Abstract
Alignment of DICOM (Digital Imaging and Communications in Medicine) capabilities among vendors is crucial to improve interoperability in the healthcare industry and advance medical imaging 2. However, a sustainable model for sharing DICOM samples is not available. To address this issue, Integrating the Healthcare Enterprise (IHE) has introduced the IHE SHARAZONE, a continuous cross-vendor DICOM data sharing test service. IHE is a highly regarded organization known for profiling standards such as DICOM, HL7 v2 (Health Level Seven, version 2), HL7 CDA (Clinical Document Architecture), and HL7 FHIR (Fast Healthcare Interoperability Resources) into practical solutions for clinical practice. The primary goal of the IHE SHARAZONE is to provide a reliable and consistent cross-vendor DICOM data sharing system. To evaluate its effectiveness, a 5-month pilot was conducted with ten imaging vendors. The pilot concluded with a participant survey, which yielded valuable insights into the initial experience with the IHE SHARAZONE. These findings can inform future improvements and developments to this important service.
Keywords: DICOM (Digital Imaging and Communications in Medicine), Interoperability, IHE (Integrating the Healthcare Enterprise), SHARAZONE, Medical imaging, Cross-vendor exchange
Introduction
According to HIMSS (Healthcare Information and Management Systems Society), in healthcare, interoperability is the ability of different information technology systems and software applications to communicate, exchange data, and use the information that has been exchanged [1]. Interoperability standards have been established to ensure that digital health information originating from creators of such information can be meaningfully consumed by every recipient.
Standards and products have inherent variations in the following: clinical application, information created, algorithms employed, workflow, and technology [2–4]. Optional and conditional elements are designed into medical informatics standards to accommodate these variations. While IHE (Integrating the Healthcare Enterprise) profiling constrains [5] standards to address specific clinical needs and minimize variation, misalignment still occurs. Misaligned products lack interoperability, impede clinical workflow, and potentially impact the safety and effectiveness of the health information technology ecosystem [6].
IHE Connectathons provide a detailed implementation and testing process to enable the adoption of standards-based interoperability by vendors and users of healthcare information systems. During a Connectathon, systems exchange information with corresponding systems in a structured and supervised peer-to-peer testing environment, performing transactions required for the roles (IHE Actors) they have selected to perform in carefully defined interoperability use cases (IHE Profiles) [7].
As the complexity of data objects advances, there is a growing requirement for robustness. Content creators need to produce objects that can be utilized by multiple recipients, while recipients must accommodate a diverse range of object implementations from creators. The IHE Connectathon, being a discrete event spanning a week, lacks the capability to provide ongoing, asynchronous testing of data objects (Table 1). In the field of medical imaging, various efforts have been made to establish a platform for exchanging DICOM samples, but none have successfully addressed the three-fold balance of economics (i.e., funding the creation and maintenance of a testing solution), openness (i.e., enabling wide participation), and confidentiality (i.e., limiting the exposure of typically irrelevant failures that are ultimately resolved). These included:
Table 1.
Image content exchange testing platforms prior to the IHE SHARAZONE
| Advantages | Disadvantages | |
|---|---|---|
|
NEMA/MITA |
• Freely available • Objects associated with DICOM supplements |
• Origin and purpose of objects unclear to those not participating in a DICOM workgroup • Obsolescence unknown • Lacks contact and feedback mechanism • Lacks creator system details and expectations for consumer display processing |
|
IHE Connectathon samples |
• Freely available (requires a Gazelle account) • Some filtering based on type and organization |
• Lacks contact and feedback mechanism outside of an IHE Connectathon • Obsolescence unknown |
| Vendor-driven DICOM Connectivity Cross-Testing | • Leveraged NEMA cross-testing guidelines |
• Contracts individually negotiated • No framework to support communication and exchange • Used by larger vendors |
IHE SHARAZONE adds a layer of specificity with an object-sharing framework that enables organizations developing applications to ensure they can consume and display DICOM (Digital Imaging and Communications in Medicine) objects coming from a variety of sources. Likewise, creators can use object sharing to ensure that their DICOM objects can be consumed by a variety of recipients. Like the IHE Connectathon, the IHE SHARAZONE simulates real-world Healthcare Delivery Organizations having a variety of different imaging products that need to interoperate by creating and consuming different DICOM objects [8].
