Abstract
The complexation of econazole with the mucoadhesive polycarbophil was found to significantly improve the therapeutic benefit of the drug in the topical treatment of experimental vaginal candidiasis in mice, while no difference in the antimycotic activity exerted by econazole and polycarbophil-econazole could be detected in vitro.
Candida albicans vaginitis has been increasing in medical importance, as a significant proportion of women suffer from acute episodes, and recurrent infections may frequently occur after therapy (19, 20). It has been estimated that 75% of all women will experience an episode of Candida vaginitis once in their lifetime, with up to 5% suffering from recurrent vaginal candidiasis (13). The increasing incidence of Candida vaginitis has highlighted the importance of establishing therapeutic strategies aimed at ensuring (i) successful eradication of the infectious agent, particularly in women prone to recurrent Candida vaginal infection; (ii) short-term treatment; (iii) achievement of high drug levels at the target site; (iv) safety; and, possibly, (v) the avoidance of the first-pass metabolism. From such a perspective, topical treatment of Candida vaginitis could represent a rational choice for the management of this localized infection, while systemic therapy could be limited to patients proven to be unresponsive or intolerant to intravaginally administered drugs. However, although antimycotic agents, such as broad-spectrum imidazole derivatives, are now available for topical, short-term therapy (21, 22), intravaginally delivered drugs may fail to achieve high concentrations at the site of infection because of their fairly prompt removal from the vaginal compartment through physiological secretions (16). Indeed, traditional vaginal delivery systems (solutions, suspensions, gels, foams, and tablets) might not ensure sufficient therapeutic efficacy, most likely because of their short residence time at the site of administration (3, 7). Therefore, current pharmacological research, focused on improving topical treatment of vaginal infections, is mainly addressed at developing suitable drug delivery systems to enable a prolonged intravaginal residence time for administered drugs.
Mucoadhesive polymers have been described as the most attractive and promising candidates to be used as drug delivery systems for topical therapy because of their capability to adhere to mucous-epithelial surfaces (4, 14). Most of them have been designed for nasal, oral, and ocular drug delivery (9, 12), and it has been shown that polycarbophil (PC), a mucoadhesive polymer of the polyacrylic acid type, remains in the vagina for 2 to 3 days (10). Such persistence is essential in order to prolong the drug residence time at the site of administration and most likely leads to a better absorption of the drug by the underlying tissue (3, 4, 7), as shown by the enhanced penetration of various drugs across the buccal, nasal, vaginal, and intestinal epithelia when delivered in systems based on mucoadhesive polyacrylic acid derivatives (8, 9, 11).
Very recently, a novel econazole delivery system based upon the complexation of the drug with PC has been developed (18). This innovative formulation has been described as a highly stable complex, from which 70% econazole is released within 18.5 h (18). The slow release of econazole from the complex, the good absorption of the drug across the vaginal epithelium (1), and the prolonged residence time of PC on vaginal tissue (10) make PC-econazole an attractive delivery system for vaginal administration of this antimycotic drug. To assess its effectiveness, a murine model of experimental vaginal candidiasis was used in the present study to investigate whether the complexation of econazole with PC would improve the therapeutic benefit of econazole in the topical treatment of C. albicans vaginitis in mice. Results obtained during the study were expressed in terms of the mean ± the standard deviation (SD), and statistical analysis was performed by the χ2 test and by the two-tailed Student’s t-test with the significance level set at a P value of <0.05.
In vitro activity of econazole and PC-econazole.
