, albendazole and ivermectin during mass drug administration for lymphatic filariasis in Haiti: results from a two-armed, open-label, cluster-randomized, community study. PLoS Negl Trop Dis. 2020;14(6):e0008298. doi: 10.1371/journal.pntd.0008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Guideline: alternative mass drug administration regimens to eliminate lymphatic filariasis. 2017. License: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/handle/10665/259381. Accessed 27 Jul 2023. [PubMed]
- 11.Njomo DW, Amuyunzu-Nyamongo M, Magambo JK, Njenga SM. The role of personal opinions and experiences in compliance with mass drug administration for lymphatic filariasis elimination in Kenya. PLoS ONE. 2012;7(11):e48395. doi: 10.1371/journal.pone.0048395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babu BV, Babu GR. Coverage of, and compliance with, mass drug administration under the programme to eliminate lymphatic filariasis in India: a systematic review. Trans R Soc Trop Med Hyg. 2014;108(9):538–549. doi: 10.1093/trstmh/tru057. [DOI] [PubMed] [Google Scholar]
- 13.Moraga P, Cano J, Baggaley RF, Gyapong JO, Njenga SM, Nikolay B, et al. Modelling the distribution and transmission intensity of lymphatic filariasis in sub-Saharan Africa prior to scaling up interventions: integrated use of geostatistical and mathematical modelling. Parasit Vectors. 2015;24(8):560. doi: 10.1186/s13071-015-1166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budge PJ, Herbert C, Andersen BJ, Weil GJ. Adverse events following single dose treatment of lymphatic filariasis: observations from a review of the literature. PLoS Negl Trop Dis. 2018;12(5):e0006454. doi: 10.1371/journal.pntd.0006454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King CL, Suamani J, Sanuku N, Cheng YC, Satofan S, Mancuso B, et al. A trial of a triple-drug treatment for lymphatic filariasis. N Engl J Med. 2018;379(19):1801–1810. doi: 10.1056/NEJMoa1706854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jambulingam P, Kuttiatt VS, Krishnamoorthy K, Subramanian S, Srividya A, Raju HKK, et al. An open label, block randomized, community study of the safety and efficacy of co-administered ivermectin, diethylcarbamazine plus albendazole vs. diethylcarbamazine plus albendazole for lymphatic filariasis in India. PLoS Negl Trop Dis. 2021;15(2):e0009069. doi: 10.1371/journal.pntd.0009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weil GJ, Bogus J, Christian M, Dubray C, Djuardi Y, Fischer PU, et al. The safety of double- and triple-drug community mass drug administration for lymphatic filariasis: a multicenter, open-label, cluster-randomized study. PLoS Med. 2019;16(6):e1002839. doi: 10.1371/journal.pmed.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavul L, Laman M, Howard C, Kotty B, Samuel A, Bjerum C, et al. Safety and efficacy of mass drug administration with a single-dose triple-drug regimen of albendazole + diethylcarbamazine + ivermectin for lymphatic filariasis in Papua New Guinea: an open-label, cluster-randomised trial. PLoS Negl Trop Dis. 2022;16(2):e0010096. doi: 10.1371/journal.pntd.0010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mugusi S, Habtewold A, Ngaimisi E, Amogne W, Yimer G, Minzi O, et al. Impact of population and pharmacogenetics variations on efavirenz pharmacokinetics and immunologic outcomes during anti-tuberculosis co-therapy: a parallel prospective cohort study in two Sub-Sahara African populations. Front Pharmacol. 2020;11:26. doi: 10.3389/fphar.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petros Z, Kishikawa J, Makonnen E, Yimer G, Habtewold A, Aklillu E. HLA-B(*)57 allele is associated with concomitant anti-tuberculosis and antiretroviral drugs induced liver toxicity in Ethiopians. Front Pharmacol. 2017;8:90. doi: 10.3389/fphar.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barry A, Olsson S, Khaemba C, Kabatende J, Dires T, Fimbo A, et al. Comparative assessment of the pharmacovigilance systems within the Neglected Tropical Diseases Programs in East Africa-Ethiopia, Kenya, Rwanda, and Tanzania. Int J Environ Res Public Health. 2021;18(4):1941. doi: 10.3390/ijerph18041941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barry A, Olsson S, Minzi O, Bienvenu E, Makonnen E, Kamuhabwa A, et al. Comparative assessment of the national pharmacovigilance systems in East Africa: Ethiopia, Kenya, Rwanda and Tanzania. Drug Saf. 2020;43(4):339–350. doi: 10.1007/s40264-019-00898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiguba R, Olsson S, Waitt C. Pharmacovigilance in low- and middle-income countries: a review with particular focus on Africa. Br J Clin Pharmacol. 2023;89(2):491–509. doi: 10.1111/bcp.15193. [DOI] [PubMed] [Google Scholar]
- 24.Mombasa County Government. Taskforce on healthcare system in Mombasa county. Available from: https://www.mombasa.go.ke/documents/. Accessed May 2023.
