Abstract
Carbapenem-resistant Klebsiella pneumoniae (CRKP) causes severe inflammation in various infectious diseases, such as bloodstream infections, respiratory and urinary tract infections, which leads to high mortality. Polydatin (PD), an active ingredient of Yinhuapinggan granule, has attracted worldwide attention for its powerful antioxidant, anti-inflammatory, antitumor, and antibacterial capacity. However, very little is known about the effect of PD on CRKP. In this research, we evaluated the inhibitory effects of PD on both the bacterial level and the bacterial-cell co-culture level on anti-biofilm and efflux pumps and the other was the inhibitory effect on apoptosis, reactive oxygen species (ROS), mitochondrial membrane potential (MMP) after CRKP induction. Additionally, we validated the mechanism of action by qRT-PCR and western blot in human lung epithelial cells. Firstly, PD was observed to have an inhibitory effect on the biofilm of CRKP and the efflux pump AcrAB-TolC. Mechanically, CRKP not only inhibited the activation of Nuclear Factor erythroid 2-Related Factor 2 (Nrf-2) but also increased the level of ROS in cells. These results showed that PD could inhibit ROS and activate Nrf-2 production. Together, our research demonstrated that PD inhibited bacterial biofilm formation and efflux pump AcrAB—TolC expression and inhibited CRKP-induced cell damage by regulating ROS and Nrf-2-regulated antioxidant pathways.
Subject terms: Cell biology, Microbiology, Physiology, Diseases
Introduction
Klebsiella pneumoniae (KP), is a Gram-negative opportunistic pathogen, which often causes hospital-acquired infections, such as bloodstream infections, respiratory and urinary tract infections, and especially underlying diseases1. In addition, KP is becoming increasingly resistant to the most effective antibiotics, such as the prevalence of imipenem-resistant KP increased each year in China, from 3.0% in 2005 to 25.0% in 20182,3. Owing to the overuse of antibiotics for decades, the emergence of Carbapenem-resistant Klebsiella pneumoniae (CRKP) poses a great challenge to the antibacterial treatment of clinical infection4. Among various resistance mechanisms, overexpression of the efflux pump is an important mechanism of KP resistance5. An important virulence characteristic of KP is the ability to form biofilms, which makes it difficult for conventional antimicrobial agents to penetrate; therefore, it repeatedly causes chronic bacterial behavior infections6,7. In recent years, biofilm and efflux pump are demonstrated to be a certain relationship, and several studies suggested that efflux pumps play at least four different roles in biofilm formation: efflux of extracellular polymeric substances (EPSs) and/or Quorum sensing (QS) and quorum quenching (QQ) molecules to facilitate biofilm matrix formation and regulate QS, respectively; indirect regulation of genes involved in biofilm formation; efflux of harmful molecules, such as antibiotics and metabolic intermediates; and influencing aggregation through promoting or preventing adhesion to surfaces and other cells8. Therefore, finding new drug candidates to treat bacterial infectious diseases and exploring their potential mechanisms is imperative.
Polydatin (PD, 3,4'-5-Trihydroxystilbene-3-β-D-glucopyranoside), a naturally occurring stilbene, owns a variety of health-promoting effects and powerful antioxidant, anti-inflammatory and anti-tumor effects (Fig. 1 A)9–11. PD is also an important component of traditional Chinese medicine compounds, Yinhuapinggan (YHPG) granules used to treat respiratory infections12–16. YHPG was composed of six herbs, which had the effect of clearing heat and detoxifying17. Investigations into the pharmacodynamics and mechanism of YHPG in animals and cells had demonstrated its antiviral, antibacterial, antipyretic, analgesic, cough relieving, and immunomodulatory effects17. In previous studies, we found that YHPG had a positive effect on the treatment of viral infectious respiratory diseases14. At the same time, recent studies had shown that oxidative stress played an important role in the pathogenesis of many inflammatory diseases and induced cell apoptosis and that high levels of reactive oxygen species produced by elements such as LPS and KP lead to oxidative stress18–20. PD had demonstrated to reduce inflammation and pulmonary fibrosis caused by Mycoplasma pneumoniae infection by blocking the NLRP3 inflammasome and NF-κB pathway21. In addition, research had demonstrated that PD could inhibit the generation of ROS and NF-κB p65 activation, suggesting that PD suppressed S. aureus lipoteichoic acid (LTA)-induced injury by attenuating ROS generation and TLR2-NFκB signalling9. However, it remained unclear whether PD possessed anti-microbial activity in bacterial pneumonia and its underlying mechanism of action.
Figure 1.
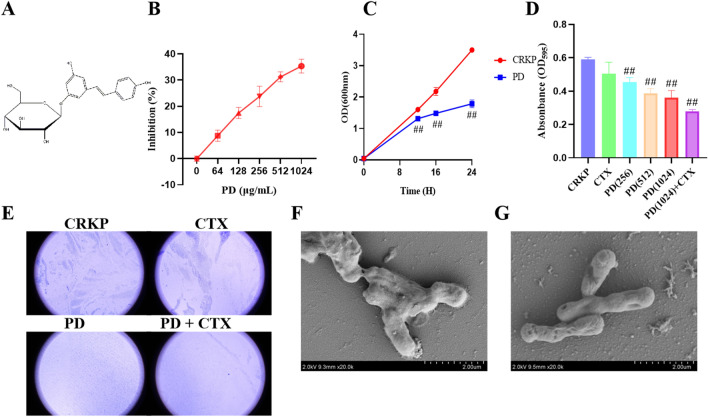
Characterization of CRKP biofilm. The structure of the polydatin (A). Growth curve analysis of bacterial growth (B) and time-killing curve of PD for CRKP (C). Inhibitory effects of PD on biofilm formation by CRKP were determined by Crystal violet assay (D). SEM images of CRKP (F). SEM images of CRKP + PD (G). CRKP morphological changes induced with or without the addition of PD were observed under an inverted microscope (E). #P < 0.05 and ##P < 0.01 vs. the CRKP alone group.
Therefore, this study mainly investigated the bacteriostatic effect of PD extracts and their protective effects against CRKP-induced cell damage. The results indicated that PD had a certain inhibitory effect on the biofilm of CRKP and the efflux pump AcrAB-TolC. Mechanically, CRKP not only inhibited the activation of Nuclear Factor erythroid 2-Related Factor 2 (Nrf-2) but also increased the level of ROS in cells. Furthermore, we used Nrf-2 inhibitors to further investigate the role of the Nrf-2 signaling pathway in CRKP-induced cell damage. These results showed that PD could inhibit ROS and activate Nrf-2 production. These findings provided valuable insights into potential novel strategies for comprehensive treatment of bacterial pneumonia.