The compositions of PC-econazole and econazole formulations were as follows: econazole (1.0 or 0.5 g), Me-paraben (methyl p-hydroxybenzoate, 0.20 g) (Nipa Laboratories LTD, Pontypridd, United Kingdom), Pr-paraben (propyl p-hydroxybenzoate, 0.02 g) (Nipa), PC (2.12 g) (BFGoodrich, Beckersville, Ohio) (included only in the PC-econazole complex), and propylene glycol to a final volume of 100 ml. Both the PC-econazole and econazole formulations were generously provided by Farmigea S.p.A. (Pisa, Italy). Preliminary experiments were designed to evaluate whether differences in the in vitro activities exerted by econazole and PC-econazole against C. albicans could be detected by using a microplate susceptibility assay, performed as described by Polonelli and Morace (15). Identical MICs (5.6 ± 0.3 μg/ml; mean ± SD of five separate experiments) of econazole and PC-econazole were obtained against two C. albicans strains (ATCC 48867 and ATCC 23866) used as reference microorganisms. A mixture containing all the excipients (i.e., PC, Me-paraben, Pr-paraben, and propylene glycol) but lacking econazole did not exert any inhibitory activity against the two C. albicans reference strains when assayed at the highest concentration used in the microdilution susceptibility test.
Experimental C. albicans vaginitis in mice.
Since vaginitis in rodents is inducible only under conditions of pseudoestrus (17), 72 h before vaginal infection, female CBA/J (H-2k) mice, aged 8 to 10 weeks, were treated subcutaneously with 0.5 mg of estradiol valerate dissolved in sesame oil. Estrogen administration continued weekly until completion of the study. The estrogen-treated mice were inoculated intravaginally (day 0) with 20 μl of a phosphate-buffered saline (PBS) suspension of 5 × 105 viable blastoconidia of C. albicans ATCC 48867, obtained from early-stationary-phase cultures in yeast nitrogen base medium (Difco Laboratories, Detroit, Mich.) supplemented with 0.5% glucose and 0.05% bovine serum albumin (pH 7.0). The time course of infection was monitored in individual mice by culturing 50 μl of undiluted and serially diluted (1:10) vaginal lavages, performed on alternate days with 100 μl of sterile PBS. The vaginal lavage fluids were seeded, in triplicate, onto Sabouraud dextrose agar plates supplemented with 400 mg of chloramphenicol/liter and 40 mg of gentamicin/liter and were incubated for 24 h at 37°C. Recovery of C. albicans from the mouse vaginas was confirmed by a germ tube test, performed on randomly selected colonies obtained from vaginal lavage cultures at various sampling times. Overt vaginitis occurred in 66% of the animals. Infected mice developed high-titer vaginal infections (4.2 × 104 ± 9.0 × 103 CFU/100 μl of vaginal lavage fluid), which decreased to 2.3 × 104 ± 7.0 × 103 CFU by week 1 and remained at approximately that value throughout a 4-week period before declining to an average of 2.1 × 102 CFU by week 10. Complete clearance of C. albicans was rarely observed, most likely because of the dominant pharmacological effect of estrogen administration, which produces an environment facilitating persistence and proliferation of Candida organisms (6).
C. albicans vaginitis in mice topically treated with econazole or PC-econazole.
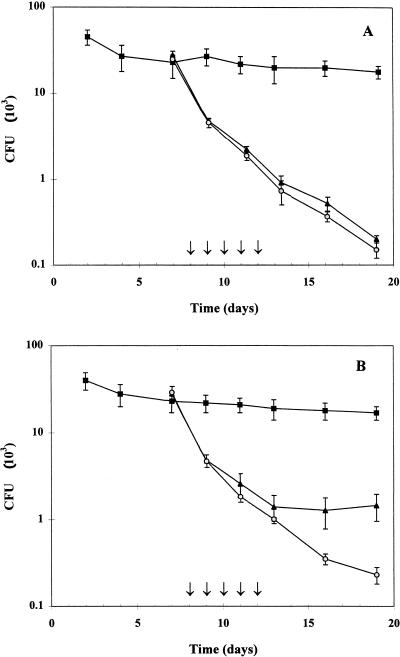
