Abstract
Triterpenoids, as the main active ingredient of Ganoderma lucidum fermented extract, exert multiple pharmacological activities, including immunomodulatory properties. Our study aimed to reveal the pharmacological effects and potential mechanisms of Ganoderic acid C2 (GAC) against cyclophosphamide (CY)-associated immunosuppression. Target genes were collected from several public databases, including the DisGeNET, Comparative Toxicogenomics Database, GeneCards, and PharmMapper. STRING database was used to construct the protein–protein interaction of network. Subsequently, molecular docking was carried out to visualize the protein-GAC interactions. Experimental validations, including ELISA and qRT-PCR were performed to confirm the pharmacological activities of GAC on CY-induced immunosuppression model. A total of 56 GAC-related targets were identified to be closely associated with CY-induced immunosuppression. Enrichment analyses results revealed that these targets were mainly involved in immune and inflammatory response-related pathways. STAT3 and TNF were identified as the core targets of GAC. Molecular docking indicated that GAC combined well with STAT3 and TNF protein. In addition, animal experiments indicated that GAC improved immunity as well as STAT3 and TNF genes expression in CY-induced immunosuppression, which further verified the prediction through bioinformatics analysis and molecular docking. We successfully revealed the potential therapeutics mechanisms underlying the effect of GAC against CY-induced immunosuppression based on the combination of bioinformatics analysis, molecular docking, and animal experiments. Our findings lay a theoretical foundation for the in-depth development and utilization of Ganoderma lucidum fermentation product in the future, and also provide theoretical guidance for the development of innovative drugs that assist in improving immunity.
Subject terms: Computational biology and bioinformatics, Immunology
Introduction
The immune system is an interactive network of cytokines, humoral factors, cells, and lymphoid organs1. As an important defense system of the body, the immune system has important physiological functions. It protects the host from the damage of pathogenic microorganisms by identifying and monitoring non-self and self substances, so as to maintain the health of the body2,3. When tumor cells are killed by drugs or radiation, the body’s immune function and bone marrow hematopoietic system will also be damaged, resulting in immunosuppression4. Cyclophosphamide (CY) is a commonly used antineoplastic drug in clinical practice. In addition, it is also a highly effective immunosuppressant, commonly used in blood and bone marrow transplantation5. It could cause oxidative stress, immunosuppression, bone marrow suppression, and other side effects, which are the primary limiting factors in clinical chemotherapy6,7. In clinical practice, in order to reduce those side effects, chemotherapy patients usually take immunoactive substances to enhance immunity8. Thus, it is very important to develop novel immunomodulators that could effectively reduce CY-induced immunodeficiency.
Many natural immunomodulators, including polypeptides, flavonoids, and polysaccharides, have the ability to boost immunity and improve CY-induced immunodeficiency9–12. Ganoderma lucidum has long been used primarily for the prevention and treatment of various diseases. More and more studies have shown that it has immunomodulatory functions13,14. Triterpenoids and polysaccharides are the major bioactive ingredients in Ganoderma lucidum15. Triterpenoids have a wide range of biological activities, including antioxidation, hepatoprotective, anti-inflammatory, anti-apoptotic, and immune restoration effects16–19. Ganoderic acid C2 (GAC) is a bioactive triterpenoid in Ganoderma lucidum fermented extract. However, the immunomodulatory effects of GAC in CY-induced immunosuppressed mice remain unclear.
Network pharmacology is a tool for systematically describing complex interactions between drugs and biological systems from a network perspective20. Molecular docking, an in silico approach, is widely recognized as one of the most prevalent and effective structure-based methods for predicting the interactions between molecules and biological targets21,22. In the present study, a network pharmacology prediction and molecular docking-based approach, combined with animal experiments verification, was carried out to reveal the potential therapeutic mechanisms and targets of GAC against the CY-induced immunodeficiency. The workflow of the research is presented in Fig. 1.
Figure 1.
Workflow of GAC in the treatment of CY-induced immunodeficiency.
Materials and methods
Collection of CY-associated targets
The CY-related targets were collected from several online databases by using “Cyclophosphamide” as keywords, including the Comparative Toxicogenomics Database (CTD, https://ctdbase.org/)23, and the SuperPred (https://prediction.charite.de/index.php)24. The Retrieve/ID mapping tool (www.uniprot.org) was used to transform protein target manes into their official gene symbols. Finally, CY-related targets were obtained by removing duplicate genes.
Prediction of GAC-related targets
The potential targets of GAC were obtained by using following databases by entering a SMILES of GAC: the SuperPred (https://prediction.charite.de/index.php) and similarity ensemble approach (https://sea.bkslab.org/)25. The Retrieve/ID mapping tool (www.uniprot.org) was used to transform protein target manes into their official gene symbols. Finally, GAC-related targets were collected by removing duplicate genes.
Screening of immunosuppression-related targets
We searched the GeneCards database (https://www.genecards.org/)26, the DISGENET database (https://www.disgenet.org/)27, and the Comparative Toxicogenomics Database (CTD, https://ctdbase.org/) to collect potential targets of immunosuppression by using “immunosuppression” or “leukopenia” as the search keywords. Finally, immunosuppression-related targets were collected by removing duplicate genes.