The IHE SHARAZONE is built on the existing IHE Gazelle Test Manager [9]. This tool hosts test campaigns, supports the development of non-IHE tests, and supports the exchange of sample test objects [10]. A dedicated Gazelle instance has been provisioned and made available globally, 24/7 to registered vendors for selecting shared objects of interest, testing their consumption, and reporting test results.
Participants are required to execute a contract that establishes terms for data usage, non-disclosure, obligations, and fees. Any entity developing DICOM software that is willing to accept the IHE SHARAZONE terms and conditions is eligible to participate. Participants self-declare as creators and/or consumers, which are defined as follows:
Creators author and upload shared test input that contains a test item and a test suite associated with the test item.
Consumers download shared test input of interest, execute the test suite on the test item, and provide a written report of results to creators.
The process is designed to safeguard both patient and vendor confidentiality. To avoid issues of Protected Health Information (PHI), non-patient or synthetic samples are exchanged. Just as with the IHE Connectathon, vendor confidentiality is ensured via a non-disclosure provision in the IHE SHARAZONE contract. Upholding confidentiality is vital for several reasons:
Ensure anti-trust compliance: in the USA, core anti-trust laws include the Sherman Act of 1890, the Clayton Act of 1914, and the Federal Trade Commission Act of 1914. In the European Union, competition law is governed by Articles 101 and 102 of the Treaty on the Functioning of the European Union, and merger control rules are covered by the EU Merger Regulation. Specific non-disclosure terms ensure vendors avoid sharing competitively sensitive information in order to promote competition and avoid unlawful business practices.
Encourage trust and respect: vendors are more inclined to participate freely and openly if they trust that their data and information will remain confidential.
Protect intellectual property: vendors are testing their latest technologies, products, and solutions. Confidentiality safeguards methods, intellectual assets, and trade secrets.
A GDPR-compliant (General Data Protection Regulation) [11] chat channel, Rocket Chat [12], is available for real-time dialogue between test participants. A dedicated IHE Moderator oversees the testing and is available to guide the participants in the IHE SHARAZONE process.
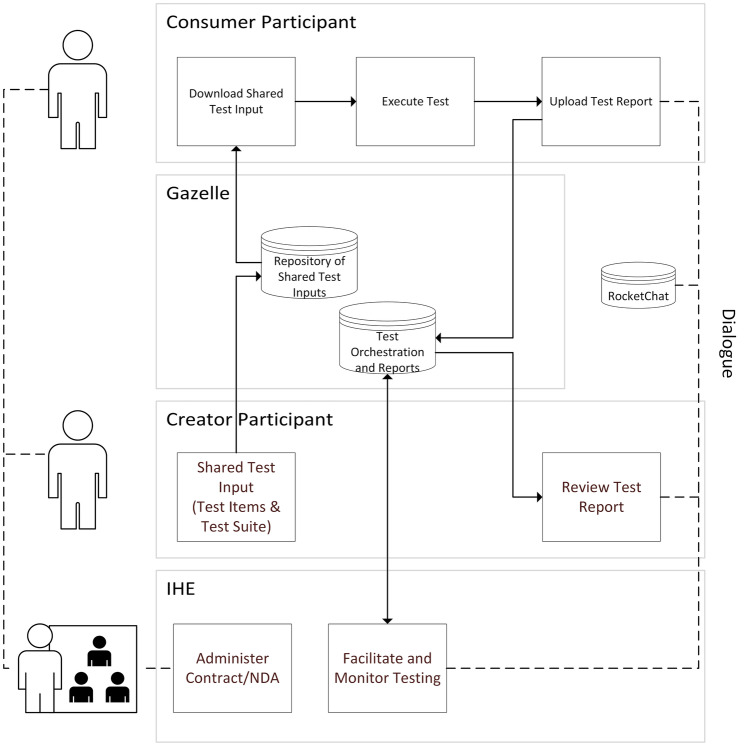
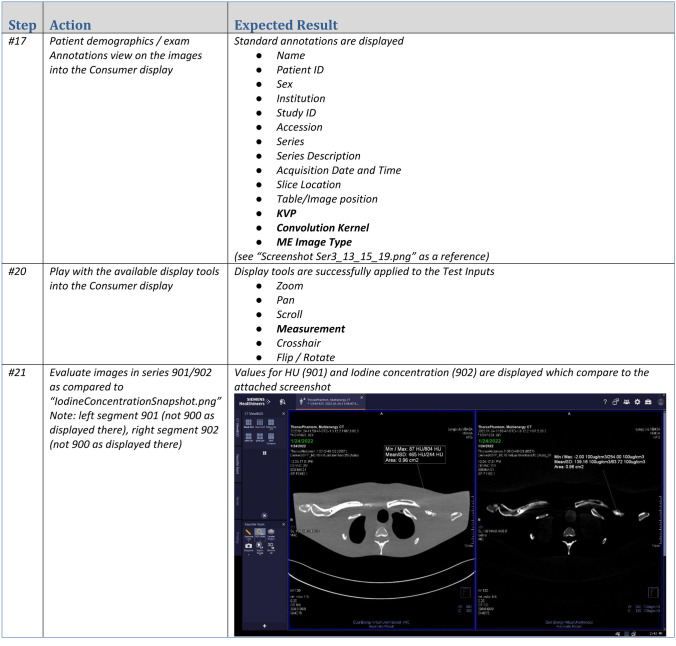
This overall process is depicted in Fig. 1, while Fig. 2 presents an example of a test suite produced by a consumer for the benefit of a creator.
