Abstract
Trastuzumab deruxtecan (T-DXd)—an antibody–drug conjugate targeting the human epidermal growth factor receptor 2 (HER2)—improved outcomes of patients with HER2-positive and HER2-low metastatic breast cancer. Guidance on monitoring and managing T-DXd–related adverse events (AEs) is an emerging unmet need as translating clinical trial experience into real-world practice may be difficult due to practical and cultural considerations and differences in health care infrastructure. Thus, 13 experts including oncologists, pulmonologists and a radiologist from the Asia-Pacific region gathered to provide recommendations for T-DXd–related AE monitoring and management by using the latest evidence from the DESTINY-Breast trials, our own clinical trial experience and loco-regional health care considerations. While subgroup analysis of Asian (excluding Japanese) versus overall population in the DESTINY-Breast03 uncovered no major differences in the AE profile, we concluded that proactive monitoring and management are essential in maximising the benefits with T-DXd. As interstitial lung disease (ILD)/pneumonitis is a serious AE, patients should undergo regular computed tomography scans, but the frequency may have to account for the median time of ILD/pneumonitis onset and access. Trastuzumab deruxtecan appears to be a highly emetic regimen, and prophylaxis with serotonin receptor antagonists and dexamethasone (with or without neurokinin-1 receptor antagonist) should be considered. Health care professionals should be vigilant for treatable causes of fatigue, and patients should be encouraged to use support groups and practice low-intensity exercises. To increase treatment acceptance, patients should be made aware of alopecia risk prior to starting T-DXd. Detailed monitoring and management recommendations for T-DXd–related AEs are discussed further.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-023-01328-x.
Key Points
| Trastuzumab deruxtecan (T-DXd) has side effects that include but are not limited to interstitial lung disease/pneumonitis, nausea and vomiting, haematological toxicities, fatigue, hair loss, and cardiac toxicities. |
| A panel of 13 experts in oncology, pulmonology and radiology developed practical recommendations for monitoring and managing these T-DXd–related side effects applicable for Asia-Pacific clinical practice. |
| While subgroup analysis of Asian (excluding Japanese) versus overall population in the DESTINY-Breast03 clinical trial uncovered no major differences in the side effects profile, proactive monitoring and management are key to maximising the benefits conferred by T-DXd for patients with HER2-positive or HER2-low metastatic breast cancer. |
| Patient education on signs and symptoms of T-DXd–related side effects is also important for maximising the benefits with this treatment. |
Introduction
The human epidermal growth factor receptor 2 (HER2) is overexpressed or amplified in approximately 20–30% of women with breast cancer [1, 2]. The eligibility for therapies targeting HER2 is largely driven by this dichotomy between patients presenting with HER2-positive and HER2-negative breast cancers [3–5]. However, clinical trials have now started to report promising anti-tumour activity with newer anti-HER2 antibody–drug conjugates (ADC) in patients who have been traditionally classified as having the HER2-negative subtype [6, 7]. These patients with HER2-low breast cancer, currently defined as having immunohistochemistry scores of 1+ or 2+ in the absence of HER2 gene amplification confirmed by in situ hybridisation, may constitute nearly 50% of patients with breast cancer [6, 7].
Trastuzumab deruxtecan (T-DXd) is an anti-HER2 ADC comprising a humanised anti-HER2 antibody, an enzymatically cleavable peptide-linker, and a topoisomerase I inhibitor [8]. The Phase II and Phase III DESTINY-Breast trials with T-DXd have yielded positive outcomes in patients with both HER2-positive and HER2-low metastatic breast cancer [9–13]. Given that these findings have resulted in swift approvals from major regulatory bodies across the world [14–17], T-DXd is poised to transform the treatment landscape for many patients with metastatic breast cancer.
However, translating experiences from randomised controlled trials into real-world practice can be challenging owing to sociocultural considerations, practicality and differences in health care infrastructures [18–20]. Findings from oncology clinical trials may not be generalisable due to an underrepresentation of minorities [21]. Indeed, while Asian women comprise 39% of breast cancer cases worldwide, pivotal Phase III randomised clinical trials of cyclin-dependent kinase 4/6 inhibitors have underrepresented this population of patients [22, 23]. In contrast, Asian women comprised 38.0%, 59.9% and 40.0% of the study population in DESTINY-Breast01, -Breast03 and -Breast04 trials, respectively [11–13].
Another issue when applying trial data in clinical practice is that patients enrolled in industry-sponsored randomised clinical trials may have greater access to health care infrastructure that is otherwise not available in real-world practice [24]. For instance, 6-weekly computed tomography (CT) scans were conducted in the DESTINY-Breast trials [25–27], but experts have recently started to highlight the impracticality of implementing such a scanning frequency in real-world clinical practice [28, 29].
Furthermore, local and regional differences in health care reimbursement may influence the management of patients with breast cancer [30–32]. Taiwan and South Korea have National Health Insurance systems, where enrolees pay income-based premiums for their coverage that includes most medical expenses for cancer treatment [32, 33]. Hong Kong has universal access to public hospitals and clinics, and the government covers all drugs in the standard formulary and provides financial assistance for some costly drugs [32, 34]. Singapore uses a mixed financing system to ensure universal health coverage, and the government-administered health insurance plan covers some cancer treatments [32, 35]. Singapore also has subsidies and financial assistance for high-cost drugs with co-payment by patients [32, 35]. Australia has government-funded universal coverage of several medical costs, and further coverage could be availed via private health insurance [36, 37].
Given the potential challenges with bridging the gap between T-DXd clinical trial experience and real-world practice, we gathered to provide recommendations for the proactive monitoring and management of T-DXd–related adverse events based on available evidence and our insights from an Asia-Pacific perspective. Moreover, we aim to compare the safety profile in the Asian population to that of the overall population in the DESTINY-Breast03 trial and provide practical recommendations for the monitoring and management of interstitial lung disease (ILD)/pneumonitis, nausea and vomiting, haematological toxicities such as neutropenia, anaemia and thrombocytopenia, fatigue, alopecia and left-ventricular ejection fraction (LVEF) decrease.
Methods
From February 2022 to April 2022, a steering committee of ten medical oncologists, two pulmonologists and one radiologist from Australia, Hong Kong, Taiwan, Singapore and South Korea gathered to develop practical recommendations on the monitoring and management of T-DXd–related adverse events in patients with HER2-positive metastatic breast cancer. This was garnered in two stages. The first stage comprised an online survey that gathered the insights, opinions and recommendations on how to monitor and manage adverse events relevant to real-world practice. The second stage involved the synthesis of the responses into recommendations or additional questions, which were further discussed by experts during a virtual meeting held on 21 April 2022. Using a modified Delphi process [38], recommendations on the monitoring and management of adverse events were developed using the responses and discussions with experts, and a literature search of reviews and guidelines on T-DXd or T-DXd–related adverse events indexed in PubMed and the National Comprehensive Cancer Network (NCCN; refer to supplementary information 1 for details on search terms used). The recommendations were subjected to four rounds of reviews (refer to supplementary information 2 for an overview on recommendation development). Summary of the Asia-Pacific multidisciplinary panel recommendations for the monitoring and management of T-DXd–related adverse events in patients with metastatic breast cancer is provided in Table 1. Figures were prepared with Adobe InDesign 2023 (version 18.1).
Table 1.
Asia-Pacific multidisciplinary panel recommendations for the monitoring and management of T-DXd–related adverse events in patients with metastatic breast cancer
| Adverse event | Recommendations | |
|---|---|---|
| ILD/pneumonitis | Prior to T-DXd treatment | Eligibility for T-DXd should be carefully evaluated by performing a chest CT scan and confirming that the patient has no current, suspected, or prior history of ILD/pneumonitis or significant pulmonary disease |
| Patients should be educated on the signs and symptoms of ILD/pneumonitis and encouraged to seek medical attention and promptly report events suggestive of ILD/pneumonitisa | ||
| Monitoring | Patients should undergo regular CT scans of the thorax (standard or high-resolution non-contrast CT), but the frequency of CT assessments may have to account for the median time of ILD/pneumonitis onset (median [range] 5.6 (1.1–20.8) months) [47], reimbursement, practicality, or accessibilityb | |
| Pulse oximetry or exertional pulse oximetry walk tests may be performed at each visit to augment ILD/pneumonitis monitoring, although their sensitivity and specificity for the detection of ILD/pneumonitis are unknown | ||
| Baseline PFTs prior to T-DXd treatment may not be recommended for patients who appear to have normal lung function, due to resource burden, cross infection potential (e.g., SARS-CoV-2), and patient inconvenience | ||
| Diagnosis | In patients with incipient pulmonary symptoms or abnormal radiological findings, T-DXd–induced ILD/pneumonitis should be differentially diagnosed from infective causes or other aetiologies with consultations with at least one supporting specialist, such as a pulmonologist, a radiologist, or an infectious disease specialist | |
| High-resolution chest CT should be the principal tool for diagnosing ILD/pneumonitis | ||
| Bronchoscopy and BAL could be considered for patients who are febrile, experience atypical radiographic patterns, or have severe ILD/pneumonitis requiring hospitalisation despite steroid initiation | ||
| Management |
It must be emphasised that the dose of steroids and length of tapering is variable according to the grade of severity, and that physicians should refer to guidelines (i.e., Swain et al 2022 [28] and Conte et al 2022 [53]) For Grade 1 ILD/pneumonitis, interrupt T-DXd, consider initiating corticosteroids (e.g., ≥ 0.5 mg/kg/day prednisolone or equivalent) to treat and prevent the risk of progression, and start empiric anti-infectives if infective aetiology is not entirely excluded |
|
| For patients with a repeated incident of Grade 1 ILD/pneumonitis, follow Grade 1 management strategies: withholding T-DXd until resolution to Grade 0 and initiating systemic corticosteroids and/or empiric anti-infectives. T-DXd rechallenge may require an evaluation of factors such as response to T-DXd and eligibility for other therapies | ||
| For Grade ≥2 ILD/pneumonitis, permanently discontinue T-DXd and promptly initiate systemic steroids for > 14 days or until complete resolution of clinical and chest CT findings, followed by a gradual taper over ≥ 4 weeksc,d | ||
| Nausea and vomiting | Prophylaxis with serotonin (5-HT3) receptor antagonists and dexamethasone (with or without neurokinin-1 receptor antagonist) should be considered for the management of nausea and vomiting associated with T-DXd | |
| Managing severe or breakthrough nausea and vomiting may require the addition of neurokinin-1 receptor antagonists (and olanzapine, if indicated) to the antiemetic regimen | ||
| Low-dose olanzapine (5 mg) could be offered to patients who experience delayed nausea, but treatment duration might need to be proactively optimised to curtail side effects | ||
| For patients with acceptable emesis control, consider either providing dexamethasone for 1 day (on day of treatment) or omitting it | ||
| Neutropenia | Primary G-CSF prophylaxis may not be necessary for most patients commencing T-DXd, but risk factors including the number of prior lines of therapy, history of neutropenia, and febrile neutropenia may need to be considered | |
| Secondary G-CSF prophylaxis to prevent cycle delays or dose reduction could be considered for patients who have experienced recurring Grade 3 neutropenia | ||
| Anaemia | CBC with differential may be considered at each visit to screen for anaemia, and a complete workup is recommended upon signs and symptoms of anaemia | |
| Dose delay and/or reduction are recommended for managing Grade ≥ 3 anaemia, and iron therapy (oral or IV) or blood transfusions should be considered, if clinically indicated | ||
| Thrombocytopenia | Dose delay and/or reduction are recommended for managing Grade ≥ 3 thrombocytopenia, and supportive platelet transfusion should be considered, if clinically indicated | |
| Fatigue | Health care professionals should be vigilant for treatable causes of fatigue (e.g., anaemia). They can encourage patients to use support groups and practice low-intensity exercises, as well as empower patients’ caregivers | |
| Alopecia | To increase treatment acceptance, patients should be made aware of the risk of alopecia prior to starting T-DXd | |
| Scalp cooling could be considered but its efficacy in patients on T-DXd is unknown | ||
| Cardiac |
ESC guidelines recommend that patients on anti-HER2 therapy should have an echocardiography at baseline and be repeated every 3 months during the first year [127]. However, for asymptomatic and low risk patients (i.e., without hypertension, borderline low LVEF, or received anthracyclines), surveillance can be reduced to every 6 months during future treatment. In high-risk patients, more frequent echocardiography and cardio-biomarkers may be considered Cardiac magnetic resonance imaging should be considered when echocardiography is unavailable or non-diagnostic |
|
BAL bronchoalveolar lavage, CBC complete blood count, CMV cytomegalovirus, CT computed tomography, G-CSF granulocyte colony-stimulating factor, ESC European Cardiology Society, ILD interstitial lung disease, IV intravenous, LVEF left ventricular ejection fraction, PFT pulmonary function test, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, T-DXd trastuzumab deruxtecan
aSigns and symptoms of ILD/pneumonitis may include dyspnoea with exertion, cough, fever and other new or worsening respiratory symptoms
bFor instance, 6-weekly CT scans may be considered for the first 6–12 months of T-DXd treatment given the known median time of ILD/pneumonitis onset and clinical trial experience. If longer intervals are chosen (9- or 12-weekly) other supplemental strategies to monitor for ILD/pneumonitis are recommended
ce.g., 1 mg/kg prednisone or equivalent for Grade 2, or 500–1000 mg/day empirical high-dose methylprednisolone IV for 3 days followed by 1 mg/kg prednisone or equivalent for > 14 days for Grade 3/4
dWhile on high-dose systemic corticosteroids (particularly pulse steroids), consider adding Pneumocystis jirovecii pneumonia prophylaxis (e.g., co-trimoxazole) and performing surveillance for CMV (e.g., CMV pp65 antigen)
Interstitial Lung Disease/Pneumonitis
Interstitial lung disease/pneumonitis is a diverse group of lung parenchymal disorders that manifest as fibrosis and/or inflammation [39, 40]. Interstitial lung disease/pneumonitis associated with drug exposure is a subtype of this disorder, and it is becoming increasingly common due to the use of novel biologics and molecular targeted therapies [41]. In the breast-cancer treatment landscape, anti-cancer therapies such as everolimus, abemaciclib, eribulin and taxanes have been reported to be associated with ILD/pneumonitis [42–45].
