Abstract
Cryptosporidium is one of the causative parasitic agents that causes gastrointestinal diseases in calves. The parasite poses a zoonotic risk to immunocompromised individuals and children. Thus, this study aimed to determine the prevalence of Cryptosporidium infection in calves in three Egyptian governorates situated in Nile Delta and assess the associated risk factors. The Cryptosporidium oocysts were detected in 81 out of 430 calves (18.84%). In addition, the univariant analysis showed that age, feeding source, hygienic status, presence of diarrhea and contact with other animals were significantly (P < 0.05) associated with Cryptosporidium prevalence in calves. Furthermore, the risk factors related with Cryptosporidium prevalence were age (OR 1.96, 95%CI 0.97–3.94), feeding on milk and pasture (OR 2.07, 95%CI 1.15–3.72), poor hygienic condition (OR 2.25, 95%CI 1.28–3.94), presence of diarrhea (OR 2.47, 95%CI 1.23–4.96) and contact with other domestic animals (OR 2.08, 95%CI 1.24–3.50). In addition, the PCR assay targeting 18srRNA showed that the most prevalent species among calves was C. parvum. Although additional researches are required to understand the most effective steps that farmers and veterinary professionals should take to decrease the occurrence of Cryptosporidium infection.
Subject terms: Parasitology, Risk factors
Introduction
Cryptosporidium is an intracellular protozoan and one of the most common enteric pathogens in claves during first two weeks of life. Four species are usually discovered infecting cattle: C. parvum, C. andersoni, C. bovis, and C. ryanae1,2. C. parvum is commonly associated with diarrhoea in susceptible hosts, causing sickness and even mortality, notably in neonatal calves3. The life cycle of this pathogen is direct, and it can grow and replicate in infected animal's gastrointestinal epithelial cells4,5. The infective stage of Cryptospordium's life cycle is the oocyst, which is secreted in the faces and contains four sporozoites. When the oocyst is ingested, sporozoites are released. These sporozoites enter the cells, forming oocysts with thick and thin walls. The thick-walled oocyst is discharged in the faces. The thin-walled oocysts can rupture, allowing the sporozoites to infect new host enterocytes and produce autoinfection, leading to relapses or persistent gut illness. These sporozoites go through several phases before creating new oocysts. Cell infection results in cell death, which causes intestinal villi to shrink and fuse6.
In addition, the parasite can be passed from person to person, animal to animal, or animal to human (zoonotic transmission)7.
Infections are usually transmitted through the faecal-oral route, with infective stages of expelled sporulated oocysts through direct or collateral contact8. The infection is known to be self-limiting in the immunocompetent hosts, but it can cause acute or severe diarrhea in young animals or in immunocompromised hosts9.
Even though bovine cryptosporidiosis has been identified as a significant contributor to newborn diarrhea and financial losses on dairy farms, it is frequently misdiagnosed10. It is characterized by anorexia, abdominal pain and diarrhea, which can cause slow growth and even death. Diarrhea usually begins 3–5 days after infection and lasts 4 to 17 days in most of infected calves11. Oocyst shedding begins four days after birth and peaks at seven to eighteen days before declining after three weeks12. During diarrhea episodes, oocyst shedding is typically increased13.
Clinically, the age, immunological, and nutritional state of animals can be used to predict how severe cryptosporidiosis will be14. Based on data of previous reports, the Cryptosporidium prevalence in cattle varies over the world and ranges from 6.25 to 39.65%15,16. Cryptosporidiosis prevalence is affected by a variety of factors, including age, hygiene, bedding type, colostrum feeding, herd management, food and water sources, diarrhea, and climate17.
Although insensitive, time-consuming, and requiring skilled personnel to detect the organism, the outdated direct microscopic diagnosis of Cryptosporidium from faecal samples using acid-fast stain is still as gold standard in many laboratories around the world18–21. Only a few studies on animals have employed microscopic methods22–24, however some have also used molecular methods23,25–29. However, there are few epidemiological data and no risk factor analyses for calve cryptosporidiosis in Egypt. The prevalence of Cryptosporidium among ruminant in Ismalia governorates was 32.7% based on PCR assay30, 14.19% among buffaloes calves raising in Dakhalia and Kafr Elsheikh governorates using microscopic examination24.
The purpose of this study was to estimate the prevalence and assess the associated risk factors for Cryptosporidium infection in newborn calve in three governorates situated at Nile Delta of Egypt.