Fig. 1.
IHE SHARAZONE process overview
Fig. 2.
Portion of a test suite to test display of multi-energy CT Images. Note the use of screenshots to facilitate communication of expected results. Courtesy of Siemens Healthineers
Is IHE SHARAZONE Different from the IHE Connectathon?
While the participants of an IHE Connectathon and the IHE SHARAZONE are similar, the scope of each is different.
Vendors participating in an IHE Connectathon follow a common test process for profiled standards to suit a particular business use case documented in the IHE Technical Framework.
Participants in the IHE SHARAZONE take part to evaluate the ability of consumer products to properly handle DICOM object content provided by creator products.
Test Process
IHE Connectathon lasts 1 week, during which systems on the same network exchange information in a controlled and monitored environment, performing IHE Profile transactions and workflows required for the Actor roles that they claim to support. IHE provides the test suites to execute and the pass/fail results are controlled and determined by IHE Monitors.
IHE SHARAZONE service is open 24/7. Creators upload test suites and test items composed of DICOM objects. Consumers then complete a test report and may also offer unsolicited feedback. In most cases, a dialogue ensues, in which both participants receive valuable input. This feedback loop establishes an opportunity for both participants to improve implementations. The evaluation of the results is strictly between the creator and the consumer participant. An independent IHE Moderator from Kereval (Rennes, France), the technical partner of IHE Europe, is on hand to supervise the testing process, ensuring its efficient and effective execution. Similar to the IHE Connectathon Monitor, the IHE SHARAZONE Moderator maintains a vendor-neutral stance. However, unlike the IHE Connectathon Monitor, the Moderator does not assess the outcome of any test [13].
The concepts of “test item” and “test suite” are rooted in the standard definitions provided by the International Software Testing Qualifications Board [14]:
A “test item” is DICOM images and other DICOM Information Objects that is exchanged.
A “test suite” is a document containing test procedures to be executed by the consumer on the test item. It also serves as a document for the consumer to record the results of the tests carried out on the test item, forming a test report [15].
To facilitate test suite creation, and establish a consistent test rigor, the IHE SHARAZONE task force created a test suite template, based on image display requirements in the IHE Basic Image Review (BIR) supplement. Creators begin with the base template, remove irrelevant test steps, and incorporate test steps specific to their product. Unlike BIR, or other IHE Profiles, each test suite incorporates evaluation criteria set by the creator based on their products’ specific intended use, intended users, use environment, and patient population. Since the IHE SHARAZONE is specific to a specific product rather than an IHE Actor, the shared test inputs often exceed the specifications of the IHE content profile.
To comprehend this differentiation, it is crucial to understand that:
IHE content profiles specify the payload content exchanged between IHE Actors, derived from a use case, without transaction dependencies.
An IHE Actor refers to a functional component or role that performs specific tasks within an interoperable healthcare environment. Each Actor has a defined set of responsibilities and capabilities outlined by IHE Profiles to ensure interoperability.
A product, on the other hand, refers to a specific implementation developed by a vendor or organization. It is a tangible software or hardware solution that can be deployed to perform certain functions or tasks within a healthcare setting.
Products can be designed to fulfill the requirements specified by IHE Profiles to ensure interoperability with other systems or devices. Vendors may develop products that serve as IHE Actors, meaning they conform to the relevant IHE specifications and can perform the defined tasks within an interoperable environment.
