Abstract
The high rates of relapse associated with current medications used to treat opioid use disorder (OUD) necessitate research that expands our understanding of the neural mechanisms regulating opioid taking to identify molecular substrates that could be targeted by novel pharmacotherapies to treat OUD. Recent studies show that activation of calcitonin receptors (CTRs) is sufficient to reduce the rewarding effects of addictive drugs in rodents. However, the role of central CTR signaling in opioid-mediated behaviors has not been studied. Here, we used single nuclei RNA sequencing (snRNA-seq), fluorescent in situ hybridization (FISH), and immunohistochemistry (IHC) to characterize cell type-specific patterns of CTR expression in the nucleus accumbens (NAc), a brain region that plays a critical role in voluntary drug taking. Using these approaches, we identified CTRs expressed on D1R- and D2R-expressing medium spiny neurons (MSNs) in the medial shell subregion of the NAc. Interestingly, Calcr transcripts were expressed at higher levels in D2R- versus D1R-expressing MSNs. Cre-dependent viral-mediated miRNA knockdown of CTRs in transgenic male rats was then used to determine the functional significance of endogenous CTR signaling in opioid taking. We discovered that reduced CTR expression specifically in D1R-expressing MSNs potentiated/augmented opioid self-administration. In contrast, reduced CTR expression specifically in D2R-expressing MSNs attenuated opioid self-administration. These findings highlight a novel cell type-specific mechanism by which CTR signaling in the ventral striatum bidirectionally modulates voluntary opioid taking and support future studies aimed at targeting central CTR-expressing circuits to treat OUD.
Subject terms: Reward, Addiction
Introduction
The United States is experiencing a national crisis of opioid overdose deaths due, in part, to the misuse and abuse of prescription opioid analgesics [1]. The Centers for Disease Control and Prevention (CDC) estimate that the total economic burden of prescription opioid misuse alone in the United States is $78.5 billion annually [2]. In 2020, ~2.3 million Americans 12 years of age and older were diagnosed with an opioid use disorder (OUD), and the number of recent initiates of prescription opioid misuse (~1.2 million) was the second highest among the illicit drugs [3]. Despite the effectiveness of current FDA-approved medications to treat OUD, there is still a high rate of relapse following detoxification [4]. Since these medications are narrowly focused on agonism (i.e., methadone, buprenorphine) or antagonism (i.e., naltrexone) of the µ-opioid receptor, there is a need to identify novel molecular substrates that regulate opioid taking and seeking in order to develop conceptually new approaches to treating OUD [1, 5, 6].
A growing body of literature indicates that neuropeptides of the gut-brain axis and their cognate receptors regulate drug-mediated behaviors in rodents and humans and, thus, could be targeted by novel pharmacotherapies to treat substance use disorders [7–10]. Recent studies demonstrate that pharmacological activation of calcitonin receptors (CTRs) is sufficient to reduce the reinforcing and rewarding effects of addictive drugs. For example, systemic administration of the CTR agonist salmon calcitonin (sCT) decreased ethanol self-administration and ethanol-induced conditioned place preference (CPP) in rodents [11, 12]. Studies investigating the role of central CTRs showed that sCT administered directly into the laterodorsal tegmental nucleus (LDTg) or ventral tegmental area (VTA) reduced ethanol-evoked dopamine release in the NAc shell of mice and ethanol consumption in rats [13]. CTR activation may also regulate behavioral responses to other addictive drugs as systemic sCT decreased cocaine- and nicotine-evoked dopamine release in the NAc shell [11, 14, 15]. While these studies indicate that pharmacological activation of CTRs is sufficient to decrease drug-induced activation of the mesolimbic dopamine system and voluntary drug taking, no studies to date have examined the role of CTRs in opioid-mediated behaviors.
The NAc shell plays an important role in regulating opioid-taking and -seeking behaviors [8, 16]. The two primary output pathways of the NAc consist of GABAergic medium spiny neurons (MSNs) expressing dopamine type 1 receptors (D1R-expressing MSNs) or dopamine type 2 receptors (D2R-expressing MSNs) [17]. Emerging evidence indicates that these two striatal cell populations may have opposing functional roles in opioid-mediated behaviors [18–23]. While CTRs are expressed at high levels in the NAc shell [24–28], their cell type-specific roles in modulating opioid-mediated behaviors have not been investigated. Thus, the overarching goal of this study was to phenotype CTR-expressing cells in the ventral striatum and determine the role of endogenous CTR signaling in D1R- and D2R-expressing MSNs in voluntary opioid taking.
In the present study, we characterized cell type-specific expression of CTRs in the medial NAc shell to expand our understanding of CTR-expressing circuits in the brain. We found that while CTRs are expressed on both D1R- and D2R-expressing MSNs, the majority of CTRs are expressed on D2R-expressing MSNs in the medial NAc shell. Next, we determined the functional role of striatal CTRs in opioid taking using viral-mediated gene delivery methods to reduce CTR expression specifically in D1R- or D2R-expressing cells in the medial NAc shell of transgenic rats. Our results indicate that reduced CTR expression in D1R- and D2R-expressing MSNs potentiated and attenuated, respectively, intravenous oxycodone self-administration in male rats. These findings indicate that endogenous CTR signaling in D1R- and D2R-expressing MSNs produces opposite effects on voluntary opioid taking. Thus, this study highlights novel cell type-specific roles of central CTRs in regulating opioid-mediated behaviors and provides insights into potential therapeutic targets for new OUD treatments.
Materials and methods
Details regarding all drugs used, sucrose self-administration, fluorescent in situ hybridization (FISH), immunohistochemistry (IHC), and verification of virus knockdown efficiency and cell type-specificity are available in the Supplement.
Animals and housing
Male transgenic Long-Evans rats (Rattus norvegicus) expressing Cre recombinase under the rat Drd1 promoter (LE-Tg(Drd1a-iCre)3Ottc) [29–31] or the rat Drd2 promoter (LE-Tg(Drd2-iCre)1Ottc) [30, 31] were purchased from the Rat Resource and Research Center (RRRC P40OD011062; Columbia, MO). Rats were housed individually with food and water available ad libitum in their home cages. A 12/12 h light/dark cycle was used with the lights on at 1900 h. All experimental procedures were performed during the dark cycle. The experimental protocols were consistent with the guidelines issued by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Single nucleus RNA sequencing (snRNA-seq)
The original snRNA-seq dataset was generated as described previously [32]. Briefly, nuclei from bilateral NAc punches were isolated and snRNA-seq libraries were prepared using the 10x Genomics Chromium Single Cell 3’ GEM Library and Gel Bead Kit v3.1. Libraries were sequenced on an Illumina NovaSeq 6000, aligned to the rat pre-mRNA transcriptome (Rnor 6.0.101) and subsequently underwent quality control to remove nuclei that were uninformative, putative multiplets, or contained >5% of transcripts of mitochondrial origin. The remaining nuclei were clustered and cell types were identified using known marker genes as described previously [32].
Count data for 95,042 nuclei from drug-naïve control NAc samples were subset from the original dataset using Seurat v3.1 and assessed for Calcr expression. 48,265 nuclei that were previously identified as MSNs were further subset for additional analyses. Counts were then normalized to 10,000 per nucleus and scaled. Highly variable genes (n = 708) were selected using the mean.var.plot method with the limits of mean scaled expression set to 0.003 and 2. The first 20 principal components (PCs) derived from these highly variable genes were used to cluster the MSN nuclei at a resolution of 0.04, producing 5 distinct cell populations. One cluster of 153 nuclei expressed putative multiplets of neuronal and glial markers and was removed, resulting in a final total of 48,112 MSN nuclei in 4 cell clusters. Clusters representing D2R-expressing neurons were identified by expression of Drd2, as described previously [32]. Due to limited capture of Drd1 transcript in this dataset, D1R-expressing neurons were identified by expression of Reln, which is highly co-expressed with Drd1 in the striatum across multiple species [33–35].