Trastuzumab deruxtecan has been associated with ILD/pneumonitis [11–13]. According to a pooled analysis of patients with metastatic breast cancer who underwent treatment, 20.6% had adjudicated drug-related ILD/pneumonitis, with 6.3%, 10.0%, 1.4%, 0% and 2.9% events classified as Grades 1, 2, 3, 4 and 5 severity, respectively [46]. In patients with HER2-positive metastatic breast cancer, the median (range) time of onset of T-DXd–related ILD/pneumonitis was 5.6 (1.1–20.8) months, with nearly all occurring within the first year of T-DXd treatment [47]. Despite evidence from a pooled analysis of patients with HER2-positive metastatic breast cancer from Phase I and Phase II trials demonstrating a low risk of developing new ILD/pneumonitis after 1 year of T-DXd treatment, the updated DESTINY-Breast03 results showed an increase in ILD/pneumonitis incidence from 10.5% to 15% with longer follow-up and increased exposure [47, 48]. In patients with HER2-low metastatic breast cancer, the median (range) time to onset of drug-related ILD/pneumonitis was 4.24 (0.85–23.3) months [13]. A study including 149 patients treated with T-DXd in a community oncology practice in the USA reported the development of ILD/pneumonitis in 4 (2.7%) patients over an average treatment duration of 5.5 months [49]. Regardless, real-world experience with T-DXd and ILD/pneumonitis is currently limited.
According to a subgroup analysis of the DESTINY-Breast03 trial, the incidence of any-grade ILD/pneumonitis was similar between Asian patients and the overall population (10.9% and 10.5%, respectively; Table 2) [50]. However, a higher incidence of any-grade ILD/pneumonitis (22.2%) was observed in the Japanese subgroup than both the overall population and Asian subgroup (Table 2) [50].
Table 2.
Incidence of adjudicated drug-related ILD/pneumonitis in the DESTINY-Breast03 trial [50]
| Population | Treatment arm | Adjudicated drug-related ILD/pneumonitis, n (%) | |||||
|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Any grade | ||
| Overall | T-DXd (n = 257) | 7 (2.7) | 18 (7.0) | 2 (0.8) | 0 | 0 | 27 (10.5) |
| T-DM1 (n = 261) | 4 (1.5) | 1 (0.4) | 0 | 0 | 0 | 5 (1.9) | |
| Asian patients | T-DXd (n = 147) | 5 (3.4) | 10 (6.8) | 1 (0.7) | 0 | 0 | 16 (10.9) |
| T-DM1 (n = 159) | 3 (1.9) | 1 (0.6) | 0 | 0 | 0 | 4 (2.5) | |
| Japanese patients | T-DXd (n = 36) | 4 (11.1) | 4 (11.1) | 0 | 0 | 0 | 8 (22.2) |
| T-DM1 (n = 31) | 2 (6.5) | 1 (3.2) | 0 | 0 | 0 | 3 (9.7) | |
ILD interstitial lung disease, T-DM1 trastuzumab emtansine, T-DXd trastuzumab deruxtecan
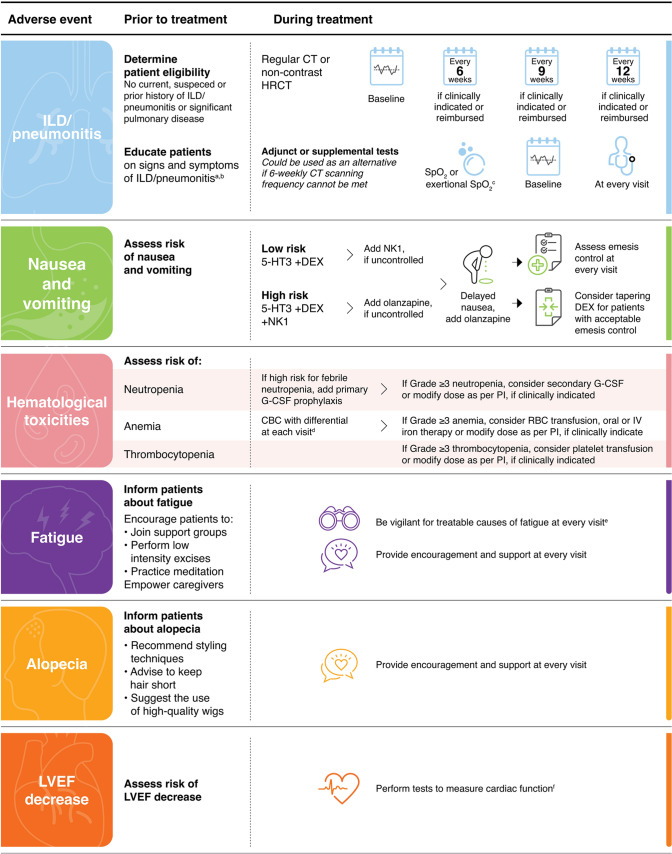
While guidelines on the management of ILD/pneumonitis associated with T-DXd and other drugs have been published [28, 29, 51–54], recommendations may need to account for regional considerations, such as reimbursement and practicality. The following recommendations aim to provide additional monitoring and management considerations for ILD/pneumonitis that aspire be more relevant to the Asia-Pacific region in the real-world setting (Figs. 1 and 2).
Fig. 1.
Graphical summary of the Asia-Pacific multidisciplinary panel recommendations for the monitoring and management of T-DXd–related adverse events in patients with metastatic breast cancer. aSigns and symptoms of ILD/pneumonitis may include cough, dyspnoea, fever and other new or worsening respiratory symptoms. bJunior doctors and breast care nurses may also be included. c6-min walk test or 1-minute sit-to-stand tests. dComplete workup is recommended upon signs and symptoms of anaemia. eSuch as anaemia. fSuch as echocardiography and other laboratory tests. 5-HT3 serotonin receptor antagonists CBC complete blood count, CT computed tomography, DEX dexamethasone, G-CSF granulocyte colony-stimulating factor, HRCT high-resolution computed tomography, ILD interstitial lung disease, IV intravenous, LVEF left ventricular ejection fraction, NK1 neurokinin 1 antagonists, PI prescribing information, RBC red blood cell, SpO2 Oxygen saturation measured by pulse oximetry, T-DXd trastuzumab deruxtecan
Fig. 2.
Summary of the Asia-Pacific multidisciplinary panel recommendations for the monitoring and management of T-DXd–related ILD/pneumonitis. The Managing ILD/pneumonitis section of this figure has been adapted from Fig. 3 of Swain SM et al 2021 [28]. aOther tests cited by guidelines and clinical trial protocol include blood culture and CBC, PFTs and pulse oximetry, arterial blood gases, PK analysis of blood sample (as clinically indicated and feasible) [28]. BAL bronchoalveolar lavage, CBC complete blood count, CMV cytomegalovirus, CT computed tomography, HRCT high-resolution computed tomography, ILD interstitial lung disease, IV intravenous, MDT multidisciplinary team, PFT pulmonary function test, PK pharmacokinetic, T-DXd trastuzumab deruxtecan
Proactive Monitoring of ILD/Pneumonitis
Determine Patient Eligibility
Recommendation 1: Eligibility for T-DXd should be carefully evaluated by performing a chest CT scan and confirming that the patient has no current, suspected, or prior history of ILD/pneumonitis or significant pulmonary disease.
Given the potential risks of developing pulmonary toxicities like ILD/pneumonitis, patients should be thoroughly evaluated. Furthermore, the benefit–risk assessment should be taken into consideration prior to commencing T-DXd. Clinical trials with T-DXd excluded patients with ILD/pneumonitis, a history of non-infectious ILD/pneumonitis that required steroids, or had suspected ILD/pneumonitis on imaging at screening [11–13]. A pooled analysis of risk factors associated with ILD/pneumonitis in T-DXd breast, lung and gastric cancer trials uncovered the following risk factors for developing T-DXd–related ILD/pneumonitis: age < 65 years, enrolment in Japan, receiving a T-DXd dose of > 6.4 mg/kg, oxygen saturation level of < 95%, moderate/severe renal impairment, presence of lung comorbidities, > 4 years since initial diagnosis [46]. Moreover, the US and European Medicines Agency (EMA) prescribing information also caution that patients with moderate/severe renal impairment may be at a heightened risk of developing ILD/pneumonitis [55, 56]. In addition to evaluating the patient’s medical history, a chest CT scan (standard or high-resolution) should be considered as an important radiological modality to detect any preexisting lung abnormalities and should serve as a baseline while the treatment progresses [28, 53, 54].
Education
Recommendation 2: Patients should be educated on the signs and symptoms of ILD/pneumonitis and encouraged to seek medical attention and promptly report events suggestive of ILD/pneumonitis.1
Patient education on the signs and symptoms of ILD/pneumonitis should be a key step for proactive monitoring of this adverse event while on T-DXd treatment. Additionally, junior doctors and breast care nurses should be educated on the signs and symptoms. Patients should self-monitor for dyspnoea with exertion (as it is often an early symptom of ILD/pneumonitis), cough, fever, or other respiratory symptoms, such as chest pain or tightness, asthenia and fatigue [53, 54]. We would like to emphasise that the patient should also be encouraged to call their treating oncologist and immediately report symptoms suggestive of ILD/pneumonitis.
In our experience, patients with respiratory symptoms would often seek help from family doctors or acute care units. Misdiagnosis and delays in initiating care may occur if patients are triaged to units without T-DXd experience or forewarned knowledge that the patient is on a therapy that poses a high risk of ILD/pneumonitis. Thus, patients may require strategies to help expedite the diagnosis of drug-related ILD/pneumonitis. For instance, in Hong Kong, health care professionals can add an alert in the electronic medical system that their patients are on a treatment prone to developing ILD/pneumonitis. In Singapore, national electronic medical records would have information regarding a patient’s medical condition and cancer treatment. Furthermore, patients would have been educated by their oncologist or oncology nurse to either return to the oncology clinic during office hours or visit the emergency department if severe symptoms occur after consultation hours. Patients may also be provided an alert card (electronic or printed) documenting the medication used, and they should be encouraged to present this card to the attending physician when they encounter acute respiratory symptoms.
CT Scanning Frequency
Recommendation 3: Patients should undergo regular CT scans of the thorax (standard or high-resolution non-contrast CT), but the frequency of CT assessments may have to account for the median time of ILD/pneumonitis onset (median [range] 5.6 (1.1–20.8) months) [47], reimbursement, practicality, or accessibility.2
According to the common terminology criteria for adverse events, symptomatic ILD/pneumonitis is classified as Grade ≥ 2 [57]. Grade 1 ILD/pneumonitis is asymptomatic and may only be determined from radiological scans [28, 53, 57]. At the same time, the US and EMA prescribing information recommend the permanent discontinuation of treatment upon confirmation of Grade ≥ 2 ILD/pneumonitis [55, 56]. Taken together, they present a particularly unique challenge for patients on T-DXd. Because mild ILD/pneumonitis is asymptomatic and severe ILD/pneumonitis may necessitate the permanent discontinuation of T-DXd, patients may require frequent CT scans to not only monitor for this potential adverse event but also maximise treatment benefits.
