Abstract
Purpose
Pollution of the environment with all kinds of plastics has become a growing problem. The problem of microplastics is mainly due to the absorption of stable organic pollutants and metals into them, and as a result, their environmental toxicity increases. The main purpose of this study is to investigate the appropriate and efficient methods of removing microplastics from aqueous environments through a systematic review.
Methods
Present study designed according to PRISMA guidelines. Two independent researchers followed all process from search to final analysis, for the relevant studies using international databases of PubMed, Scopus and ISI/WOS (Web of Science), without time limit. The search strategy developed based on the main axis of “microplastics”, “aqueous environments” and “removal”. This research was carried out from 2017 until the March of 2022. All relevant observational, analytical studies, review articles, and a meta-analysis were included.
Results
Through a comprehensive systematic search we found 2974 papers, after running the proses of refining, 80 eligible papers included to the study. According to the results of the review, the methods of removing microplastics from aquatic environments were divided to physical (12), chemical (18), physicochemical (27), biological (12) and integrated (11) methods. In different removal methods, the most dominant group of studied microplastics belonged to the four groups of polyethylene (PE), polystyrene (PS), polypropylene (PP) and polyethylene tetra phthalate (PET). Average removal efficiency of microplastics in different processes in each method was as: physical method (73.76%), chemical method (74.38%), physicochemical method (80.44%), biological method (75.23%) and integrated method (88.63%). The highest removal efficiency occurred in the processes based on the integrated method and the lowest efficiency occurred in the physical method. In total, 80% of the studies were conducted on a laboratory scale, 18.75% on a full scale and 1.25% on a pilot scale.
Conclusion
According to the findings; different processes based on physical, chemical, physicochemical, biological and integrated methods are able to remove microplastics with high efficiency from aqueous environments and in order to reduce their hazardous effects on health and environment, these processes can be easily used.
Keywords: Microplastic, Nanoplastic, Microplastic removal methods, Aqueous environment
Introduction
Recently, microplastics (MPs) have become a controversial issue, mainly due to the adsorption of persistent organic pollutants and metals to them, and as a result, their environmental toxicity is intensified [1]. In fact, plastic waste is currently considered as one of the biggest environmental problems because millions of tons of plastic are produced annually in the world and many of the plastic wastes that pollute the aquatic environment are microplastics [2]. Microplastics are small plastic parts, fibers and granules that are defined in different sizes with a diameter less than 5 millimeters [3]. The main concern about these particles is related to their ability to collect large amounts of two pathogenic substances called PCBs and PAHs and adsorption of these substances by the tissues of the digestive system. Due to the long shelf life and bioaccumulation of microplastics in nature, the World Health Organization has classified these particles as emerging pollutants [4]. Today, there are two main categories for microplastics, which are defined as primary and secondary microplastics. The first category is plastic pieces or particles that are less than 5 mm in size before entering the environment, such as microfibers from clothes, small grains, and plastic tablets. When larger pieces of plastic materials enter the environment through natural weather changes, they are affected by the sun’s UV rays and physical factors, etc., after which physical, mechanical, photolytic or biological decomposition occurs, so they create the secondary type of microplastics [3, 5]. The widespread presence of MPs in various water bodies, for example oceans and urban waters (lakes, rivers, sewage and drinking water), has caused scientific and public concern due to their adverse effects on aquatic organisms [6]. Many effects of plastic waste have been reported on the marine environment. Every year, 5800 artificial waste particles are swallowed by each person, most of which comes from tap water. Both micro- and nanoplastics (NPs) may have severe consequences of chronic toxicity in aquatic life; However, due to their ability to penetrate the membranes of living organisms, NPs may be carriers for many pathogens and sorbents for many toxic [7, 8] and hydrophobic organic pollutants such as heavy metals, pesticides, polychlorinated biphenyls, polyaromatic hydrocarbons due to their high level and have more threat potential [9–11]. Review of previous studies show that in general, different methods have been used to remove microplastics from water sources. In recent years, many researches have been conducted all over the world to remove microplastics from ocean water, replace microbeads with natural materials and use less plastic materials, but there is still little information about the removal of microplastics from drinking water. However, considering that the size of these particles is between 1 and 5 microns, water treatment with new filtration methods such as: ultrafiltration (UF), membrane (MBR), reverse osmosis (RO) and carbon filters can separate these harmful substances from drinking water, effectively [12].
With respect to investigations on various methods show the application of a wide range of different physical, chemical and biological processes to remove microplastics from aqueous environments, which are mainly carried out on a laboratory and full scales. Due to different harmful effects of MPs on the human health and environment such as; environmental toxicity, long shelf life and bioaccumulation of microplastics in nature, and public concern due to their adverse effects on aquatic organisms and may be carriers for many pathogens and sorbents for many toxic, the main purpose of this study is to investigate various appropriate and efficient methods of removing microplastics from aqueous environments through a systematic review.
Materials and methods
This study is a systematic review that examines “methods for removing microplastics in aqueous environments”. In this research, a comprehensive and complete search of scientific documents published in PubMed, Scopus and ISI/WOS (Web of Science) databases was conducted. The main criteria are based on the words “microplastics”, “aqueous environments” and “removal”, and all related keywords are based on these three axes were considered. The strategy of searching articles according to the keywords selected in this research, is depicted in Table 1. Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) strategy was used in this study. The PRISMA statement was published in 2009 to improve the quality of systematic review and meta-analysis reports [13]. PRISMA strategy consists of four stages including identification, screening, eligibility and inclusion. in the first step, the articles related to the subject are identified in the relevant databases. In the second stage (screening), articles that are not related to the topic in terms of the title and abstract are removed from the study. In the third stage (eligibility), articles are carefully examined from the point of view of content, and articles that are not related to the subject in terms of materials, methods, and results are discarded. And finally, in the last stage (Included), the articles that do not match the subject in terms of data extraction and compliance with the qualitative evaluation criteria will be removed and the remaining articles will be chosen as selected articles. The keywords used in this search were combined with the medical subject indexes (Mesh) and with the abstract and title. To retrieve all related articles, searching in databases was combined with selected keywords and their synonyms by “AND” and “OR” operators. In order to increase the sensitivity in the search, the operator “OR” was used between synonyms of keywords. Therefore, for this reason, more articles were found in the initial search from the selected databases, which at first glance, some of them had duplicate and unrelated titles. In the next step, in order to increase the specificity or to make the searched titles specific, all the titles were carefully checked twice from the point of view of repetition and relevance. At this stage, many articles that were not related to the research topic were removed from the list of searched sources, and only articles with related titles (methods for removing microplastics from aqueous environments) were selected for the next stage (abstract review). In this study, in order to determine the inclusion and exclusion criteria of various experimental studies in screening the full text of articles, the scoring method proposed by Cho et al. and Timmer et al. was used as a model for qualitative evaluation of quantitative studies [14].