Surgery
Transgenic rats were handled daily and allowed one week to acclimate to their home cages upon arrival. Rats were then anesthetized using a mixture of 80 mg/kg ketamine (Midwest Veterinary Supply, Valley Forge, PA) and 12 mg/kg xylazine (Sigma Aldrich/RBI, St. Louis, MO). An indwelling catheter (SAI Infusion Technologies, Lake Villa, IL) was inserted into the right jugular vein and sutured in place. The catheter was routed to a mesh backmount platform that was implanted subcutaneously dorsal to the shoulder blades. To prevent infection and maintain patency, catheters were flushed daily with 0.2 ml of the antibiotic Timentin (0.93 mg/ml; Fisher, Pittsburgh, PA) dissolved in heparinized 0.9% saline (Butler Schein, Dublin, OH). When not in use, catheters were sealed with plastic obturators.
We developed a microRNA (miRNA) sequence [5′-TCCAGTTCTTCAGGCTCCTACCAATCTCA-3′] to selectively target CTR transcripts based on our prior studies using viral-mediated delivery of shRNA to knockdown CTR [36, 37]. The miRNA sequence was cloned and packaged into an adeno-associated virus (AAV; serotype 1) downstream of the CB7 promoter. For Cre recombinase-dependent expression, the virus was modified to include a FLEX switch (AAV1.CB7.CI.FLEX.miR-CTRA-EGFP.WPRE.bGH, herein referred to as CTRa KD virus). Splice variants of the CTR yield two subtypes: CTRa and CTRb [38]. We targeted CTRa transcripts because CTRa is expressed at much higher levels than CTRb in the NAc shell [24, 26]. A GFP-expressing AAV1 (AAV1.CAG.FLEX.EGFP.WRPE.bGH) was used as a control virus. Viruses were manufactured and validated in collaboration with the Viral Vector Core at the University of Pennsylvania. Immediately after catheter implantation, rats were mounted in a stereotaxic apparatus (Kopf Instruments, CA). Bilateral stainless-steel guide cannulas (14 mm, 26 gauge; Plastics One, Roanoke, VA) were placed 2 mm dorsal to the medial NAc shell and cemented in place by affixing dental acrylic to stainless steel screws secured in the skull. The coordinates for the ventral ends of the guide cannulas, relative to bregma, according to the atlas of Paxinos and Watson [39], were as follows: +1.0 mm A/P, ±1.0 mm M/L, −5.0 mm D/V. Next, 33-gauge stainless steel microinjectors (16.0 mm, Plastics One) were inserted and control or CTRa KD virus was infused bilaterally into the medial NAc shell (1 µl over 5 min). Microinjectors were left in place for an additional 5 min after infusion to allow for diffusion away from the injection site. Rats were then returned to their home cages for 3 weeks to ensure maximum viral expression [40].
Oxycodone self-administration
Three weeks after surgery, rats were placed in operant conditioning chambers and allowed to lever-press for intravenous infusions of oxycodone (0.06 or 0.15 mg/kg/59 µl saline, infused over 5 s) under a fixed-ratio 1 (FR1) schedule of reinforcement using a between-subjects design [41]. Acquisition of opioid taking was assessed over 10 consecutive days. Self-administration test sessions were 3-h in duration. Each oxycodone infusion was paired with a 20-s contingent light cue illuminated directly above the active lever (i.e., drug-paired lever). A 20-s time-out period followed each oxycodone infusion, during which time active lever responses were tabulated but had no scheduled consequences. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during the self-administration test sessions.
Drug-naïve rats that self-administered sucrose were used to study the effects of CTR knockdown on the motivation to self-administer different unit doses of oxycodone. Initially, rats were allowed to acquire oxycodone taking on an FR1 schedule as described above. After ten consecutive days of self-administering oxycodone (0.15 mg/kg/infusion) on an FR1 schedule, rats were switched to a progressive ratio (PR) schedule of reinforcement. Using a counterbalanced design, rats were allowed to respond for two different unit doses of oxycodone (0.06 and 0.15 mg/kg/infusion) similar to our previously published PR studies [42]. Rats self-administered each unit dose of oxycodone for two consecutive days. The breakpoint was operationally defined as the last response requirement completed before the termination of the PR test session. Total active and inactive lever responses, total infusions, and breakpoints were averaged across test days.
Statistics
Statistical comparisons were performed using GraphPad Prism (La Jolla, CA). For oxycodone self-administration experiments, total daily active and inactive lever responses and total daily oxycodone infusions over the acquisition phase were analyzed with separate repeated measures (RM) two-way analyses of variance (ANOVAs). For sucrose self-administration studies, total active and inactive lever response and total sucrose pellets earned were analyzed with separate RM two-way ANOVAs. For oxycodone PR studies, total active and inactive lever responses, total infusions, and breakpoints were analyzed with separate RM two-way ANOVAs. Pairwise comparisons were made with Bonferroni post hoc tests (p < 0.05). Total number of infusions were analyzed using unpaired t-tests (p < 0.05). Unpaired t-tests were also used to analyze CTRa KD in D1R- and D2R-expressing MSNs in the medial NAc shell. All data are presented as mean ±SEM.
Results
snRNA-seq identifies cell type-specific patterns of CTR expression in the ventral striatum
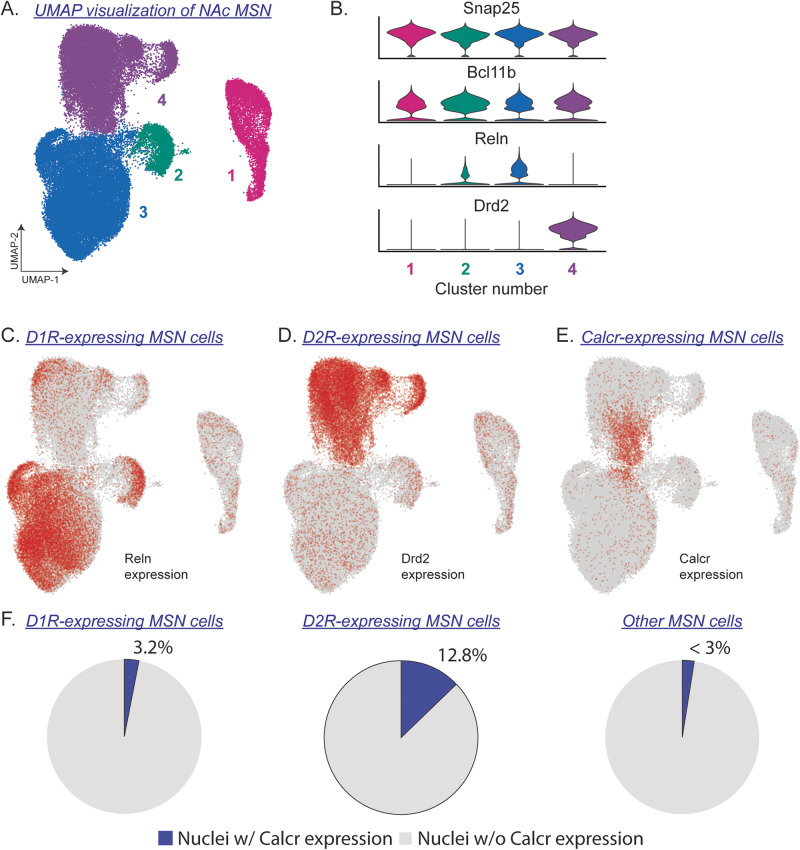
NAc snRNA-seq data from drug-naïve control rats were obtained from our previously published dataset [32]. Expression of Calcr, the gene encoding the CTR protein, was identified in 4.17% of all nuclei and was present in all cell populations (Table 1). However, Calcr expression was most prevalent in neurons. The highest expression of Calcr transcripts was found in a subtype of D2R-expressing MSNs (Table 1; Drd2 MSN2; 59.88% Calcr+).
Table 1.