In clinical trials, CT scans of the chest, abdomen and pelvis were performed every 6 weeks to assess treatment response [25–27]. Outside of clinical trials, conducting such frequent CT scans may be difficult owing to patient considerations, such as inconvenience, financial costs and concerns of radiation exposure [58–60]. Furthermore, in our opinion, the heterogeneity of health care infrastructure in the Asia-Pacific region may also impact the frequency of CT scans for patients on T-DXd. In Taiwan, due to the reimbursement regulations set by National Health Insurance, CT scans may only be covered every 12 weeks. In Hong Kong, imaging services are subject to the availability of resources, and patients may need to pay out-of-pocket at private facilities for additional CT scans. In Singapore, CT scans are readily available for patients in both private and public health care institutions, although out-of-pocket costs may be incurred for some patients. For instance, private insurance may cover the cost of CT scans, and government health care schemes may provide additional coverage for those without private insurance depending on eligibility, such as age and financial means.
Given the absence of clinical data on the optimal CT scanning frequency for patients on T-DXd, the oncologist selecting a CT scanning frequency may have to balance practicality and evidence from clinical trials. Hence, performing CT scans (regular or high-resolution) every 6 weeks for patients receiving T-DXd could be considered if reimbursement is feasible, and patients accept the potential risks and inconveniences. Such a monitoring strategy may be more pertinent for the first 6–12 months of treatment with T-DXd given the median (range) time of onset of ILD/pneumonitis of 5.6 (1.1–20.8) months [47]. However, recent evidence from the DESTINY-Breast03 trial has demonstrated an increase in ILD/pneumonitis incidence with longer follow-up and increased exposure [48]. Furthermore, if 9- or 12-weekly CT scans are selected, supplementary or adjunct investigations may be considered (see 3.1.5 Pulse oximetry and exercise tolerance).
Chest X-Rays
The use of chest X-rays to monitor ILD/pneumonitis was discussed by our expert panel. Compared with CT scans, chest X-rays are affordable, widely accessible and have a lower ionizing radiation risk [28, 61, 62]. However, there is a dearth of clinical evidence on the specificity and sensitivity of chest X-rays for the detection of T-DXd–related ILD/pneumonitis, particularly asymptomatic cases. Few studies have demonstrated that chest X-rays may have the requisite specificity and accuracy for the diagnosis of ILD/pneumonitis [63, 64]. Nevertheless, the evaluation of chest X-rays for ILD/pneumonitis may be challenging for non-pulmonologists [65, 66]. Serial chest X-rays can be useful for the identification of lung abnormalities, such as diffuse reticulonodular patterns or ground-glass opacities, and perhaps, confirmation of progressive lung disease [66]. However, repeated chest X-rays may not be applicable for patients having lung metastasis and hence require regular CT scans for the assessment of response.
Pulse Oximetry and Exercise Tolerance
Recommendation 4: Pulse oximetry or exertional pulse oximetry walk tests may be performed at each visit to augment ILD/pneumonitis monitoring, although their sensitivity and specificity for the detection of ILD/pneumonitis are unknown.
Pulse oximetry is a non-invasive and convenient option to monitor ILD/pneumonitis [28, 29]. Oxygen desaturation should prompt the suspicion of ILD/pneumonitis in patients treated with T-DXd [28, 29]. However, hypoxemia can be absent in patients with ILD/pneumonitis at rest, and thus, exertional pulse oximetry walking test can be considered [28, 29]. The 6-min walk test (6MWT) is a well-established method to measure exercise tolerance and exercise-induced desaturation in patients with various chronic lung diseases [67]. However, 6MWT may not be feasible in routine clinical practice given that it is time-consuming and requires a long corridor; in that case, other tests like the 1-min sit-to-stand tests may be more suitable [68].
Remote monitoring of patient-recorded pulse oximetry might be an alternative approach to hospital-based monitoring of ILD/pneumonitis. An observational study demonstrated the feasibility of daily recording of pulse oximetry, as it showed high adherence in patients with ILD/pneumonitis (82% maintained adherence to pulse oximetry on ≥ 70% of study days) [69]. Furthermore, a systematic review demonstrated that the use of pulse oximetry as a monitoring tool for patients at home with COVID-19 helped guide care escalation [70]. However, it must be acknowledged that there are limited data on the sensitivity and specificity of pulse oximetry and exercise tolerance tests in patients with metastatic breast cancer; thus, these tests should not replace regular CT scans for diagnosis or surveillance. Furthermore, exertional tests might not be applicable in patients with poor performance scores.
Pulmonary Function Tests
Recommendation 5: Baseline pulmonary function tests (PFTs) prior to T-DXd treatment may not be recommended for patients who appear to have normal lung function due to resource burden, cross infection potential (i.e., severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) and patient inconvenience.
Pulmonary function tests can provide accurate assessment of a patient’s respiratory status [71]. For patients on T-DXd, some physicians in the USA reportedly find it helpful to measure the diffusing capacity of the lungs for carbon monoxide (DLCO) in between CT/positron emission tomography (PET) scans (e.g., every 3 months, alternating with scans) [29]. It has been reported that reduced DLCO (< 80%) may have a sensitivity of 83.6% and a specificity of 45.8% for the early diagnosis of ILD in patients with inflammatory rheumatic diseases [72]. On the other hand, this same study reported that forced vital capacity (FVC) appeared to have an opposite sensitivity and specificity when compared with DLCO, with a sensitivity of 47.5% and specificity of 78.7% [72]. Significant improvements in the diagnostic value of DLCO were observed when combined with chest X-ray and chest HRCT scans (sensitivity of 95.2% and specificity of 77.4%) [72]. With regard to drug-induced ILD, findings from a meta-analysis did not support routine PFTs for the early detection of drug-induced ILD/pneumonitis, although most studies included in this review had a small sample size and were focused on bleomycin- and amiodarone-related ILD/pneumonitis [73]. Moreover, PFTs require access to specialised equipment and skilled technicians; additionally, they may be inconvenient to the patient [74, 75]. Furthermore, considering the coronavirus disease 2019 (COVID-19) pandemic, patients and health care professionals may be at risk of being exposed to the virus during the tests [76, 77]. Given these considerations, baseline PFTs may not be available or advisable for patients with metastatic breast cancer who are starting treatment with T-DXd. However, PFTs could be used to monitor patients with pre-existing lung disease and to potentially help identify patients susceptible to developing ILD/pneumonitis [28, 29, 53].
Diagnosis of ILD/Pneumonitis
Recommendation 6: In patients with incipient pulmonary symptoms or abnormal radiological findings, T-DXd–induced ILD/pneumonitis should be differentially diagnosed from infective causes or other aetiologies with consultations with at least one supporting specialist, such as a pulmonologist, a radiologist, or an infectious disease specialist.
Interstitial lung disease/pneumonitis must be considered if the patient experiences the onset of new or worsening pulmonary signs or symptoms or if there are radiographic changes that are consistent with this adverse event [28, 29, 55, 56]. In accordance with current guidelines and prescribing information, if ILD/pneumonitis is suspected, T-DXd treatment should be withheld until diagnostic confirmation [28, 29, 55, 56]. Given that drug-induced ILD/pneumonitis is a diagnosis of exclusion, further tests should be performed to eliminate other causes, such as pneumonia, infections, lung metastases and pulmonary embolisms [28, 29, 53].
Consulting a multidisciplinary team can improve diagnostic confidence, and this is considered the gold standard for ILD/pneumonitis diagnosis [78]. However, the use of formal multidisciplinary teams for the adjudication of ILD/pneumonitis may not be feasible or practical in some clinical settings [78]. Nevertheless, given the benefits of collaborative decision making, particularly for a multifactorial adverse event like ILD/pneumonitis [78], we recommend that the evaluation of suspected cases of ILD/pneumonitis should include at least an oncologist, a pulmonologist, a radiologist and an infectious disease specialist.
High-Resolution CT
Recommendation 7: High-resolution chest CT should be the principal tool for diagnosing ILD/pneumonitis.
The gold standard for diagnosing ILD/pneumonitis is non-contrast high-resolution CT [28, 29, 53, 79]. The quality of the images depends largely on the choice of scanning protocol, with the optimal slice thickness being ≤ 1.5 mm (overlapping or contiguous slices) [79]. High-resolution CT scans may not need to be repeated if a patient has already had a regular CT thorax scan to assess the treatment response. If the source images for the regular CT thorax scan are available, the radiologist could reconstruct these images into thinner (high resolution) slices for review. Like other reports, we have noticed various radiological patterns of ILD/pneumonitis in patients treated with T-DXd, but more clinical data on their frequency are warranted [28]. COVID-19 pneumonia may present with a radiological pattern resembling drug-related ILD/pneumonitis, and thus, SARS-CoV-2 infection should be considered when attempting to distinguish T-DXd–related ILD/pneumonitis [28, 29]. Thus, high-resolution CT findings should be interpreted in the context of potential infections and specialised tests, particularly when radiological patterns are inconclusive [28, 29, 53].
Bronchoscopy, Bronchoalveolar Lavage (BAL) and Other Tests
Recommendation 8: Bronchoscopy and BAL could be considered for patients who are febrile, experience atypical radiographic patterns, or have severe ILD/pneumonitis requiring hospitalisation despite steroid initiation.
The use of specialised examinations such as bronchoscopy and BAL for the differential diagnosis of ILD/pneumonitis may vary across institutions and regions [80]. In our experience, bronchoscopy and BAL could be used to exclude infectious causes, particularly if the patient is febrile, is suspected to have metastases or alveolar haemorrhage, or does not present a typical radiological pattern of ILD/pneumonitis on high-resolution CT scans [53, 81, 82]. Similar to the European Society for Medical Oncology (ESMO) recommendations, we suggest that BAL can be considered for individuals with a lack of improvement after the withdrawal of the causative drug despite corticosteroid therapy [53].
Additional evaluations listed by guidelines include complete blood count (CBC) with differential, PFTs (if severity allows for testing), liver and kidney function and markers for inflammation or infection [28, 29, 53]. Serum markers associated with ILD/pneumonitis include Krebs von den Lungen-6, pulmonary surfactant protein-A, and pulmonary surfactant protein-D; however, their use in a clinical diagnostic setting may need to be validated [28, 53, 83, 84]. Lung biopsies may seldom be needed and could be reserved for the diagnosis of clinically and radiologically unclassifiable ILD/pneumonitis when required [53].
Managing ILD/Pneumonitis
The management of ILD/pneumonitis has been detailed in the prescribing information and guidelines for T-DXd [28, 29, 54–56]. Briefly, it is recommended that T-DXd should be interrupted or permanently discontinued depending on the severity of ILD/pneumonitis, and patients should receive corticosteroids and supportive care to manage this adverse event [28, 29, 54–56]. Proactive surveillance and management of ILD/pneumonitis may have contributed to reducing the risk of severe ILD/pneumonitis associated with T-DXd [29, 46]. However, despite these measures, it has been noted that Japanese patients appeared to have a higher incidence of ILD/pneumonitis with T-DXd (22.2%) when compared with other Asian populations (10.9%) and the overall population (10.5%) in the DESTINY-Breast03 trial [50]. Pooled analysis of the DESTINY trials has also demonstrated that patients treated with T-DXd in Japan had a higher risk of ILD/pneumonitis when compared with patients treated outside of Japan [46]. It is unclear whether this disparity is due to differences in monitoring and management practices or biological factors [46]. In this section, we will detail key recommendations that might be appropriate to real-world clinical practice in the Asia-Pacific region.
Grade 1 ILD/Pneumonitis
Recommendation 9: For Grade 1 ILD/pneumonitis, interrupt T-DXd, consider initiating corticosteroids (e.g., ≥ 0.5 mg/kg/day prednisolone or equivalent) to treat and prevent the risk of progression, and start empiric anti-infectives if infective aetiology is not entirely excluded.
If a patient has Grade 1 ILD/pneumonitis, T-DXd should be withheld until the adverse event is fully resolved to Grade 0 [28, 29, 54–56]. Prescribing information leaves the initiation of systemic corticosteroids at the discretion of the health care provider [55, 56]. According to Swain et al, experts suggested that corticosteroids should be reserved for Grade 1 events that demonstrate extensive lung involvement or for patients who are at increased risk for progression of ILD/pneumonitis [28]. In our cumulative clinical experience, we have tended to initiate systemic steroids for most, if not all, instances of Grade 1 ILD/pneumonitis followed by a gradual taper over at least 4 weeks. It remains to be seen whether the initiation of corticosteroids for all Grade 1 events may result in different outcomes to those who did not receive this therapy. In our opinion, empiric anti-infectives may also be added while commencing systemic corticosteroids [53]. This strategy might be useful in situations where infective aetiology is not completely excluded, particularly if culture results are pending.