Table 1.
The strategy of searching articles according to the keywords selected in this research
| Pubmed |
|---|
| ((((((“MPs“[Abstract]) OR Nanoplastic*[Abstract]) OR Plastic*[Abstract]) OR Microplastics [MeSH Major Topic])) AND (((“aquatic environment“[Abstract]) OR “Aqueous Environment“[Abstract]) OR water[Abstract])) AND ((((Removal[Abstract]) OR Elimination[Abstract]) OR Degradation[Abstract]) OR treatment[Abstract]) |
| Scopus |
| ( ( ( TITLE-ABS-KEY ( microplastic* ) OR TITLE-ABS-KEY ( “MPs” ) OR TITLE-ABS-KEY ( nanoplastic* ) ) ) AND ( ( TITLE-ABS-KEY ( “aquatic environment” ) OR TITLE-ABS-KEY ( “Aqueous Environments” ) OR TITLE-ABS-KEY ( water ) ) ) ) AND ( ( TITLE-ABS-KEY ( removal ) OR TITLE-ABS-KEY ( elimination ) OR TITLE-ABS-KEY ( degradation ) OR TITLE-ABS-KEY ( treatment ) ) ) AND ( EXCLUDE ( SUBJAREA , “COMP” ) OR EXCLUDE ( SUBJAREA , “DENT” ) OR EXCLUDE ( SUBJAREA , “NEUR” ) OR EXCLUDE ( SUBJAREA , “NURS” ) OR EXCLUDE ( SUBJAREA , “ARTS” ) ) |
| ISI/WOS |
|
TOPIC: (Microplastic*) OR TS=(“MPs”) OR TS=(Nanoplastic*) Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI Timespan = All years TOPIC: (“aquatic environment”) OR TOPIC: (“Aqueous Environments”) OR TOPIC: (water) Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI Timespan = All years TOPIC: (Removal) OR TOPIC: (Elimination) OR TOPIC: (Degradation) OR TOPIC: (treatment) Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI Timespan = All years Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
As mentioned in the introduction section, in this research, the methods of removing microplastics in aqueous environments are divided into four main methods; physical, chemical, physicochemical, biological and a combination of these methods (e.g., integrated method). Then the results were written in the respective tables and according to the divided methods. In these tables, the information extracted from the studies were divided into columns including: the purpose of the study, the name of the first author and the year of publication, the name of the country, the model and design of the study, the type of microplastic, the treatment method, the removal efficiency, the summary of the study and the points obtained.
Results and discussion
The review of the studies conducted on the methods and processes of removing microplastics from aqueous environments shows that different physical, chemical, physicochemical, biological and integrated methods have been used to remove them. Among the important and major processes used in removing microplastics from aqueous environments, can be mentioned to physicochemical processes like, filtration [12, 15–17], adsorption [18, 19, 8], adsorption with biochar [9, 20] and flotation with dissolved air [21], chemical coagulation [9, 22–30], electrocatalysis, carbon nanotubes [7], electrocoagulation [31], application of radiation [32, 33], electro-oxidation [34]; coagulation and filtration [4, 10, 35, 36], adsorption and thermal degradation [37], coagulation and flocculation [4, 38], coagulation and sedimentation [39], precipitation [40], photo-catalysts [41–45], centrifuge [46], flotation [1, 47, 48], coagulation and clarification [49] and carbon nanotubes [50]; Biological methods include activated sludge [51–53], use of fungi [5, 54], microbial consortium [55], microalgae [56], sea shells [57, 58], biofilm [59], Biofilter [60], and wetlands [61, 62] and integrated processes such as water and wastewater treatment plants [63–66].
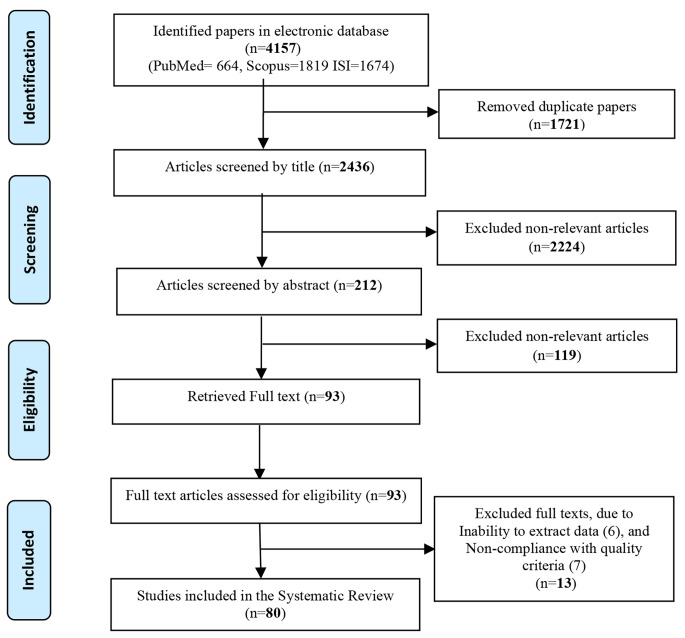
First, an advanced search was performed from the three databases mentioned above and according to the truth table designed based on valid keywords, and a total of 4157 articles were found. All found articles were entered in EndNote software. After first stage removal of duplicate studies in the EndNote software, the number of articles decreased to 2974. Then, the number of articles was reduced to 2436 in the second stage removal of duplicate studies in EndNote. In the next step, unrelated articles were screened according to the available titles and reduced to 212 according to the related titles. At the stage of review of abstracts, the number of selected articles for full text review reached 93 articles. Then, in the review of complete articles, the number of selected articles by checking the desired references, finally reached 80, which were selected as the selected articles of the present study. The steps of selection and screening of articles are given in Fig. 1 by PRISMA method.