Calc+ cell types in the nucleus accumbens.
| Cluster name | Marker genes | Total nuclei | Calcr+ nuclei | % Calcr+ |
|---|---|---|---|---|
| Astro1 | Gja1 | 856 | 8 | 0.93% |
| Astro2 | Gja1 | 7524 | 95 | 1.26% |
| Astro3 | Gja1 | 569 | 7 | 1.23% |
| Cholinergic | Slc5a7 | 448 | 5 | 1.12% |
| Drd1 MSN1 | Bcl11b, Ebf1, Nr4a1 | 472 | 35 | 7.42% |
| Drd1 MSN2 | Bcl11b, Ebf1 | 18636 | 463 | 2.48% |
| Drd1 MSN3 | Bcl11b, Ebf1 | 3217 | 167 | 5.19% |
| Drd2 MSN1 | Bcl11b, Drd2, Ntng1 | 1274 | 24 | 1.88% |
| Drd2 MSN2 | Bcl11b, Drd2 | 2575 | 1542 | 59.88% |
| Drd2 MSN3 | Bcl11b, Drd2 | 11422 | 577 | 5.05% |
| Drd2 MSN4 | Bcl11b, Drd2 | 1707 | 82 | 4.80% |
| Endo | Cldn5 | 1157 | 25 | 2.16% |
| Grm8 MSN | Grm8 | 1654 | 47 | 2.84% |
| Interneuron1 | Kit, Kcnc2 | 895 | 21 | 2.35% |
| Interneuron2 | Kcnc2 | 2410 | 145 | 6.02% |
| Interneuron3 | Kcnc2 | 187 | 6 | 3.21% |
| Micro | Arhgap15 | 1769 | 26 | 1.47% |
| MSN1 | Bcl11b,Ntng1 | 486 | 33 | 6.79% |
| MSN2 | Bcl11b, Grm8, Ntng1 | 3465 | 69 | 1.99% |
| MSN3 | Bcl11b, Grm8 | 3357 | 97 | 2.89% |
| Oligo1 | Mag | 857 | 24 | 2.80% |
| Oligo2 | Mag | 2821 | 44 | 1.56% |
| Oligo3 | Mag | 16556 | 244 | 1.47% |
| Oligo4 | Mag | 4983 | 64 | 1.28% |
| OPC | Pdgfra | 2858 | 42 | 1.47% |
| PVALB Inter | Kit, Kcnc2 | 1705 | 40 | 2.35% |
| SST Inter | Sst, Kcnc2 | 1182 | 29 | 2.45% |
Astro astrocyte, Oligo oligodendrocyte, OPC oligodendrocyte precursor cell, Micro microglia, MSN medium spiny neuron, Endo endothelial cell, PVALB Inter parvalbumlin positive neuron, SST Inter somatostatin positive neuron, Grm8 glutamate metabortropic receptor 8 positive neuron.
To compare Calcr expression in D1R- and D2R-expressing MSNs, our previously published data [32] were subset to neurons (Snap25-expressing cells) and only MSNs (Bcl11b-expressing cells) [34, 43]. Re-clustering MSN nuclei generated four major cell populations (Fig. 1A), including individual clusters representing D1R (Reln-expressing cells; clusters 2 and 3) and D2R (Drd2-expressing cells; cluster 4) -expressing MSNs (Fig. 1B) and one additional MSN clusters that did not express Reln or Drd2 (Fig. 1B; cluster 1). Calcr transcripts were detected in 3.2% of D1R-expressing MSNs, 12.8% of D2R-expressing MSNs, and <3% of all other MSN cell types (Fig. 1C–F).
Fig. 1. Expression of Calcr in D1R- and D2R-expressing striatal neurons.
A A uniform manifold approximation and projection (UMAP) identifying four subtypes of medium spiny neurons (MSNs) in the NAc of drug-naïve rats. B MSNs were identified based on expression of Snap25 and Bcl11b. D1R-expressing and D2R-expressing MSNs were identified based on expression of Reln and Drd2, respectively. Nuclei highlighted in red expressed at least one transcript for (C) Reln, (D) Drd2, or (E) Calcr. F The percentage of neurons containing Calcr transcripts was ~4-fold higher in D2R-expressing MSNs compared to D1R-expressing MSNs (12.8% of D2R-expressing MSNs vs 3.2% of D1R-expressing MSNs).
Differential expression of CTRs in medial NAc shell D1R- and D2R-expressing cells
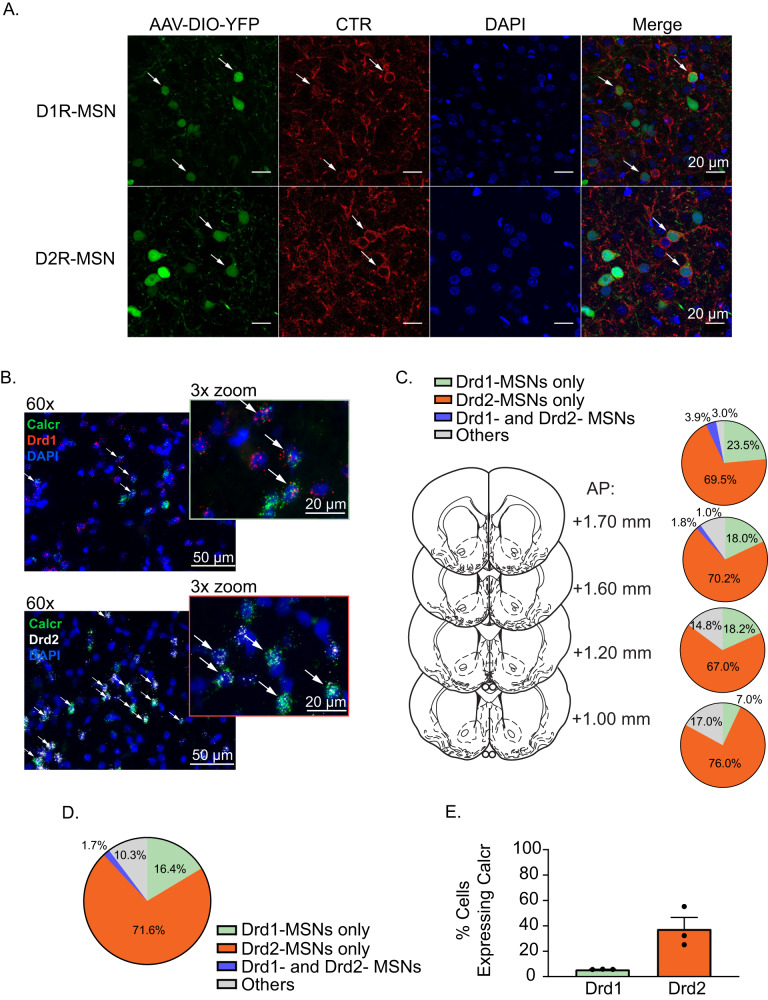
To validate our snRNA-seq findings, D1R- and D2R-expressing MSNs were labeled by infusing AAV-DIO-YFP bilaterally into the medial NAc shell of transgenic rats expressing Cre recombinase under the Drd1 or Drd2 promoter (Fig. 2A). IHC revealed CTR protein expression (red fluorescence) colocalized with YFP expression (green fluorescence). These results indicate that CTRs are expressed on both D1R- and D2R-expressing MSNs in the medial NAc shell. FISH was then used to quantify the percentage of D1R- and D2R-expressing MSNs that co-express CTR using probes against Drd1, Drd2, and Calcr, respectively. Figure 2B shows that Calcr transcripts (green fluorescence) are colocalized with Drd1 (red fluorescence; top panel) and Drd2 (white fluorescence; bottom panel) transcripts, further confirming our snRNA-seq and IHC results. We found that Calcr expression is consistent throughout the rostral/caudal gradient of the medial NAc shell (Fig. 2C). Importantly, we discovered that 16.4 ± 4.7% of Calcr-expressing cells in the medial NAc shell co-expressed Drd1 transcripts, and 71.6 ± 5.6% of Calcr-expressing cells in the medial NAc shell co-expressed Drd2 transcripts. Furthermore, 1.7 ± 1.2% of Calcr-expressing cells co-expressed both Drd1 and Drd2 transcripts, and 10.3 ± 2.1% of Calcr-expressing cells did not express Drd1 or Drd2 transcripts (Fig. 2D). Consistent with our snRNA-seq data, we found greater expression of CTRs in D2R- versus D1R-expressing MSNs. Specifically, 5.7 ± 0.05% of Drd1-expressing cells co-expressed Calcr transcripts and 37.5 ± 9.1% of Drd2-expressing cells co-expressed Calcr transcripts in the medial NAc shell (Fig. 2E). Taken together, these studies identified cell type-specific patterns of CTR expression in the ventral striatum and revealed that CTRs are expressed at higher levels in D2R- vs D1R-expressing MSNs in the medial NAc shell.