The protein–protein interaction (PPI) network construction
First, the Venn diagram tool (https://bioinfogp.cnb.csic.es/tools/venny/index.html) was used to identify the intersection of GAC, CY, and immunosuppression targets. Then, those intersection targets were imported into the STRING database (https://cn.string-db.org/) to construct the PPI network; the protein interaction selection score was set to > 0.4. The TSV file was downloaded and used to visualize the PPI network results by using the Cytoscape software (version 3.2.1) (http://cytoscape.org/). The cytoHubba plugin of Cytoscape software was used to identify the core targets of GAC28.
Functional enrichment analysis
We identified the potential pathways by the GO and KEGG enrichment analyses to further clarify the role of the potential anti-immunosuppression targets of GAC. The enrichment analyses were performed and results were visualized by using R packages, including enrichplot, ggplot2, and clusterProfiler. The p < 0.05 was considered statistically significant.
Molecular docking
We performed molecular docking analysis to investigate the potential binding mode of GAC to a macromolecular receptor. The core genes were selected for molecular docking analysis. The 3D structures of targets protein (TNF and STAT3) were downloaded from PDB (https://www.rcsb.org) databases, and the 3D conformations of proteins with a crystal resolution < 3 Å were selected. For the preparation of receptor macromolecule: firstly, the proteins were removed from solvent and proto-ligand small molecules using PyMOL software and saved as PDB format. Then using AutoDock software, the protein was subjected to de-watering, hydrogenation, charge calculation and atom type addition operations, and finally saved as a PDBQT format file. For the preparation of small molecule ligand: firstly, the small molecule ligand was opened using AutoDock software, in which the charge was adjusted and finally saved as a PDBQT file. The AutoDockTools 1.5.6 software was used to open the receptor macromolecule and ligand PDBQT files. The size of the grid was designed to cover the binding pocket, with XYZ dimensions of 80 Å × 80 Å × 80 Å. The spacing between grid points was set to 0.6 Å. The docking process utilized the Vina force field and the Lamarckian GA (4.2) algorithm. The results of molecular docking were visualized by using the PyMOL software.
Animals
The specific-pathogen-free Kunming mice (22–25 g and 6-week-old) were purchased from the Harbin Medical University. Mice were kept with tap water and food ad libitum, and housed in air-conditioned room (relative humidity 45–65% and temperature 20–24 °C) with a 12 h dark/light cycle. The animal experimental protocol was approved by the Ethics Committee of the Harbin Normal University.
Acute toxicity study
The mice were divided into two groups, namely the vehicle control group and GAC control group, with 6 animals in each group. GAC was dissolved in normal saline and administered to the animals at a dose of 2000 mg/kg body weight through oral gavage. The mice were monitored for 14 days to detect any signs of toxicity or mortality. The animals' behavior was closely observed for the first 4 h, and their body weight was recorded at the beginning of the experiment, on the 7th day, and at the end of the experiment.
Drug administration
After 7 days of acclimation, all mice were divided into five groups (n = 10 per group): control group (Control), CY-induced immunosuppression group (CY), CY group of mice received with GAC at a dose of 10 mg/kg (LGAC), CY group of mice received with GAC at a dose of 20 mg/kg (MGAC), CY group of mice received with GAC at a dose of 40 mg/kg (HGAC). CY-induced immunodeficiency mice were given intragastrically with GAC (10, 20, or 40 mg/kg body weight) for consecutive 14 days. Control group was given intragastrically with normal saline. The immunodeficiency model was induced by injecting intraperitoneally with CY (50 mg/kg/d) at day 8, 10, 12 and 14 based on the previously study29. Control and CY groups were given saline alone with the same operation. GAC was obtained from Shanghai Standard Technology Co Ltd (Shanghai, China). After the experiments, the mice were fasted without water overnight. Then, the mice were weighted and killed by cervical dislocation. We collected spleen and thymus tissues and weighted immediately. The immune organ index was calculated based on the following formula: organ index (mg/g) = organ weight (mg) / body weight (g).
Inflammatory cell counts
After the last GAC treatment, the whole blood sample was collected into EDTA-2 K tubes. After mixing 100 μL of blood sample with 900 μL of phosphate buffer solution, the resulting mixture was homogenized and centrifuged at 3000 r/min for 10 min at 4 °C. Finally, the supernatant was collected. The white blood cell (WBC), neutrophil (NEUT), and lymphocyte (LYMPH) were counted using a hematology analyzer (Siemens, ADVIA 2120i, Germany).
Detection of serum inflammatory cytokines and immunoglobulin
After the last GAC treatment, the whole blood sample was collected into EDTA-2 K tubes. After mixing 100 μL of blood sample with 900 μL of phosphate buffer solution, the resulting mixture was homogenized and centrifuged at 3000 r/min for 10 min at 4 °C. Finally, the supernatant was collected. The level of serum inflammatory cytokines (TNF-α, IL-12, IL-4, IFN-γ) and immunoglobulin (lgA and lgG) were measure by Enzyme Linked Immunosorbent Assay (ELISA). The blood samples were collected from orbital venous plexus after the last GAC treatment. We also measured the serum inflammatory cytokines and immunoglobulin levels by ELISA kit (Jiancheng, Nanjing) based on the manufacturer’s instruction.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from spleen tissues using TRIzol Reagent (Invitrogen, CA, USA) based on the manufacturer’s protocol. In addition, the RNA was measured using spectrophotometry at a wavelength of 260 nm to determine its concentration, while its purity was assessed by calculating the absorbance ratio at 260/280 nm. It is recommended that the absorbance ratios of RNA samples fall within the range of 1.8 to 2.0 for optimal purity. The purified RNA was synthesized into the cDNA using the cDNA synthesis kits (Takara Bio). Then, the qRT-PCR was performed on a StepOnePlus Real-time PCR system (Applied Biosystems, CA, USA). The 2−ΔΔCt method was used to measure the mRNA expression levels. The primers were presented in Table S1.