Results
Characteristics of CRKP biofilm
The bacterial growth curve concluded that the MIC value of PD for CRKP was greater than 1024 μg/mL, and PD showed antibacterial activity against CRKP strain at 1024 μg/mL (Fig. 1B,C). Biofilm formation abilities of CRKP detected by crystal violet staining were shown in Fig. 1D and Table 1, the results proved that 1024 μg/mL PD was effective in biofilm inhibition. This finding was similar to the results under an inverted microscope, PD had an inhibitory effect on the biofilm formation of CRKP, and combined with cefotaxime sodium (CTX) had an inhibitory effect on the biofilm formation of CRKP (Fig. 1E). Further, Scanning Electron Microscope (SEM) was used to observe the thallus shape of CRKP, and it was found that the thallus of CRKP without drug addiction was full and the surface did not collapse (Fig. 1F), but the group with PD was not full and its surface collapsed (Fig. 1G).
Table 1.
Biofilm formation abilities of different treatment groups.
| CRKP | CTX | PD | PD + CTX | |
|---|---|---|---|---|
| Biofilm formation capability (%) | 100.00% | 85.45% | 61.01% | 47.11% |
Relationship of biofilm formation and efflux pump in CRKP
Additionally, to elucidate the relationship between biofilm and efflux pump in CRKP, we further quantified them by qRT-PCR based on biofilm detection. The relative transcription levels of AcrA, AcrB, and TolC were significantly increased in the CTX group. However, after CTX was combined with PD, the transcript levels of AcrA, AcrB, and TolC were decreased compared with the same concentration of the CTX alone group, which was statistically significant (Fig. 2A).
Figure 2.
Effect of PD on the expression of PCR and proteins in CRKP. The mRNA expression of Acr A, Acr B and Tol C (A). MarA, RobA, SoxS, and RamA (B), Ompk35 (C), KPC, NDM, and OXA-48 (D) of CRKP infected cells were assessed by RT-PCR in the presence or absence of PD after 16 h incubation. #P < 0.05 and ##P < 0.01 vs. the CRKP alone group. *P < 0.05 and **P < 0.01 vs. the CTX group.
MarA and RamA were stress regulators, which regulated the expression of AcrAB-TolC by adjusting their expression levels when the environment changed22. The relative transcription levels of MarA, RobA, and SoxS were significantly upregulated in the CTX group (Fig. 2B). Similarly, the expressions of MarA, RobA, and SoxS were significantly down-regulated after combined treatment with PD and CTX (compared with the CTX group) (Fig. 2B). Ompk35 was a protein gene, and the expression of Ompk35 could increase the sensitivity of CRKP to antibiotics. The relative transcription levels of Ompk35 were significantly upregulated in the group exposed to PD compared with the CRKP group (Fig. 2C). Similarly, the results were identical for KPC, NDM, and OXA-48(Fig. 2D).
In conclusion, the addition of CRKP to CTX increased the expression of AcrAB-TolC, leading to the excretion of CTX, which reduced the bactericidal capacity of CTX. However, after CRKP was combined with PD, the expression of AcrAB-TolC was inhibited, thus reducing the excretion of drugs and toxins.
Effect of PD on protein
Herein, we determined the effect of PD on the protein of CRKP by SDS-PAGE. Many protein bands appeared significantly shallower compared to the CRKP group after CTX and CTX + PD treatment (Fig. 3).
Figure 3.
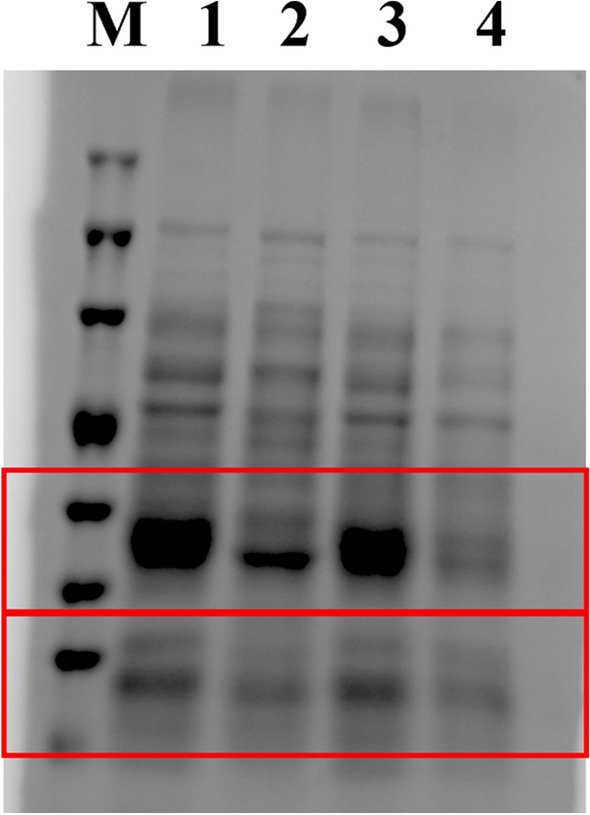
Effect of PD on proteins in CRKP. Total protein profile of SDS-PAGE cells. M: Molecular weight marker; 1: CRKP of the control group; 2: CRKP treated with CTX; 3: CRKP treated with PD; 4; CRKP treated with PD + CTX.
PD reduced cell damage caused by CRKP infection
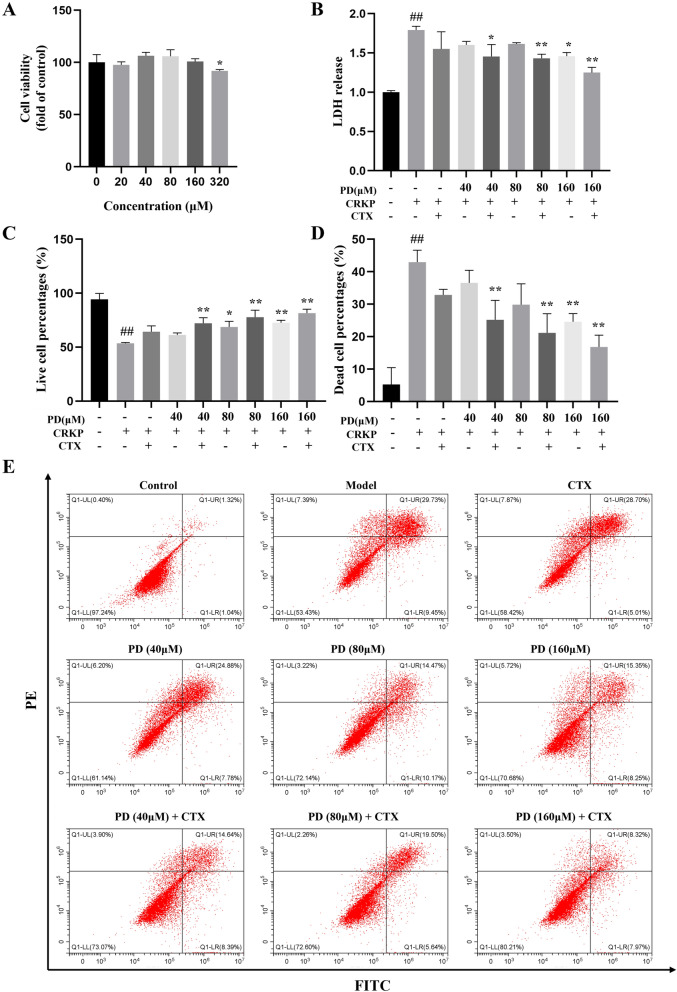
To investigate the effect of PD on CRKP-infected A549 cells. This study initially evaluated the cytotoxicity of PD. The results proved no significant change in cell viability when PD-induced cell damage was less than 160 μM (62 μg/mL) (Fig. 4A). Therefore, a PD concentration of 40–160 μM was used in the follow-up experiment. To elucidate whether PD could protect cells from CRKP damage, we further researched the survival status of cells by Annexin V-FITC and PI. The analysis showed that PD reduced the proportion of dead cells induced by CRKP and increased the proportion of living cells, as well as showed a synergistic effect with CTX (Fig. 4C,E). In addition, we conducted an analysis and the LDH assay corroborated the results that PD functions with the same results obtained by flow cytometry (Fig. 4B).