Fig. 2. Differential expression of CTRs in D1R- and D2R-expressing MSNs in the medial NAc shell.
A Representative images showing CTRs (red fluorescence) expressed in D1R- and D2R-expressing cells (green fluorescence) in the medial NAc shell. White arrows indicate cells co-labeled with YFP (green fluorescence) and CTR-A/B (red fluorescence). B Calcr, Drd1, and Drd2 mRNA transcripts were labeled using fluorescent in situ hybridization (FISH). DAPI-labeled cell nuclei are indicated by blue fluorescence. White arrows indicate cells co-labeled with Drd1 (red fluorescence) and Calcr (green fluorescence) in the top panel and cells co-labeled with Drd2 (white fluorescence) and Calcr (green fluorescence) in the bottom panel. C Calcr was expressed in Drd1- and Drd2-expressing cells throughout the anterior and posterior regions of the medial NAc shell (n = 3/group). D 16.4 ± 4.7% of Calcr-positive cells in the medial NAc shell express Drd1 and 71.6 ± 5.6% of Calcr-positive cells express Drd2 (n = 3/group). E Percent of Drd1- and Drd2-expressing cells that express Calcr (n = 3/group).
Chronic knockdown of CTRa in D1R-expressing MSNs potentiates/augments oxycodone taking
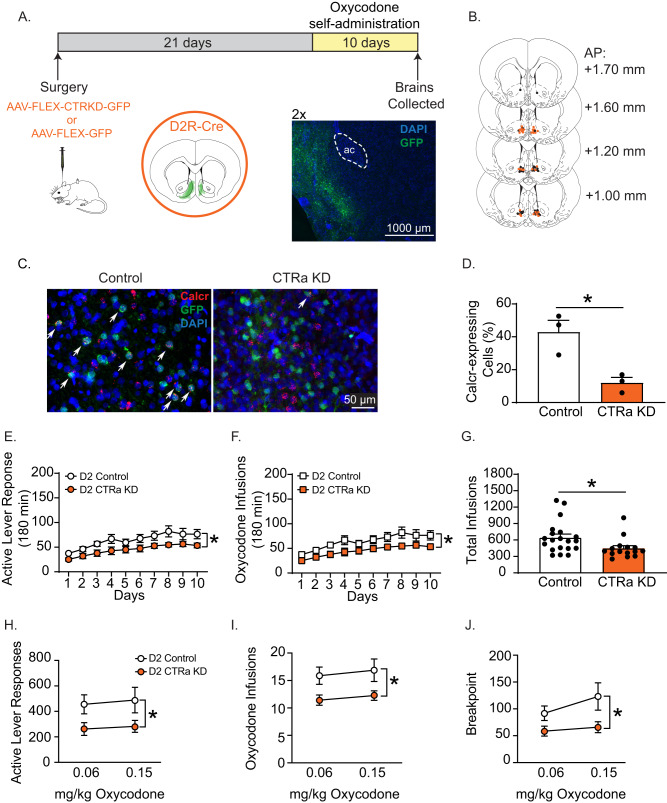
To assess the cell type-specific role of CTRs expressed on D1R-expressing MSNs on voluntary opioid taking, transgenic rats expressing Cre-recombinase under the Drd1 promoter were infused with Control or Cre-dependent CTRa KD virus into the medial NAc shell (Fig. 3A). Infusion sites are shown in Fig. 3B. The knockdown efficiency of the CTRa KD virus in D1R-expressing MSNs was evaluated using a combination of FISH and IHC which showed that the number of cells expressing CTR transcripts was significantly lower in D1 CTRa KD rats compared to D1 Control rats (Fig. 3C). Specifically, Calcr transcript expression was reduced ~51% in rats infused with the CTRa KD virus versus rats that were infused with the Control virus [t(5) = 7.80, p < 0.001] (Fig. 3D). The percentage of D1R-expressing cells that co-expressed Calcr in control rats (~6.7%, Fig. 3D) was consistent with findings from both the snRNA-seq (3.4%, Fig. 1F) and FISH (~5.7%, Fig. 2E) studies.
Fig. 3. Reduced CTRa expression in D1R-expressing MSNs increases the acquisition of oxycodone taking.
A Transgenic rats expressing Cre-recombinase under the Drd1 promoter were infused with control or CTRa KD virus into the medial NAc shell. Acquisition of oxycodone self-administration (0.06 mg/kg/infusion) was assessed three weeks later. Green shading in the coronal section indicates viral spread within the NAc shell. Viral spread was confined to the medial NAc shell as shown in the representative fluorescent micrograph. B Infusions sites within the medial NAc shell of rats that received control (black dots) or CTRa KD (green dots) virus. C Calcr expression (red fluorescence) is decreased in D1R-expressing MSNs (green fluorescence) of CTRa KD rats compared to control rats. Cell nuclei were stained with DAPI (blue fluorescence). White arrows indicate cells co-labeled with eGFP (green fluorescence) and Calcr (red fluorescence). D Calcr expression in D1R-expressing MSNs of CTRa KD rats (n = 3) is 51% lower than that in control rats (n = 4). Over the 10-day acquisition phase, daily active lever responses (E) and oxycodone infusions (F) as well as cumulative number of oxycodone infusions (G) in CTRa KD rats (n = 8) were significantly higher compared to control rats (n = 10). The effects of CTR knockdown on the oxycodone dose-response curve were evaluated in separate cohorts of rats self-administering two different unit doses of oxycodone on a PR schedule. Total number of active lever responses (H), total oxycodone infusions (I), and breakpoints (J) were significantly increased for both unit doses of oxycodone in CTRa KD rats (n = 12) compared to control rats (n = 7). Data are mean ± SEM. (t-test, *p < 0.05; Bonferroni, #p < 0.05; 2-way ANOVA, significant main effect of treatment, *p < 0.05).
The effects of selective CTRa KD in D1R-expressing neurons on the acquisition of oxycodone taking were assessed three weeks after viral infusions into the medial NAc shell (control: n = 10, CTRa KD: n = 8, Fig. 3A). Total daily active lever responses were analyzed with a RM two-way ANOVA, which revealed a significant treatment x day interaction [F(9,144) = 2.56, p < 0.01] (Fig. 3E). Subsequent post hoc tests revealed significant increases in active lever responses in D1 CTRa KD rats compared to D1 Control rats on self-administration days 3, 4, 6, 8, and 9 (Bonferroni, p < 0.05). No significant differences were found on inactive lever responding between treatments (data not shown). Total daily oxycodone infusions were analyzed with a RM two-way ANOVA, which revealed a significant treatment x day interaction [F(9,144) = 2.03, p < 0.05] (Fig. 3F). Subsequent post hoc tests revealed significant increases in total oxycodone infusions in D1 CTRa KD rats versus D1 Control rats on self-administration days 4, 6, and 8 (Bonferroni, p < 0.05). Consistent with these results, total oxycodone infusions during the acquisition phase were significantly increased in D1 CTRa KD rats compared to D1 Control rats [t(16) = 3.90, p < 0.05] (Fig. 3G). Similar differences were found in rats self-administering a higher dose of oxycodone (see Suppl Results & Suppl Fig. 1). To generate an oxycodone dose-response curve, separate cohorts of D1 CTRa KD (n = 12) and D1 Control (n = 7) rats were allowed to self-administer two different unit doses of oxycodone (0.06 and 0.15 mg/kg/infusion) on a PR schedule of reinforcement. Total active lever responses (Fig. 3H), total number of oxycodone infusions (Fig. 3I), and breakpoints (Fig. 3J) were analyzed with separate RM two-way ANOVAs, which revealed significant main effects of treatment for all three variables (active lever responses, [F(1,10) = 9.87, p < 0.05]; oxycodone infusions, [F(1,10) = 9.87, p < 0.05]; breakpoints, [F(1,10) = 9.87, p < 0.05]). No significant differences were found on inactive lever responding between treatments (data not shown). The effects of CTRa KD in D1R-expressing neurons were reinforcer-specific as there were no effects of this cell type-specific manipulation on the acquisition of sucrose self-administration in drug-naïve rats (see Supplementary Results & Supplementary Fig. 2). Further validation of CTR protein knockdown in D1R-expressing cells is shown in Supplementary Fig. 3. These results indicate that decreased CTR signaling in medial NAc shell D1R-expressing neurons potentiates/augments the acquisition of oxycodone taking and increases the motivation to self-administer oxycodone.