Statistical analysis
The GraphPad Prism 8.0 software was used to perform the data analysis. Experimental data were shown as mean ± standard deviation (SD). One-way analysis of variance was used to compare the group differences. p < 0.05 was indicated statistically significant.
Ethics approval
All experiments protocols and animal use were approved by the Laboratory Animal Ethics Committee of Harbin Normal University.
Results
Screening potential targets of GAC in the treatment of CY-induced immunodeficiency
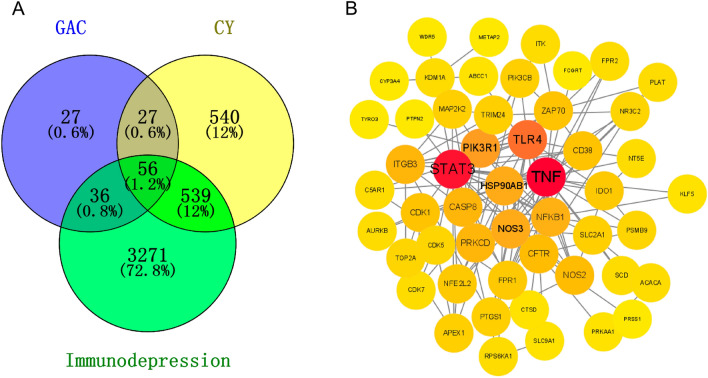
In Fig. 2A, we utilized the Comparative Toxicogenomics Database and SuperPred to collect a total of 1162 targets for CY. From the SuperPred and similarity ensemble approach databases, we obtained 146 GAC-related targets. Additionally, 3902 targets associated with immunodeficiency were gathered from the GeneCards, DISGENET, and CTD databases. As a result, we identified a total of 56 intersecting genes, which were considered potential targets of GAC in treating CY-induced immunodeficiency. To visualize the interactions among these overlapping genes, we employed Cytoscape software to construct a PPI network. As depicted in Fig. 2B, the PPI network consisted of 51 nodes and 145 edges. Notably, from the PPI network, we observed that TNF, STAT3, TLR4, PIK3R1, and HSP90AB1 were significant targets for GAC intervention in CY-induced immunodeficiency.
Figure 2.
Screening potential targets of GAC in the treatment of CY-induced immunodeficiency. (A) The intersection of GAC-, CY- and immunodepression-related genes. (B) PPI network of 56 intersection targets.
GO and KEGG enrichment analyses
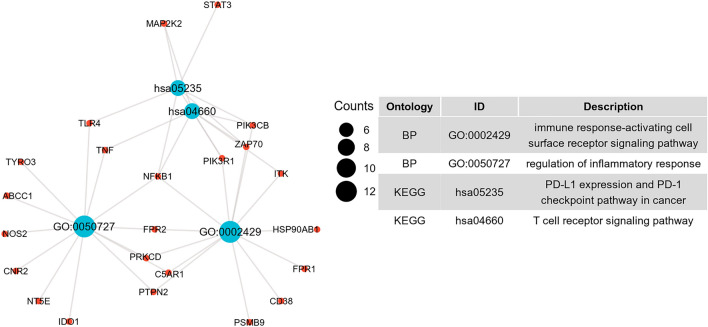
In our study, we conducted functional enrichment analyses to gain further insights into the biological functions of the potential genes targeted by GAC in the treatment of CY-induced immunodeficiency. In the category of biological processes (BP), the target genes exhibited significant enrichment in various pathways, including regulation of inflammatory response, immune response-activating cell surface receptor signaling pathway, cellular response to peptide, regulation of small molecule metabolic process, and activation of protein kinase activity (Fig. 3A). Regarding the cellular component (CC) category, the target genes were primarily associated with cell–cell junction, the apical part of the cell, membrane region, membrane microdomain, and membrane raft (Fig. 3B). In the molecular function (MF) category, the target genes were mainly involved in protein serine/threonine kinase activity, tetrapyrrole binding, heme binding, protein tyrosine kinase activity, and cyclin-dependent protein kinase activity (Fig. 3C). In the KEGG category, the target genes were mainly involved in fluid shear stress and atherosclerosis, insulin resistance, sphingolipid signaling pathway, HIF-1 signaling pathway, yersinia infection, T cell receptor signaling pathway, Toll-like receptor signaling pathway, PD-L1 expression and PD-1 checkpoint pathway in cancer, etc. (Fig. 3D). Notably, the potential mechanisms underlying the therapeutic effects of GAC against CY-induced immunosuppression involve various immune-related pathways. These pathways include the regulation of inflammatory response, immune response-activating cell surface receptor signaling pathway, PD-L1 expression and PD-1 checkpoint pathway in cancer, and T cell receptor signaling pathway (Fig. 4).
Figure 3.
Functional enrichment analysis of 56 intersection targets. Bubble diagrams depicted the top 10 BP (A), CC (B), MF (C) terms and top 10 KEGG pathways.
Figure 4.
The immune-related pathways of GAC intervention CY-induced immunodeficiency. The red circular nodes represent target genes, the blue circular nodes represent immune-related pathways.