Characteristics of IHE SHARAZONE
Before launching a product in the market, an IHE SHARAZONE creator can conduct tests to identify problems with newly developed or modified support of DICOM objects. This includes making changes to existing DICOM objects, incorporating new DICOM modules (e.g., CT multi-energy), or implementing intricate DICOM objects (e.g., ophthalmic, breast tomosynthesis, structured reports, microscopy). Likewise, consumers can ensure that their existing products are capable of handling such objects, thereby preventing unforeseen failures among their customer base.
IHE SHARAZONE is capable of hosting shared test inputs from multiple generations of products that are available to all participants for testing with newer products. This better reflects the mix of product generations present in imaging departments.
IHE SHARAZONE service fosters collaboration between participants. For example, a consumer vendor may request specific DICOM objects from creators. In turn, all consumers benefit from testing with the requested test item.
Consumers ensure that presentation states properly control the display of source images; providing the desired transformations, annotations, overlays and greyscale or color pipeline. Consumers also ensure that AI (Artificial Intelligence) results, such as structured reports, segmentation objects, and parametric maps are associated with the original study, properly appear in the PACS (Picture Archive and Communications System) worklist, and correctly apply overlays, color, and real-world values. Consumers also make certain that AI results do not create conflicts with existing hanging protocols.
Note: the IHE SHARAZONE is not intended as a repository for AI inferencing inputs. AI accelerators, such as the Medical Imaging and Data Resource Center (MIDRC) or the American College of Radiology AI-LAB™, address this need.
Strict DICOM conformance is encouraged through the use of external DICOM validators, such as the Gazelle External Validation Service (EVS) [16], but not a precondition for shared test input. Despite being a standards organization, IHE acknowledges the frequent presence of semi-valid DICOM objects [17]. When conformance errors surface during testing, consumers are presented an opportunity to enhance error handling, recovery, and user experience; creators are driven to provide corrections. Early detection of conformance errors through the IHE SHARAZONE accelerates improvement of DICOM conformance.
The IHE Connectathon and the IHE SHARAZONE are complementary. Vendors typically participate in the Connectathon to evaluate interface robustness in later development phases, after DICOM encoding and network services are established, have been modified, or when additional claims are added to the IHE Integration Statement. Throughout the product development lifecycle (i.e., prior to and between Connectathon events), vendors leverage the IHE SHARAZONE to evaluate prototype encoding of images and objects, released product modifications, or product enhancements that include adoption of DICOM modules, macros, or controlled terminology.
Evaluation of IHE SHARAZONE
Compared to the IHE Connectathon that was established in 1998, the IHE SHARAZONE is in its early stages of development. To assess the IHE SHARAZONE concept, and evaluate the adaptation of Gazelle, we elected to hold a small-scale test of tooling and procedures.
In October 2019, the SHARAZONE Task Force was formed by IHE Europe, comprising volunteer members from the IHE Europe General Assembly. This task force includes a chairperson from IHE Catalyst (formerly IHE Services), a neutral participant from Kereval (the technical partner of IHE Europe), and three vendor participants: one from Siemens (Erlangen, Germany), one from Agfa HealthCare (Rueil Malmaison, France), and one from GE HealthCare (Waukesha, Wisconsin, USA). The task force operates under the governance of Committee Procedures, as outlined in the IHE International Principles of Governance.
Prior to initiating the IHE SHARAZONE, a stakeholder group, consisting of task force members, vendors, consultants, and IHE test monitors, gathered at the IHE North American Connectathon, held from January 20–24, 2020, to conduct a needs assessment for image content exchange. In March of the same year, a project proposal that detailed roles, process flow, confidentiality terms, and pricing was circulated for public feedback to IHE International, all IHE Domains, and all IHE Deployments. The comments received were incorporated into the initial framework of the IHE SHARAZONE, which was launched in June 2021.
Following the launch, the task force coordinated a small-scale pilot involving 10 vendors for a duration of 5 months. The pilot aimed to evaluate the workflow, tools, and contractual terms of the IHE SHARAZONE Participants were recruited from IHE Member Organizations, and eligible if they created or consumed DICOM objects. Each of the 10 participants signed a common contract that includes terms of use, data usage policy, anti-trust rules, and non-disclosure terms. Seventeen systems were registered: 13 creator systems and 12 consumer systems. Ten sets of shared test inputs were uploaded, resulting in 23 test reports. Shared test inputs included visible light, visible light video, dose reports, CT multi-energy, Grayscale Softcopy Presentation State (GSPS), and ophthalmology. Most shared test inputs contained one image; one contained a series of 255 images. Participants, the IHE SHARAZONE Task Force, and the IHE Moderator met every other week, between June 15, 2021, and November 18, 2021, for a total of ten meetings, to review test progression, tooling, documentation conventions, and communication channels.