Figure 4.
PD attenuated cellular apoptosis induced by CRKP. (A) CCK8 analyzed the cell viability after 24 h incubation with various concentrations of PD. CRKP-induced cellular injury induced by PD was detected by LDH (B). (E) A representative result for the changes in alive cell proportion and dead cell proportion was assessed by flow cytometry, and the statistical outcomes were shown (C-D). ##P < 0.01 vs. the control group; *P < 0.05 and **P < 0.01 vs. the CRKP alone group. Model: CRKP (MOI = 30) alone group.
PD reduced the adhesion rate and invasion rate of CRKP
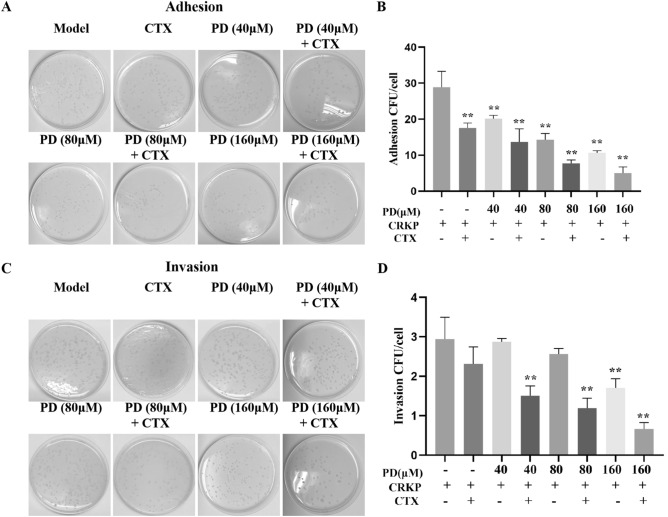
Next, we investigated the effect of PD on CRKP on cell adhesion and invasion. The results proved that the adhesion and invasion of CRKP to cells were observed to be rapid, while PD decreased the adhesion and invasion capacity of CRKP (Fig. 5A-D). In addition, compared with CTX alone, PD and CTX synergistically protected cells from CRKP adhesion and invasion. These results indicated that PD could inhibit adhesion and invasion of CRKP.
Figure 5.
Effects of PD on the adhesion and invasion rate of CRKP-infected A549 cells. Cells were infected by CRKP with or without PD or CTX for 12 h, and then an appropriate amount of cell lysate was applied to LB solid medium for 24 h (A), and the statistical results were shown for adhesion (B). On the other hand, infected-cell were cultured in F12K complete medium containing antibiotics, and extracellular CRKP was washed with PBS. The appropriate amount of CRKP-infected A549 cell lysate was applied to LB solid medium for 24 h and counted (C), and the statistical results were shown for invasion (D). *P < 0.05 and **P < 0.01 vs. the CRKP alone group.
PD decreased the production of proinflammatory cytokines induced by CRKP infection
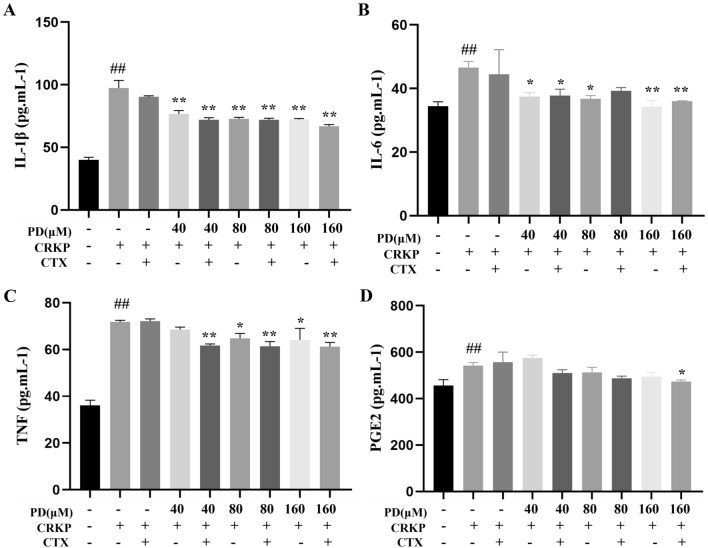
In this study, Enzyme-linked immunosorbent assay (ELISA) was performed to investigate the effect of PD on CRKP-infected proinflammatory cytokines. As shown in Fig. 6A-D, Interleukin-1β (IL-1β), Tumor necrosis factor-α (TNF-α), Interleukin-6 (IL-6), and Prostaglandin E2 (PGE2) concentrations were significantly higher in the CRKP group than in the control group, while the expression of these cytokines (except PGE2) was inhibited in the PD group. These results indicated that PD suppressed the CRKP-stimulated inflammatory expression.
Figure 6.
PD ameliorated the cellular inflammatory response brought on by CRKP stimulation. The production of IL-1β (A), IL-6 (B), TNF-α (C), and PGE2 (D) of CRKP-infected cells with or without PD or CTX in the supernatant were analyzed by ELISA. ##P < 0.01 vs. the control group; *P < 0.05 and **P < 0.01 vs. the CRKP alone group.