Core targets identification
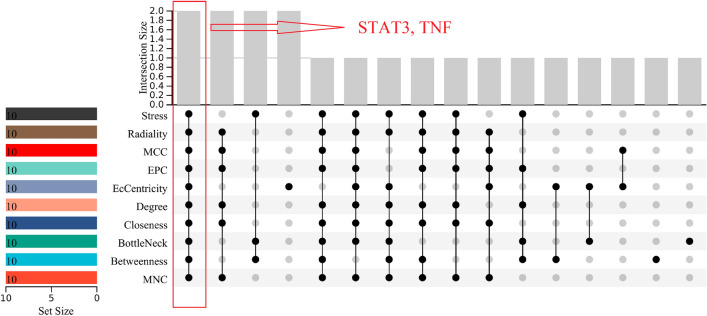
Based on the results shown in Table 1, the top 10 genes were identified using 10 different topological analysis algorithms in cytoHubba. Subsequently, we employed the R package "UpSet" to determine the two core genes, namely STAT3 and TNF (Fig. 5).
Table 1.
Top 10 genes by 10 ranked methods in cytoHubba.
| Rank methods in cytoHubba | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| MNC | Betweenness | BottleNeck | Closeness | Degree | EcCentricity | EPC | MCC | Radiality | Stress |
| Top 10 genes | |||||||||
| PRKCD | KDM1A | KDM1A | PRKCD | PRKCD | ZAP70 | PRKCD | PRKCD | PRKCD | KDM1A |
| PIK3R1 | SLC2A1 | PIK3R1 | PIK3R1 | PIK3R1 | SLC2A1 | PIK3R1 | PIK3R1 | PIK3R1 | PIK3R1 |
| NOS3 | PIK3R1 | NOS3 | NOS3 | NOS3 | NOS3 | NOS3 | NOS3 | NOS3 | NOS3 |
| HSP90AB1 | HSP90AB1 | HSP90AB1 | HSP90AB1 | HSP90AB1 | HSP90AB1 | ITGB3 | NFE2L2 | HSP90AB1 | HSP90AB1 |
| TLR4 | ITGB3 | ITGB3 | TLR4 | ITGB3 | FPR1 | TLR4 | TLR4 | TLR4 | ITGB3 |
| CASP8 | TLR4 | RPS6KA1 | CASP8 | TLR4 | NFE2L2 | CASP8 | CASP8 | CASP8 | TLR4 |
| STAT3 | ABCC1 | STAT3 | STAT3 | CASP8 | TNF | STAT3 | STAT3 | STAT3 | STAT3 |
| NFKB1 | STAT3 | TNF | NFKB1 | STAT3 | STAT3 | NFKB1 | NFKB1 | NFKB1 | TNF |
| TNF | TNF | IDO1 | TNF | NFKB1 | IDO1 | TNF | TNF | TNF | NFKB1 |
| NOS2 | CFTR | CFTR | NOS2 | TNF | NOS2 | NOS2 | NOS2 | NOS2 | CFTR |
Figure 5.
Core targets identification. Ten algorithms (stress, radiality, MCC, EPC, EcCentricity, degree, closeness, bottleneck, betweenness, and MNC) were used to identify hub genes based on R package “UpSet”.
Molecular docking
In this study, we conducted molecular docking analysis on GAC using two hub proteins, STAT3 and TNF, to further investigate the potential targets of GAC against CY-induced immunosuppression. The key binding sites between GAC and the amino acid residues of the target proteins were illustrated in Fig. 6, demonstrating a strong binding affinity between GAC and the target proteins (STAT3 and TNF). The binding energy between GAC and STAT3 was calculated to be − 12.2 kcal/mol, while for TNF it was − 9.29 kcal/mol. These results from the molecular docking analysis were consistent with the findings from network pharmacology, confirming the accuracy of the predicted results obtained through network pharmacology.
Figure 6.
Molecular docking analysis of GAC binding to two hub targets. Protein (A) STST3 (6TLC), (B) TNF (1TNR) were exhibited interacting with the GAC small molecular. The yellow stick model represents GAC. The light dashed line represents hydrogen bond. The green stick model represents residue in the binding sites. The pink represents large molecular protein.
Acute toxicity study
There were no observed alterations in behavioral parameters in mice administered with a dose of 2000 mg/kg of GAC orally. Additionally, there were no significant changes in body weight recorded throughout the duration of the study (Table S2). Moreover, the mice administered a dose of 2000 mg/kg of GAC exhibited no signs of toxicity and survived, suggesting that a single dose of GAC at this concentration is well-tolerated by mice.