- 25.Khaemba C, Barry A, Omondi WP, Bota K, Matendechero S, Wandera C, et al. Safety and tolerability of mass diethylcarbamazine and albendazole administration for the elimination of lymphatic filariasis in Kenya: an active surveillance study. Pharmaceuticals. 2021;14(3):264. doi: 10.3390/ph14030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. A practical handbook on the pharmacovigilance of antiretroviral medicines. 2009. https://apps.who.int/iris/handle/10665/44236. Accessed 27 Jul 2023.
- 27.World Health Organization. Preventive chemotherapy in human helminthiasis : coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. 2006. https://apps.who.int/iris/handle/10665/43545. Accessed 27 Jul 2023.
- 28.Evidence Action. Charting the path for a new evidence-based approach to combating lymphatic filariasis piloting triple-drug therapy in Kenya. https://www.evidenceaction.org/wp-content/uploads/2020/01/Charting-the-path-for-a-new-evidence-based-approach-to-combating-Lymphatic-Filariasis-in-Kenya.pdf. Accessed 26 Feb 2023.
- 29.Fimbo AM, Minzi OM, Mmbando BP, Gurumurthy P, Kamuhabwa AAR, Aklillu E. Safety and tolerability of ivermectin and albendazole mass drug administration in lymphatic filariasis endemic communities of Tanzania: a cohort event monitoring study. Pharmaceuticals. 2022;15(5):594. doi: 10.3390/ph15050594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabatende J, Barry A, Mugisha M, Ntirenganya L, Bergman U, Bienvenu E, et al. Safety of praziquantel and albendazole coadministration for the control and elimination of schistosomiasis and soil-transmitted helminths among children in Rwanda: an active surveillance study. Drug Saf. 2022;45(8):909–922. doi: 10.1007/s40264-022-01201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf. Accessed 27 Jul 2023.
- 32.Bjerum CM, Ouattara AF, Aboulaye M, Kouadio O, Marius VK, Andersen BJ, et al. Efficacy and safety of a single dose of ivermectin, diethylcarbamazine, and albendazole for treatment of lymphatic filariasis in Cote d'Ivoire: an open-label randomized controlled trial. Clin Infect Dis. 2020;71(7):e68–75. doi: 10.1093/cid/ciz1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edi C, Bjerum CM, Ouattara AF, Chhonker YS, Penali LK, Meite A, et al. Pharmacokinetics, safety, and efficacy of a single co-administered dose of diethylcarbamazine, albendazole and ivermectin in adults with and without Wuchereria bancrofti infection in Cote d'Ivoire. PLoS Negl Trop Dis. 2019;13(5):e0007325. doi: 10.1371/journal.pntd.0007325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomsen EK, Sanuku N, Baea M, Satofan S, Maki E, Lombore B, et al. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of Bancroftian filariasis. Clin Infect Dis. 2016;62(3):334–341. doi: 10.1093/cid/civ882. [DOI] [PubMed] [Google Scholar]
- 35.Report on active surveillance for adverse events following the use of drug co-administrations in the global programme to eliminate lymphatic filariasis. Wkly Epidemiol Rec. 2003;78(36):315–7. [PubMed]
- 36.Colombo D, Zagni E, Nica M, Rizzoli S, Ori A, Bellia G. Gender differences in the adverse events' profile registered in seven observational studies of a wide gender-medicine (MetaGeM) project: the MetaGeM safety analysis. Drug Des Devel Ther. 2016;10:2917–2927. doi: 10.2147/DDDT.S97088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebreyesus TD, Makonnen E, Tadele T, Gashaw H, Degefe W, Gerba H, et al. Safety surveillance of mass praziquantel and albendazole co-administration in school children from southern Ethiopia: an active cohort event monitoring. J Clin Med. 2022;11(21):6300. doi: 10.3390/jcm11216300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson S, Caster O, Rochon PA, den Ruijter H. Reported adverse drug reactions in women and men: aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine. 2019;17:100188. doi: 10.1016/j.eclinm.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapeyre-Mestre M. Considering sex-specific adverse drug reactions should be a priority in pharmacovigilance and pharmacoepidemiological studies. EClinicalMedicine. 2019;17:100216. doi: 10.1016/j.eclinm.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunawardena S, Sri Ranganathan S, Fernandopulle R. Pharmacovigilance through consumer feedback (reporting) in the mass treatment of lymphatic filariasis using diethylcarbamazine and albendazole in two districts of Sri Lanka. Trop Med Int Health. 2008;13(9):1153–1158. doi: 10.1111/j.1365-3156.2008.02120.x. [DOI] [PubMed] [Google Scholar]
- 41.Moulia-Pelat JP, Nguyen LN, Glaziou P, Chanteau S, Ottesen EA, Cardines R, et al. Ivermectin plus diethylcarbamazine: an additive effect on early microfilarial clearance. Am J Trop Med Hyg. 1994;50(2):206–209. doi: 10.4269/ajtmh.1994.50.206. [DOI] [PubMed] [Google Scholar]
- 42.Vuong M, Gonzalez Aragon C, Montarroyos SS. Common food and drug interactions. Pediatr Rev. 2023;44(2):68–80. doi: 10.1542/pir.2022-005641. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization . Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030 Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization; 2020. [Google Scholar]