Upon concluding the pilot phase, the participants conducted a consensus vote, based on individual perceptions and judgments, to endorse the public launch of the IHE SHARAZONE. The vote passed, following IHE International voting protocols [12]. In addition to the vote, an anonymous survey was executed to assess the participants’ pilot experience. To increase the sample size, we encouraged representatives who were part of the pilot from each vendor to respond to the survey (Tables 2 and 3).
Table 2.
IHE SHARAZONE pilot survey
| # | Question | Respondents | Response constraint |
|---|---|---|---|
| Q1 | What is your IHE SHARAZONE participant role | All | Creator, consumer or both |
| Q2 | The IHE SHARAZONE adds benefits to my company’s product development process | All |
Scale of 1–5 1 = Strongly Agree, 5 = Strongly Disagree |
| Q3 | What is the percentage of test instances in which you engaged in a dialogue with your test partner? | All | 0–100, increments of 10 |
| Q4 | Did you require support from the IHE SHARAZONE moderator? | All | Yes, No |
| Q5 | The Shared Test Input test instructions from creators that I executed were clear and effective | Consumers |
Scale of 1–5 1 = Strongly Agree, 5 = Strongly Disagree |
| Q6 | What could be improved with Shared Test Input instructions? | Consumers | Free text response |
| Q7 | On average, in hours, how long did it take you to execute a test report? | Consumers | Number (decimal) |
| Q8 | The test reports that I received from Consumers were clear and effective | Creators |
Scale of 1–5 1 = Strongly Agree, 5 = Strongly Disagree |
| Q9 | What could be improved with test reports? | Creators | Free text response |
| Q10 | On average, in hours, how long did it take you to prepare a shared test input? | Creators | Number (decimal) |
Table 3.
IHE SHARAZONE pilot survey results
| Question # | Respondents | Results |
|---|---|---|
| Q1 | 13 | Both = 7, Creator = 3, Consumer = 3 |
| Q2 | 13 | Median = 1, Min = 1, Max = 3 |
| Q3 | 13 | Median = 80%, Min = 10%, Max = 100% |
| Q4 | 13 | Yes = 3, No = 10 |
| Q5 | 10 | Median = 1, Min = 1, Max = 3 |
| Q6 | 4 | 4 responses received |
| Q7 | 10 | Median = 1 h, Min = 0.5 h, Max = 4 h |
| Q8 | 9 | Median = 1, Min = 1, Max = 4 |
| Q9 | 3 | 3 responses received |
| Q10 | 7 | Median = 2.25, Min = 1, Max = 3, Average = 2.6 |
Analysis
Due to the small sample size and categorical data types, the ability to provide a detailed statistical analysis is limited. When feasible, chi-square goodness-of-fit test was performed to identify differences in groups of responses by role (Minitab 20.4 Statistical Software, State College, PA).
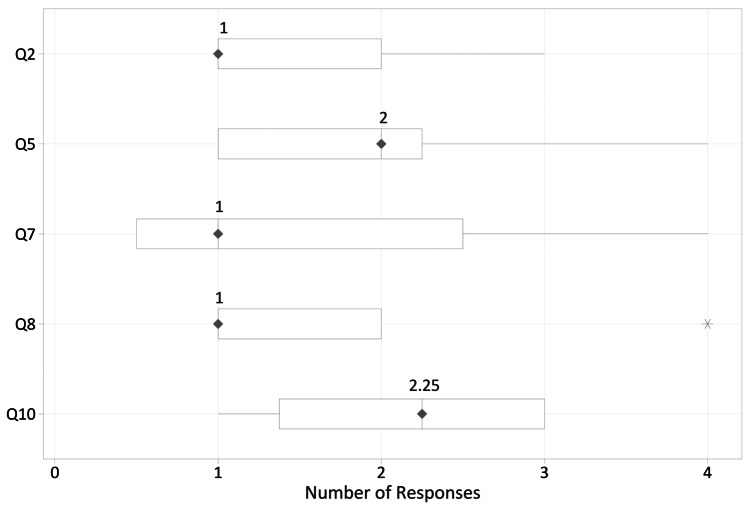
Each participating vendor had at least 1 survey response. All participants indicated that the IHE SHARAZONE benefits the product development process (median = 1 = Strongly Agree, see Q2 and Fig. 3). Further segmentation by role of creator (n = 3), consumer (n = 3), or both creator and consumer (n = 7) indicates that the both creator and consumer group provided different responses (p = 0.041), due to a single neutral (neither Agree nor Disagree) response. Participants indicated that they engaged in dialogue with their test partners 80% of the time (median = 80%). The both creator and consumer group engaged in dialogue less frequently than participants registered as only creators, or only consumers (median = 70% and median = 95% respectively). Three participants required moderator assistance.