Effect of GAC on body weight and immune organ index in CY-induced immunodeficiency
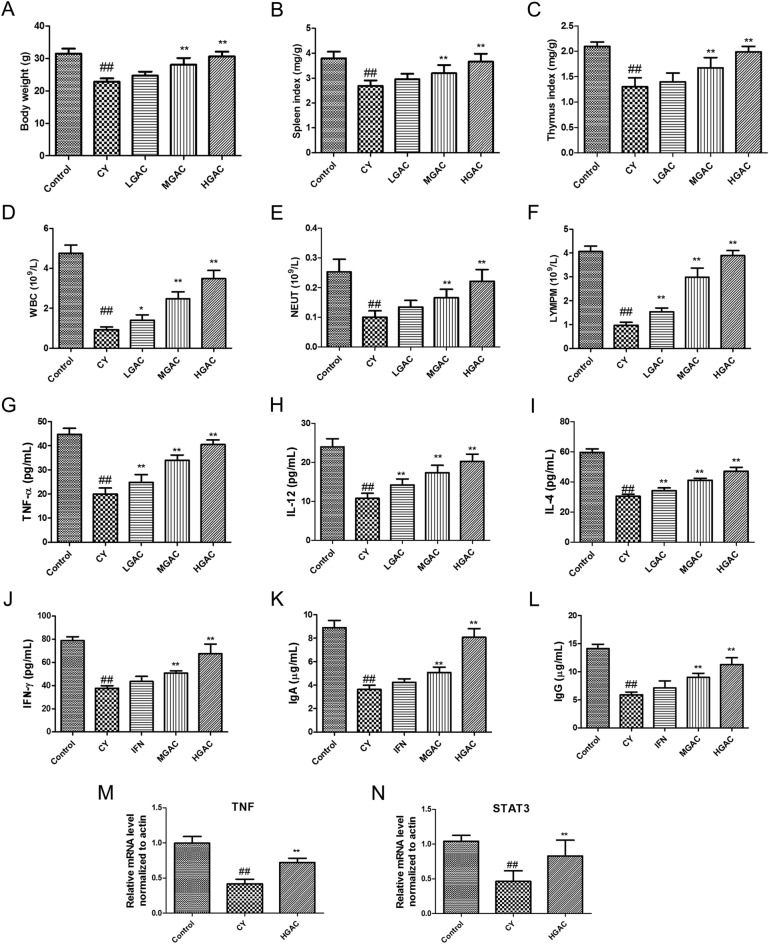
Figure 7A illustrates that the bodyweight of mice in the CY group decreased significantly compared to the control group (p < 0.05). However, after treatment with GAC, the mice in the MGAC and HGAC groups exhibited a significant increase in body weight when compared to the CY group (p < 0.05). Furthermore, the spleen and thymus indices of mice in the CY group decreased significantly compared to the control group (p < 0.05), as shown in Fig. 7B,C. However, after a 14-day treatment with GAC (20 or 40 mg/kg), the reduction in immune organ indices induced by CY was alleviated (p < 0.05).
Figure 7.
GAC exhibits a protective effect on mice with immunosuppression induced by CY. Effect of GAC on body weight (A), spleen index (B), thymus index (C), WBC (D), NEUT (E), and LYMPM (F) in CY-treated mice. Effect of GAC on the level of TNF-α (G), IL-12 (H), IL-4 (I), IFN-γ (J), lgA (K) and lgG (L) in CY-treated mice. Effect of GAC on the mRNA expression of TNF (M) and STAT3 (N) in CY-treated mice. Data were shown as mean ± standard deviation (SD). ##p < 0.01, compared with the control group; *p < 0.05, compared with the CY group; **p < 0.01, compared with the CY group.
Effects of GAC on inflammatory cell counts in CY-induced immunodeficiency
According to the data depicted in Fig. 7D–F, the administration of CY resulted in a significant decrease in the counts of WBC, NEUT, and LYMPH in mice (p < 0.05). However, after a 14-day treatment with GAC (20 or 40 mg/kg), the decline in inflammatory cell counts induced by CY was significantly improved (p < 0.05). These results demonstrate the immunomodulatory activity of GAC in CY-treated mice.
Effects of GAC on the level of inflammatory cytokines in CY-treated mice
According to the data illustrated in Fig. 7G–J, the administration of CY resulted in a significant decrease in the levels of inflammatory cytokines (TNF-α, IL-12, IL-4, IFN-γ) in mice (p < 0.05). However, after a 14-day treatment with GAC (20 or 40 mg/kg), the decline in inflammatory cytokine levels induced by CY was significantly alleviated (p < 0.05).
GAC treatment promoted the generation of immunoglobulin in CY-induced immunodeficiency
According to the findings presented in Fig. 7K,L, the administration of CY led to a significant decrease in the levels of immunoglobulins (IgA and IgG) in mice (p < 0.05). However, after a 14-day treatment with GAC (20 or 40 mg/kg), the decline in immunoglobulin levels induced by CY was effectively reversed (p < 0.05).
GAC up-regulated core genes expression levels in CY-induced immunodeficiency
To confirm the findings of the molecular docking analysis, a qRT-PCR analysis was conducted. As depicted in Fig. 7M,N, the expressions of TNF and STAT3 were markedly up-regulated in the CY-induced immunodeficiency after a 14-day treatment with GAC (20 or 40 mg/kg) (p < 0.01).
Discussion
The immunosuppressive environment plays a critical role in facilitating the development and advancement of tumors. In clinical practice, certain chemotherapeutic drugs, like cyclophosphamide (CY), not only target cancer cells, but also hinder the immune system of patients, ultimately leading to a lower rate of tumor recovery5. Simultaneously, prior research has indicated that prolonged chemotherapy can result in the depletion of T cells and lymphopenia30. As a result, the discovery of new medications that can safeguard against the immunosuppressive impact of CY is of utmost importance. Chinese herbal medicine and natural products offer a complementary and alternative approach to combating CY-induced immunodeficiency due to their ability to target multiple pathways and mechanisms31–33. Ganoderma lucidum has been utilized for a considerable period to prevent and treat a diverse range of diseases owing to its immunomodulatory properties13,14. Ganoderic acid C2 (GAC) is a significant bioactive component found in Ganoderma lucidum. However, the therapeutic effects and mechanisms of GAC in treating immunodeficiency remain unexplored. Network pharmacology, as an advanced technology, is primarily employed to predict the intricate interaction between drugs and diseases. It has proven useful in researching novel drug targets, understanding drug mechanisms, and discovering new medications34,35. In our study, we integrated network pharmacology, molecular docking, and animal experiments to investigate the potential therapeutic targets and mechanisms of GAC in combating cyclophosphamide-induced immunosuppression.