Recommendations for the monitoring of patients with Grade 1 ILD/pneumonitis have also been described in current guidelines [28, 29, 54]. Monitoring for the onset of clinical symptoms and performing pulse oximetry are recommended 2–7 days after the diagnosis of ILD/pneumonitis, and follow-up CT scans (high-resolution or regular) should be repeated within 2 weeks [28, 29, 53, 54]. Computed tomography scans (high-resolution or regular) should be used to monitor response after T-DXd treatment cessation and systemic corticosteroid initiation [28, 29, 53, 54]. Other tests like pulse oximetry and PFTs are also useful for monitoring [28, 29, 53, 54].
Guidance on the resumption of T-DXd after the resolution of Grade 1 ILD/pneumonitis are detailed in the prescription information [55, 56]. Specifically, if the adverse event fully resolves to Grade 0 by 28 days from onset, T-DXd can be resumed at the same dose [55, 56]. If it takes more than 28 days to resolve, the dose should be reduced by one dose level [55, 56].
Repeated Grade 1 ILD/Pneumonitis
Recommendation 10: For patients with a repeated incident of Grade 1 ILD/pneumonitis, follow Grade 1 management strategies: withholding T-DXd until resolution to Grade 0 and initiating systemic corticosteroids and/or empiric anti-infectives. Trastuzumab deruxtecan rechallenge may require an evaluation of factors such as response to T-DXd and eligibility for other therapies.
Given the possibility that patients may receive multiple cycles of T-DXd, we identified that patients might encounter another Grade 1 event after the resolution of the initial event. The frequency of repeated Grade 1 ILD/pneumonitis was not documented in clinical trials, and there is no guidance on how to manage such an event. In general, we suggest that if a patient experiences another episode of Grade 1 event after successful recovery from the initial Grade 1 event, the management advice for a Grade 1 event in the prescription information and section above can be followed (see Grade 1 ILD/pneumonitis section).
However, we suggest that certain factors may need to be evaluated. For example, if a patient’s response to T-DXd and ILD/pneumonitis is mild, we would consider rechallenging with T-DXd but recommend more frequent monitoring. If a patient has failed other treatments, then T-DXd may be continued at a reduced dose specified in the dose-modification schedule of the prescribing information [55, 56]. On the other hand, if other treatments are available or ILD/pneumonitis recovery is lengthy, cessation of T-DXd should be considered. We acknowledge that these recommendations are based on our clinical judgement and not from the prescribing information, and as such, more data are required to validate such a strategy.
Grade 2 and 3/4 ILD/Pneumonitis
Recommendation 11: For Grade ≥ 2 ILD/pneumonitis, permanently discontinue T-DXd and promptly initiate systemic steroids for > 14 days or until complete resolution of clinical and chest CT findings, followed by a gradual taper over ≥ 4 weeks.3,4
Trastuzumab deruxtecan should be discontinued permanently upon the diagnosis of Grade ≥2 ILD/pneumonitis and steroids should be promptly initiated and gradually tapered over at least 4 weeks [28, 29, 54–56]. Similar to Grade 1 recommendations, starting an empiric antibiotic course may be considered [53]. The prescribing information and recently published guidelines have provided comprehensive management strategies in the event of Grade ≥2 ILD/pneumonitis, and as such, are not elaborated further in this publication [28, 53–56]. However, we would like to add that for patients receiving high-dose systemic corticosteroids, particularly pulse steroids, Pneumocystis jirovecii pneumonia prophylaxis should be considered and the patient should be monitored for cytomegalovirus infection (e.g., CMV pp65 antigen) [85–87].
Nausea and Vomiting
Trastuzumab deruxtecan has been categorised as a moderately emetogenic drug by the American Society of Clinical Oncology (ASCO), but recently, the National Comprehensive Cancer Network (NCCN) guidelines have reclassified this agent as having a high emetic risk [88, 89]. In the DESTINY-Breast01 trial, any grade nausea and vomiting occurred in 77.7% and 45.7% of patients, respectively [11]. The incidence of these events was similar in the DESTINY-Breast03 trial [48]. However, the proportion of patients with any=grade nausea and vomiting was notably higher in the T-DXd arm than in the trastuzumab emtansine (T-DM1) arm (77% vs 30% and 52% vs 11%, respectively) [48]. When analysed by race, there appeared to be a slightly lower incidence of nausea and vomiting in Asian patients than in the overall population (65.3% vs 72.8% and 42.9% vs 44.0%, respectively; Table 3) [50]. Chemotherapy-induced nausea and vomiting can compromise the compliance of patients [90] and hence, should be proactively managed.
Table 3.
Most common drug-related adverse events in the DESTINY-Breast03 trial by overall population and Asian subgroup [50]
| Event, n (%) | Overall | Asian patients | ||||||
|---|---|---|---|---|---|---|---|---|
| T-DXd (n = 257) | T-DM1 (n = 261) | T-DXd (n = 147) | T-DM1 (n = 159) | |||||
| Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | Any grade | Grade ≥ 3 | |
| Blood and lymphatic system disorders | ||||||||
| Neutropeniaa | 110 (42.8) | 49 (19.1) | 29 (11.1) | 8 (3.1) | 70 (47.6) | 36 (24.5) | 23 (14.5) | 8 (5.0) |
| Anaemiab | 78 (30.4) | 15 (5.8) | 37 (14.2) | 11 (4.2) | 47 (32.0) | 12 (8.2) | 22 (13.8) | 6 (3.8) |
| Thrombocytopeniac | 64 (24.9) | 18 (7.0) | 135 (51.7) | 65 (24.9) | 49 (33.3) | 17 (11.6) | 103 (64.8) | 55 (34.6) |
| Gastrointestinal disorders | ||||||||
| Nausea | 187 (72.8) | 17 (6.6) | 72 (27.6) | 1 (0.4) | 96 (65.3) | 6 (4.1) | 34 (21.4) | 1 (0.6) |
| Vomiting | 113 (44.0) | 4 (1.6) | 15 (5.7) | 1 (0.4) | 63 (42.9) | 2 (1.4) | 7 (4.4) | 1 (0.6) |
| General disorders | ||||||||
| Fatigued | 115 (44.7) | 13 (5.1) | 77 (29.5) | 2 (0.8) | 63 (42.9) | 8 (5.4) | 38 (23.9) | 1 (0.6) |
| Skin and subcutaneous tissue disorders | ||||||||
| Alopecia | 93 (36.2) | 1 (0.4) | 6 (2.3) | 0 | 56 (38.1) | 0 | 4 (2.5) | 0 |
ILD interstitial lung disease, T-DM1 trastuzumab emtansine, T-DXd trastuzumab deruxtecan
aThis category includes the preferred terms neutrophil count decreased and neutropenia [50]
bThis category includes the preferred terms haemoglobin decreased, red blood cell count decreased, anaemia and haematocrit decreased [50]
cThis category includes the preferred terms platelet count decreased and thrombocytopenia [50]
dThis category includes the preferred terms fatigue, asthenia and malaise [50]
Prophylaxis with Antiemetics
Recommendation 12: Prophylaxis with serotonin (5-HT3) receptor antagonists and dexamethasone (with or without neurokinin-1 receptor antagonist) should be considered for the management of nausea and vomiting associated with T-DXd.
Based on our clinical experience and in line with current guidelines as well as the EMA prescribing information, a two- or three-drug regimen of 5-HT3 receptor antagonists and dexamethasone with or without neurokinin-1 receptor antagonist is a suitable option for most patients with metastatic breast cancer starting on the first cycle of T-DXd [29, 54, 55, 91]. An Italian expert panel preferred the use of palonosetron over other 5-HT3 receptor antagonists, such as ondansetron, because of the longer half-life, patient convenience and potentially superior symptom control [91]. The NCCN guidelines recommend a three-drug regimen but also suggest the inclusion of olanzapine (5–10 mg) on Days 2, 3 and 4 for patients on highly emetic anticancer agents like T-DXd [89].
Some patients may be more prone to nausea and vomiting from chemotherapies. These possible patient-related risk factors include being female, having a history of nausea and vomiting, morning sickness or anxiety, younger age and low alcohol intake [92]. Expectancy of nausea and vomiting after chemotherapies was another risk factor [92]. Thus, such patients could start their first cycle of T-DXd with the addition of neurokinin-1 receptor antagonist, such as aprepitant, to 5-HT3 receptor antagonist and dexamethasone [54, 91].
Managing Suboptimal or Breakthrough Cases
Recommendation 13: Managing severe or breakthrough nausea and vomiting may require the addition of neurokinin-1 receptor antagonists (and olanzapine, if indicated) to the antiemetic regimen.
For patients with suboptimal control of nausea and vomiting after the first cycle of T-DXd, we suggest the addition of neurokinin-1 receptor antagonist if that patient is on a two-drug regimen of 5-HT3 receptor antagonist and dexamethasone [29, 54, 91]. Patients already on an escalated three-drug regimen may need the addition of olanzapine on Days 1–4 after each T-DXd cycle [29, 54, 91]. Olanzapine has been shown to benefit patients who are highly prone to this adverse event and to control delayed nausea and vomiting (see section Managing Delayed Nausea) [29, 54]. Using low-dose olanzapine (5 mg vs 10 mg) might balance its benefits over the concerns of somnolence and could be cost effective for patients in Asia receiving emetic regimens [93, 94]. From our experience, breakthrough cases of nausea and vomiting have been reported while on trial. Therefore, we recommend the provision of as-needed medication including metoclopramide or transdermal granisetron patches for the control of breakthrough symptoms [91, 95].
Managing Delayed Nausea
Recommendation 14: Low-dose olanzapine (5 mg) could be offered to patients who experience delayed nausea, but treatment duration might need to be proactively optimised to curtail side effects.
Delayed nausea is a particularly challenging adverse event to manage [96]. Although its incidence may not have been reported in clinical trials, we have encountered a few cases where delayed nausea posed an issue for patients on T-DXd. From our experience and in line with recommendations, olanzapine (5 mg) may benefit patients for whom delayed nausea is a concern [29, 54].
Recommendation 15: For patients with acceptable emesis control, consider either providing dexamethasone for 1 day (on day of treatment) or omitting it.
Experts from Italy have highlighted that dexamethasone plays a critical role in controlling delayed nausea, and thus, recommended 4 mg of dexamethasone from Day 2 to Day 3 for patients who did not experience nausea after the first cycle and after each cycle for some patients without risk factors for nausea and vomiting [91]. However, steroid use is associated with undesirable side effects [97]. Thus, if patients do not suffer from this side effect while on other powerful antiemetic agents, treating oncologists can consider reducing dexamethasone to 1 day on the day of chemotherapy or omitting this drug altogether. Nevertheless, we acknowledge that there is no clinical evidence examining the efficacy of steroid-sparing regimens for the control of T-DXd–related nausea and vomiting.
Dose Modification
We recommend that nausea and vomiting control should be optimised as best as possible prior to considering dose modifications as suggested in the prescribing information of T-DXd [54–56, 91].
Haematological Toxicities
Based on our experience in trials and real-world practice, we found that haematological toxicities, such as neutropenia and anaemia, were common among patients on T-DXd. In the DESTINY-Breast01 trial, all-grade neutropenia and anaemia occurred in 34.8% and 29.9% of patients receiving T-DXd, respectively [11]. In the DESTINY-Breast03 trial, the incidence of any grade neutropenia and anaemia were much higher in patients on T-DXd than those on T-DM1 (31% vs 11% and 37% vs 20%, respectively) [48]. Grade ≥ 3 neutropenia and anaemia were generally more frequent with T-DXd than with T-DM1 (16% vs 3% and 9% vs 7%, respectively) [48]. Thrombocytopenia was associated with T-DXd but the incidence was generally lower than that with T-DM1 (any grade: 25% vs 44%; grade ≥ 3: 8% vs 20%, respectively) [48]. When analysed per subgroup, neutropenia (any grade: 47.6% vs 42.8%; grade ≥ 3: 24.5% vs 19.1%), anaemia (any grade: 32.0% vs 30.4%; grade ≥ 3: 8.2% vs 5.8%), and thrombocytopenia (any grade: 33.3% vs 24.9%; grade ≥ 3: 11.6% vs 7.0%) were slightly higher in Asian patients than in the overall population receiving T-DXd (Table 3) [50].