Fig. 3.
IHE SHARAZONE pilot survey responses depicted in a box and whisker plot
Consumers (n = 10) spent approximately 1 h testing downloaded shared test inputs (Q7 and Fig. 3) and indicated that test suites from creators were clear and effective (median = 2 = Agree, see Q5 and Fig. 3).
Creators (n = 9) spent over 2 h to prepare shared test input (median = 2.25, see Q10 and Fig. 3) and indicated that test reports from creators were clear and effective (median = 1 = Strongly Agree, see Q10 and Fig. 3).
Study Limitations
The following limitations should be considered when interpreting the findings of the study:
The study was conducted with only 10 imaging vendors. This small sample size may not be representative of all imaging vendors, which limits the generalizability of the findings.
Due to the small sample size and categorical data types, the ability to provide a detailed statistical analysis is limited.
The study relied on self-reported data from the participants, which can be subject to bias.
The IHE SHARAZONE is in its early stages of development. Therefore, the findings of the study may not hold true as the system evolves and matures.
Discussion
The IHE SHARAZONE pilot study demonstrated the potential to significantly contribute to the product development process in the healthcare industry. The pilot study included acquisition modalities, image manager/archives and image displays, representing the radiology, endoscopy, eye care IHE domains. Image displays were often grouped with image archives to assess both image networking and display. The participants’ feedback indicates a high level of satisfaction with the service, highlighting its role in facilitating the testing of established and novel DICOM features, providing quick and profound feedback, and creating an always-available, collaborative environment.
The survey results suggest that the IHE SHARAZONE is a low-burden service, with consumers spending approximately 1 h testing downloaded test packages and creators spending over 2 h preparing test packages. This indicates that the service is efficient and manageable for both creators and consumers.
The dialogue between test partners, which occurred 80% of the time, is a testament to the collaborative nature of the IHE SHARAZONE. This peer-to-peer dialogue is a cornerstone of interoperability testing, connecting stakeholders before connecting machines. The minimal reliance on the moderator suggests that the tooling and documentation are intuitive and user-friendly.
Future
The survey has demonstrated a good level of satisfaction from the current participants with an environment that meets their needs and provides a clear return on their investments. The likely expansion of IHE SHARAZONE will involve:
New participants: the IHE SHARAZONE's participation is on the rise, aiming to reach 30 participants by the conclusion of 2023. In order to align the participant profile more closely with the imaging market, IHE is seeking to attract participants from small and mid-sized companies.
Additional systems: new participants will register new systems both as creators and consumers and contribute their shared test input. Existing participants will also expand the number of their participating systems, both with newer systems and older systems that need to be verified for interoperability with newer systems.
Such a steady growth will require improvement in the IHE SHARAZONE infrastructure tooling offered, to address better ways to navigate a much larger choice of shared test inputs as well as a larger set of test reports being produced by consumers that need to be processed by the creators.
The survey also identified that the creation of test suites and their fulfillment in creating test reports could be further facilitated by a choice of test suite forms and test report creation tools that are semi-automatically derived.
In addition to engaging a broader set of participants and systems which is the natural growth expected, IHE SHARAZONE is exploring the engagements of other communities than the community of product and solutions developers. The standards and profile development activities are a natural area of growth. As new standards and profiles are introduced, the IHE imaging-related domains such as radiology, radiation oncology, cardiology, endoscopy, or pathology could leverage the IHE SHARAZONE to offer “sandboxes” where early version of these new standardized or profiled objects could be made accessible to early implementers. Through their testing, they could identify areas where the standard or profile could be further improved before being released in final form. IHE SHARAZONE has extended its contract to offer to Profile/Standards Development Organizations such as IHE Radiology to become creators of newly standardized or profiled objects and use the IHE SHARAZONE testing process to promote their implementation.