Initially, we identified 56 target genes that serve as therapeutic targets for GAC in combating cyclophosphamide-induced immunosuppression. Notably, two hub genes (TNF and STAT3) were identified as playing a pivotal role in the PPI network. Moreover, molecular docking results revealed that hydrogen bonding is the primary mode of interaction between target proteins and GAC, providing further validation to the network pharmacology findings. Tumor necrosis factor (TNF), an inflammatory cytokine, plays a crucial role in disease pathogenesis and maintaining homeostasis36. The TNF family members and their receptors are involved in activating, differentiating, proliferating, or promoting the survival of immune cells37. Furthermore, recent research has shown a decrease in TNF levels in mice with CY-induced immunosuppression31,38. Signal transducer and activator of transcription 3 (STAT3), a transcriptional regulator, plays a vital role in mature tissue function and vertebrate development including control of immunity and inflammation39,40. STAT3 involved in tumor immunity and inflammation through contributing to pro-oncogenic inflammatory pathways, such as IL-6-GP130-JAK and NF-kappaB pathways41. Moreover, recent studies showed that the JAK2/STAT3 signaling pathway was inactivated in CY-induced hematopoietic dysfunction42–44. In this study, it was observed that GAC could increase the expression levels of TNF and STAT3 in mice with CY-induced immunodeficiency. This suggests that the TNF and STAT3 genes are likely to be crucial in the development of CY-induced immunodeficiency, and targeting them could be a potential therapeutic approach for GAC in treating immunodeficiency.
Neutrophils, lymphocytes, and white blood cells, along with immune organs such as the thymus and spleen, as well as immunologic active materials like interleukins, interferon, and immunoglobulin, are vital components of the immune system45,46. Previous research has shown that natural compounds have immunomodulatory effects by activating immune cells and increasing levels of inflammatory cytokines and immunoglobulins9,11. In our current study, we discovered that GAC can enhance immune response in mice treated with CY, providing further confirmation of the network pharmacology findings.
Conclusion
By utilizing network pharmacology, molecular docking, and experimental validation, our study demonstrates that GAC possesses immunomodulatory properties by activating TNF and STAT3 expression. This research provides a comprehensive understanding of the therapeutic targets and mechanisms of GAC in the treatment of immunodeficiency, integrating network pharmacology with experimental validation.
Supplementary Information
Author contributions
Y.L. wrote the manuscript and designed the study. D.T. and H.C. analyzed the data and perpared the figures. J.W. reviewed and edited the manuscript. All authors reviewed the manuscript.
Funding
This work was supported by Graduate Innovation Fund of Harbin Normal University (HSDBSCX2021-107), Domestication and Technology Promotion of BF2020, a Coldland Wild Morel Fungus Strain, 2023 Harbin Normal University, “Digital Economy, Bio-economy, Dual Carbon Strategy and Creative Design” (Project No.: 1305123258), and Scientific Research Project on Basic Research Operating Costs of Provincial Higher Education Institutions in Heilongjiang Province (2021-KYYWF-0172).
Data availability
The data used in our study are available from the corresponding authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-44394-y.
References
- 1.Parkin J, Cohen B. An overview of the immune system. Lancet (London, England) 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 2.Kumar D, Arya V, Kaur R, Bhat ZA, Gupta VK, Kumar V. A review of immunomodulators in the Indian traditional health care system. J. Microbiol. Immunol. Infect. 2012;45:165–184. doi: 10.1016/j.jmii.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 3.Tomar N, De RK. A brief outline of the immune system. Methods Mol. Biol. 2014;1184:3–12. doi: 10.1007/978-1-4939-1115-8_1. [DOI] [PubMed] [Google Scholar]
- 4.Phan TA, Halliday GM, Barnetson RS, Damian DL. Spectral and dose dependence of ultraviolet radiation-induced immunosuppression. Front. Biosci. 2006;11:394–411. doi: 10.2741/1807. [DOI] [PubMed] [Google Scholar]