Infected mice, harboring an average vaginal level of 2.3 × 104 CFU of C. albicans 1 week post inoculation, were topically treated with 20 μl of 1% econazole or 1% PC-econazole or with 20 μl of a blend of excipients (0.20 g of Me-paraben, 0.02 g of Pr-paraben, and 2.12 g of PC in 100 ml of propylene glycol) once daily for five consecutive days, starting from day 8 postinoculation with C. albicans. No appreciable decrease in the vaginal C. albicans burden was observed in control mice receiving only excipients (Fig. 1A). In contrast, infected mice receiving econazole or PC-econazole showed similar sharp declines in C. albicans levels immediately after day 1 postadministration and displayed indistinguishable infection kinetics during and after drug administration. One week after cessation of therapy both groups of mice exhibited a 120-fold decrease in the C. albicans burden (0.2 × 103 ± 20 and 0.15 × 103 ± 30 CFU/100 μl of vaginal fluid), which was significantly lower than that recorded in control animals (1.8 × 104 ± 3.0 × 103 CFU) (P < 0.001) at the same sampling time (Fig. 1A). This finding was not surprising, since both econazole and PC-econazole exert the same in vitro antimycotic activity on the C. albicans strain used to induce vaginal candidiasis, and econazole, unlike other antimicrobial agents, was proven to be successfully absorbed through the vaginal wall in rodents (1). The possibility cannot be excluded, however, that the rapid absorption of econazole across the vaginal epithelium could have masked a therapeutic benefit achieved by prolonging the intravaginal residence time of econazole, when delivered as a PC complex. Therefore, infected mice were subjected to deliberate vaginal washing after every drug administration, in an attempt to increase the loss of intravaginally delivered drugs through physiological secretions, which in women are discharged from the vagina at a measured rate of 3 to 4 g/h (2) and are claimed to explain the failure of topical treatment of vaginal infection (7).
FIG. 1.
Effect of topical administration of econazole and PC-econazole on C. albicans vaginal infection in mice. Groups of 10 estrogen-treated mice were intravaginally inoculated with 5.0 × 105 C. albicans blastoconidia. Drugs were administered once daily for five consecutive days as indicated by the arrows. The numbers of C. albicans CFU are given as the means ± SD of three separate experiments and were quantitated by culturing serial dilutions of vaginal lavage fluid collected from each mouse on alternate days. (A) time course of infection in mice receiving 20 μl of 1.0% econazole (▴), 1.0% PC-econazole (○), and excipients (■). (B) C. albicans infection kinetics in mice treated as described for panel A and subjected to deliberate vaginal washing with 100 μl of PBS 4 h after each administration of drugs.
C. albicans vaginitis in mice subjected to vaginal washing after econazole or PC-econazole administration.
Vaginal washing was performed by using a vaginal irrigator, especially set up for the mouse vagina during this study. Deliberate washings were performed with 1.0 ml of sterile PBS 4 h after every administration of drugs. Vaginal infection kinetics were quite similar in excipient-treated control mice, whether subjected to vaginal washing or not, clearly showing that such a procedure did not lead to any appreciable decrease in the C. albicans burden in the absence of specific antimycotic treatment (Fig. 1). With the administration of drugs, similar infection kinetics were observed in econazole-treated mice as well as in PC-econazole-treated mice, both exhibiting numbers of C. albicans CFU (an average of 1.0 × 103 to 1.4 × 103 CFU) significantly lower (P < 0.01) than those recorded in control animals (1.9 × 104 ± 5.0 × 103 CFU) on the last day of drug administration (day 13) (Fig. 1B). The effect exerted by washing on the time course of infection became evident after cessation of therapy, from day 13 to day 19 (the last day of observation) (Fig. 1B). While mice receiving PC-econazole exhibited up to a 70-fold decrease in C. albicans levels, achieving vaginal infection titers as low as 2.3 × 102 ± 58 CFU at day 19, the econazole-treated mice did not show any further decline in vaginal titers of C. albicans CFU, which remained at approximately the value quantitated at day 13 (1.4 × 103 ± 75 CFU); this resulted in titers significantly higher (P < 0.05) than