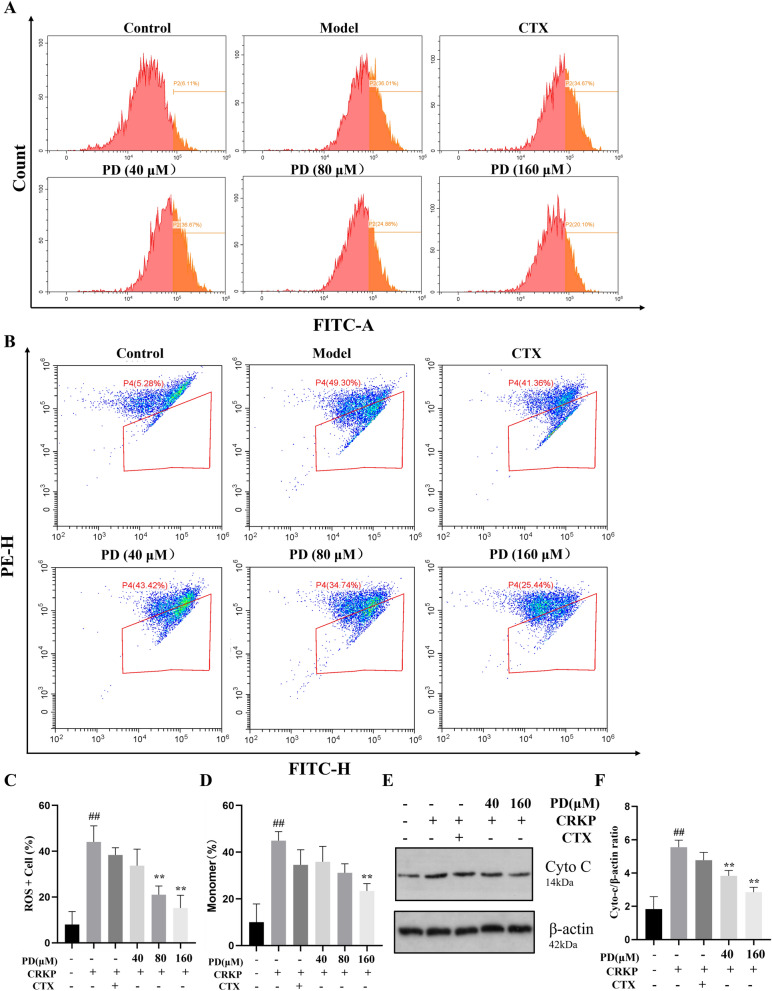
PD inhibited the downregulation of MMP in CRKP-induced cells by reducing ROS levels
Oxidative stress may oxidize and destroy KP's biofilm, resulting in the loss of major membrane proteins and KP activity23. To confirm whether PD could modulate ROS expression induced by CRKP infection, we measured ROS concentration and found that CRKP responded significantly more to cell stimulation than the control group. Meanwhile, PD exhibited antioxidant effects, as demonstrated by a dose-dependent reduction in ROS generation (Fig. 7A,C). Next, we wondered whether the changes in the influence of CRKP infection on mitochondrial membrane potential (MMP) might be induced by ROS. Compared with the control group, flow cytometry results noted that MMP was significantly down-regulated after CRKP stimulation, while MMP down-regulated after PD treatment was improved (Fig. 7B and D). The above results indicated that PD suppressed CRKP-induced MMP downregulation in cells, thereby inhibiting the production of intracellular oxidative stress. In addition to MMP, Cyto-c was also an important component of the mitochondrial respiratory chain, which could activate the downstream cysteine-regulated apoptotic pathway. Therefore, we also analyzed the regulation of Cyto-c in PD by WB. As shown in Fig. 7E,F, the expression of Cyto-c in the CRKP group was higher than that in the control group. However, the high concentration of the PD group had an inhibitory effect on the expression of Cyto-c. These results confirmed that PD could reduce oxidative stress in CRKP infection and inhibit the corresponding downregulation of MMP.
Figure 7.
PD reduced mitochondrial dysfunction by inhibiting cellular damage induced by CRKP. A549 cell cultured with CRKP and with PD for 12 h was collected to evaluate intracellular ROS levels using a DCFH-DA probe. A representative flow cytometric result of ROS expression in various groups was analyzed (A), and a statistical graph was built (C). A549 cells incubated with CRKP for 12 h were collected for assessment of MMP using JC-1 staining (B, D). A representative result of the protein expression of Cyto-c among all groups was analyzed by western blot, and a statistical result was calculated (E–F). ##P < 0.01 vs. the control group; *P < 0.05 and **P < 0.01 vs. the CRKP alone group.
PD inhibited oxidative stress damage in CRKP-induced cells by activating the Nrf-2 pathway
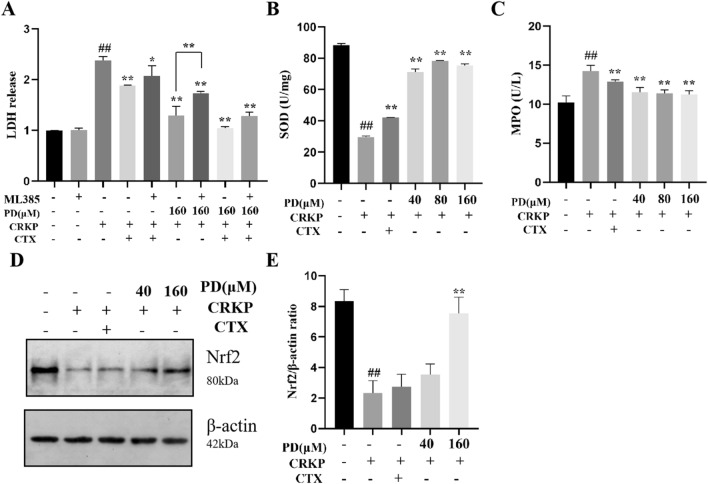
Nrf-2 was a transcription factor that coordinated the expression of cellular protective genes to maintain homeostasis24. In the above experiments, we know that PD could protect against CRKP-induced cell damage by activating Nrf-2 production. Next, we combined an Nrf-2 inhibitor (ML385) to further investigate the effect of phase on cell survival. First, the lung epithelial cells were pretreated with ML385 and then treated with PD and CRKP for 12 h. As shown in Fig. 8A, cell viability decreased with the ML385 combination compared to the group treated with PD alone. This result further indicated that PD could activate the Nrf-2 signaling pathway to promote cell survival. Activation of Nrf-2 activates the production of downstream antioxidant factors (Superoxide dismutase (SOD). As shown in Fig. 8B, compared with the control group, SOD was inhibited by CRKP infection, but PD interference reversed SOD production. In contrast, the CRKP-stimulated Myeloperoxidase (MPO), an oxidized protein, was produced in reduced amounts upon PD intervention (Fig. 8C). Here, we confirmed whether PD could activate the Nrf-2 pathway by WB analysis. As shown in Fig. 8D,E, the expression of Nrf-2 at the protein level was inhibited by CRKP, but its expression could be reversed by PD. In conclusion, PD could inhibit CRKP-induced oxidative stress by up-regulating Nrf-2.
Figure 8.