- 5.Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009;6:638–647. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- 6.Feng L, Huang Q, Huang Z, Li H, Qi X, Wang Y, Liu Z, Liu X, Lu L. Optimized animal model of cyclophosphamide-induced bone marrow suppression. Basic Clin. Pharmacol. Toxicol. 2016;119:428–435. doi: 10.1111/bcpt.12600. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y, Li S, Li C, Shao Y, Chen A. Polysaccharides from spores of cordyceps cicadae protect against cyclophosphamide-induced immunosuppression and oxidative stress in mice. Foods (Basel, Switzerland) 2022;11:515. doi: 10.3390/foods11040515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JG, Kim YS, Lee YJ, Ahn HY, Kim M, Kim M, Cho MJ, Cho Y, Lee JH. Effect of immune-enhancing enteral nutrition enriched with or without beta-glucan on immunomodulation in critically Ill patients. Nutrients. 2016;8:336. doi: 10.3390/nu8060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai J, Chen J, Qi J, Ding M, Liu W, Shao T, Han J, Wang G. Konjac Glucomannan from Amorphophallus konjac enhances immunocompetence of the cyclophosphamide-induced immunosuppressed mice. Food Sci. Nutr. 2021;9:728–735. doi: 10.1002/fsn3.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Shen M, Wu T, Yu Y, Yu Q, Chen Y, Xie J. Mesona chinensis Benth polysaccharides protect against oxidative stress and immunosuppression in cyclophosphamide-treated mice via MAPKs signal transduction pathways. Int. J. Biol. Macromol. 2020;152:766–774. doi: 10.1016/j.ijbiomac.2020.02.318. [DOI] [PubMed] [Google Scholar]
- 11.Chang Y, Guo A, Jing Y, Lin J, Sun Y, Kong L, Zheng H, Deng Y. Immunomodulatory activity of puerarin in RAW264.7 macrophages and cyclophosphamide-induced immunosuppression mice. Immunopharmacol. Immunotoxicol. 2021;43:223–229. doi: 10.1080/08923973.2021.1885043. [DOI] [PubMed] [Google Scholar]
- 12.Berköz M, Yalın S, Özkan-Yılmaz F, Özlüer-Hunt A, Krośniak M, Francik R, Yunusoğlu O, Adıyaman A, Gezici H, Yiğit A, Ünal S, Volkan D, Yıldırım M. Protective effect of myricetin, apigenin, and hesperidin pretreatments on cyclophosphamide-induced immunosuppression. Immunopharmacol. Immunotoxicol. 2021;43:353–369. doi: 10.1080/08923973.2021.1916525. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Lin Z. Immunomodulating effect of Ganoderma (Lingzhi) and possible mechanism. Adv. Exp. Med. Biol. 2019;1182:1–37. doi: 10.1007/978-981-32-9421-9_1. [DOI] [PubMed] [Google Scholar]
- 14.Lin ZB. Cellular and molecular mechanisms of immuno-modulation by Ganoderma lucidum. J. Pharmacol. Sci. 2005;99:144–153. doi: 10.1254/jphs.crj05008x. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad MF, Ahmad FA, Khan MI, Alsayegh AA, Wahab S, Alam MI, Ahmed F. Ganoderma lucidum: A potential source to surmount viral infections through β-glucans immunomodulatory and triterpenoids antiviral properties. Int. J. Biol. Macromol. 2021;187:769–779. doi: 10.1016/j.ijbiomac.2021.06.122. [DOI] [PubMed] [Google Scholar]
- 16.Chiu HF, Fu HY, Lu YY, Han YC, Shen YC, Venkatakrishnan K, Golovinskaia O, Wang CK. Triterpenoids and polysaccharide peptides-enriched Ganoderma lucidum: A randomized, double-blind placebo-controlled crossover study of its antioxidation and hepatoprotective efficacy in healthy volunteers. Pharm. Biol. 2017;55:1041–1046. doi: 10.1080/13880209.2017.1288750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kou RW, Gao YQ, Xia B, Wang JY, Liu XN, Tang JJ, Yin X, Gao JM. Ganoderterpene A, a new triterpenoid from Ganoderma lucidum, attenuates LPS-induced inflammation and apoptosis via suppressing MAPK and TLR-4/NF-κB pathways in BV-2 Cells. J. Agric. Food Chem. 2021;69:12730–12740. doi: 10.1021/acs.jafc.1c04905. [DOI] [PubMed] [Google Scholar]
- 18.Wu YL, Han F, Luan SS, Ai R, Zhang P, Li H, Chen LX. Triterpenoids from Ganoderma lucidum and their potential anti-inflammatory effects. J. Agric. Food Chem. 2019;67:5147–5158. doi: 10.1021/acs.jafc.9b01195. [DOI] [PubMed] [Google Scholar]
- 19.Radwan FF, Perez JM, Haque A. Apoptotic and Immune restoration effects of ganoderic acids define a new prospective for complementary treatment of cancer. J. Clin. Cell. Immunol. 2011;S3:4. doi: 10.4172/2155-9899.S3-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boezio B, Audouze K, Ducrot P, Taboureau O. Network-based approaches in pharmacology. Mol. Inform. 2017;36:1700048. doi: 10.1002/minf.201700048. [DOI] [PubMed] [Google Scholar]
- 21.Hosen, M. A., El Bakri, Y., Rehman, H. M., Hashem, H. E., Saki, M. & Kawsar, S. M. A. A computational investigation of galactopyranoside esters as antimicrobial agents through antiviral, molecular docking, molecular dynamics, pharmacokinetics, and bioactivity prediction. J. Biomol. Struct. Dyn. (2023) 1–16. [DOI] [PubMed]
- 22.Rehman HM, Sajjad M, Ali MA, Gul R, Irfan M, Naveed M, Bhinder MA, Ghani MU, Hussain N, Said ASA, Al Haddad AHI, Saleem M. Identification of NS2B-NS3 protease inhibitors for therapeutic application in ZIKV infection: A pharmacophore-based high-throughput virtual screening and MD simulations approaches. Vaccines. 2023;11:131. doi: 10.3390/vaccines11010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis AP, Wiegers TC, Johnson RJ, Sciaky D, Wiegers J, Mattingly CJ. Comparative toxicogenomics database (CTD): Update 2023. Nucleic Acids Res. 2022;51:D1257–D1262. doi: 10.1093/nar/gkac833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallo K, Goede A, Preissner R, Gohlke BO. SuperPred 3.0: Drug classification and target prediction-a machine learning approach. Nucleic Acids Res. 2022;50:726–731. doi: 10.1093/nar/gkac297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Liang L, Yin Z, Lin J. Improving chemical similarity ensemble approach in target prediction. J. Cheminformatics. 