Chronic knockdown of CTRa in D2R-expressing MSNs attenuates oxycodone taking
To assess the cell type-specific role of CTRs expressed on D2R-expressing neurons on voluntary opioid taking, transgenic rats expressing Cre-recombinase under the Drd2 promoter were infused with control or Cre-dependent CTRa KD virus into the medial NAc shell (Fig. 4A). Infusion sites are shown in Fig. 4B. The knockdown efficiency of the CTRa KD virus in D2R-expressing MSNs was evaluated using a combination of FISH and IHC which showed that the number of cells expressing CTR transcripts was lower in D2 CTRa KD rats compared to D2 Control rats (Fig. 4C). Specifically, CTR transcript expression was reduced ~73% in rats infused with the CTRa KD virus versus rats infused with the control virus [t(4) = 3.93, p < 0.05] (Fig. 4D). The percentage of D2R-expressing cells that co-express Calcr in control rats (~42.9%, Fig. 4D) was consistent with findings from both the snRNA-seq (12.8%, Fig. 1F) and FISH (~37.5%, Fig. 2E) studies. The lower absolute percentage of Calcr-positive D1R- and D2R-expressing MSNs in the single nuclei studies compared to the FISH studies is likely due to the zero-inflated nature of snRNA-seq data [44].
Fig. 4. Reduced CTRa expression in D2R-expressing MSNs decreases the acquisition of oxycodone taking.
A Transgenic rats expressing Cre-recombinase under the Drd2 promoter were infused with control or CTRa KD virus into the medial NAc shell. Acquisition of oxycodone self-administration (0.06 mg/kg/infusion) was assessed three weeks later. Green shading in the coronal section indicates viral spread within the NAc shell. Viral spread was confined to the medial NAc shell as shown in the representative fluorescent micrograph. B Infusions sites within the medial NAc shell of rats that received control (black dots) or CTRa KD (orange dots) virus. C Calcr expression (red fluorescence) is decreased in D2R-expressing MSNs (green fluorescence) of CTRa KD rats compared to control rats. Cell nuclei were stained with DAPI (blue fluorescence). White arrows indicate cells co-labeled with eGFP (green fluorescence) and Calcr (red fluorescence). D Calcr expression in D2R-expressing MSNs of CTRa KD rats (n = 3) is 72% lower than that in control rats (n = 3). Over the 10-day acquisition phase, daily active lever responses (E) and oxycodone infusions (F) as well as cumulative number of oxycodone infusions (G) in CTRa KD rats (n = 15) were significantly lower compared to control rats (n = 20). The effects of CTR knockdown on the oxycodone dose-response curve were evaluated in separate cohorts of rats self-administering two different unit doses of oxycodone on a PR schedule. Total number of active lever responses (H), total oxycodone infusions (I), and breakpoints (J) were significantly decreased for both unit doses of oxycodone in CTRa KD rats (n = 6) compared to control rats (n = 4). Data are mean ± SEM. (t-test, *p < 0.05; 2-way ANOVA, significant main effect of treatment, *p < 0.05).
The effects of selective CTRa KD in D2R-expressing neurons on the acquisition of oxycodone taking were assessed three weeks after viral infusions into the medial NAc shell (control: n = 20, CTRa KD: n = 15, Fig. 4A). Total daily active lever responses were analyzed with a RM two-way ANOVA, which revealed significant main effects of treatment [F(1,33) = 5.25, p < 0.05] and day [F(9,297) = 12.20, p < 0.01], but no significant treatment x day interaction, so no post-hoc analyses were performed (Fig. 4E). No significant differences were found on inactive lever responding between treatments (data not shown). Total daily oxycodone infusions were analyzed with a RM two-way ANOVA, which revealed significant main effects of treatment [F(1,33) = 5.15, p < 0.05] and day [F(9,297) = 12.37, p < 0.0001] but no significant treatment x day interaction, so no post-hoc analyses were performed (Fig. 4F). Consistent with these results, total oxycodone infusions during the acquisition phase were significantly decreased in D2 CTRa KD rats compared to D2 Control rats [t(33) = 2.29, p < 0.05] (Fig. 4G). Similar differences were found in rats self-administering a higher dose of oxycodone (see Suppl Results & Supplementary Fig. 1). To generate an oxycodone dose-response curve, separate cohorts of D2 CTRa KD (n = 6) and D2 Control (n = 4) rats were allowed to self-administer two different unit doses of oxycodone (0.06 and 0.15 mg/kg/infusion) on a PR schedule of reinforcement. Total active lever responses (Fig. 4H), total number of oxycodone infusions (Fig. 4I), and breakpoints (Fig. 4J) were analyzed with separate RM two-way ANOVAs, which revealed significant main effects of treatment for all three variables (active lever responses, [F(1,10) = 9.87, p < 0.05]; oxycodone infusions, [F(1,10) = 9.87, p < 0.05]; breakpoints, [F(1,10) = 9.87, p < 0.05]). No significant differences were found on inactive lever responding between treatments (data not shown). The effects of CTRa KD in D2R-expressing neurons were reinforcer-specific as there were no effects of this cell type-specific manipulation on the acquisition of sucrose self-administration in drug-naïve rats (see Supplementary Results & Supplementary Fig. 2). Further validation of CTR protein knockdown in D2R-expressing cells is shown in Supplementary Fig. 3. These results indicate that decreased CTR signaling in medial NAc shell D2R-expressing neurons attenuates acquisition of oxycodone taking and reduces the motivation to self-administer oxycodone.
Discussion
Using snRNA-seq and IHC, we discovered that CTRs are expressed on both D1R- and D2R-expressing MSNs in the medial NAc shell. We confirmed these findings with FISH and showed that Calcr expression is higher in Drd2- versus Drd1-expressing MSNs. To determine the functional roles of CTRs expressed on these two MSN cell types in opioid taking, we used viral-mediated gene delivery techniques in transgenic rats to reduce CTR expression specifically in D1R- and D2R-expressing MSNs in the medial NAc shell. We found that selectively reducing CTR expression in D1R-expressing MSNs potentiated oxycodone taking and increased the motivation to self-administer oxycodone. In contrast, selectively reducing CTR expression in D2R-expressing MSNs attenuated oxycodone taking and reduced the motivation to self-administer oxycodone. Taken together, these results indicate that endogenous CTR signaling in the ventral striatum produces bidirectional effects on opioid taking that are cell type-specific. Specifically, activation of CTRs on D1R-expressing MSNs functions to limit or reduce opioid taking, whereas activation of CTRs on D2R-expressing MSNs facilitates opioid taking.
Differential expression of CTRs in D1R- and D2R-expressing MSNs
Previous studies identified dense expression of CTRs in the ventral striatum with higher expression of CTRs in the NAc shell compared to the NAc core [25–28]. Our detailed mapping of CTR expression in the medial NAc shell extends these findings and reveals cell type-specific patterns of CTR expression. Using snRNA-seq, we discovered Calcr expression in multiple clusters of D1R- and D2R-expressing cells. Expression of Calcr was ~4 times greater in D2R-expressing MSNs compared to D1R-expressing MSNs. These results were confirmed by our FISH studies, which found that 71.6% of Calcr-positive cells in the medial NAc shell co-express Drd2 and 16.4% of Calcr-positive cells in the medial NAc shell co-express Drd1. The snRNA-seq results further indicate that this differential expression is driven primarily by high levels of Calcr transcript in a single distinct subpopulation of D2R-expressing MSNs (Table 1). This subpopulation warrants further study, as it may play a central role in the effects of CTR activation on voluntary drug-taking and -seeking behaviors. Interestingly, 10.3% of Calcr-positive cells did not express Drd1 or Drd2. Our snRNA-seq results also revealed that, in addition to D1R- and D2R-expressing MSNs, other cell types, such as interneurons, astrocytes, oligodendrocytes, and microglia, express Calcr transcripts. The prevalence of Calcr-positive nuclei was low (<3%) in all glial cell populations. In contrast, expression was more common (>5%) in specific clusters of D1R-expressing MSNs, D2R-expressing MSNs, and interneurons, suggesting that these neuronal subtypes may be more relevant to the behavioral responses produced by CTR activation in the ventral striatum. Future studies are required to understand the functional significance of CTR activation on other types of CTR-expressing cell populations in drug-mediated behaviors. Taken together, these neuroanatomical and sequencing studies characterize Calcr-expressing cells in the NAc and expand our understanding of the central mechanisms mediating the effects of CTRs on motivated behaviors.