Neutropenia
Primary Granulocyte Colony-Stimulating Factor (G-CSF) Prophylaxis
Recommendation 16: Primary granulocyte colony-stimulating factor
G-CSF prophylaxis may not be necessary for most patients commencing T-DXd, but risk factors including the number of prior lines of therapy, history of neutropenia and febrile neutropenia may need to be considered.
American Society of Clinical Oncology (ASCO) and ESMO guidelines recommend the prophylactic use of G-CSFs if the risk of febrile neutropenia is 20% or higher [98, 99]. Based on the DESTINY trials, however, the incidence of febrile neutropenia was generally low (DESTINY-Breast01: 1.6%; DESTINY-Breast04: 0.3%) [11, 13]. In our clinical experience, we did not routinely initiate primary prophylaxis with G-CSFs in patients starting T-DXd. However, we recognise that certain patient populations may benefit from primary G-CSF prophylaxis. Patient-specific risk factors may include older age (aged ≥ 65 years), advanced-disease stage, and febrile neutropenia occurrence with previous chemotherapy [100].
Dose Modification and Secondary Prophylaxis with G-CSF
Recommendation 17: Secondary G-CSF prophylaxis to prevent cycle delays or dose reduction could be considered for patients who have experienced recurring Grade 3 neutropenia.
Dose delay and/or reduction are the recommended strategies for the management of severe neutropenia (Grade ≥ 3) [55, 56]. However, it is unclear to what extent these dose modifications can impact clinical outcomes, and prescribing information and clinical trial protocols lack guidance on how G-CSFs might be integrated into the management of neutropenia [25–27, 55, 56].
Secondary prophylaxis with G-CSF is a guideline-recommended strategy to prevent recurrent neutropenia [98, 99]. Maintaining dose intensity can potentially maximise the clinical benefits of anti-cancer therapy [98, 99, 101]. Hence, similar to the recommendations by Bardia and colleagues from the USA, the administration of short-acting G-CSF for 2–3 days to elevate the white blood cell may allow the patient to continue T-DXd without delays [29]. Clinical experience suggested that some patients would need a prolonged course of daily G-CSF for a week to maintain an optimal white cell count level. Alternatively, long-acting G-CSF, such as pegfilgrastim, given on Day 2 could be considered [29]. Like Bardia and colleagues, we acknowledge that patients with poor bone marrow counts may not respond adequately to G-CSF and should be managed through a dose delay and/or reduction [29].
Anaemia
Essential Tests for Anaemia
Recommendation 18: Complete blood count with differential may be considered at each visit to screen for anaemia, and a complete workup is recommended upon signs and symptoms of anaemia.
We recommend that a CBC with differential to monitor for anaemia should be performed during treatment. If the cause of anaemia is unclear, a workup that includes the iron profile, vitamin B12 and folate levels and excluding gastrointestinal tract bleeding should be considered to help select an appropriate anaemia management plan [102–105].
Dose Modification, Iron Therapy and Blood Transfusions
Recommendation 19: Dose delay and/or reduction are recommended for managing Grade ≥ 3 anaemia, and iron therapy (oral or intravenous [IV]) or blood transfusions should be considered, if clinically indicated.
According to clinical trial protocols, dose modifications and blood transfusions were recommended as the key strategies for managing T-DXd–related anaemia [25–27]. For Grade 3 or 4 anaemia (haemoglobin < 8.0 g/dL), T-DXd should be interrupted until it is resolved to Grade 2 or lower [25–27]. For Grade 4 events, dose can be reduced by one level in subsequent cycles [25–27]. Blood transfusions have been indicated for those who experienced Grade 3 or worse anaemia [25–27]. The impact of maintaining dose intensity while providing support through red blood cell transfusions are unclear, thus this approach needs to be balanced against potential transfusion-related complications [106, 107]. Oral or IV iron therapy has also been recommended by guidelines from ASCO, ESMO and NCCN [104, 105, 108], but the route chosen may depend upon several factors such as the onset and grade of anaemia, extent of iron deficiency, likelihood of oral absorption due to the presence of gastrointestinal comorbidities and potential for drug–drug interactions [106, 107].
Erythropoiesis Stimulating Agents (ESAs)
Guidelines from ASCO, ESMO and NCCN have suggested that ESAs may be offered to patients with chemotherapy-associated anaemia whose treatment is not curative and who have haemoglobin levels of less than 10 g/dL [104, 105, 108]. By increasing haemoglobin concentrations to the lowest acceptable levels, ESAs can reduce the need for transfusions and, thus, could be useful in patients who require frequent red blood cell transfusions [104, 107]. However, clinicians are advised to weigh the risk of thromboembolisms associated with ESAs [104, 108]. In fact, most members of the Asia-Pacific multidisciplinary panel do not use ESAs for the management of anaemia caused by anti-cancer therapy, but we acknowledge that other oncologists may require them in certain cases.
Thrombocytopenia
Dose Modification, Platelet Transfusion and Novel Agents
Recommendation 20: Dose delay and/or reduction are recommended for managing Grade ≥ 3 thrombocytopenia, and supportive platelet transfusion should be considered, if clinically indicated.
Prescribing information recommends the delay of T-DXd for Grade 3 thrombocytopenia (platelet count ≤ 50–25 × 109/L) until its resolution to Grade 1 or lower [56]. The management of Grade 4 thrombocytopenia is similar but requires dose reduction by one level [56]. Although not described in the prescribing information, the NCCN and ASCO guidelines recommend the use of platelet transfusion for chemotherapy-induced thrombocytopenia (CIT) according to clinical indication [105, 109]. However, platelet transfusions may cause infusion-related complications and could be used when maintaining dose intensity is vital for response [110].
Evidence suggests that thrombopoietin receptor agonists (TPO-RAs) such as romiplostim and eltrombopag are effective in alleviating CIT [111, 112]. In Hong Kong, TPO-RAs have not been approved for CIT but is occasionally used if patients can afford the treatment. It is unknown whether such agents confer an overall benefit to patients on T-DXd.
Fatigue
In our experience, fatigue was a common complaint in patients on T-DXd. In the DESTINY-Breast01 trial, fatigue was observed in 49.5% of participants, with 6% recording Grade 3 in severity [11]. A similar proportion of patients (31%) experienced this side effect in the DESTINY-Breast03 trial, with 6% reporting Grade ≥ 3 [48]. Notably, fatigue was much lower with T-DM1 (any grade: 20%; Grade ≥ 3: < 1%) [48]. When analysed per subgroup of patients receiving T-DXd, there appeared to be no difference in the incidence of fatigue in Asian patients compared to the overall population (all grades: 42.9% vs 44.7%; Grade ≥ 3: 5.4% versus 5.1%, respectively; Table 3) [50].
Fatigue Management
Recommendation 21: Health care professionals should be vigilant for treatable causes of fatigue (e.g., anaemia). They can encourage patients to use support groups and practice low-intensity exercises, as well as empower patients’ caregivers.
The management of fatigue may be complicated owing to its underestimation by both patients and physicians and the lack of universally effective strategies [113]. Nonetheless, we advocate that it should be an important aspect of care while patients are on T-DXd.
Patients with cancer can have numerous reasons for fatigue [113–115]. Discerning the provenance of this complaint is important. For instance, supportive blood transfusion can improve fatigue related to anaemia [113]. Moreover, patients should be educated about this side effect before treatment and be reassured that it could be manageable [116, 117]. Non-pharmacological approaches, such as support groups, have been associated with a reduction in cancer-related fatigue [117, 118]. Low-intensity exercise like Tai Chi and Qi Gong are effective in alleviating fatigue while on chemotherapy [119, 120] and is generally accepted in Asian cultures. For patients with impaired mobility, meditation has been shown to improve the quality of life in patients with metastatic breast cancer on chemotherapy [121]. Empowering caregivers may also help alleviate cancer-related fatigue [117].
Alopecia
Although T-DXd and T-DM1 are both anti-HER2 ADCs, the incidence of alopecia appeared to be more frequent with T-DXd than with T-DM1. Indeed, the DESTINY-Breast03 trial demonstrated that alopecia was more common in patients on T-DXd than those on T-DM1 (40% vs 3%, respectively) [48]. Furthermore, the DESTINY-Breast01 and DESTINY-Breast04 trials reported similar incidences of alopecia with T-DXd [11, 13]. Overall, the frequency of alopecia was comparable between Asian patients and the overall population receiving T-DXd (38.1% vs 36.2%, respectively; Table 3) [50].
Alopecia Management
Recommendation 22: To increase treatment acceptance, patients should be made aware of the risk of alopecia prior to starting T-DXd.
While alopecia rarely represents a serious medical concern, the psychological impact of this side effect could be overwhelming [122]. Thus, prior to treatment, we recommend that patients should be made aware of the risk of alopecia with T-DXd, as psychological preparedness may increase acceptance [123]. In our experience, patients can be encouraged to keep their hair short, be provided with styling techniques like the use of hats or scarfs and be provided advice on where to find high-quality wigs.
Recommendation 23: Scalp cooling could be considered but its efficacy in patients on T-DXd is unknown.
Scalp cooling has been shown to reduce hair loss caused by chemotherapies like taxanes [124]. However, this device can cause headaches, nausea and vomiting and scalp and neck pain [125]. In our experience, Asian cultures believe that wearing a chilled cap could induce “Tou Feng,” literally meaning “wind in the head,” which could potentially reduce the acceptance of this technique. Most importantly, it is unclear whether scalp cooling could prevent hair loss in patients treated with T-DXd. In our experience, scalp cooling is only available in private practice with additional cost, and as such, would not be accessible or recommended to patients.
Cardiac Toxicities
Similar to other HER2-targeted therapies, T-DXd is associated with a risk of left-ventricular (LV) dysfunction [55, 56, 126]. In the DESTINY-Breast01 trial, LVEF decrease occurred in 1.6% of patients and all were asymptomatic [11]. There were no events of cardiac failure associated with the LVEF decrease [11]. In the DESTINY-Breast03 trial, a decrease in ejection fraction was reported in 6 patients (2.3%) and 1 patient (0.4%) in the T-DXd and T-DM1 groups, respectively [12]. In the DESTINY-Breast04 trial, LV dysfunction was observed in 17 patients (4.6%) [13].
Monitoring
Recommendation 24: European Society of Cardiology (ESC) guidelines recommend that patients on anti-HER2 therapy should have an echocardiograph at baseline, which should be repeated every 3 months during the first year [127]. However, for asymptomatic and low-risk patients (i.e., without hypertension, borderline low LVEF, or received anthracyclines), surveillance can be reduced to every 6 months during future treatment. In high-risk patients, more frequent echocardiography and cardio-biomarkers may be considered. Cardiac magnetic resonance imaging should be considered when echocardiography is unavailable or non-diagnostic.
As anti-HER2 therapies can cause cardiac toxicities, we recommend that cardiac function should be evaluated at baseline and during T-DXd treatment [55, 56]. According to guidelines, the recommended frequency for monitoring cardiac toxicities with T-DXd was every 3 months [128, 129]. However, the incidence of cardiac adverse events is generally low (1.6–4.6%) [11–13]. Thus, in asymptomatic, patients without history or recent LVEF decreases, being low risk, clinicians may extend surveillance cardiac imaging to 6-monthly intervals [29, 130]. Such an approach would also be applicable in resource-limited settings. On the other hand, routine echocardiography every 3 months should be considered if patients have had prior exposure to anthracyclines or are at high risk because of concomitant hypertension or have borderline low LVEF [130]. According to the 2022 ESC guidelines, cardiac magnetic resonance imaging should be considered when echocardiography is unavailable or non-diagnostic [127]. Nevertheless, oncologists should be vigilant for cardiac symptoms and repeat cardiac function tests if the patient becomes symptomatic. Laboratory tests for troponin might also be useful for monitoring cardiac toxicities with anti-HER2 therapies, particularly in doubtful cases [128, 129]. Prescribing information has detailed recommendations on managing LVEF decrease associated with T-DXd [55, 56]. Additionally, referral to cardiac specialists may be considered at the discretion of the oncologist.
Discussion
Trastuzumab deruxtecan has the potential to markedly improve the outcomes of patients with HER2-positive metastatic breast cancer and a new group of patients who are now classified to be HER2-low. To maximise the benefits of this treatment, proactive monitoring and management of side effects with T-DXd will be required. As T-DXd advances from clinical trials to real-world practice, it is imperative to educate health care professionals on the effective management of its adverse events. Our goal with this publication is to leverage our clinical trial experience with this agent and provide practical recommendations on the monitoring and management of T-DXd–related adverse events in the context of Asia-Pacific health care practice, and our recommendations are summarised in Figs. 1 and 2. Since these recommendations are mainly based on expert consensus, we acknowledge that there are many aspects that require validation and confirmation with clinical trials.