Fig. 1.
Flowchart of selection and screening of selected articles in this study (PRISMA Flowchart)
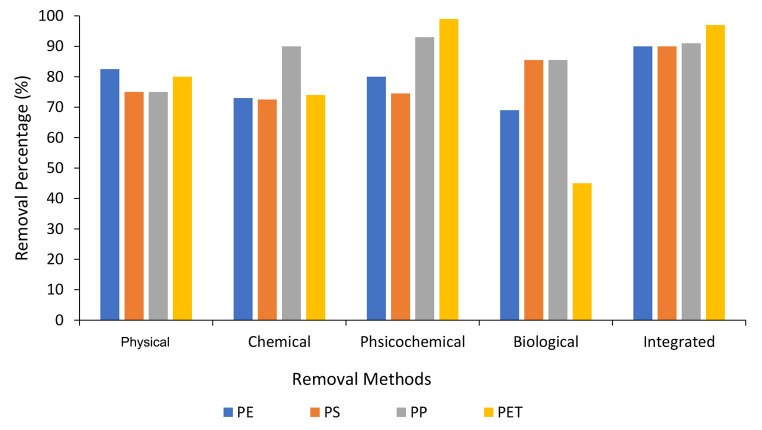
The number of articles and types of methods for removing microplastics from the aqueous environment in this study are given in an overview in different years in Table 2; Fig. 2, respectively. By examining the available studies in this research, the main microplastics removed in different removal methods with the processes used were in four groups: polyethylene (PE), polystyrene (PS), polypropylene (PP), and polyethylene tetraphthalate (PET) (Table 3). Average removal percentages of different MPs from aqueous environment in different removal methods is shown in Fig. 3.
Table 2.
The number of articles according to the types of methods for removing microplastics from the aqueous environment in the present study
| No. | Removal Method | Removal process | Major microplastic | Year of publication | Country | Number of articles |
|---|---|---|---|---|---|---|
| 1 | Physical | Adsorption, filtration, flotation with dissolved air | Polystyrene, polyethylene, polyamide | 2020–2022 | India, China, Spain, Indonesia, Switzerland, Finland, Taiwan | 12 |
| 2 | Chemical | Coagulation, electrocatalysis, electrooxidation, nanocarbon, UV | Polystyrene, polyethylene, polypropylene, polyvinyl chloride | 2018–2022 | China, Australia, South Korea, Switzerland, Sweden, America, Canada | 18 |
| 3 | Physico-chemical | Coagulation and filtration, adsorption and thermal degradation, photocatalyst, magnetic carbon nanotubes | Polystyrene, polyethylene, polypropylene, polyvinyl chloride, polyamide | 2019–2021 | China, Sweden, America, Canada, Malaysia, India, Mexico, Germany, Spain, Iran | 27 |
| 4 | Biological | Using fungi, bacterial consortium, microalgae, biofilter, biofilm | Polystyrene, polyethylene, polypropylene, polyvinyl chloride, polyamide, polyethylene tetraphthalate | 2017–2021 | China, South Korea, Canada, Iceland, Denmark, Italy, Saudi Arabia | 12 |
| 5 | Integrated | biological membrane (MBR), Rapid sand filter (RSF), oxidation channel system and rapid sand filter (RSF), oxidation channel (OD) and membrane bioreactor (MBR), A2/O, secondary sedimentation, denitrification, UF, O3, UV | Polystyrene, polyethylene, polypropylene, polyvinyl chloride, polyamide, polyethylene tetraphthalate | 2017–2021 | China, South Korea, Finland, Italy, Spain, Belgium, Turkey | 11 |
Fig. 2.
Number of selected articles in different years according to different removal methods
Table 3.
Comparison of the removal methods of polyethylene, polystyrene, polypropylene and polyethylene terephthalate microplastics in different removal processes
| Removal Method | Removal process | Removal efficiency (%) | |||
|---|---|---|---|---|---|
| PE | PS | PP | PET | ||
| Physical | Adsorption | NA | 81 | NA | 100 |
| Filteration | 96 | 90 | NA | NA | |
| Adsorption using biochar | NA | 54 | NA | NA | |
| flotation with dissolved air | 69 | NA | NA | 61 | |
| Chemical | Coagulation | 64 | 85 | NA | 74 |
| Electrocoagulation | 82 | NA | 90 | - | |
| Electrooxidaition | NA | 60 | NA | NA | |
| VU radiation of zinc oxide nanotubes | NA | NA | NA | NA | |
| Physico-chemical | Coagulation and filtration | 57 | NA | NA | NA |
| Coagulation and sedimentation | NA | 80 | NA | NA | |
| Coagulation and flotation | 89 | NA | NA | NA | |
| Coagulation and flocculation and sedimentation and filtration | 90 | 90 | NA | NA | |
| Adsorption and thermal degradation | NA | 97 | NA | NA | |
| Photocatalytic | 83 | NA | NA | NA | |
| Thermophotocatalytic | NA | NA | 89 | NA | |
| Afran coagulating gas | NA | 94 | NA | NA | |
| Magnetic carbon nanotubes | 100 | NA | NA | NA | |
| Organosyls | 97 | 58 | 97 | NA | |
| Laser beam and sunlight | NA | 54 | NA | NA | |
| Ferrofluid | NA | NA | NA | 99 | |
| Nano ferrofluid | 49 | 49 | NA | NA | |
| Biological | Activated sludge process | 98 | NA | 98 | 17 |
| Zalerion maritimum mushroom | 43 | NA | NA | NA | |
| Wetland | NA | 73 | 73 | 73 | |
| Shell | 66 | NA | NA | NA | |
| Integrated | Membrane biological reactor | 86 | 84 | 86 | 98 |
| Rapid sand filter (RSF) | 75 | 75 | 75 | NA | |
| Extended activated sludge | 90 | NA | 90 | NA | |
| Oxidation ditch and RSF | 97 | 97 | 97 | 97 | |
| Wetland with vertical flow | 98 | 98 | 98 | NA | |
| Adsorption and electrocoagulation | 92 | NA | 92 | NA | |
| A2/O, secondary sedimentation, denitrification, UF, O3, UV | NA | 95 | 95 | 95 | |
PE: Polyethylene; PS: Polystyrene; PP: Polypropylene; PET: Polyethylene terephthalate; NA: Not available
Fig. 3.