PD ameliorated oxidative stress by up-regulating the Nrf-2 signaling pathway in CRKP-infected A549 cells. CRKP-induced cellular injury induced by PD and ML385 was detected by LDH (A). The cells were incubated with CRKP and PD for 12 h and the concentration of SOD (B), MPO (C) were assessed by corresponding kits. A representative result of the protein expression of Nrf-2 among all groups was analyzed by western blot, and a statistical result was calculated (D-E). ##P < 0.01 vs. the control group; *P < 0.05 and **P < 0.01 vs. the CRKP alone group.
Discussion
Over the past few years, there have been affected by infections caused by enterobacteria, including the MDR and CRKP, which have been increasingly detected in healthcare facilities25. KP adopted different molecular strategies to develop resistance to carbapenem, including (1) production of carbapenemase (blaKPC, blaOXA, and metal-beta-lactamase); (2) nonexpression or mutation of OmpK35 porin genes prevented bacteria acquisition of carbapenems through the outer membrane of carbapenems; and (3) up-regulation of the efflux system (AcrB), the latter two mechanisms often combined with high levels of other types of beta-lactamases25,26. Studies had shown that efflux pumps were associated with the formation of biofilms of pathogenic microorganisms, which was an important factor for the resistance of pathogenic microorganisms to antibiotics27,28. That could be attributed to the constitutive stress of antibiotics, which enhanced induced gene regulation and provided fitness advantages for resistant strains, leading to biofilm formation29. Resveratrol had shown its potential value in antibacterial therapy, which had been reported to enhance the antimicrobial efficacy of aminoglycosides against Staphylococcus aureus. PD, as the most abundant derivative of resveratrol in nature. Although the antioxidant and anti-inflammatory effects of PD had been well established, little was known about the nature of its antibacterial action30. At the same time, we found that PD had an inhibitory effect on biofilm and that the inhibitory effect on biofilm was greatly enhanced by combining with PD. PD exhibited antibacterial activity based on the killing time curve. Some studies had found that Rama overproduction and porin loss both decreased envelope permeability, and that porin loss had been demonstrated to raise carbapenem MICs for KP isolate-producing ESBLs or AmpC cephalosporinases, this was observed31. Proteomic analyses showed that this enhancement was mainly achieved by activating efflux pump production32. Therefore, we determined that PD could control AcrAB-TolC by regulating the regulatory factors MarA, RamA, RobA, and SoxS in AcrAB-TolC in CRKP, reducing the effects of CRKP efflux drugs. In addition, total bacterial protein concentrations were determined by SDS-PAGE. The results indicated that PD + CTX could reduce the total cellular protein content of CRKP, implying the expression of certain proteins in CRKP was suppressed by PD + CTX. In general, this research preliminarily clarified that PD might exhibit anti-CRKP activity by disrupting cell membrane integrity, affecting protein expression, and inhibiting AcrAB-TolC. However, the specific mechanism of action of PD on CRKP remained to be further studied in the future.
Oxidative stress was an imbalance between oxidative and antioxidant effects in the body that could lead to chronic inflammation, which activated multiple transcription factors, such as Nrf-2 and NF-κB/AP-1was an important node in the inflammatory response. Nrf-2 was an important transcription factor regulating oxidative stress, which up-regulated the production of many antioxidant proteins (such as SOD) once activated33. In the present study, we also investigated the beneficial effect of PD on the oxidative stress damage induced by CRKP in A549 cells. Mechanism studies revealed that PD increased the protein levels of Nrf-2 and up-regulated the protein levels of Cyto-c. In this study, we also found that PD reduced the content of MPO in lung epithelial cells after CRKP induction, and increased the activity of antioxidants such as SOD to combat oxidative stress response. This study speculated that PD might achieve a protective effect through the activation of the Nrf-2 pathway. In order to evaluate this possibility, we further adopted an Nrf-2 inhibitor to verify the role of Nrf-2 in CRKP-induced lung epithelial cells. We found that PD did not inhibit the oxidative stress of CRKP-induced lung epithelial cells inhibited by Nrf-2. These results suggested that Nrf-2 played a key role in the protective effect of PD on CRKP-induced lung epithelial cells. In addition, mitochondria were the main sites for the production of ROS, which were crucial in fighting infections; however, mitochondrial damage could lead to ROS increase, MMP changes, and morphological damage34–36. We measured ROS concentrations using flow cytometry and found a significant increase in CRKP response to cellular stimuli compared to controls. At the same time, PD exhibited antioxidant effects, which could be demonstrated by reducing ROS production. Meanwhile, compared with the control group, flow cytometry showed that MMP was significantly down-regulated after CRKP stimulation, while MMP down-regulated after PD treatment was improved. These results proved that PD could reduce CRKP-induced oxidative stress and inhibit the corresponding decline in MMP by regulating Nrf-2 levels.
At the same time, cytokines were required for immunity, and the initiation, continuation, and resolution of inflammation depended on a complex network of pro-inflammatory and anti-inflammatory cytokines; however, if their activity was overwhelming, it could cause sepsis, which could lead to multiple organ damage37. Therefore, controlling the production of proinflammatory cytokines was one of the strategies to treat infectious diseases. Previous clinical studies had reported that circulating levels of pro-inflammatory cytokines, including TNF-α, IL-6, and IL-8, were generally elevated in patients with pneumonia38–41. To further clarify the anti-inflammatory mechanism of PD, we used an ELISA kit to detect the expression levels of IL-1β, IL-6, TNF-α, and PEG2, and the results consistently showed that CRKP infection increased the levels of IL-1β, IL-6 and TNF-α in human lung epithelial cells. Moreover, the expression levels of IL-1β, IL-6, and TNF-α were decreased under the intervention of PD, compared with the CRKP group. PD reduced the expression of inflammatory cytokines during CRKP infection, but its anti-inflammatory effects need to be further verified.
In summary, our study was the first to report that the first antimicrobial effects of PD and PD alleviate CRKP-induced cell damage by activating the Nrf-2 signaling pathway. Given that PD had many outstanding advantages (low toxicity, no respiratory depression, and low addictive potential), it might become a new and interesting drug for the treatment of CRKP-induced bacterial pneumonia.
Conclusion
In the present study, PD showed significant antibacterial activity against CRKP by inhibiting the activity of the efflux pump AcrAB-TolC and biofilm integrity. Further, we identified an underlying intracellular mechanism by which PD triggered the activation of Nrf-2 to counteract the oxidative stress caused by CRKP, thus restoring dysfunctional mitochondria and reducing inflammation (Fig. 9). There were still limitations to the study that need to be considered. First, we showed that PD regulation of Nrf-2 exerts an antioxidant role in CRKP-induced cell damage in vitro. However, models of CRKP infection were not evaluated due to biosafety level limitations. Even though PD was able to suppress inflammation and oxidative stress in CRKP infection, the relationship between inflammation and antioxidants was not clear. Third, there were no animal experiments or primary experiments to evaluate the effect of PD on CRKP. We speculated that PD suppressed the secretion of inflammatory cytokines by reducing ROS, but its associated inflammatory signaling pathways should be further confirmed.
Figure 9.