Endogenous cell type-specific CTR signaling in the NAc exerts bidirectional effects on opioid self-administration
An emerging body of evidence indicates that activating CTRs attenuates drug-mediated behaviors. Systemic administration of sCT reduced oral ethanol intake in male rats and ethanol-induced CPP and dopamine release in the NAc shell of male mice [11, 12]. Systemic sCT also decreased the locomotor-activating effects of amphetamine [45, 46], cocaine [14], nicotine [15], and ethanol [11], effects associated with decreased drug-evoked dopamine release in the NAc shell [11, 14, 15]. These behavioral responses are likely due, in part, to activation of central CTRs as infusions of sCT directly into the NAc shell or VTA reduced the locomotor-activating effects of ethanol in mice [13]. In addition, sCT infused directly into the VTA or LDTg reduced ethanol-evoked dopamine release in the NAc shell of mice and ethanol intake in rats [13]. These findings suggest that CTR agonists attenuate drug-mediated behaviors via activation of central CTRs and subsequent modulation of the mesolimbic dopamine system.
The present study builds upon and expands these findings by examining the functional significance of endogenous CTR signaling in the NAc on opioid taking. We showed that selectively reducing CTR expression in D1R- and D2R-expressing cells potentiated and attenuated, respectively, the acquisition of oxycodone taking. The effects of the cell type-specific reductions in CTR expression on the acquisition of oxycodone taking were consistent across two unit doses of oxycodone and schedules of reinforcement. Our PR studies showed that reduced CTR expression in D1R- and D2R-expressing cells increased and decreased, respectively, motivation to self-administer oxycodone. Interestingly, these effects were reinforcer specific and not due to operant learning deficits as selectively reducing CTR expression in these two striatal cell populations did not alter the acquisition of sucrose self-administration. These findings highlight a cell type-specific mechanism by which endogenous CTR signaling in the NAc bidirectionally modulates voluntary opioid taking. The mechanisms underlying these behavioral responses, however, are not clear and likely complex. The effects of CTR knockdown in D2R-expressing cells may be due to reduced expression of CTRs in both D2R-expressing MSNs and D2R-expressing cholinergic interneurons. High levels of Calcr transcript were found in a single distinct cluster of D2R-expressing MSNs. Since reduced CTR expression in D2R-expressing cells attenuated opioid taking, these findings highlight a potential cluster of D2R-expressing MSNs that may function to promote drug taking. Consistent with this hypothesis, recent studies indicate that different clusters of D2R-expressing MSNs play distinct roles in regulating motivated behaviors [47–49]. In addition to MSNs, more than 80% of cholinergic interneurons in the striatum express D2Rs [50–53]. Despite comprising only 2% of the total cell population in the NAc [54], cholinergic interneurons robustly modulate striatal dopamine release and drug-mediated behaviors [55–60]. Therefore, the effects of CTR knockdown in D2R:Cre rats are likely due to a combination of reduced endogenous CTR signaling on D2R-expressing MSNs and D2R-expressing cholinergic interneurons. Although CTRs are expressed mainly on D2R-expressing cells, reduced CTR expression in D1R-expressing cells produced a more robust effect on oxycodone taking compared to reduced CTR expression in D2R-expressing cells. It is provocative to think that endogenous CTR signaling in a specific cluster of D1R-expressing cells functions to inhibit opioid taking [61–63]. Systematic evaluation of the role of CTR signaling in specific clusters of D1R-expressing MSNs, D2R-expressing MSNs, and interneurons in opioid taking will be important for determining how endogenous CTR signaling regulates NAc output and behavior. Strategies aimed at selectively increasing or reducing CTR signaling on D1R- and D2R-expressing cells, respectively, could correct the imbalance in NAc activity associated with opioid taking [22, 23] and decrease the reinforcing effects of opioids [23, 64].
The role of CTRs in drug-mediated behaviors
Elucidating the precise mechanisms underlying the effects of CTR activation on drug-mediated behaviors has been difficult because sCT binds to and activates both CTRs and amylin receptors [65, 66]. Amylin receptors are protein complexes consisting of a CTR coupled to a receptor activity-modifying protein (RAMP) [66–68]. In addition to high CTR expression, the NAc contains high levels of high-affinity amylin binding sites [69, 70], further supporting the possibility that activation of amylin receptors contributes to the actions of sCT. Therefore, it is not possible to determine if the behavioral responses to sCT in the aforementioned studies are mediated via CTRs alone, amylin receptors alone, or both. Given that a core component of amylin receptors is CTR, decreased CTRa expression in the NAc shell likely reduced expression of both CTRs and amylin receptors in the present study. Thus, it is difficult to draw firm conclusions as to whether opioid taking is altered via disruption of calcitonin signaling, amylin signaling, or both. Adding to the complexity, CTRs are activated by several endogenous ligands including calcitonin, and calcitonin gene-related peptide (CGRP) [65]. Therefore, reduced CTRa expression likely disrupts the signaling of multiple neuromodulators. Disentangling the relative contributions of CTRs and amylin receptors as well as their endogenous ligands in reducing opioid-mediated behaviors will be critical for designing novel therapeutic approaches to treating OUD.
Conclusion
The present study expands our understanding of the role of central CTR signaling in drug-mediated behaviors and highlights novel CTR-expressing striatal cell populations that could be targeted to reduce voluntary opioid taking. Differential expression of CTRs on D1R- and D2R-expressing MSNs suggests that CTR agonists may shift the balance of NAc activity and alter NAc output to affect motivated behaviors. Future studies investigating cell type-specific changes following CTR activation are required to understand how exactly CTR activation alters striatal cell function and opioid-mediated behaviors. One limitation of the current study is that only male rats were studied. Since previous studies have shown sex differences in behavioral responses to CTR/amylin receptor agonists [71] and opioids [72], it will be important to extend these findings to female rats. It will also be important to determine how activation of cell type-specific CTR-expressing striatal circuits affects withdrawal phenotypes including drug seeking during abstinence.
Supplementary information
Acknowledgements
The authors would like to thank Antonia Caffrey, Suditi Rahematpura and Kael Ragnini for their technical contributions to this project.
Author contributions
YZ, JB, and RM contributed to the acquisition and analyses of the data as well as drafted the manuscript. AM and MWK also contributed to data collection. BCR and RCC collected and analyzed snRNA-seq data and helped draft the manuscript. MRH contributed to control and CTRa KD virus design. HDS was responsible for the study concept and design, supervised the acquisition of the data, and helped draft the manuscript. All authors reviewed content and approved the final version for publication.
Funding
This work was supported by the following grants from the National Institutes of Health: R01 DA037897 (HDS), R01 DK105155 (HDS and MRK), R21 DA045792 (HDS) and R01 DA044015 (RCC). HDS and JB were partially supported by the Velay Women’s Science Research Fellowship and a Benjamin Franklin Scholars Summer Research Grant from the Center for Undergraduate Research & Fellowships (CURF) at the University of Pennsylvania. HDS and AM were partially supported by a Pincus-Magaziner Family Undergraduate Research Award, a Mary L. and Matthew S. Santirocco Undergraduate Research Award, and an Ernest M. Brown, Jr. College Alumni Society Undergraduate Research Grant from CURF at the University of Pennsylvania. HDS and RM were partially supported by a Pincus-Magaziner Family Undergraduate Research Award from CURF at the University of Pennsylvania. HDS and MWK were partially supported by a Louis H Castor, M.D., C’48 Undergraduate Research Grant from CURF at the University of Pennsylvania. RCC was partially supported by a State of Pennsylvania Department of Health Nonformula Tobacco Settlement Act Grant, Pharmacogenetics of Opioid Use Disorder.