Removal percentages of different MPs from aqueous environment in different removal methods
The purpose of this systematic review study was to investigate various methods of removing microplastics from aqueous environments. From the point of view of the scale of the studies conducted in this research, it shows that 64 of the studies are on a laboratory scale (80%), 15 are on a full scale (18.75%) and one is on a pilot scale (1.25%). Among the 80 selected articles during frame times from 2017 to 2022, 15% of articles were assigned to the physical, 22.5% to the chemical, 33.75% to the physicochemical, 15% to the biological, and 13.75% to the integrated methods. Therefore, it can be concluded that, on the one hand, research on the methods of removing microplastics is new and has accelerated in recent years due to their health importance (e.g., 2.5% in 2017, 3.75% in 2018, 12.5% in 2019, 30% in 2020, 47.5% in 2021 and 3.75% in 2022). It should be noted that the low number of articles in 2022 is due to the completion of the search by the end of March 2022. On the other hand, the methods used are diverse and include physical, chemical, physicochemical, biological and integrated methods with different processes (Table 2; Fig. 3).
Comparing the removal efficiency of various types of microplastics from the aqueous environment
According to the studies conducted in this research, among the various studied microplastics (including: polyethylene, polystyrene, polypropylene and polyethylene tetra phthalate, microfibers, polyamines, polyvinyl chlorides, polymethyl methacrylate, cellulose acetate) in different methods of removal, the most dominant group of removed microplastics belonged into four groups: polyethylene, polystyrene, polypropylene and polyethylene tetraphthalates.
In the methods used to remove polyethylene microplastic, the removal efficiency of integrated, physical, physicochemical, chemical, and biological methods were 90%, 82.5%, 81%, 72%, and 69%, respectively. In the study that was done by Kim and Park (2020) for the advanced removal of polyethylene microplastics from wastewater using electrocoagulation and granular activated carbon with thermal regeneration; Granular activated carbon (GAC) has been able to remove more than 92% of all polyethylene particles in the influent. On the other hand, electrocoagulation was able to increase the removal efficiency in 30 min after coagulation. The results of this study show the good efficiency of the integrated method in removing polyethylene microplastics [67].
In the methods used to remove polystyrene microplastic, the removal efficiency of integrated, biological, physical, physicochemical and chemical methods were 90%, 85.5%, 75%, 74.5%, and 72%, respectively. The study conducted by Wang et al. (2021) for the removal of polyethylene and polystyrene microplastics using lagoons constructed with vertical flow showed that this process as an integrated method was succeeded in removing polyethylene microplastics with the efficiency 98% [68].
In the methods used to remove polyethylene tetraphthalate microplastic, the removal efficiency of physicochemical, integrated, physical, chemical, and biological methods were 99%, 97%, 80%, 74%, and 45%, respectively. In a study by Hamzah et al. (2021) for the removal of polyethylene tetra phthalate microplastics using ferrofluid function; this method is considered as a physico-chemical method with an efficiency of over 99%, a high efficiency process to remove this microplastic [69].
In the methods used to remove polypropylene microplastics, the removal efficiency of physicochemical, integrated, biological and chemical methods were 93%, 90%, 85.5% and 77%, respectively. In a study conducted by Sturm et al. (2021) for the removal of polypropylene microplastics, it was shown that the new method of organosilanes has a great potential to remove this microplastic on a technical scale, and the chemical composition and surface chemistry of microplastics have a great impact on removal and physical interaction with organosilanes process [70]. Therefore, this process as a physicochemical method has been highly effective in removing this microplastic.
Comparing the removal of microplastics from the aqueous environment based on removal methods
In comparison of the removal efficiency of the processes based on physical methods, the filtration, the adsorption, the adsorption with biochar, and the flotation with dissolved air have the highest removal efficiency of a set of dominant microplastics, respectively. In the processes based on chemical methods, the highest removal efficiency were electrocoagulation, coagulation, electrocatalysis, zinc oxide nanotubes visible light irradiation process, electrooxidation process and carbon nanotubes process, respectively. The removal efficiency of processes based on physicochemical methods, the highest removal efficiency were magnetic carbon nanotubes, sedimentation, ferrofluid, adsorption and thermal degradation process, artificial foams, afran coagulant gas, filtration and centrifugation, Coagulation and flotation, thermophotocatalytic process, photocatalytic process, coagulation and flocculation process with sedimentation and filtration, coagulation and sedimentation process, coagulation and filtration process, organocells, laser beam and sunlight and nano-ferrofluid, respectively. In the processes based on biological methods, the highest removal efficiency were sequencing batch reactor, activated sludge process, wetland process, oyster, periphytic biofilter and Zalarion-maritimum mushroom, respectively. Also, in the comparison of the removal efficiencies of the processes based on integrated methods, the highest removal efficiency were the wetland with vertical flow, A2/O, secondary clarifier, denitrification, UF, O3, UV, flotation with dissolved air, adsorption and electrocoagulation, activated sludge, extended aeration, membrane biological reactor process, oxidation channel system and rapid sand filter, trash removal, granulation and conventional activated sludge with screening and grit removal, rapid sand filter and disc filters, respectively.
By calculating the removal efficiency of microplastics in each of the physical, chemical, physicochemical, biological and integrated methods for different and major groups of microplastics, the removal efficiency in the integrated, physicochemical, physical, biological and chemical, methods were 88.63%, 80.44%, 76.73%, 74.38%, 75.23%, respectively;that the highest efficiency of removing microplastics occurred in the processes based on the integrated method and the lowest efficiency occurred in the physical method.