ILD/Pneumonitis
While T-DXd–related ILD/pneumonitis has emerged as an important safety concern, clinical trials have demonstrated that proactive monitoring and management can mitigate its risk [29, 46]. Through our discussions, we uncovered that health care systems in the Asia-Pacific region may impose limitations in the frequent use of CT scan monitoring. With constraints in CT scan use across the region, studies comparing the sensitivity and specificity of rapid and affordable techniques such as chest X-rays, pulse oximetry and PFTs are required. Novel technologies leveraging machine learning and artificial intelligence for the diagnosis of ILD/pneumonitis from radiological scans might be a much-needed tool for monitoring [131]. Perhaps at a more fundamental level, knowledge of the mechanism of how T-DXd induces lung damage in patients with metastatic breast cancer is urgently required. A study conducted in cynomolgus monkeys suggested that T-DXd-related ILD/pneumonitis might be due to the involvement of alveolar macrophages [132].
We believe that there may be a need to address a subpopulation of patients for whom steroids are contraindicated or for cases where ILD/pneumonitis does not respond to steroids. Some forms of ILD/pneumonitis, such as unclassifiable idiopathic interstitial pneumonia and interstitial pneumonia with autoimmune features, may be associated with poor response to steroids [133]. Steroid-refractory ILD/pneumonitis might also be common with immune-checkpoint inhibitors, but its incidence in patients on T-DXd is unclear [134].
Adverse Events in Asian and Other Patient Populations
Evidence has started to uncover that biological variables like race may affect drug efficacy and safety profile [135]. However, based on the results presented in the manuscript, there appeared to be no major differences in the adverse event profile between Asian patients and the overall population in the DESTINY-Breast03 trial [50]. A notable exception might be an increased incidence of ILD/pneumonitis in Japanese patients when compared with Asian patients and overall population [50]. Studies have demonstrated that Japanese patients may have a slightly reduced rate of T-DXd clearance [136]. Furthermore, an exposure–safety analysis uncovered a higher rate of safety concerns, such as discontinuations and dose delays due to adverse events, in Japanese patients than in non-Asian patients [136, 137]. On the other hand, it is possible that the increased toxicity of T-DXd in Japanese patients when compared with other populations may be attributed to the differences in clinical practices, such as proactive patient monitoring [28].
Balancing Dose Modification and Managing Other Adverse Events
During our discussions, we noted that the clinical decisions on opting for dose modification (delay or reduction) or to treat adverse events like neutropenia and anaemia may require additional evidence. In particular, knowledge of the clinical outcomes in patients who experienced dose reductions to control such adverse events might help oncologists make more informed decisions on the management of adverse events.
Strengths and Limitations
We believe that the key strength of this publication is that it strives to provide recommendations that are timely and applicable to real-world practice. Moreover, the methods used to develop these recommendations, particularly, the pre-meeting survey and live video meeting, may have enabled us to discuss the challenges with the monitoring and management of T-DXd–related adverse events and their potential solutions in more depth than a traditional expert consensus panels. Nonetheless, it must be stressed that these recommendations are mostly based on our clinical experience and, thus, will require further validation through post-marketing trials. Furthermore, despite the diversity of our expert panel, we acknowledge that recommendations might not be applicable to other countries in the Asia-Pacific region. Finally, the clinical utility of these recommendations may have been even more relevant if we had investigated rates of adverse events in the Asian population enrolled in other DESTINY trials, although subgroup analyses were not available as of this writing.
Conclusion
The outcomes from the DESTINY-Breast trials are practice changing. As T-DXd advances from clinical trials to real-world practice, clinicians may have questions on how best to balance its benefits with side effects in a practical manner. To address this emerging unmet need, we have developed recommendations for real-world practice using published guidelines and our own clinical experience with T-DXd. In this manuscript, we hope to provide practical suggestions on the management of ILD/pneumonitis, nausea and vomiting, haematological toxicities such as neutropenia, anaemia and thrombocytopenia, fatigue, alopecia and LVEF decrease. In essence, proactive monitoring and management are key to maximising the benefits conferred by T-DXd for patients with HER2-positive or HER2-low metastatic breast cancer. Future research is required to uncover risk factors for adverse events such as ILD/pneumonitis, new tools for monitoring and diagnosing ILD/pneumonitis and advances in supportive care for the management of fatigue and alopecia.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Polina Gavrilova, Yulia Abbasova, Sara Frau and Natalia Denisova from MphaR; and Charlotte White, Celestine Quek and Isuru Wijesoma, PhD, from McCann Health Singapore for supporting the implementation of the Online Survey and Live Video Meeting. We would like to acknowledge Isuru Wijesoma, PhD, from McCann Health Singapore for medical writing assistance. Finally, the authors would like to thank the patients and families; the staff and investigators at all the trials sites; and members of the independent data monitoring committees. AstraZeneca and Daiichi Sankyo provided funding to assist with the preparation of this article.
Declarations
Funding
AstraZeneca and Daiichi Sankyo provided funding to assist with the preparation of this article.
Conflicts of Interest
Joanne Wing Yan Chiu received honorarium from AstraZeneca for consulting services and lectures related to the drug of interest; Soo Chin Lee received grants from Pfizer, Eisai, Taiho, ACT Genomics, Bayer, Karyopharm; consulting fees or honorarium from Pfizer, Novartis, AstraZeneca, ACT Genomics, Eli Lilly, MSD, Roche, Eisai; payments for lectures from Pfizer, Novartis, Astra Zeneca, ACT Genomics, MSD, Roche and Eisai; James Chung-man Ho received consulting fees or honorarium and lectures from AstraZeneca; Yeon Hee Park received grants AstraZeneca, Pfizer; consulting fees or honorarium from AstraZeneca, Pfizer, Lilly, MSD, Eisai, Roche, Daiichi Sankyo, MENARINI, Bixink, Everest and Novartis Pharmaceuticals; Payment for writing or review or reviewing manuscript for Lancet Oncology; and payment for lectures from AstraZeneca, Pfizer, Lilly, MSD, Eisai, Roche and Novartis Pharmaceuticals; Ta-Chung Chao has no conflicts of interest to disclose; Sung-Bae Kim received grants to their institution from Novartis, Sanofi-Aventis and DongKook Pharm Co; consulting fees or honorarium from Novartis, AstraZeneca, Lilly, Dae Hwa Pharmaceutical Co Ltd, ISU Abxis, OBI Pharma, Beigene and Daiichi Sankyo; and holds stock/stock options from Genopeaks and NeogeneTC; Elgene Lim received consulting fees or honorarium for advisory boards from AstraZeneca; provision of writing assistance (manuscript) by Medical Pharma Services; and payment for lectures by AstraZeneca; Ching-Hung Lin has no conflicts of interest to disclose; Sherene Loi receives research funding to her institution from Novartis, Bristol Myers Squibb, Merck, Puma Biotechnology, Eli Lilly, Nektar Therapeutics, AstraZeneca and Seattle Genetics. She has acted as consultant (not compensated) to Seattle Genetics, Novartis, Bristol Myers Squibb, Merck, AstraZeneca, Eli Lilly, Pfizer, Gilead Therapeutics and Roche-Genentech. She has acted as consultant (paid to her institution) to Aduro Biotech, Novartis, GlaxoSmithKline, Roche-Genentech, AstraZeneca, Silverback Therapeutics, G1 Therapeutics, PUMA Biotechnologies, Pfizer, Gilead Therapeutics, Seattle Genetics, Daiichi Sankyo, Merck, Amunix, Tallac Therapeutics, Eli Lilly and Bristol Myers Squibb; Sherene Loi is supported by the National Breast Cancer Foundation of Australia Endowed Chair and the Breast Cancer Research Foundation, New York; Su Ying Low received grants received from Boehringer Ingelheim; consulting fees or honorarium from Roche and Boehringer Ingelheim; payment for writing or reviewing manuscript from AstraZeneca; Lynette Teo consulting fees or honorarium and provision of writing assistance from AstraZeneca; Winnie Yeo consulting fees or honorarium from AstraZeneca and Daiichi Sankyo; payment for lectures from AstraZeneca and Daiichi Sankyo; Rebecca Dent has no conflicts of interest to disclose.
Availability of Data and Material
Not applicable.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Author Contributions
Conceptualisation and supervision: JWYC, RD; Writing—original draft preparation: all authors; Writing—review and editing: all authors. All authors read and approved the final manuscript.
Footnotes
Signs and symptoms of ILD/pneumonitis may include dyspnoea with exertion, cough, fever and other new or worsening respiratory symptoms.
For instance, 6-weekly CT scans may be considered for the first 6–12 months of T-DXd treatment given the known median time of ILD/pneumonitis onset and clinical trial experience. If longer intervals are chosen (9- or 12-weekly) other supplemental strategies to monitor for ILD/pneumonitis are recommended.
e.g., 1 mg/kg prednisone or equivalent for Grade 2, or 500–1000 mg/day empirical high-dose methylprednisolone intravenous (IV) for 3 days followed by 1 mg/kg prednisone or equivalent for >14 days for Grade 3/4.
While on high-dose systemic corticosteroids (particularly pulse steroids), consider adding Pneumocystis jirovecii pneumonia prophylaxis (e.g., co-trimoxazole) and performing surveillance for CMV (e.g., CMV pp65 antigen).
Soo Chin Lee, James Chung-man Ho, Yeon Hee Park have contributed equally to the manuscript development.
Contributor Information
Joanne Wing Yan Chiu, Email: jwychiu@hku.hk.
Soo Chin Lee, Email: csilsc@nus.edu.sg.
James Chung-man Ho, Email: jhocm@hku.hk.
Yeon Hee Park, Email: yhparkhmo@skku.edu.
Ta-Chung Chao, Email: tcchao@vghtpe.gov.tw.
Sung-Bae Kim, Email: sbkim712@gmail.com.
Elgene Lim, Email: e.lim@garvan.org.au.
Ching-Hung Lin, Email: chinghlin@ntu.edu.tw.
Sherene Loi, Email: sherene.loi@petermac.org.
Su Ying Low, Email: low.su.ying@singhealth.com.sg.
Lynette Li San Teo, Email: dnrtlsl@nus.edu.sg.
Winnie Yeo, Email: winnie@clo.cuhk.edu.hk.
Rebecca Dent, Email: rebecca.dent@duke-nus.edu.sg.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2011;9(1):16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 3.Dieci MV, Miglietta F. HER2: a never ending story. Lancet Oncol. 2021;22(8):1051–1052. doi: 10.1016/S1470-2045(21)00349-1. [DOI] [PubMed] [Google Scholar]
- 4.Giordano SH, Franzoi MAB, Temin S, et al. Systemic therapy for advanced human epidermal growth factor receptor 2–positive breast cancer: ASCO guideline update. J Clin Oncol. 2022;40(23):2612–2635. doi: 10.1200/JCO.22.00519. [DOI] [PubMed] [Google Scholar]
- 5.Gennari A, André F, Barrios CH, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Miglietta F, Griguolo G, Bottosso M, et al. HER2-low-positive breast cancer: evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer. 2022;8(1):66. doi: 10.1038/s41523-022-00434-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eiger D, Agostinetto E, Saúde-Conde R, de Azambuja E. The exciting new field of HER2-low breast cancer treatment. Cancers (Basel). 2021;13(5):1015. doi: 10.3390/cancers13051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, A novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 9.Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18(11):1512–1522. doi: 10.1016/S1470-2045(17)30604-6. [DOI] [PubMed] [Google Scholar]
- 10.Tamura K, Tsurutani J, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):816–826. doi: 10.1016/S1470-2045(19)30097-X. [DOI] [PubMed] [Google Scholar]
- 11.Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortés J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 13.Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food & Drug Administration. FDA grants regular approval to fam-trastuzumab deruxtecan-nxki for breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-fam-trastuzumab-deruxtecan-nxki-breast-cancer. Accessed Dec 2022.
- 15.US Food & Drug Administration. FDA approves fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2-positive-breast-cancer. Accessed 11 July 2023.
- 16.US food & Drug Administration. FDA Approves First Targeted Therapy for HER2-Low Breast Cancer. https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-her2-low-breast-cancer. Accessed 11 July 2023.
- 17.European Medicines Agency. Enhertu. https://www.ema.europa.eu/en/medicines/human/EPAR/enhertu#authorisation-details-section. Accessed 11 July 2023.