those exhibited by PC-econazole-treated mice and only 11-fold lower than those quantitated in excipient-treated mice (1.7 × 104 ± 1.5 × 103 CFU) at day 19 (Fig. 1B). These observations suggested that although topically administered econazole might control vaginal Candida infection efficiently, the efficacy of therapeutic treatment appeared to rely upon frequent drug administration, which was required to replace the drug deliberately removed by washing. In contrast, the improvement in the therapeutic efficacy of econazole when delivered as PC-econazole could be convincingly explained by assuming that the prolonged residence time of PC on the vaginal mucosal surface (10) leads to an enhancement in drug availability at the site of infection. Such a hypothesis was supported by the finding that successful clearance of C. albicans was achieved only in mice receiving PC-econazole, making the therapeutic benefit of PC-econazole significantly higher (P = 0.0371) than that exerted by econazole alone at a drug concentration as low as 1% (Table 1). Indeed, infected mice receiving econazole never showed eradication of C. albicans from the vagina, and up to 10 and 20% of the mice receiving 0.5 and 1.0% econazole, respectively, were insensitive to the treatment. In addition, more than 50% of the animals receiving 1% econazole exhibited high-titer infections, the average C. albicans burden being 100 < CFU < 1,000. In contrast, none of the infected mice were insensitive to PC-econazole; at least one half of the animals exhibited infection titers lower than 100 CFU, and complete clearance of C. albicans infection was achieved in 10 and about 20% of infected mice treated with 0.5 and 1.0% PC-econazole, respectively (Table 1). Eradication of vaginal candidiasis in about 20% of the mice may be considered of value since (i) spontaneous clearance of Candida vaginitis has rarely been observed in estrogen-treated mice (5, 21) and (ii) eradication of vaginal infection has been reported to be achieved only by using high concentrations of topically administered drugs, even in the absence of deliberate vaginal washing (21, 22).
TABLE 1.
Effect of econazole and PC-econazole in the topical treatment of C. albicans vaginitis in mice subjected to deliberate vaginal washing
| Administered druga | Mice (%)b
|
|||
|---|---|---|---|---|
| Insensitive | Improved | Markedly improved | Cured | |
| Excipients | 80.2 | 19.8 | 0 | 0 |
| Econazole (0.5%) | 11.1 | 66.7 | 22.2 | 0 |
| Econazole (1.0%) | 18.8 | 56.2 | 25.0 | 0 |
| PC-econazole (0.5%) | 0 | 40.0 | 50.0 | 10.0 |
| PC-econazole (1.0%) | 0 | 31.2 | 50.0 | 18.8 |
PC-econazole, econazole, and excipients (0.20 g of Me-paraben, 0.02 g of Pr-paraben, and 2.12 g of PC in 100 ml of propylene glycol) were administered intravaginally (20 μl/mouse) to infected mice, carrying an average of 2.3 × 104 CFU of C. albicans, once daily from day 8 to day 12 postinoculation. The numbers in parentheses refer to the drug concentration.
Percentage of animals in each category was evaluated at day 16 postinoculation with C. albicans. Mice were considered insensitive, improved, markedly improved, or cured when C. albicans CFU numbers were >1,000, 999 to 100, 99 to 1, and 0, respectively. A total of 150 mice were used.
The overall results obtained during this investigation suggest that the improved therapeutic benefit of PC-econazole in the topical treatment of vaginal candidiasis in mice is due to the combined effects of the slow release of econazole from PC (18), the prolonged residence time of PC on the vaginal mucosal surface (10), and the good absorption of econazole across vaginal tissue (1). Based on these considerations, it can be inferred that the innovative PC-econazole formulation (18) represents an attractive and promising candidate for the topical treatment of Candida vaginitis in women.