In a study conducted by Olmos et al. in Spain in 2019, the effectiveness of combined processes for removing low-density and high-density polyethylene, polypropylene, and nylon were investigated. These processes were a combination of extended aeration activated sludge process (ASP), rapid sand filter (RSF) and membrane bioreactor (MBR). The reduction of microplastics from primary effluent to final effluent was 90.2% for ASP, 93.8% for RSF, and 96.2% for MBR, respectively [71]. In a study conducted by Yang et al. in 2019 under the title “removal of microplastics in urban wastewater from China’s largest water treatment plant”, the most common microplastics of polyethylene tetra phthalate, polystyrene and polypropylene were removed by the A2/O process, secondary sedimentation, denitrification, UF, O3, UV with an efficiency of over 95% [66]. These two studies show two types of studies of integrated methods with the highest efficiency in removing microplastics from the aqueous environment.
Examining the performance of different processes in removing different types of microplastics from aqueous environments shows the different efficiency of these processes in removing these pollutants. For example, in the study of “Performance of single media rapid sand filter to remove microplastics” that uses rapid sand filter (RSF) with silica sand to remove plastic bags and pieces of rubber, with sizes from 10 to more than 500 micrometer; the removal efficiency in this method for different effective sizes (ES) of filter media varied from 90.6 to 97.7% [15]. Also, in a study conducted by Wang et al. in 2020 on the use of filters containing biochar and sand filters in the filtration of spherical polystyrene microplastics; for all biochars, the filter efficiency for removing spherical microplastics was higher than 95% [17]. The study conducted by Shen et al. in 2022 on “Removing microplastics from wastewater by electrocoagulation process” showed that the electrocoagulation is used to remove microplastics of polyethylene, polymethyl methacrylate, cellulose acetate and poly Propylene with efficiencies of 82%, 74%, 92% and 90%, respectively, introduced this method as a high efficiency process to remove microplastics [31]. The study of Tang et al. in 2021 on the use of magnetic carbon nanotubes (M-CNT) method to remove microplastics from aqueous solutions showed that the efficiency of removing microplastics along with increasing the dose of M- CNTs increased and reached nearly 100% within 180 min. The analysis of the mechanism clearly showed that the adsorption of M-CNTs by polyethylene is due to the strong hydrophobicity of microplastics. Therefore, according to the specified characteristics of M-CNT, it shows that they can be used as an efficient, economical and environmentally friendly material to remove microplastics in aqueous environment recovery and wastewater treatment [50]. In a study conducted by Lee and Kim in 2018 in biological wastewater treatment facilities to remove microplastics; showed that more than 98% of microplastics were removed in the A2/O, SBR and bioreactor [52].
Among the strengths of this study compared to previous studies, the following can be mentioned:
In the present study, all the processes used in the selected articles to remove microplastics in aqueous environments are classified according to the type of process into physical, chemical, physicochemical, biological, and integrated methods, and different methods were compared based on the efficiency of microplastic removal;
In this study, the most appropriate and practical processes and methods for removing microplastics from aqueous environments until 2022 were investigated, summarized and presented.
Conclusion
The removal efficiency of the four dominant types of microplastics, polyethylene, polystyrene, polypropylene and polyethylene tetraphthalate, were compared in selected studies, and the most effective methods used to remove polyethylene microplastics were integrated, physical, physicochemical, chemical and biological methods, respectively. Regarding the removal of polystyrene microplastics, the methods of integrated, biological, physical, physicochemical and chemical were highly efficient, respectively. In the removal of polyethylene tetraphthalate microplastic, the methods of physicochemical, integrated, physical, chemical and biological and for the removal of polypropylene microplastic, the methods of physicochemical, integrated, biological and chemical showed high performance, respectively.
Examining the average removal efficiency of microplastics in each of the physical, chemical, physicochemical, biological and integrated methods for different groups of microplastics, the removal efficiency in the processes based on the integrated method is 88.63%, in the physicochemical method 80.44%, in the biological method 75.23%, in the chemical method 74.38% and in the physical method equal to 73.76%. Also, due to lack of quantitative data information for some subgroups of removal method categories, it will be difficult to summarize the best removal method in all categories. Therefore, with respect to that point, we have concluded that the “integrated methods” as the best removal method based on the average removal efficiencies in different types of MPs.
Therefore, highest efficiency of removing microplastics was in the processes based on the integrated method (PE, PS, PP using wetland with vertical flow and PET using membrane biological reactor), and the lowest efficiency was in the physical method. Finally, it can be concluded that different processes in physical, chemical, physicochemical, biological and integrated methods are able to remove different microplastics with high efficiency from aqueous environments and in order to reduce their hazardous effects on health and environment, these processes can be easily used.
Limitations and strengths of the study
Some of the limitations of the study are given below:
Publication bias resulting from the exclusion of some types of study designs from the systematic review;
Lack of access to articles or full version of articles in databases.
Among the strengths of this study compared to previous studies, the following can be mentioned:
In the present study, all the processes used in the selected articles to remove microplastics in aqueous environments were categorized based on the type of process into physical, chemical, physicochemical, biological and integrated methods, and their removal efficiency was compared ;
In this study, the best processes and different methods for removing microplastics from aqueous environments were summarized and presented by comparing the efficiency of removing processes;
In this study, the latest technologies used in the removal of microplastics from aqueous environments in the world and Iran were examined in detail;
Low cost of the study process.
Acknowledgements
The authors gratefully acknowledge the assistant of Department of Environmental Health Engineering, School of Health, Iran University of Medical Sciences (IUMS).
Authors’ Contributions
A.A. conducted the experiments and wrote the manuscript, M.Gh. supervised and supported and edited the manuscript, Sh.Dj. designed methodology and advised epidemiological and statistical methods, M.F. observed and advised the scientific content of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Data Availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
this study has been approved by the ethical committee of The Iran University of Medical Sciences (IUMS).
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mitra Gholami, Email: gholamim@iums.ac.ir, Email: gholamimitra32@gmail.com.
Shirin Djalalinia, Email: shdjalalinia@gmail.com.