- 18.Averitt AJ, Weng C, Ryan P, Perotte A. Translating evidence into practice: eligibility criteria fail to eliminate clinically significant differences between real-world and study populations. NPJ Digit Med. 2020;3:67. doi: 10.1038/s41746-020-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018;33(34):213. doi: 10.3346/jkms.2018.33.e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boissel JP, Cogny F, Marko N, Boissel FH. From clinical trial efficacy to real-life effectiveness: why conventional metrics do not work. Drugs Real World Outcomes. 2019;6(3):125–132. doi: 10.1007/s40801-019-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duma N, Vera Aguilera J, Paludo J, et al. Representation of minorities and women in oncology clinical trials: review of the past 14 Years. J Oncol Pract. 2018;14(1):e1–10. doi: 10.1159/000441818. [DOI] [PubMed] [Google Scholar]
- 22.Fan L, Goss PE, Strasser-Weippl K. Current status and future projections of breast cancer in Asia. Breast Care (Basel). 2015;10(6):372–378. doi: 10.1159/000441818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirko KA, Rocque G, Reasor E, et al. The impact of race and ethnicity in breast cancer-disparities and implications for precision oncology. BMC Med. 2022;20(1):72. doi: 10.1186/s12916-022-02260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LoCasale RJ, Pashos CL, Gutierrez B, et al. Bridging the gap between RCTs and RWE through endpoint selection. Ther Innov Regul Sci. 2021;55(1):90–96. doi: 10.1007/s43441-020-00193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daiichi Sankyo. DESTINY-Breast01. Clinical Study Protocol.
- 26.Daiichi Sankyo. DESTINY-Breast03. Clinical Study Protocol.
- 27.Daiichi Sankyo. DESTINY-Breast04. Clinical Study Protocol.
- 28.Swain SM, Nishino M, Lancaster LH, et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-Focus on proactive monitoring, diagnosis, and management. Cancer Treat Rev. 2022;106:102378. doi: 10.1016/j.ctrv.2022.102378. [DOI] [PubMed] [Google Scholar]
- 29.Bardia A, Harnden K, Mauro L, Pennisi A, Armitage M, Soliman H. Clinical practices and institutional protocols on prophylaxis, monitoring, and management of selected adverse events associated with trastuzumab deruxtecan. Oncologist. 2022;27(8):637–645. doi: 10.1093/oncolo/oyac107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi E, Lee S, Nhung BC, et al. Cancer mortality-to-incidence ratio as an indicator of cancer management outcomes in Organization for Economic Cooperation and Development countries. Epidemiol Health. 2017;39:e2017006. doi: 10.4178/epih.e2017006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trapani D, Curigliano G, Eniu A. Breast cancer: Reimbursement policies and adoption of new therapeutic agents by national health systems. Breast Care (Basel). 2019;14(6):373–381. doi: 10.1159/000502637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ithimakin S, Parinyanitikul N, Kim SB, et al. Disparities in access to systemic treatment for breast cancer in Thailand and major Asian territories. J Breast Cancer. 2022;25(3):207–217. doi: 10.4048/jbc.2022.25.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smullen A, Hong PK. Comparing the health care systems of high-performing Asian countries. Asia Pac Policy Stud. 2015;2(2):347–355. doi: 10.1002/app5.76. [DOI] [Google Scholar]
- 34.Leung GM, Wagstaff A, Lindelow M, Lu JR. China, Hong Kong and Taiwan, Health Systems of. Int Encycl Public Health. 2008 doi: 10.1016/B978-012373960-5.00305-1. [DOI] [Google Scholar]
- 35.Tan CC, Lam CSP, Matchar DB, Zee YK, Wong JEL. Singapore's health-care system: Key features, challenges, and shifts. Lancet. 2021;398(10305):1091–1104. doi: 10.1016/S0140-6736(21)00252-X. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Acevedo AJ, Chan RJ, Olsen CM, Pandeya N, Whiteman DC, Gordon LG. Out-of-pocket medical expenses compared across five years for patients with one of five common cancers in Australia. BMC Cancer. 2021;21(1):1055. doi: 10.1186/s12885-021-08756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldsbury DE, Yap S, Weber MF, et al. Health services costs for cancer care in Australia: Estimates from the 45 and Up Study. PLoS ONE. 2018;13(7):e0201552. doi: 10.1371/journal.pone.0201552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasa P, Jain R, Juneja D. Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol. 2021;11(4):116–129. doi: 10.5662/wjm.v11.i4.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guler SA, Corte TJ. Interstitial lung disease in 2020: A history of progress. Clin Chest Med. 2021;42(2):229–239. doi: 10.1016/j.ccm.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Ryerson CJ, Corte TJ, Myers JL, Walsh SLF, Guler SA. A contemporary practical approach to the multidisciplinary management of unclassifiable interstitial lung disease. Eur Respir J. 2021;58(6):2100276. doi: 10.1183/13993003.00276-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spagnolo P, Bonniaud P, Rossi G, Sverzellati N, Cottin V. Drug-induced interstitial lung disease. Eur Respir J. 2022;60(4):2102776. doi: 10.1183/13993003.02776-2021. [DOI] [PubMed] [Google Scholar]
- 42.Willemsen AECAB, Tol J, van Erp NP, et al. Prospective study of drug-induced interstitial lung disease in advanced breast cancer patients receiving everolimus plus exemestane. Target Oncol. 2019;14(4):441–451. doi: 10.1007/s11523-019-00656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nawa H, Niimura T, Yagi K, Goda M, Zamami Y, Ishizawa K. Evaluation of potential complication of interstitial lung disease with abemaciclib and palbociclib treatments. Cancer Rep (Hoboken). 2022;5(1):e1402. doi: 10.1002/cnr2.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakami M, Kanemura H, Tomishima Y, Nakano E, Tamura T. Eribulin-induced interstitial pneumonia: A case series and retrospective cohort study. Intern Med. 2020;59(4):563–567. doi: 10.2169/internalmedicine.2779-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anoop TM, Joseph R, Unnikrishnan P, Thomas F, Venugopal M. Taxane-induced acute interstitial pneumonitis in patients with breast cancer and outcome of taxane rechallenge. Lung India. 2022;39(2):158–168. doi: 10.4103/lungindia.lungindia_126_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell CA, Modi S, Iwata H, et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open. 2022;7(4):100554. doi: 10.1016/j.esmoop.2022.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powell CA, Modi S, Iwata H, et al. 92O Analysis of study drug-related interstitial lung disease (ILD) in patients (pts) with HER2+ metastatic breast cancer (mBC) treated with trastuzumab deruxtecan (T-DXd). Ann Oncol. 2021;32:S61–2. https://www.annalsofoncology.org/article/S0923-7534(21)00984-4/fulltext. Accessed 11 July 2023.
- 48.Hurvitz SA, Hegg R, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105–117. doi: 10.1016/S0140-6736(22)02420-5. [DOI] [PubMed] [Google Scholar]
- 49.Kish JK, Mehta S, Kwong J, et al. Monitoring and management of interstitial lung disease/pneumonitis among patients with metastatic breast cancer treated with trastuzumab deruxtecan. J Clin Oncol. 2022;40(16_suppl):1036. doi: 10.1016/S0140-6736(22)02420-5. [DOI] [PubMed] [Google Scholar]
- 50.Im SA, Xu B, Kim SB, et al. Trastuzumab deruxtecan vs T-DM1 in HER2+ mBC in Asian subgroup: Results of the randomized phase 3 study DESTINY-Breast03 (PS2-1). In Japanese Society of Medical Oncology Annual Meeting. 2022. Kyoto, Japan. https://www.c-linkage.co.jp/jsmo2022/en/contents/program.html. Accessed 11 July 2023.
- 51.Kubo K, Azuma A, Kanazawa M, et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig. 2013;51(4):260–277. doi: 10.1016/j.resinv.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Tarantino P, Modi S, Tolaney SM, et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review. JAMA Oncol. 2021;7(12):1873–1881. doi: 10.1001/jamaoncol.2021.3595. [DOI] [PubMed] [Google Scholar]
- 53.Conte P, Ascierto PA, Patelli G, et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open. 2022;7(2):100404. doi: 10.1016/j.esmoop.2022.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rugo HS, Bianchini G, Cortes J, Henning JW, Untch M. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open. 2022;7(4):100553. doi: 10.1016/j.esmoop.2022.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.European Medicines Agency. Summary of product characteristics. ENHERTU. https://www.ema.europa.eu/en/documents/product-information/enhertu-epar-product-information_en-0.pdf Accessed 11 July 2023.
- 56.US Food & Drug Administration. Prescribing Information. ENHERTU. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761139s011lbl.pdf. Accessed 11 July 2023.
- 57.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed 11 July 2023.
- 58.Liu H, Liu Y, Zhao L, Li X, Zhang W. Preprocedural fasting for contrast-enhanced CT: when experience meets evidence. Insights Imaging. 2021;12(1):180. doi: 10.1186/s13244-021-01131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Houston R, Mahato B, Odell T, Khan YR, Mahato D. The financial and radiation burden of early reimaging in neurosurgical patients: an original study and review of the literature. Cureus. 2021;13(8):e17383. doi: 10.7759/cureus.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Power SP, Moloney F, Twomey M, James K, O'Connor OJ, Maher MM. Computed tomography and patient risk: facts, perceptions and uncertainties. World J Radiol. 2016;8(12):902–915. doi: 10.4329/wjr.v8.i12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin EC. Radiation risk from medical imaging. Mayo Clin Proc. 2010;85(12):1142–1146. doi: 10.4065/mcp.2010.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Beek EJ, Mirsadraee S, Murchison JT. Lung cancer screening: computed tomography or chest radiographs? World J Radiol. 2015;7(8):189–193. doi: 10.4329/wjr.v7.i8.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vizioli L, Ciccarese F, Forti P, et al. Integrated use of lung ultrasound and chest X-ray in the detection of interstitial lung disease. Respiration. 2017;93(1):15–22. doi: 10.1159/000452225. [DOI] [PubMed] [Google Scholar]
- 64.Afzal F, Raza S, Shafique M. Diagnostic accuracy of X-ray chest in interstitial lung disease as confirmed by high resolution computed tomography (HRCT) chest. Pak Armed Forces Med J. 2017;67(4):593–98. https://www.pafmj.org/index.php/PAFMJ/article/view/699. Accessed 11 July 2023.
- 65.Karayama M, Aoshima Y, Yasui H, et al. Simple method for detecting idiopathic interstitial pneumonias by measuring vertical lung length on chest X-ray. Sci Rep. 2021;11(1):7669. doi: 10.1038/s41598-021-87452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walsh SLF, Devaraj A, Enghelmayer JI, et al. Role of imaging in progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27(150):180073. doi: 10.1183/16000617.0073-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 68.Oishi K, Matsunaga K, Asami-Noyama M, et al. The 1-minute sit-to-stand test to detect desaturation during 6-minute walk test in interstitial lung disease. NPJ Prim Care Respir Med. 2022;32(1):5. doi: 10.1038/s41533-022-00268-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barth S, Edwards C, Saini G, et al. P33 What affects acceptability of remote digital monitoring of spirometry in patients with interstitial lung disease? Thorax. 2022;77:A97–A98. [Google Scholar]
- 70.Alboksmaty A, Beaney T, Elkin S, et al. Effectiveness and safety of pulse oximetry in remote patient monitoring of patients with COVID-19: a systematic review. Lancet Digit Health. 2022;4(4):e279–e289. doi: 10.1016/S2589-7500(21)00276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gold WM, Koth LL. Pulmonary function testing. In: Murray and Nadel's Textbook of respiratory medicine. 2016:407–35. 10.1016/B978-1-4557-3383-5.00025-7.