As previously stated, the imaging AI community remains keen on utilizing the IHE SHARAZONE, not only to enhance their access to imaging data, but also to ensure seamless integration of AI results into the imaging enterprise in a manner that supports day-to-day clinical practice [18]. Multiple Shared Test Inputs with AI results have been added to the IHE SHARAZONE, making them some of the most thoroughly tested packages.
Participating in the IHE SHARAZONE
Physicians, clinicians, medical staff, and healthcare executives participate in the IHE process by identifying and defining integration issues [2]. These users can encourage their vendors to test specific DICOM integration issues that they have encountered in their practice through the IHE SHARAZONE. Due to the NDA (non-disclosure agreement) obligations of the IHE SHARAZONE contract, vendors are not able to reveal test details to users; however, they are permitted to provide the names of participating products and the number of test instances each product has participated in. As previously mentioned, details pertaining to test peers and test results cannot be disclosed to maintain anti-trust compliance and ensure that candid engagement of participants is not curtailed.
Healthcare Delivery Organizations engaged in the development of software for creating or consuming DICOM images and other objects are invited to take part in the IHE SHARAZONE [19]. To join, organizations are required to review and sign the IHE SHARAZONE Non-Disclosure Agreement (NDA) contract and submit the participation fee. As of June 2023, participant fees are structured based on the size of the organization, categorized as follows [20]:
Small < 250 full-time employees (FTE)
Mid-sized 250–2500 FTE, and
Large (> 2500 FTE)
The fees are entirely used for hosting, maintenance, and feature development of Gazelle, as well as contract administration, and staffing the IHE Moderator. Although the small tier exceeds the average size of most startups, the pricing remains favorable for startups, as demonstrated by the involvement of three startups during the preparation of this manuscript. Feedback received following the pilot study has indicated that the current pricing model is also acceptable for open-source developers. However, open-source projects encounter a distinct challenge, as they lack a legal entity capable of signing the contract.
Note: This same limitation often prevents open-source developers from participating in the IHE Connectathon.
As of June 2023, thirty-six products have participated in more than eighty tests in the IHE SHARAZONE [20]. These thirty-six systems are distributed among twelve vendors as shown in Table 4.
Table 4.
IHE SHARAZONE participant profile at time of draft of this manuscript
| Creator systems | Consumer systems | |
|---|---|---|
| Small participant | 4 | 3 |
| Mid-sized participant | 3 | 1 |
| Large participant | 5 | 3 |
Conclusion
Interoperability continues to require mobilization and representation from users and vendors. Users have a powerful role in influencing vendor behavior by insisting on participation in IHE interoperability improvement initiatives. This has been clearly demonstrated through tenders or RFPs (request for proposal) that require proof of participation in the IHE Connectathon, in addition to the submission of IHE Integration Statements. As the IHE SHARAZONE adds a meaningful layer of specificity to the IHE Connectathon testing; the IHE User community can continue to drive interoperability by motivating vendors to participate in the IHE SHARAZONE and request proof of participation.
Author Contribution
All authors designed the study. SN collected and analyzed the data. SN wrote the following sections of the manuscript: Abstract, Introduction, Evaluation of IHE SHARAZONE, Analysis, Discussion. BL wrote the following sections of the manuscript:Is IHE SHARAZONE different from the IHE Connectathon?,Test process, Key benefits of IHE SHARAZONE.CP wrote the following sections of the manuscript: Future, Participating in the IHE SHARAZONE. All authors reviewed and approved the final manuscript.
Data Availability
Data are available from Steven Nichols (steven.nichols@ge.com) at GE HealthCare.
Declarations
Ethics Approval
Ethics approval was not required for this study as it did not involve human subjects or protected health information from human participants. The research was conducted in accordance with all applicable laws and regulations.
Consent to Participate
All survey participants were informed of the purpose of the study and provided verbal consent for their responses to be used in the analysis and reporting of findings.
Competing Interests
Steven Nichols is an Employee of GE HealthCare, a member of the IHE Europe Steering Committee, and a member of the DICOM Executive Committee. Bruno Laffin is an Employee of Agfa HealthCare, a member of the IHE Europe Steering Committee, and a member of the DICOM Executive Committee. Charles Parisot is the Principal and Chief Executive of InteropEhealth, a member of the IHE Europe Steering Committee, member-at-large of IHE International, a member of the General Assembly of IHE-Catalyst, and a member of the DICOM Executive Committee.