References
- 1.Esfandiari A, Mowla D. Investigation of microplastic removal from greywater by coagulation and dissolved air flotation. Process Saf Environ Prot. 2021;151:341–54. doi: 10.1016/j.psep.2021.05.027. [DOI] [Google Scholar]
- 2.Moreschi AC, Callil CT, Christo SW, Ferreira Junior AL, Nardes C, de Faria É, et al. Filtration, assimilation and elimination of microplastics by freshwater bivalves. Case Stud Chem Environ Eng. 2020;2. 10.1016/j.cscee.2020.100053.
- 3.Bayo J, López-Castellanos J, Olmos S. Abatement of microplastics from municipal effluents by two different wastewater treatment technologies. WIT Trans Ecol Environ. 2020;242:15–26. doi: 10.2495/WP200021. [DOI] [Google Scholar]
- 4.Kankanige D, Babel S. Contamination by ≥ 6.5 µm-sized microplastics and their removability in a conventional water treatment plant (WTP) in Thailand. J Water Process Eng. 2021;40. 10.1016/j.jwpe.2020.101765.
- 5.Paco A, Duarte K, da Costa JP, Santos PSM, Pereira R, Pereira ME, et al. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci Total Environ. 2017;586:10–5. doi: 10.1016/j.scitotenv.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Xu Q, Huang QS, Luo TY, Wu RL, Wei W, Ni BJ. Coagulation removal and photocatalytic degradation of microplastics in urban waters. Chem Eng J. 2021;416. 10.1016/j.cej.2021.129123.
- 7.Kang J, Zhou L, Duan XG, Sun HQ, Ao ZM, Wang SB. Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings. Matter. 2019;1(3):745–58. doi: 10.1016/j.matt.2019.06.004. [DOI] [Google Scholar]
- 8.Sun CZ, Wang ZG, Chen LY, Li FM. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem Eng J. 2020;393. 10.1016/j.cej.2020.124796.
- 9.Ganie ZA, Khandelwal N, Tiwari E, Singh N, Darbha GK. Biochar-facilitated remediation of nanoplastic contaminated water: Effect of pyrolysis temperature induced surface modifications. J Hazard Mater. 2021;417. 10.1016/j.jhazmat.2021.126096. [DOI] [PubMed]
- 10.Ma BW, Xue WJ, Hu CZ, Liu HJ, Qu JH, Li LL. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment (vol 359, pg 159, 2019). Chem Eng J. 2021;405. 10.1016/j.cej.2020.126983.
- 11.Zhang Y, Zhou G, Yue J, Xing X, Yang Z, Wang X, et al. Enhanced removal of polyethylene terephthalate microplastics through polyaluminum chloride coagulation with three typical coagulant aids. Sci Total Environ. 2021;800. 10.1016/j.scitotenv.2021.149589. [DOI] [PubMed]
- 12.Pizzichetti ARP, Pablos C, Álvarez-Fernández C, Reynolds K, Stanley S, Marugán J. Evaluation of membranes performance for microplastic removal in a simple and low-cost filtration system. Case Stud Chem Environ Eng. 2021;3. 10.1016/j.cscee.2020.100075.
- 13.Panic NLE, De Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE. 2013;8(12). [DOI] [PMC free article] [PubMed]
- 14.Kmet LMCL, Lee RC. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. 2004.
- 15.Sembiring E, Fajar M, Handajani M. Performance of rapid sand filter - single media to remove microplastics. Water Supply. doi:10.2166/ws.2021.060.
- 16.Shen M, Hu T, Huang W, Song B, Zeng G, Zhang Y. Removal of microplastics from wastewater with aluminosilicate filter media and their surfactant-modified products: performance, mechanism and utilization. Chem Eng J. 2021;421. 10.1016/j.cej.2021.129918.
- 17.Wang Z, Sedighi M, Lea-Langton A. Filtration of microplastic spheres by biochar: removal efficiency and immobilisation mechanisms. Water Res. 2020;184. 10.1016/j.watres.2020.116165. [DOI] [PubMed]
- 18.Arenas LR, Gentile SR, Zimmermann S, Stoll S. Nanoplastics adsorption and removal efficiency by granular activated carbon used in drinking water treatment process. Sci Total Environ. 2021;791. 10.1016/j.scitotenv.2021.148175. [DOI] [PubMed]
- 19.Sun C, Wang Z, Zheng H, Chen L, Li F. Biodegradable and re-usable sponge materials made from chitin for efficient removal of microplastics. J Hazard Mater. 2021;420. 10.1016/j.jhazmat.2021.126599. [DOI] [PubMed]
- 20.Siipola V, Pflugmacher S, Romar H, Wendling L, Koukkari P. Low-cost Biochar Adsorbents for Water Purification Including Microplastics removal. Appl Sciences-Basel. 2020;10(3). 10.3390/app10030788.
- 21.Wang Y, Li Y, Tian L, Ju L, Liu Y. The removal efficiency and mechanism of microplastic enhancement by positive modification dissolved air flotation. Water Environ Res. 2021;93(5):693–702. doi: 10.1002/wer.1352. [DOI] [PubMed] [Google Scholar]
- 22.Arenas LR, Gentile SR, Zimmermann S, Stoll S. Coagulation of TiO2, CeO2 nanoparticles, and polystyrene nanoplastics in bottled mineral and surface waters. Effect of water properties, coagulant type, and dosage. Water Environ Res. 2020;92(8):1184–94. doi: 10.1002/wer.1313. [DOI] [PubMed] [Google Scholar]
- 23.Lapointe M, Farner JM, Hernandez LM, Tufenkji N. Understanding and improving Microplastic removal during Water Treatment: impact of Coagulation and Flocculation. Environ Sci Technol. 2020;54(14):8719–27. doi: 10.1021/acs.est.0c00712. [DOI] [PubMed] [Google Scholar]
- 24.Lee PS, Jung SM. Quantitative analysis of microplastics coagulation-removal process for clean sea salt production. Int J Environ Sci Technol. doi:10.1007/s13762-021-03469-x.
- 25.Lu S, Liu L, Yang Q, Demissie H, Jiao R, An G, et al. Removal characteristics and mechanism of microplastics and tetracycline composite pollutants by coagulation process. Sci Total Environ. 2021;786. 10.1016/j.scitotenv.2021.147508.