REFERENCES
- 1.Benzinger D P, Edelson J. Adsorption from the vagina. Drug Metab Rev. 1983;14:137–168. doi: 10.3109/03602538308991387. [DOI] [PubMed] [Google Scholar]
- 2.Bergh P A. Vaginal changes with aging. In: Breen J L, editor. The gynecologist and the older patient. Rockville, Md: Aspen Publishers; 1988. pp. 299–311. [Google Scholar]
- 3.Brannon-Peppas L. Novel vaginal drug release application. Adv Drug Delivery Rev. 1993;11:169–177. [Google Scholar]
- 4.Duchene D, Touchard F, Peppas N A. Pharmaceutical and medical aspects of bioadhesive system for drug administration. Drug Dev Ind Pharm. 1988;14:283–318. [Google Scholar]
- 5.Fidel P L, Jr, Lynch M E, Sobel J D. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:1990–1995. doi: 10.1128/iai.61.5.1990-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinsman O S. Conference on Candida. Washington, D.C: American Society for Microbiology; 1987. Effect of mammalian steroid hormone and luteinizing hormone on germination of Candida albicans, abstr. 27; p. 13. [Google Scholar]
- 7.Knuth K, Amiji M, Robinson J R. Hydrogel delivery system for vaginal and oral applications. Adv Drug Delivery Rev. 1993;11:137–167. [Google Scholar]
- 8.Lehr C M, Bouwstra J A, Kok W. Effects of the mucoadhesive polymer polycarbophil on the intestinal absorption of a peptide drug. J Pharm Pharmacol. 1992;44:402–407. doi: 10.1111/j.2042-7158.1992.tb03633.x. [DOI] [PubMed] [Google Scholar]
- 9.Lehr C M, Lee Y H, Lee V H L. Improved ocular penetration of gentamicin by mucoadhesive polymer polycarbophil in the pigmented rabbit. Investig Ophthalmol Vis Sci. 1994;35:2809–2914. [PubMed] [Google Scholar]
- 10.Leung S-H S, Robinson J R. Bioadhesives in drug delivery. Polym News. 1990;15:333–342. [Google Scholar]
- 11.Morimoto K, Iwamoto T, Morisaka K. Possible mechanisms for the enhancement of rectal absorption of hydrophilic drugs and polypeptides by aqueous poly acrylic acid gel. J Pharmacobio Dyn. 1987;10:85–91. doi: 10.1248/bpb1978.10.85. [DOI] [PubMed] [Google Scholar]
- 12.Nagai T. Adhesive topical drug delivery system. J Control Release. 1985;2:121–134. [Google Scholar]
- 13.Odds F C. Candida and candidosis. 2nd ed. London, England: Balliere Tindall; 1988. [Google Scholar]
- 14.Park H, Robinson J R. Physico-chemical properties of water insoluble polymers important in the mucin-epithelial adhesion. J Control Release. 1985;2:47–57. [Google Scholar]
- 15.Polonelli L, Morace G. A microautomated dilution method for susceptibility testing with antifungal drugs. Mycopathologia. 1984;86:21–28. doi: 10.1007/BF00437225. [DOI] [PubMed] [Google Scholar]
- 16.Richardson J L, Illum L. Routes of delivery: case studies. The vaginal route of peptide and protein drug delivery. Adv Drug Delivery Rev. 1992;8:341–366. [Google Scholar]
- 17.Ryley J F, McGregor S. Quantification of vaginal Candida albicans infections in rodents. J Med Vet Mycol. 1986;24:455–460. [PubMed] [Google Scholar]
- 18.Saettone, M. F., L. Panichi, B. Giannaccini, E. Boldrini, and P. Bianchini. July 1997. Bioadhesive complexes of polycarbophil and azole antifungal or antiprotozoal drugs. International patent application PCT IT97/00187.
- 19.Schröppel K, Rotman M, Galask R, Mac K, Soll D R. Evolution and replacement of Candida albicans strains during recurrent vaginitis demonstrated by DNA fingerprinting. J Clin Microbiol. 1994;32:2646–2654. doi: 10.1128/jcm.32.11.2646-2654.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soll D R, Galask R, Isley S, Gopala Rao T V, Stone D, Hicks J, Schmid G, Mac K, Hanna C. Switching of Candida albicans during successive episodes of recurrent vaginitis. J Clin Microbiol. 1989;27:681–690. doi: 10.1128/jcm.27.4.681-690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valentin A, Bernard C, Mallié M, Huerre M, Bastide J-M. Control of Candida albicans vaginitis in mice by short-duration butoconazole treatment in situ. Mycoses. 1993;36:379–384. doi: 10.1111/j.1439-0507.1993.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 22.Van Cutsem J. The in vitro activity of terconazole against yeasts: its topical long-acting therapeutic efficacy in experimental vaginal candidiasis in rats. Am J Obstet Gynecol. 1991;165:1200–1206. doi: 10.1016/s0002-9378(12)90727-9. [DOI] [PubMed] [Google Scholar]