Data availability
Single-nuclei RNA-sequencing data are available at the NCBI Gene Expression Omnibus (GEO) under accession number GSE171165.
Competing interests
The authors declare no competing interests. MRH and BCR receive research funds from Boehringer Ingelheim and Novo Nordisk that were not used to support these studies.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01634-z.
References
- 1.Volkow ND, Blanco C. The changing opioid crisis: development, challenges and opportunities. Mol Psychiatry. 2021;26:218–33. doi: 10.1038/s41380-020-0661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54:901–6. doi: 10.1097/MLR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SAMHSA. 2021. https://www.samhsa.gov/data/report/2020-nsduh-annual-national-report.
- 4.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014:CD002207. [DOI] [PubMed]
- 5.Humphreys K, Shover CL, Andrews CM, Bohnert ASB, Brandeau ML, Caulkins JP, et al. Responding to the opioid crisis in North America and beyond: recommendations of the Stanford-Lancet Commission. Lancet. 2022;399:555–604. doi: 10.1016/S0140-6736(21)02252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow ND, Jones EB, Einstein EB, Wargo EM. Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry. 2019;76:208–16. [DOI] [PubMed]
- 7.Jerlhag E. Gut-brain axis and addictive disorders: a review with focus on alcohol and drugs of abuse. Pharmacol Ther. 2019;196:1–14. doi: 10.1016/j.pharmthera.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Merkel R, Moreno A, Zhang Y, Herman R, Ben Nathan J, Zeb S, et al. A novel approach to treating opioid use disorders: dual agonists of glucagon-like peptide-1 receptors and neuropeptide Y(2) receptors. Neurosci Biobehav Rev. 2021;131:1169–79. doi: 10.1016/j.neubiorev.2021.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez NS, Schmidt HD. Central GLP-1 receptors: novel molecular targets for cocaine use disorder. Physiol Behav. 2019;206:93–105. doi: 10.1016/j.physbeh.2019.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farokhnia M, Faulkner ML, Piacentino D, Lee MR, Leggio L. Ghrelin: From a gut hormone to a potential therapeutic target for alcohol use disorder. Physiol Behav. 2019;204:49–57. doi: 10.1016/j.physbeh.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Kalafateli AL, Vallöf D, Jerlhag E. Activation of amylin receptors attenuates alcohol-mediated behaviours in rodents. Addict Biol. 2019;24:388–402. doi: 10.1111/adb.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalafateli AL, Vallöf D, Colombo G, Lorrai I, Maccioni P, Jerlhag E. An amylin analogue attenuates alcohol-related behaviours in various animal models of alcohol use disorder. Neuropsychopharmacology. 2019;44:1093–102. doi: 10.1038/s41386-019-0323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalafateli AL, Satir TM, Vallöf D, Zetterberg H, Jerlhag E. An amylin and calcitonin receptor agonist modulates alcohol behaviors by acting on reward-related areas in the brain. Prog Neurobiol. 2021;200:101969. doi: 10.1016/j.pneurobio.2020.101969. [DOI] [PubMed] [Google Scholar]
- 14.Kalafateli AL, Aranäs C, Jerlhag E. Activation of the amylin pathway modulates cocaine-induced activation of the mesolimbic dopamine system in male mice. Horm Behav. 2021;127:104885. doi: 10.1016/j.yhbeh.2020.104885. [DOI] [PubMed] [Google Scholar]
- 15.Aranäs C, Vestlund J, Witley S, Edvardsson CE, Kalafateli AL, Jerlhag E. Salmon calcitonin attenuates some behavioural responses to nicotine in male mice. Front Pharmacol. 2021;12:685631. doi: 10.3389/fphar.2021.685631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y. Relapse to opioid seeking in rat models: behavior, pharmacology and circuits. Neuropsychopharmacology. 2019;44:465–77. doi: 10.1038/s41386-018-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graziane NM, Sun S, Wright WJ, Jang D, Liu Z, Huang YH, et al. Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci. 2016;19:915–25. doi: 10.1038/nn.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, et al. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci USA. 2016;113:757–62. doi: 10.1073/pnas.1519248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell SE, Puttick DJ, Sawyer AM, Potter DN, Mague S, Carlezon WA, Jr, et al. Nucleus accumbens AMPA receptors are necessary for morphine-withdrawal-induced negative-affective states in rats. J Neurosci. 2016;36:5748–62. doi: 10.1523/JNEUROSCI.2875-12.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y, Wienecke CF, Nachtrab G, Chen X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530:219–22. doi: 10.1038/nature16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Neal TJ, Bernstein MX, MacDougall DJ, Ferguson SM. A conditioned place preference for heroin is signaled by increased dopamine and direct pathway activity and decreased indirect pathway activity in the nucleus accumbens. J Neurosci. 2022;42:2011–24. doi: 10.1523/JNEUROSCI.1451-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Neal TJ, Nooney MN, Thien K, Ferguson SM. Chemogenetic modulation of accumbens direct or indirect pathways bidirectionally alters reinstatement of heroin-seeking in high- but not low-risk rats. Neuropsychopharmacology. 2020;45:1251–62. doi: 10.1038/s41386-019-0571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mietlicki-Baase EG, Rupprecht LE, Olivos DR, Zimmer DJ, Alter MD, Pierce RC, et al. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology. 2013;38:1685–97. doi: 10.1038/npp.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baisley SK, Bremer QZ, Bakshi VP, Baldo BA. Antipsychotic-like actions of the satiety peptide, amylin, in ventral striatal regions marked by overlapping calcitonin receptor and RAMP-1 gene expression. J Neurosci. 2014;34:4318–25. doi: 10.1523/JNEUROSCI.2260-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becskei C, Riediger T, Zünd D, Wookey P, Lutz TA. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res. 2004;1030:221–33. doi: 10.1016/j.brainres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Hendrikse ER, Rees TA, Tasma Z, Le Foll C, Lutz TA, Siow A, et al. Calcitonin receptor antibody validation and expression in the rodent brain. Cephalalgia. 2022;42:815–26. doi: 10.1177/03331024221084029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamoto H, Soeda Y, Takami S, Minami M, Satoh M. Localization of calcitonin receptor mRNA in the mouse brain: coexistence with serotonin transporter mRNA. Brain Res Mol Brain Res. 2000;76:93–102. doi: 10.1016/s0169-328x(99)00335-6. [DOI] [PubMed] [Google Scholar]
- 29.Job MO, Chojnacki MR, Daiwile AP, Cadet JL. Chemogenetic inhibition of dopamine D1-expressing neurons in the dorsal striatum does not alter methamphetamine intake in either male or female long evans rats. Neurosci Lett. 2020;729:134987. doi: 10.1016/j.neulet.2020.134987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strong CE, Hagarty DP, Brea Guerrero A, Schoepfer KJ, Cajuste SM, Kabbaj M. Chemogenetic selective manipulation of nucleus accumbens medium spiny neurons bidirectionally controls alcohol intake in male and female rats. Sci Rep. 2020;10:19178. doi: 10.1038/s41598-020-76183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Keller C, Scofield MD, Neuhofer D, Varanasi S, Reeves MT, Hughes B, et al. Relapse-associated transient synaptic potentiation requires integrin-mediated activation of focal adhesion kinase and cofilin in D1-expressing neurons. J Neurosci. 2020;40:8463–77. doi: 10.1523/JNEUROSCI.2666-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiner BC, Zhang Y, Stein LM, Perea ED, Arauco-Shapiro G, Ben Nathan J, et al. Single nucleus transcriptomic analysis of rat nucleus accumbens reveals cell type-specific patterns of gene expression associated with volitional morphine intake. Transl Psychiatry. 2022;12:374. doi: 10.1038/s41398-022-02135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savell KE, Tuscher JJ, Zipperly ME, Duke CG, Phillips RA, 3rd, Bauman AJ, et al. A dopamine-induced gene expression signature regulates neuronal function and cocaine response. Sci Adv. 2020;6:eaba4221. doi: 10.1126/sciadv.aba4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He J, Kleyman M, Chen J, Alikaya A, Rothenhoefer KM, Ozturk BE, et al. Transcriptional and anatomical diversity of medium spiny neurons in the primate striatum. Curr Biol. 2021;31:5473–86.e6. doi: 10.1016/j.cub.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Guglielmo G, Iemolo A, Nur A, Turner A, Montilla-Perez P, Martinez A, et al. Reelin deficiency exacerbates cocaine-induced hyperlocomotion by enhancing neuronal activity in the dorsomedial striatum. Genes Brain Behav. 2022;21:e12828. doi: 10.1111/gbb.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiner DJ, Mietlicki-Baase EG, Olivos DR, McGrath LE, Zimmer DJ, Koch-Laskowski K, et al. Amylin acts in the lateral dorsal tegmental nucleus to regulate energy balance through gamma-aminobutyric acid signaling. Biol Psychiatry. 2017;82:828–38. [DOI] [PMC free article] [PubMed]
- 37.Mietlicki-Baase EG, Reiner DJ, Cone JJ, Olivos DR, McGrath LE, Zimmer DJ, et al. Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology. 2015;40:372–85. doi: 10.1038/npp.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999;56:235–42. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press: New York; 1997.