A schematic diagram showed that the antimicrobial mechanism of PD and the protection of mitochondrial integrity through the Nrf-2 pathway against the damage induced by CRKP in cells.
Materials and methods
Reagents and materials
PD was purchased from Chengdu Alfa Biotechnology Co., LTD. (HPLC ≥ 98%, Chengdu, China). The IL-1β, TNF-α, IL-6, and PGE2 ELISA kits were from Enzyme-labeled organisms (Jiangsu, China). The MPO, SOD were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The crystal violet was obtained from Beyotime (Shanghai, China).
Bacteria culture
CRKP strain was isolated from Hangzhou First People's Hospital, and previous studies tested the resistance of CRKP to antibiotics20. CRKP strain was inoculated in LB broth under 200 rpm, 37 ℃.
Crystal violet assay
The ability of biofilm formation was evaluated using the crystal violet method; specifically, bacterial suspensions of 108 CFU/mL were added to a 96-well polystyrene microtiter plate. The absorbance at 595 nm was measured in accordance with the 0.1% crystal violet kit instructions. At the same time, the concentration of bacteria was observed with a scanning electron microscope.
Gene expression testing by quantitative reverse transcription-PCR (qRT-PCR)
To detect the transcriptional expression levels of regulator genes RamA, MarA, RobA, and SoxS, as well as efflux pump genes AcrA, AcrB, and TolC, qRT-PCR was utilized. cDNA was prepared with ReverTra Ace® qPCR kit according to the instructions and prepared with SYBR® Green Realtime PCR Master Mix was amplified by QuantStudio 12 K Flex Real-Time PCR Syste. The relative expression of the gene was calculated by 2−ΔΔCt. The primer sequence is shown in Table 2.
Table 2.
The primer sequences for qRT-PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Acr A | GGCAAACATGGATCAACTG | GGCGGTATCGTAGTCTTG |
| Acr B | GGAAGATACACCGCAGTT | TGTTAATGTCGCTGATGGA |
| Tol C | CTACGCTGTATAACGCTAA | CTAACGCCGACTTAATGT |
| Mara | ATGATGTCCAGACGTAATAATGA | GGCGATTCCAGGTTATCC |
| Roba | TATTCTATACCACCGCGCTGAC | GTGCCGTAGACGGTCAGGAT |
| Soxs | CTTAACATTGATATAGTCGCCAGA | CATCACGGTACGGAACATC |
| KPC | ATGTCACTGTATCGCCGTCT | TTTTCAGAGCCTTACTGCCC |
| NDM | GGTTTGGCGATCTGGTTTTC | CGGAATGGCTCATCACGATC |
| OXA-48 | GCGTGGTTAAGGATGAACAC | CATCAAGTTCAACCCAACCG |
| Rama | GCTGCGTATTGATGATAT | TCTCCCTTGTACTGTAAA |
| Ompk35 | GGATGGAAAGATGCCTTCAG | CATGACGAGGTTCCATTGT |
| 16sDNA | AGGCCTAACACATGCAAGTC | GTGCAATATTCCCCACTGCTG |
SDS-PAGE analysis
Changes in protein expression were determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). All of the cellular proteins were denatured at 100 ºC for 10 min, then put through electrophoresis on a 5.0% stacked gel at 80 V for half an hour and a 10% resolving gel at 120 V for 70 min. After that, the gel was stained with Coomassie brilliant blue G250 until the protein bands were distinct. The image of the protein bands was obtained using the gel imaging system.
Cell viability was determined by LDH, Annexin V and PI, and CCK8 assay
Culturing of A549 cells was conducted in F12K complete medium that comprised of 10% fetal bovine serum (FBS, GIBCO, Poncho) and 100 U/mL penicillin–streptomycin solution(Beyotime, Shanghai, China), at 37 ℃, 5% CO2.
CCK8 was used to assess cell viability. Seeding A549 cells in 96-well plates for 24 h, cells were exposed to different concentrations of PD (20, 40, 80, or 160 μM), then 10 μL of CCK8 was added to each well for 1–2 h. Cell viability was assessed by absorbance (optical density) with a microplate reader at 450 nm. The effect of PD on CRKP-induced cells was determined by the LDH assay. The absorbance at 490 nm was measured using a microplate reader, according to the LDH kit (Source leaf, Shanghai, China). Apoptosis was detected by using the FITC Annexin V Apoptosis Assay kit (BD, Guangzhou, China). The role of Nrf-2 in CRKP-induced cell damage was further investigated using Nrf-2 inhibitors (ML385). The experiments were tested with the LDH kit.
ROS and MMP were measured by flow cytometry
Intracellular ROS levels as a general indicator of oxidative stress were determined using a dichloro-dihydro-fluorescein diacetate (DCFH-DA) probe. MMP was evaluated using a fluorescent indicator JC-1 kit (Beyotime, Shanghai, China).
Determination of oxidative protein/antioxidant protein and inflammatory cytokines
The contents of MPO, SOD in CRKP-infected cells were analyzed. MPO activity was determined using an MPO assay kit. SOD activity in cell lysates was measured using SOD kits, respectively.
The cells were treated with CRKP and PD for 12 h. The concentrations of TNF-α, IL-6, IL-1β, and PGE2 in cell-free supernatant were determined by the quantitative factor ELISA ki.
Adhesion assay and invasion assay
For the adhesion experiment, after 12 h treatment, each group was washed 3 times with PBS and soaked in 0.25% pancreatic enzyme100 μL and 0.5% TritonX-400 μL for 10 min. Then, 100 μL of the diluted solution was placed on LB medium and incubated at 37℃. Finally, the cells were counted and analyzed. In the invasion experiment, infected cells were treated as described above for 12 h, and 500 μL of tigecycline (TIG) cell basal medium was added to each group to kill extracellular bacteria, washed in PBS, and treated according to steps in the adhesion test.
Western blot
Protein concentration was determined by using a BCA kit. Then, the same amount of protein was electrophoresed in 12% polyacrylamide gel, transferred to the PVDF membrane, and sealed with 5% fat-free milk powder for 1 h. The membranes were then incubated with rabbit anti-human β-actin (Abcam, China), Nrf-2 (Abcam, China), and Cyto-c (CST, China) antibodies at 4 ℃ overnight. Tris–HCl-Tween (TBST) was used for 10 min and incubated with an anti-rabbit IgG secondary antibody (ThermoPierce, China) at room temperature for 1 h. Ultimately, the gel Imager (Bio-Red, Shanghai, China) was used to visualize the membrane using an enhanced chemiluminescence (ECL) agent. The results were analyzed by ImageJ software. β-actin was used as endogenous control and normalization.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Differences between the groups were followed by a one-way ANOVA multiple comparison test. There were significant differences at P < 0.05 (significant) or P < 0.01 (highly significant).