- 26.Park JW, Lee SJ, Hwang DY, Seo S. Removal of microplastics: via tannic acid-mediated coagulation and in vitro impact assessment. RSC Adv. 2021;11(6):3556–66. doi: 10.1039/d0ra09645h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peydayesh M, Suta T, Usuelli M, Handschin S, Canelli G, Bagnani M, et al. Sustainable removal of Microplastics and Natural Organic Matter from Water by Coagulation-Flocculation with protein amyloid fibrils. Environ Sci Technol. 2021;55(13):8848–58. doi: 10.1021/acs.est.1c01918. [DOI] [PubMed] [Google Scholar]
- 28.Skaf DW, Punzi VL, Rolle JT, Kleinberg KA. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chem Eng J. 2020;386. 10.1016/j.cej.2019.123807.
- 29.Zhang Y, Zhao J, Liu Z, Tian S, Lu J, Mu R, et al. Coagulation removal of microplastics from wastewater by magnetic magnesium hydroxide and PAM. J Water Process Eng. 2021;43. 10.1016/j.jwpe.2021.102250.
- 30.Zhou G, Wang Q, Li J, Li Q, Xu H, Ye Q, et al. Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: performance and mechanism. Sci Total Environ. 2021;752. 10.1016/j.scitotenv.2020.141837. [DOI] [PubMed]
- 31.Shen M, Zhang Y, Almatrafi E, Hu T, Zhou C, Song B, et al. Efficient removal of microplastics from wastewater by an electrocoagulation process. Chem Eng J. 2022;428. 10.1016/j.cej.2021.131161.
- 32.Cai L, Wang J, Peng J, Wu Z, Tan X. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci Total Environ. 2018;628–629:740–7. doi: 10.1016/j.scitotenv.2018.02.079. [DOI] [PubMed] [Google Scholar]
- 33.Uheida A, Mejia HG, Abdel-Rehim M, Hamd W, Dutta J. Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. J Hazard Mater. 2021;406. 10.1016/j.jhazmat.2020.124299. [DOI] [PubMed]
- 34.Kiendrebeogo M, Estahbanati MRK, Mostafazadeh AK, Drogui P, Tyagi RD. Treatment of microplastics in water by anodic oxidation: a case study for polystyrene. Environ Pollut. 2021;269. 10.1016/j.envpol.2020.116168. [DOI] [PubMed]
- 35.Shahi NK, Maeng M, Kim D, Dockko S. Removal behavior of microplastics using alum coagulant and its enhancement using polyamine-coated sand. Process Saf Environ Prot. 2020;141:9–17. doi: 10.1016/j.psep.2020.05.020. [DOI] [Google Scholar]
- 36.Wang Z, Lin T, Chen W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci Total Environ. 2020;700. 10.1016/j.scitotenv.2019.134520. [DOI] [PubMed]
- 37.Wang J, Sun C, Huang QX, Chi Y, Yan JH. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J Hazard Mater. 2021;419. 10.1016/j.jhazmat.2021.126486. [DOI] [PubMed]
- 38.Zhang Y, Diehl A, Lewandowski A, Gopalakrishnan K, Baker T. Removal efficiency of micro- and nanoplastics (180 nm–125 µm) during drinking water treatment. Sci Total Environ. 2020;720. 10.1016/j.scitotenv.2020.137383. [DOI] [PMC free article] [PubMed]
- 39.Chen ZY, Liu JH, Chen CY, Huang ZJ. Sedimentation of nanoplastics from water with Ca/Al dual flocculants: characterization, interface reaction, effects of pH and ion ratios. Chemosphere. 2020;252. 10.1016/j.chemosphere.2020.126450. [DOI] [PubMed]
- 40.Batool A, Valiyaveettil S. Coprecipitation - an efficient method for removal of Polymer Nanoparticles from Water. ACS Sustainable Chemistry and Engineering. 2020;8(35):13481–7. doi: 10.1021/acssuschemeng.0c04511. [DOI] [Google Scholar]
- 41.Ariza-Tarazona MC, Villarreal-Chiu JF, Barbieri V, Siligardi C, Cedillo-Gonzalez EI. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram Int. 2019;45(7):9618–24. doi: 10.1016/j.ceramint.2018.10.208. [DOI] [Google Scholar]
- 42.Fadli MH, Ibadurrohman M, Slamet S, editors. Microplastic Pollutant degradation in Water using modified TiO photocatalyst under UV-Irradiation2021: IOP Publishing Ltd.
- 43.Maulana DA, Ibadurrohman M, Slamet, editors. Synthesis of Nano-Composite Ag/TiO for Polyethylene Microplastic Degradation Applications2021: IOP Publishing Ltd.
- 44.Razali NA, Abdullah WRW, Zikir NM, EFFECT OF THERMO-PHOTOCATALYTIC PROCESS USING ZINC OXIDE, ON DEGRADATION OF MACRO/MICRO-PLASTIC IN AQUEOUS ENVIRONMENT J Sustain Sci Manage. 2020;15(6):1–14. doi: 10.46754/jssm.2020.08.001. [DOI] [Google Scholar]
- 45.Tofa TS, Ye F, Kunjali KL, Dutta J. Enhanced visible light photodegradation of microplastic fragments with plasmonic platinum/zinc oxide nanorod photocatalysts. Catalysts. 2019;9(10). 10.3390/catal9100819.
- 46.Murray A, Ormeci B. Removal effectiveness of nanoplastics (< 400 nm) with separation processes used for Water and Wastewater Treatment. Water. 2020;12(3). 10.3390/w12030635.