- 72.Hoffmann T, Oelzner P, Franz M, et al. Assessing the diagnostic value of a potential screening tool for detecting early interstitial lung disease at the onset of inflammatory rheumatic diseases. Arthritis Res Ther. 2022;24(1):107. doi: 10.1186/s13075-022-02786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bui A, Han S, Alexander M, Toner G, Irving L, Manser R. Pulmonary function testing for the early detection of drug-induced lung disease: a systematic review in adults treated with drugs associated with pulmonary toxicity. Intern Med J. 2020;50(11):1311–1325. doi: 10.1111/imj.14647. [DOI] [PubMed] [Google Scholar]
- 74.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Resp J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 75.Cheung HJ, Cheung L. Coaching patients during pulmonary function testing: a practical guide. Can J Respir Ther. 2015;51(3):65–68. doi: 10.1016/j.chest.2020.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kouri A, Gupta S, Yadollahi A, et al. Addressing reduced laboratory-based pulmonary function testing during a pandemic. Chest. 2020;158(6):2502–2510. doi: 10.1016/j.chest.2020.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McGowan A, Laveneziana P, Bayat S, et al. International consensus on lung function testing during the COVID-19 pandemic and beyond. ERJ Open Res. 2022;8(1):00602–2021. doi: 10.1183/23120541.00602-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richeldi L, Launders N, Martinez F, et al. The characterisation of interstitial lung disease multidisciplinary team meetings: a global study. ERJ Open Res. 2019;5(2):00209–2018. doi: 10.1183/23120541.00209-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 80.Mikolasch TA, Garthwaite HS, Porter JC. Update in diagnosis and management of interstitial lung disease. Clin Med (Lond) 2017;17(2):146–153. doi: 10.7861/clinmedicine.17-2-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185(9):1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 82.Kebbe J, Abdo T. Interstitial lung disease: the diagnostic role of bronchoscopy. J Thorac Dis. 2017;9(Suppl 10):S996–1010. doi: 10.21037/jtd.2017.06.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamakawa H, Hagiwara E, Kitamura H, et al. Serum KL-6 and surfactant protein-D as monitoring and predictive markers of interstitial lung disease in patients with systemic sclerosis and mixed connective tissue disease. J Thorac Dis. 2017;9(2):362–371. doi: 10.21037/jtd.2017.02.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takahashi H, Kuroki Y, Tanaka H, et al. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am J Respir Crit Care Med. 2000;162(1):258–263. doi: 10.1164/ajrccm.162.1.9903014. [DOI] [PubMed] [Google Scholar]
- 85.Malpica L, Moll S. Practical approach to monitoring and prevention of infectious complications associated with systemic corticosteroids, antimetabolites, cyclosporine, and cyclophosphamide in nonmalignant hematologic diseases. Hematol Am Soc Hematol Educ Program. 2020;2020(1):319–327. doi: 10.1182/hematology.2020000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamamoto Y, Shiroyama T, Hirata H, et al. Prolonged corticosteroid therapy and cytomegalovirus infection in patients with severe COVID-19. J Med Virol. 2022;94(3):1067–1073. doi: 10.1002/jmv.27421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sato Y, Motoyama S, Maruyama K, et al. CMV reactivation caused by methylprednisolone therapy for ARDS after esophagectomy. Esophagus. 2012;9(3):141–146. doi: 10.1007/s10388-012-0316-x. [DOI] [Google Scholar]
- 88.Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: ASCO guideline update. J Clin Oncol. 2020;38(24):2782–2797. doi: 10.1200/JCO.20.01296. [DOI] [PubMed] [Google Scholar]
- 89.National Comprehensive Cancer Network. Antiemesis. 2023. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed 11 July 2023.
- 90.Aapro M. Searching for perfection: Further progress in management of chemotherapy-induced nausea and vomiting-introduction. Support Care Cancer. 2018;26(Suppl 1):3–4. doi: 10.1007/s00520-018-4147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bianchini G, Arpino G, Biganzoli L, et al. Emetogenicity of antibody-drug conjugates (ADCs) in solid tumors with a focus on trastuzumab deruxtecan: insights from an Italian expert panel. Cancers (Basel). 2022;14(4):1022. doi: 10.3390/cancers14041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mosa ASM, Hossain AM, Lavoie BJ, et al. Patient-related risk factors for chemotherapy-induced nausea and vomiting: a systematic review. Front Pharmacol. 2020;11:329. doi: 10.3389/fphar.2020.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hashimoto H, Abe M, Tokuyama O, et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(2):242–249. doi: 10.1016/S1470-2045(19)30678-3. [DOI] [PubMed] [Google Scholar]
- 94.Chanthawong S, Lim YH, Subongkot S, et al. Cost-effectiveness analysis of olanzapine-containing antiemetic therapy for managing highly emetogenic chemotherapy in Southeast Asia: a multinational study. Support Care Cancer. 2019;27(3):1109–1119. doi: 10.1007/s00520-018-4400-1. [DOI] [PubMed] [Google Scholar]
- 95.Sun S, Ko YH, Jin JY, et al. Efficacy of the granisetron transdermal system for the control of nausea and vomiting induced by highly emetogenic chemotherapy: a multicenter, randomized, controlled trial. Korean J Intern Med. 2022 doi: 10.3904/kjim.2020.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rapoport BL. Delayed chemotherapy-induced nausea and vomiting: pathogenesis, incidence, and current management. Front Pharmacol. 2017;8:19. doi: 10.3389/fphar.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kondo T, Amano K. Era of steroid sparing in the management of immune-mediated inflammatory diseases. Immunol Med. 2018;41(1):6–11. doi: 10.1080/09114300.2018.1451593. [DOI] [PubMed] [Google Scholar]
- 98.Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–3212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
- 99.Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2016;27(suppl 5):v111–v118. doi: 10.1093/annonc/mdw325. [DOI] [PubMed] [Google Scholar]
- 100.Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42(15):2433–2453. doi: 10.1016/j.ejca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Choi JH, Geum MJ, Kang JE, Park NG, Oh YK, Rhie SJ. Clinical outcomes of secondary prophylactic granulocyte colony-stimulating factors in breast cancer patients at a risk of neutropenia with doxorubicin and cyclophosphamide-based chemotherapy. Pharmaceuticals (Basel) 2021;14(11):1200. doi: 10.3390/ph14111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cascio MJ, DeLoughery TG. Anemia: Evaluation and diagnostic tests. Med Clin N Am. 2017;101(2):263–284. doi: 10.1016/j.mcna.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 103.Snook J, Bhala N, Beales ILP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021;70(11):2030–2051. doi: 10.1136/gutjnl-2021-325210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aapro M, Beguin Y, Bokemeyer C, et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29(Suppl 4):iv96–110. doi: 10.1093/annonc/mdx758. [DOI] [PubMed] [Google Scholar]
- 105.National Comprehensive Cancer Network. Hematopoietic growth factors. 2023. https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed 11 July 2023.
- 106.Madeddu C, Neri M, Sanna E, Oppi S, Macciò A. Experimental drugs for chemotherapy- and cancer-related anemia. J Exp Pharmacol. 2021;13:593–611. doi: 10.2147/JEP.S262349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gilreath JA, Rodgers GM. How I treat cancer-associated anemia. Blood. 2020;136(7):801–813. doi: 10.1182/blood.2019004017. [DOI] [PubMed] [Google Scholar]
- 108.Bohlius J, Bohlke K, Castelli R, et al. Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH clinical practice guideline update. J Clin Oncol. 2019;37(15):1336–1351. doi: 10.1200/JCO.18.02142. [DOI] [PubMed] [Google Scholar]
- 109.Schiffer CA, Bohlke K, Delaney M, et al. Platelet transfusion for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(3):283–299. doi: 10.1200/JOP.2017.028902. [DOI] [PubMed] [Google Scholar]
- 110.Kuter DJ. Treatment of chemotherapy-induced thrombocytopenia in patients with non-hematologic malignancies. Haematologica. 2022;107(6):1243–1263. doi: 10.3324/haematol.2021.279512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Al-Samkari H, Parnes AD, Goodarzi K, Weitzman JI, Connors JM, Kuter DJ. A multicenter study of romiplostim for chemotherapy-induced thrombocytopenia in solid tumors and hematologic malignancies. Haematologica. 2021;106(4):1148–1157. doi: 10.3324/haematol.2020.251900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Winer ES, Safran H, Karaszewska B, et al. Eltrombopag for thrombocytopenia in patients with advanced solid tumors receiving gemcitabine-based chemotherapy: a randomized, placebo-controlled phase 2 study. Int J Hematol. 2017;106(6):765–776. doi: 10.1007/s12185-017-2319-9. [DOI] [PubMed] [Google Scholar]
- 113.Horneber M, Fischer I, Dimeo F, Rüffer JU, Weis J. Cancer-related fatigue: epidemiology, pathogenesis, diagnosis, and treatment. Dtsch Arztebl Int. 2012;109(9):161–171. doi: 10.3238/arztebl.2012.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weber D, O'Brien K. Cancer and cancer-related fatigue and the interrelationships with depression, stress, and inflammation. J Evid Based Complementary Altern Med. 2017;22(3):502–512. doi: 10.1177/2156587216676122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32(17):1840–1850. doi: 10.1016/j.annonc.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fabi A, Bhargava R, Fatigoni S, et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol. 2020;31(6):713–723. doi: 10.1016/j.annonc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 118.Mardanian-Dehkordi L, Kahangi L. The Relationship between perception of social support and fatigue in patients with cancer. Iran J Nurs Midwifery Res. 2018;23(4):261–266. doi: 10.4103/ijnmr.IJNMR_63_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang LL, Wang SZ, Chen HL, Yuan AZ. Tai Chi exercise for cancer-related fatigue in patients with lung cancer undergoing chemotherapy: a randomized controlled trial. J Pain Symptom Manag. 2016;51(3):504–511. doi: 10.1016/j.jpainsymman.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 120.Wang R, Huang X, Wu Y, Sun D. Efficacy of Qigong exercise for treatment of fatigue: a systematic review and meta-analysis. Front Med (Lausanne). 2021;8:684058. doi: 10.3389/fmed.2021.684058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gok Metin Z, Karadas C, Izgu N, Ozdemir L, Demirci U. Effects of progressive muscle relaxation and mindfulness meditation on fatigue, coping styles, and quality of life in early breast cancer patients: an assessor blinded, three-arm, randomized controlled trial. Eur J Oncol Nurs. 2019;42:116–125. doi: 10.1016/j.ejon.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 122.Moattari CR, Jafferany M. Psychological aspects of hair disorders: Consideration for dermatologists, cosmetologists, aesthetic, and plastic surgeons. Skin Appendage Disord. 2022;8(3):186–194. doi: 10.1159/000519817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim IR, Cho J, Choi EK, et al. Perception, attitudes, preparedness and experience of chemotherapy-induced alopecia among breast cancer patients: a qualitative study. Asian Pac J Cancer Prev. 2012;13(4):1383–1388. doi: 10.7314/apjcp.2012.13.4.1383. [DOI] [PubMed] [Google Scholar]
- 124.Coolbrandt A, T'Jonck A, Blauwens K, et al. Scalp cooling in breast cancer patients treated with docetaxel-cyclophosphamide: patient- and nurse-reported results. Breast Cancer Res Treat. 2021;186(3):715–722. doi: 10.1007/s10549-020-06063-w. [DOI] [PubMed] [Google Scholar]
- 125.Shen XF, Ru LX, Yao XB. Efficacy of scalp cooling for prevention of chemotherapy induced alopecia: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(16):5090–5103. doi: 10.26355/eurrev_202108_26520. [DOI] [PubMed] [Google Scholar]
- 126.Copeland-Halperin RS, Liu JE, Yu AF. Cardiotoxicity of HER2-targeted therapies. Curr Opin Cardiol. 2019;34(4):451–458. doi: 10.1097/HCO.0000000000000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 128.Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl 7):vii155–vii166. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 129.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(36):2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 130.Collier P, Hussain M, Popovic ZB, Griffin BP. Clevel Clin J Med. 2021;88(2):110–116. doi: 10.3949/ccjm.88a.19150. [DOI] [PubMed] [Google Scholar]
- 131.Soffer S, Morgenthau AS, Shimon O, et al. Artificial intelligence for interstitial lung disease analysis on chest computed tomography: a systematic review. Acad Radiol. 2022;29(Suppl 2):S226–S235. doi: 10.1016/j.acra.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 132.Kumagai K, Aida T, Tsuchiya Y, Kishino Y, Kai K, Mori K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys. Cancer Sci. 2020;111(12):4636–4645. doi: 10.1111/cas.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wiertz IA, van Moorsel CHM, Vorselaars ADM, Quanjel MJR, Grutters JC. Cyclophosphamide in steroid refractory unclassifiable idiopathic interstitial pneumonia and interstitial pneumonia with autoimmune features (IPAF) Eur Respir J. 2018;51(4):1702519. doi: 10.1183/13993003.02519-2017. [DOI] [PubMed] [Google Scholar]
- 134.Balaji A, Hsu M, Lin CT, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer. 2021;9(1):e001731. doi: 10.1136/jitc-2020-001731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.O'Donnell PH, Dolan ME. Cancer pharmacoethnicity: Ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res. 2009;15(15):4806–4814. doi: 10.1158/1078-0432.CCR-09-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yin O, Xiong Y, Endo S, et al. Population pharmacokinetics of trastuzumab deruxtecan in patients with HER2-positive breast cancer and other solid tumors. Clin Pharmacol Ther. 2021;109(5):1314–1325. doi: 10.1002/cpt.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yin O, Iwata H, Lin CC, et al. Exposure-response relationships in patients with HER2-positive metastatic breast cancer and other solid tumors treated with trastuzumab deruxtecan. Clin Pharmacol Ther. 2021;110(4):986–996. doi: 10.1002/cpt.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.