Ethics approval
The carbapenem-resistant Klebsiella pneumoniae (CRKP) strain was reviewed and approved by the Hangzhou First People's Hospital ethics committee, Hangzhou, China. We confirmed that informed consent was obtained from the study participants. We confirmation that the guidelines outlined in the Declaration of Helsinki were followed.
Supplementary Information
Abbreviations
- KP
Klebsiella pneumoniae
- CRKP
Carbapenem-resistant Klebsiella pneumoniae
- PD
Polydatin
- ROS
Reactive oxygen species
- YHPG
Yinhuapinggan
- Nrf-2
Nuclear factor erythroid 2-related factor 2
- CTX
Cefotaxime sodium
- SEM
Scanning electron microscope
- MMP
Mitochondrial membrane potential
- IL-1β
Interleukin-1β
- TNF-α
Tumor necrosis factor-α
- IL-6
Interleukin-6
- PGE2
Prostaglandin E2
- ELISA
Enzyme-linked immunosorbent assay
- MPO
Myeloperoxidase
- SOD
Superoxide dismutase
- TIG
Tigecycline
- TBST
Tris–HCl-tween
- ECL
Enhanced chemiluminescence
- SD
Standard deviation
Author contributions
X.G. and L.J. conceived and designed the experiment. X.G. performed the experiments, analyzed and interpreted the data, and drafted the manuscript. H.Z., J.C., H.W., Y.B., J.H.Y., D.Y., and H.W. substantively revised the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (grant number 81930111), and the research was also supported by the biosafety laboratory of integrated Chinese and Western medicine at Zhejiang Chinese Medicine University (BSL20205713156), and Zhejiang Province Traditional Chinese Medicine Science and Technology project (2023ZF157), and the Research Project of Zhejiang Chinese Medical University (2021RCZXZK23, 2022GJYY025). The funders have a role in study design, data collection and analysis, the decision to publish, and the preparation of the manuscript.
Data availability
All data generated or analyzed during this study are included in the Supplementary Information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiaodan Guan and Liang Jin.
Contributor Information
Daojun Yu, Email: yudaojun98@163.com.
Haitong Wan, Email: whtong@126.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-44836-7.
References
- 1.Moo CL, Osman MA, Yang SK, Yap WS, Ismail S, Lim SH, Chong CM, Lai KS. Antimicrobial activity and mode of action of 1,8-cineol against carbapenemase-producing Klebsiella pneumoniae. Sci. Rep. 2021;11(1):20824. doi: 10.1038/s41598-021-00249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 2016;80(3):629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Y, Liu C, Shen Z, Zhou H, Cao J, Chen S, Lv H, Zhou M, Wang Q, Sun L, Sun Q, Hu F, Wang Y, Zhang R. Prevalence, risk factors and molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in patients from Zhejiang, China, 2008–2018. Emerg. Microb. Infect. 2020;9(1):1771–1779. doi: 10.1080/22221751.2020.1799721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassetti M, Righi E, Carnelutti A, Graziano E, Russo A. Multidrug-resistant Klebsiella pneumoniae: challenges for treatment, prevention and infection control. Expert. Rev. Anti Infect. Ther. 2018;16(10):749–761. doi: 10.1080/14787210.2018.1522249. [DOI] [PubMed] [Google Scholar]
- 5.Xu Q, Sheng Z, Hao M, Jiang J, Ye M, Chen Y, Xu X, Guo Q, Wang M. RamA upregulates multidrug resistance efflux pumps AcrAB and OqxAB in Klebsiella pneumoniae. Int. J. Antimicrob. Agents. 2021;57(2):106251. doi: 10.1016/j.ijantimicag.2020.106251. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Yu K, Chen L, Zheng X, Huang N, Lin Y, Jia H, Liao W, Cao J, Zhou T. Synergistic activity and biofilm formation effect of colistin combined with PFK-158 against colistin-resistant gram-negative bacteria. Infect. Drug Res. 2021;14:2143–2154. doi: 10.2147/IDR.S309912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adeosun IJ, Baloyi IT, Cosa S. Anti-biofilm and associated anti-virulence activities of selected phytochemical compounds against Klebsiella pneumoniae. Plants. 2022;11(11):1429. doi: 10.3390/plants11111429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alav I, Sutton JM, Rahman KM. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018;73(8):2003–2020. doi: 10.1093/jac/dky042. [DOI] [PubMed] [Google Scholar]
- 9.Zhao G, Jiang K, Wu H, Qiu C, Deng G, Peng X. Polydatin reduces staphylococcus aureus lipoteichoic acid-induced injury by attenuating reactive oxygen species generation and TLR2-NFκB signalling. J. Cell Mol. Med. 2017;21(11):2796–2808. doi: 10.1111/jcmm.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao XJ, Yu HW, Yang YZ, Wu WY, Chen TY, Jia KK, Kang LL, Jiao RQ, Kong LD. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox. Biol. 2018;18:124–137. doi: 10.1016/j.redox.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu T, Fei Z, Su H, Xie R, Chen L. Polydatin inhibits proliferation and promotes apoptosis of doxorubicin-resistant osteosarcoma through LncRNA TUG1 mediated suppression of Akt signaling. Toxicol. Appl. Pharmacol. 2019;371:55–62. doi: 10.1016/j.taap.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Wang X, Li Y, Xue Z, Shao R, Li L, Zhu Y, Zhang H, Yang J. Xuanfei Baidu decoction reduces acute lung injury by regulating infiltration of neutrophils and macrophages via PD-1/IL17A pathway. Pharmacol. Res. 2022;176:106083. doi: 10.1016/j.phrs.2022.106083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu XI, Yujing SH, Jie SU, Friedemann T, Zhenggang TA, Lu Y, Yun LI, Lv Y, Ronghua ZH, Zihan GE, Xiaolan CU. Shufeng Jiedu, a promising herbal therapy for moderate COVID-19: Antiviral and anti-inflammatory properties, pathways of bioactive compounds, and a clinical real-world pragmatic study. Phytomedicine. 2021;85:153390. doi: 10.1016/j.phymed.2020.153390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng XQ, Zhou HF, Lu YY, Chen JK, Wan HT, Zhang YY. Protective effects of Yinhuapinggan granule on mice with influenza viral pneumonia. Int. Immunopharmacol. 2016;30:85–93. doi: 10.1016/j.intimp.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Jin L, Zhang Y, Yang J, Zhou H, Jia G, He Y, Wan H. Investigation of pharmacological mechanisms of Yinhua Pinggan granule on the treatment of pneumonia through network pharmacology and In Vitro. Biomed. Res. Int. 2022;2022:1602447. doi: 10.1155/2022/1602447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo P, Jin L, Zhou H, Bao Y, Yang J, Chen J, He Y, Yu D, Wan H. Glycyrrhetinic acid protects against Multidrug-resistant Acinetobacter baumannii-induced lung epithelial cells injury by regulating inflammation and oxidative stress. BMC Pharmacol. Toxicol. 