- 47.Pramanik BK, Pramanik SK, Monira S. Understanding the fragmentation of microplastics into nano-plastics and removal of nano/microplastics from wastewater using membrane, air flotation and nano-ferrofluid processes. Chemosphere. 2021;282. 10.1016/j.chemosphere.2021.131053. [DOI] [PubMed]
- 48.Zhang M, Yang J, Kang Z, Wu X, Tang L, Qiang Z, et al. Removal of micron-scale microplastic particles from different waters with efficient tool of surface-functionalized microbubbles. J Hazard Mater. 2021;404. 10.1016/j.jhazmat.2020.124095. [DOI] [PubMed]
- 49.Sarkar DJ, Sarkar SD, Das BK, Praharaj JK, Mahajan DK, Purokait B, et al. Microplastics removal efficiency of drinking water treatment plant with pulse clarifier. J Hazard Mater. 2021;413. 10.1016/j.jhazmat.2021.125347. [DOI] [PubMed]
- 50.Tang Y, Zhang S, Su Y, Wu D, Zhao Y, Xie B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem Eng J. 2021;406. 10.1016/j.cej.2020.126804.
- 51.Lares M, Ncibi MC, Sillanpaa M, Sillanpaa M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018;133:236–46. doi: 10.1016/j.watres.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 52.Lee H, Kim Y. Treatment characteristics of microplastics at biological sewage treatment facilities in Korea. Mar Pollut Bull. 2018;137:1–8. doi: 10.1016/j.marpolbul.2018.09.050. [DOI] [PubMed] [Google Scholar]
- 53.Torena P, Alvarez-Cuenca M, Reza M. Biodegradation of polyethylene terephthalate microplastics by bacterial communities from activated sludge. Can J Chem Eng. 2020 doi: 10.1002/cjce.24015. [DOI] [Google Scholar]
- 54.Corona E, Martin C, Marasco R, Duarte CM. Passive and active removal of Marine Microplastics by a mushroom coral (Danafungia scruposa). Front Mar Sci. 2020;7. 10.3389/fmars.2020.00128.
- 55.Gao RR, Sun CM. A marine bacterial community capable of degrading poly(ethylene terephthalate) and polyethylene. J Hazard Mater. 2021;416. 10.1016/j.jhazmat.2021.125928. [DOI] [PubMed]
- 56.Cunha C, Silva L, Paulo J, Faria M, Nogueira N, Cordeiro N. Microalgal-based biopolymer for nano- and microplastic removal: a possible biosolution for wastewater treatment. Environ Pollut. 2020;263. 10.1016/j.envpol.2020.114385. [DOI] [PubMed]
- 57.Arossa S, Martin C, Rossbach S, Duarte CM. Microplastic removal by Red Sea giant clam (Tridacna maxima) Environ Pollut. 2019;252:1257–66. doi: 10.1016/j.envpol.2019.05.149. [DOI] [PubMed] [Google Scholar]
- 58.Zhou H, Mayorga-Martinez CC, Pumera M. Microplastic removal and degradation by mussel-inspired Adhesive Magnetic/Enzymatic microrobots. Small Methods. 2021 doi: 10.1002/smtd.202100230. [DOI] [PubMed] [Google Scholar]
- 59.Shabbir S, Faheem M, Ali N, Kerr PG, Wang LF, Kuppusamy S, et al. Periphytic biofilm: an innovative approach for biodegradation of microplastics. Sci Total Environ. 2020;717. 10.1016/j.scitotenv.2020.137064. [DOI] [PubMed]
- 60.Liu F, Nord NB, Bester K, Vollertsen J. Microplastics removal from treated wastewater by a biofilter. Water (Switzerland). 2020;12(4). 10.3390/W12041085.
- 61.Wei S, Luo H, Zou J, Chen J, Pan X, Rousseau DPL, et al. Characteristics and removal of microplastics in rural domestic wastewater treatment facilities of China. Sci Total Environ. 2020;739. 10.1016/j.scitotenv.2020.139935. [DOI] [PubMed]
- 62.Zhang L, Liu J, Xie Y, Zhong S, Gao P. Occurrence and removal of microplastics from wastewater treatment plants in a typical tourist city in China. J Clean Prod. 2021;291. 10.1016/j.jclepro.2021.125968.
- 63.Bayo J, López-Castellanos J, Olmos S. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar Pollut Bull. 2020;156. 10.1016/j.marpolbul.2020.111211. [DOI] [PubMed]
- 64.Lv X, Dong Q, Zuo Z, Liu Y, Huang X, Wu WM. Microplastics in a municipal wastewater treatment plant: Fate, dynamic distribution, removal efficiencies, and control strategies. J Clean Prod. 2019;225:579–86. doi: 10.1016/j.jclepro.2019.03.321. [DOI] [Google Scholar]
- 65.Vardar S, Onay TT, Demirel B, Kideys AE. Evaluation of microplastics removal efficiency at a wastewater treatment plant discharging to the sea of Marmara. Environ Pollut. 2021;289. 10.1016/j.envpol.2021.117862. [DOI] [PubMed]
- 66.Yang L, Li K, Cui S, Kang Y, An L, Lei K. Removal of microplastics in municipal sewage from China’s largest water reclamation plant. Water Res. 2019;155:175–81. doi: 10.1016/j.watres.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 67.Kim KT, Park S. Enhancing microplastics removal from wastewater using electro-coagulation and granule-activated carbon with thermal regeneration. Processes. 2021;9(4). 10.3390/pr9040617.
- 68.Wang QT, Hernandez-Crespo C, Du BB, Van Hulle SH, Rousseau DPL. Fate and removal of microplastics in unplanted lab-scale vertical flow constructed wetlands. Sci Total Environ. 2021;778. 10.1016/j.scitotenv.2021.146152. [DOI] [PubMed]
- 69.Hamzah S, Ying LY, Azmi AAAR, Razali NA, Hairom NHH, Mohamad NA, et al. Synthesis, characterisation and evaluation on the performance of ferrofluid for microplastic removal from synthetic and actual wastewater. J Environ Chem Eng. 2021;9(5). 10.1016/j.jece.2021.105894.
- 70.Sturm MT, Horn H, Schuhen K. Removal of microplastics from waters through agglomeration-fixation using organosilanes—effects of polymer types, water composition and temperature. Water (Switzerland) 2021;13(5):1–15. doi: 10.3390/w13050675. [DOI] [Google Scholar]
- 71.Olmos S, López-Castellanos J, Bayo J. Are advanced wastewater treatment technologies a solution for total removal of microplastics in treated effluents? WIT Trans Ecol Environ. 2019;229:109–16. doi: 10.2495/WRM190111. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.