- 40.Hernandez NS, Weir VR, Ragnini K, Merkel R, Zhang Y, Mace K, et al. GLP-1 receptor signaling in the laterodorsal tegmental nucleus attenuates cocaine seeking by activating GABAergic circuits that project to the VTA. Mol Psychiatry. 2021;26:4394–408. doi: 10.1038/s41380-020-00957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Kahng MW, Elkind JA, Weir VR, Hernandez NS, Stein LM, et al. Activation of GLP-1 receptors attenuates oxycodone taking and seeking without compromising the antinociceptive effects of oxycodone in rats. Neuropsychopharmacology. 2020;45:451–61. doi: 10.1038/s41386-019-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2016;41:1917–28. doi: 10.1038/npp.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci. 2008;28:622–32. doi: 10.1523/JNEUROSCI.2986-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang R, Sun T, Song D, Li JJ. Statistics or biology: the zero-inflation controversy about scRNA-seq data. Genome Biol. 2022;23:31. doi: 10.1186/s13059-022-02601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Twery MJ, Kirkpatrick B, Lewis MH, Mailman RB, Cooper CW. Antagonistic behavioral effects of calcitonin and amphetamine in the rat. Pharmacol. Biochem Behav. 1986;24:1203–7. doi: 10.1016/0091-3057(86)90171-1. [DOI] [PubMed] [Google Scholar]
- 46.Clementi G, Valerio C, Emmi I, Prato A, Drago F. Behavioral effects of amylin injected intracerebroventricularly in the rat. Peptides. 1996;17:589–91. doi: 10.1016/0196-9781(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 47.Soares-Cunha C, Domingues AV, Correia R, Coimbra B, Vieitas-Gaspar N, de Vasconcelos NAP, et al. Distinct role of nucleus accumbens D2-MSN projections to ventral pallidum in different phases of motivated behavior. Cell Rep. 2022;38:110380. doi: 10.1016/j.celrep.2022.110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cole SL, Robinson MJF, Berridge KC. Optogenetic self-stimulation in the nucleus accumbens: D1 reward versus D2 ambivalence. PLOS One. 2018;13:e0207694. doi: 10.1371/journal.pone.0207694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soares-Cunha C, Coimbra B, Domingues AV, Vasconcelos N, Sousa N, Rodrigues AJ. Nucleus accumbens microcircuit underlying D2-MSN-driven increase in motivation. eNeuro. 2018;5:ENEURO.0386-18. [DOI] [PMC free article] [PubMed]
- 50.Aubry JM, Schulz MF, Pagliusi S, Schulz P, Kiss JZ. Coexpression of dopamine D2, and substance P (neurokinin-1) receptor messenger RNAs by a subpopulation of cholinergic neurons in the rat striatum. Neuroscience. 1993;53:417–24. doi: 10.1016/0306-4522(93)90205-t. [DOI] [PubMed] [Google Scholar]
- 51.Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, et al. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci. 2004;24:10289–301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan Z, Song W-J, Surmeier DJ. D2 dopamine receptors reduce N-Type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol. 1997;77:1003–15. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- 53.Alcantara AA, Chen V, Herring BE, Mendenhall JM, Berlanga ML. Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain Res. 2003;986:22–9. doi: 10.1016/s0006-8993(03)03165-2. [DOI] [PubMed] [Google Scholar]
- 54.Kimura H, McGeer PL, Peng F, McGeer EG. Choline acetyltransferase-containing neurons in rodent brain demonstrated by immunohistochemistry. Science. 1980;208:1057–9. doi: 10.1126/science.6990490. [DOI] [PubMed] [Google Scholar]
- 55.Cachope R, Cheer JF. Local control of striatal dopamine release. Front Behav Neurosci. 2014;8:188. doi: 10.3389/fnbeh.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cragg SJ. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–31. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Collins AL, Aitken TJ, Huang IW, Shieh C, Greenfield VY, Monbouquette HG, et al. Nucleus accumbens cholinergic interneurons oppose cue-motivated behavior. Biol Psychiatry. 2019;86:388–96. doi: 10.1016/j.biopsych.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JH, Ribeiro EA, Kim J, Ko B, Kronman H, Jeong YH, et al. Dopaminergic regulation of nucleus accumbens cholinergic interneurons demarcates susceptibility to cocaine addiction. Biol Psychiatry. 2020;88:746–57. doi: 10.1016/j.biopsych.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis RG, Serra M, Radl D, Gori M, Tran C, Michalak SE, et al. Dopaminergic control of striatal cholinergic interneurons underlies cocaine-induced psychostimulation. Cell Rep. 2020;31:107527. doi: 10.1016/j.celrep.2020.107527. [DOI] [PubMed] [Google Scholar]
- 60.Collins AL, Aitken TJ, Greenfield VY, Ostlund SB, Wassum KM. Nucleus accumbens acetylcholine receptors modulate dopamine and motivation. Neuropsychopharmacology. 2016;41:2830–38. doi: 10.1038/npp.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Z-D, Han X, Chen R, Liu Y, Bhattacherjee A, Chen W, et al. A molecularly defined D1 medium spiny neuron subtype negatively regulates cocaine addiction. Sci Adv. 2022;8:eabn3552. doi: 10.1126/sciadv.abn3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, et al. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron. 2015;87:1063–77. doi: 10.1016/j.neuron.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soares-Cunha C, de Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, et al. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol Psychiatry. 2020;25:3241–55. doi: 10.1038/s41380-019-0484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hay DL, Garelja ML, Poyner DR, Walker CS. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br J Pharmacol. 2018;175:3–17. doi: 10.1111/bph.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: pharmacology, physiology, and clinical potential. Pharmacol Rev. 2015;67:564–600. doi: 10.1124/pr.115.010629. [DOI] [PubMed] [Google Scholar]
- 67.Masi L, Brandi ML. Calcitonin and calcitonin receptors. Clin Cases Miner Bone Metab. 2007;4:117–22. [PMC free article] [PubMed] [Google Scholar]
- 68.Bower RL, Hay DL. Amylin structure-function relationships and receptor pharmacology: implications for amylin mimetic drug development. Br J Pharmacol. 2016;173:1883–98. doi: 10.1111/bph.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beaumont K, Kenney MA, Young AA, Rink TJ. High affinity amylin binding sites in rat brain. Mol Pharmacol. 1993;44:493–7. [PubMed] [Google Scholar]
- 70.Sexton PM, Paxinos G, Kenney MA, Wookey PJ, Beaumont K. In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience. 1994;62:553–67. doi: 10.1016/0306-4522(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 71.Kalafateli AL, Vestlund J, Raun K, Egecioglu E, Jerlhag E. Effects of a selective long-acting amylin receptor agonist on alcohol consumption, food intake and body weight in male and female rats. Addict Biol. 2021;26:e12910. doi: 10.1111/adb.12910. [DOI] [PubMed] [Google Scholar]
- 72.Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E. Oxycodone self-administration in male and female rats. Psychopharmacology. 2017;234:977–87. doi: 10.1007/s00213-017-4536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Single-nuclei RNA-sequencing data are available at the NCBI Gene Expression Omnibus (GEO) under accession number GSE171165.