Footnotes
Patents and Intellectual Property
There are no patents to disclose
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.HIMSS: HIMSS Dictionary of Health Information Technology Terms, Acronyms, and Organizations, Fourth Edition, Boca Raton, FL, Taylor & Francis Group, 2017
- 2.Channin D, Parisot C, Wanchoo V, Leontiv A, Siegel E: Integrating the Healthcare Enterprise: A Primer Part 3. What Does IHE Do for ME?. Radiographics 21:1351–1358, 2001 [DOI] [PubMed]
- 3.Clunie DA, Dennison DK, Cram D, et al. Technical Challenges of Enterprise Imaging: HIMSS-SIIM Collaborative White Paper. J Digit Imaging. 2016;29:583–614. doi: 10.1007/s10278-016-9899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boufahja A, Nichols S, Pangon V. Quantitative Evaluation of PACS Query/Retrieve Capabilities. J Digit Imaging. 2021;34:1302–1315. doi: 10.1007/s10278-021-00511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oemig F, Snelick R. Healthcare Interoperability Standards Compliance Handbook: Conformance and Testing of Healthcare Data Exchange Standards. Switzerland: Springer International Publishing; 2016. [Google Scholar]
- 6.Design Considerations and Premarket Submission Recommendations for Interoperable Medical Devices, U.S. Food and Drug Administration, https://www.fda.gov/media/95636/download. Accessed February 13, 2023.
- 7.Connectathon, IHE International, https://www.ihe.net/participate/connectathon/. Accessed February 13, 2023.
- 8.Noumeir R. Integrating the healthcare enterprise process. Int. J. Healthcare Technology and Management. 2008;9:167–180. doi: 10.1504/IJHTM.2008.017371. [DOI] [Google Scholar]
- 9.Hussey P, Kennedy MA: Introduction to Nursing Informatics, Fifth Edition, Switzerland, Springer Nature Switzerland AG, 2021
- 10.IHE Europe Gazelle https://www.ihe-europe.net/testing-IHE/gazelle. Accessed May 31, 2023.
- 11.General Data Protection Regulation (GDPR) Compliance Guidelines, Complete Guide to GDPR Compliance, Proton AG, 2022, https://gdpr.eu/. Accessed February 13, 2023.
- 12.Rocket.chat Is GDPR Compliant, Rocket.chat, https://docs.rocket.chat/legal/gdpr. Accessed February 13, 2023.
- 13.IHE Europe Monitors 2023, https://connectathon.ihe-europe.net/monitors-2023. Accessed May 31, 2023.
- 14.International Software Testing Qualifications Board, Standard Glossary of Terms Used in Software Testing Version 3.01, http://www.itqb.org/sites/default/files/glossary/ISTQB_glossary_Foundation_v3.01%28EN%29.pdf. Accessed May 31, 2023
- 15.IHE Services SHARAZONE user manual, https://sharazone.ihe-europe.net/user-manual/IHE-SHARAZONE-USER_MANUAL-1_1.pdf. Accessed May 31, 2023.
- 16.External Validation Service Front-end, https://gazelle.ihe.net/EVSClient/home.seam. Accessed May 31, 2023.
- 17.Pianykh O. Digital Imaging and Communications in Medicine (DICOM) A Practical Introduction and Survival Guide. 2. Germany: Springer-Verlag, Berlin Heidelberg; 2012. [Google Scholar]
- 18.Pierce JD, Rosipko B, Youngblood L, Gilkeson RC, Gupta A, Bittencourt LK. Seamless Integration of Artificial Intelligence Into the Clinical Environment: Our Experience With a Novel Pneumothorax Detection Artificial Intelligence Algorithm. J Am Coll Radiol. 2021;18:1497–1505. doi: 10.1016/j.jacr.2021.08.023. [DOI] [PubMed] [Google Scholar]
- 19.IHE SHARAZONE, IHE Europe, Accessed June 19, 2023, https://www.ihe-europe.net/IHE_SHARAZONE
- 20.IHE SHARAZONE Statistics (DICOM), IHE Catalyst, https://sharazone.ihe-europe.net/statistics.html. Accessed March 13, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from Steven Nichols (steven.nichols@ge.com) at GE HealthCare.