2016;8:20. doi: 10.1186/s13321-016-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safran M, Dalah I, Alexander J, Rosen N, Iny Stein T, Shmoish M, Nativ N, Bahir I, Doniger T, Krug H, Sirota-Madi A, Olender T, Golan Y, Stelzer G, Harel A, Lancet D. GeneCards Version 3: The human gene integrator. Database J. Biol. Databases Curation. 2010;2010:baq020. doi: 10.1093/database/baq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, Ronzano F, Centeno E, Sanz F, Furlong LI. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020;48:D845–d855. doi: 10.1093/nar/gkz1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014;8(Suppl 4):S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Wu X, Jin W, Guo Y. Immunomodulatory effects of a low-molecular weight polysaccharide from enteromorpha prolifera on RAW 264.7 macrophages and cyclophosphamide- induced immunosuppression mouse models. Mar. Drugs. 2020;18:340. doi: 10.3390/md18070340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karachi A, Yang C, Dastmalchi F, Sayour EJ, Huang J, Azari H, Long Y, Flores C, Mitchell DA, Rahman M. Modulation of temozolomide dose differentially affects T-cell response to immune checkpoint inhibition. Neuro-oncology. 2019;21:730–741. doi: 10.1093/neuonc/noz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan H, Lu J, Wang J, Chen L, Wang Y, Li L, Miao L, Zhang H. Prevention of cyclophosphamide-induced immunosuppression in mice with traditional chinese medicine Xuanfei baidu decoction. Front. Pharmacol. 2021;12:730567. doi: 10.3389/fphar.2021.730567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shu G, Xu D, Zhao J, Yin L, Lin J, Fu H, Tang H, Fang J, Peng X, Zhao X. Protective effect of Polygonatum sibiricum polysaccharide on cyclophosphamide-induced immunosuppression in chickens. Res. Vet. Sci. 2021;135:96–105. doi: 10.1016/j.rvsc.2020.12.025. [DOI] [PubMed] [Google Scholar]
- 33.Zheng S, Zheng H, Zhang R, Piao X, Hu J, Zhu Y, Wang Y. Immunomodulatory effect of ginsenoside Rb2 against cyclophosphamide-induced immunosuppression in mice. Front. Pharmacol. 2022;13:927087. doi: 10.3389/fphar.2022.927087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo TT, Lu Y, Yan SK, Xiao X, Rong XL, Guo J. Network pharmacology in research of chinese medicine formula: Methodology, application and prospective. Chin. J. Integr. Med. 2020;26:72–80. doi: 10.1007/s11655-019-3064-0. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Wang ZY, Zheng JH, Li S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021;19:1–11. doi: 10.1016/S1875-5364(21)60001-8. [DOI] [PubMed] [Google Scholar]
- 36.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juhász K, Buzás K, Duda E. Importance of reverse signaling of the TNF superfamily in immune regulation. Expert Rev. Clin. Immunol. 2013;9:335–348. doi: 10.1586/eci.13.14. [DOI] [PubMed] [Google Scholar]
- 38.Cheng Y, Liu L, Mo S, Gao J, Zhang H, Zhang H, Zhang C, Song X, Li L, Geng Z. The immunomodulatory effects of phellodendri cortex polysaccharides on cyclophosphamide-induced immunosuppression in mice. Evid. Based Complement. Altern. Med. eCAM. 2021;2021:3027708. doi: 10.1155/2021/3027708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016;31:1–15. doi: 10.1016/j.cytogfr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Y, Mao R, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu W, Jia D, An S, Mu M, Qiao X, Liu Y, Li X, Wang D. Calf Spleen Extractive Injection protects mice against cyclophosphamide-induced hematopoietic injury through G-CSF-mediated JAK2/STAT3 signaling. Sci. Rep. 2017;7:8402. doi: 10.1038/s41598-017-08970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Chu Q, Jiang X, Yu Y, Wang L, Cui Y, Lu J, Teng L, Wang D. Sarcodon imbricatus polysaccharides improve mouse hematopoietic function after cyclophosphamide-induced damage via G-CSF mediated JAK2/STAT3 pathway. Cell Death Dis. 2018;9:578. doi: 10.1038/s41419-018-0634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mu W, Ao J, Li Y, Zhang J, Duan C. Exploring the protective mechanisms of total tannins from Geum japonicum var. chinense F.Bolle in mice with hematopoietic dysfunction via the JAK2/STAT3/5 signaling pathway. J. Ethnopharmacol. 2022;296:115507. doi: 10.1016/j.jep.2022.115507. [DOI] [PubMed] [Google Scholar]
- 45.Sadighi Akha AA. Aging and the immune system: An overview. J. Immunol. Methods. 2018;463:21–26. doi: 10.1016/j.jim.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Bonilla FA, Oettgen HC. Adaptive immunity. J. Allergy Clin. Immunol. 2010;125:S33–40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in our study are available from the corresponding authors upon request.