2023;24(1):5. doi: 10.1186/s40360-023-00648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Hu H, Du H, Luo M, Cao Y, Xu J, Chen T, Guo Y, Li Q, Chen W, Zhang Y, Han J, Wan H. Clinical efficacy protocol of Yinhuapinggan Granules: A randomized, double-blind, parallel, and controlled clinical trial program for the intervention of community-acquired drug-resistant bacterial pneumonia as a complementary therapy. Front. Pharmacol. 2022;13:852604. doi: 10.3389/fphar.2022.852604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122(6):877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almuntashiri S, Han Y, Zhu Y, Dutta S, Niazi S, Wang X, Siddiqui B, Zhang D. CC16 Regulates inflammation, ROS generation and apoptosis in bronchial epithelial cells during Klebsiella pneumoniae infection. Int. J. Mol. Sci. 2021;22(21):11459. doi: 10.3390/ijms222111459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan X, Jin L, Yu D, He Y, Bao Y, Zhou H, Wan H. Glycyrrhetinic acid prevents carbapenem-resistant Klebsiella pneumoniae-induced cell injury by inhibiting mitochondrial dysfunction via Nrf-2 pathway. Microb. Pathog. 2023;177:105825. doi: 10.1016/j.micpath.2022.105825. [DOI] [PubMed] [Google Scholar]
- 21.Tang J, Li Y, Wang J, Wu Q, Yan H. Polydatin suppresses the development of lung inflammation and fibrosis by inhibiting activation of the NACHT domain-, leucine-rich repeat-, and pyd-containing protein 3 inflammasome and the nuclear factor-κB pathway after Mycoplasma pneumoniae infection. J. Cell. Biochem. 2019;120(6):10137–10144. doi: 10.1002/jcb.28297. [DOI] [PubMed] [Google Scholar]
- 22.Buffet-Bataillon S, Le Jeune A, Le Gall-David S, Bonnaure-Mallet M, Jolivet-Gougeon A. Molecular mechanisms of higher MICs of antibiotics and quaternary ammonium compounds for Escherichia coli isolated from bacteraemia. J. Antimicrob. Chemother. 2012;67(12):2837–2842. doi: 10.1093/jac/dks321. [DOI] [PubMed] [Google Scholar]
- 23.Yang SK, Yusoff K, Ajat M, Thomas W, Abushelaibi A, Akseer R, Lim SH, Lai KS. Disruption of KPC-producing Klebsiella pneumoniae membrane via induction of oxidative stress by cinnamon bark (Cinnamomum verum J. Presl) essential oil. PloS one. 2019;14(4):e0214326. doi: 10.1371/journal.pone.0214326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He F, Ru X, Wen T. NRF2, A transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020;21(13):4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vuotto C, Longo F, Pascolini C, Donelli G, Balice MP, Libori MF, Tiracchia V, Salvia A, Varaldo PE. Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J. Appl. Microbiol. 2017;123(4):1003–1018. doi: 10.1111/jam.13533. [DOI] [PubMed] [Google Scholar]
- 26.Durante-Mangoni E, Andini R, Zampino R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2019;25(8):943–950. doi: 10.1016/j.cmi.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 27.De Kievit TR, Parkins MD, Gillis RJ, Srikumar R, Ceri H, Poole K, Iglewski BH, Storey DG. Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2001;45(6):1761–1770. doi: 10.1128/AAC.45.6.1761-1770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 2002;49(6):973–980. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 29.Eze EC, Chenia HY, El Zowalaty ME. Acinetobacter baumannii biofilms: effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect. Drug Res. 2018;11:2277–2299. doi: 10.2147/IDR.S169894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang KF, Zhao G, Deng GZ, Wu HC, Yin NN, Chen XY, Qiu CW, Peng XL. Polydatin ameliorates Staphylococcus aureus-induced mastitis in mice via inhibiting TLR2-mediated activation of the p38 MAPK/NF-κB pathway. Acta Pharmacol. Sin. 2017;38(2):211–222. doi: 10.1038/aps.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai YK, Fung CP, Lin JC, Chen JH, Chang FY, Chen TL, Siu LK. Klebsiella pneumoniae outer membrane porins OmpK35 and OmpK36 play roles in both antimicrobial resistance and virulence. Antimicrob. Agents Chemother. 2011;55(4):1485–1493. doi: 10.1128/AAC.01275-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G, Zhao G, Chao X, Xie L, Wang H. The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health. 2020;17:6278. doi: 10.3390/ijerph17176278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rojo de la Vega M, Dodson M, Gross C, Mansour HM, Lantz RC, Chapman E, Wang T, Black SM, Garcia JG, Zhang DD. Role of Nrf2 and autophagy in acute lung injury. Curr. Pharmacol. Rep. 2016;2(2):91–101. doi: 10.1007/s40495-016-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gómez-Crisóstomo NP, López-Marure R, Zapata E, Zazueta C, Martínez-Abundis E. Bax induces cytochrome c release by multiple mechanisms in mitochondria from MCF7 cells. J. Bioenerg. Biomembr. 2013;45(5):441–448. doi: 10.1007/s10863-013-9508-x. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Wang J, Xie R, Liu R, Lu Y. Mitochondria-derived reactive oxygen species play an important role in Doxorubicin-induced platelet apoptosis. Int. J. Mol. Sci. 2015;16(5):11087–11100. doi: 10.3390/ijms160511087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrieux P, Chevillard C, Cunha-Neto E, Nunes JP. Mitochondria as a cellular hub in infection and inflammation. Int. J. Mol. Sci. 2021;22(21):11338. doi: 10.3390/ijms222111338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch. Intern. Med. 2007;167(15):1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puren AJ, Feldman C, Savage N, Becker PJ, Smith C. Patterns of cytokine expression in community-acquired pneumonia. Chest. 1995;107(5):1342–1349. doi: 10.1378/chest.107.5.1342. [DOI] [PubMed] [Google Scholar]
- 39.Calbo E, Alsina M, Rodríguez-Carballeira M, Lite J, Garau J. Systemic expression of cytokine production in patients with severe pneumococcal pneumonia: effects of treatment with a beta-lactam versus a fluoroquinolone. Antimicrob. Agents Chemother. 2008;52(7):2395–2402. doi: 10.1128/AAC.00658-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glynn P, Coakley R, Kilgallen I, Murphy N, O'Neill S. Circulating interleukin 6 and interleukin 10 in community acquired pneumonia. Thorax. 1999;54(1):51–55. doi: 10.1136/thx.54.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bordon J, Aliberti S, Fernandez-Botran R, Uriarte SM, Rane MJ, Duvvuri P, Peyrani P, Morlacchi LC, Blasi F, Ramirez JA. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int. J. Infect. Dis. IJID: Off. Publ. Int. Soc. Infect. Dis. 2013;17(2):e76–83. doi: 10.1016/j.ijid.2012.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the Supplementary Information files.