Materials and methods
Ethical statement
The ethical committee for animal research at Benha University approved the entire study's methodology and procedures. Informed consent was obtained from owners of examined claves. The Faculty of Veterinary Medicine's ethical committee ensured that all procedures were carried out in accordance with the relevant laws and guidelines. The ARRIVE criteria were followed during research procedure.
Study area
The study was performed in three governorates situated at Nile Delta of Egypt. The governorates selected for the study are Kafer ElSheikh, Qalyubia, and Gharbia, which are located in latitudes of 31° 06′ 42′′ N, 30.41° N, and 30.867° N, respectively, and longitudes of 30° 56′ 45′′ E, 31.21° E, and 31.028° E, Fig. 1.
Figure 1.
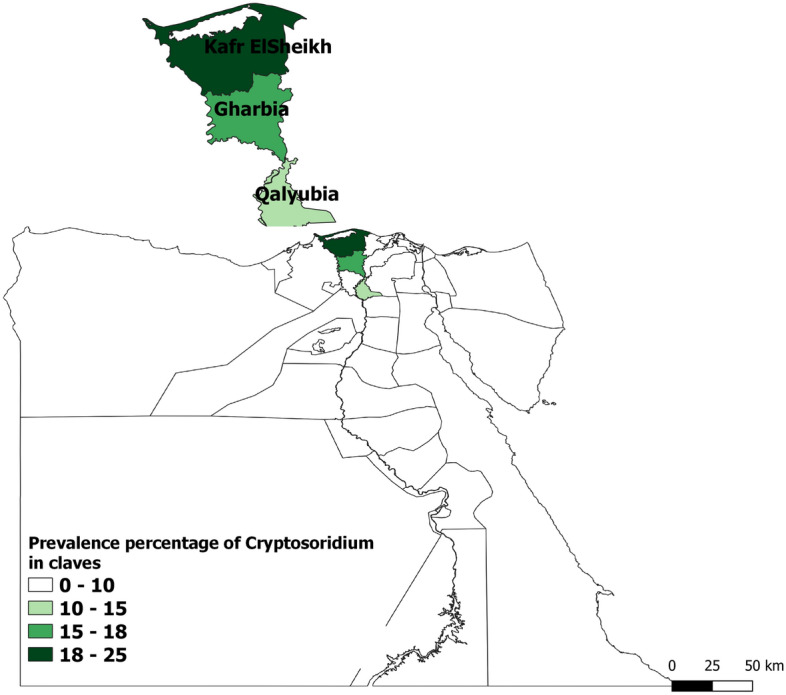
Prevalence of Cryptosporidium in calves in different studied areas (MAP generated by QGIS software, https://qgis.org/).
The Delta is the driest region in the country, has relatively mild temperatures, which increase to 38 °C in summer season. On average, the delta receives 100–200 mm of rain each year, with the majority of this falling during the winter months. These research areas focus mostly on agriculture, livestock husbandry, and have a high number of farms and pastures.
Sample size and sampling
A cross sectional study was conducted during October 2020 to September 2021 using simple random sampling approach to achieve the forementioned goals. Based on Thrusfield's formula31, the sample size was calculated using an expected prevalence of 19.2%30 at a 95% confidence interval and a 5% precision value. Consequently, 238 cow calves were included in the sample. However, 430 cow calves in total were enlisted to gather the necessary faeces samples. Using sterile plastic gloves, each calf's individual faeces was collected directly from the rectum and preserved at 4 °C before being transported to the laboratory.
Questionnaire
At the time of sampling, the farmer completed the provided questionnaire, which mostly asked about animal-related information including breed, age, sex, and body condition. Data on management, including feed source (pasture and milk or pasture), hygienic status (good or poor), the presence of diarrhea (yes or no), and contact with other domestic animals (yes or no), were also gathered.
Sample analysis
The samples were transferred to the laboratory for further processing on the same day they were collected. After that, the samples were treated via faecal flotation in a Sheather's sugar solution24. Then, fecal smear was prepared and stained by modified Ziehl–Neelsen stain32. According to Anderson33, the severity of the infection was determined by counting the cryptosporidial oocysts in a field at a 1000× magnification; the levels were mild (1–5 oocysts/field), moderate (6–20 oocysts/field), and severe (more than 20 oocysts/field), Fig. 2.
Figure 2.

Microscopy of Cryptosporidium oocysts in diarrheal calves' faeces stained with Ziehl–Neelsen stain (×1000).
Molecular identification of cryptosporidiosis
The QIAamp DNA stool Mini Kit (QIAGEN, Hilden, Germany) was used to extract direct DNA from 220 mg of faeces samples according to the manufacturer's instructions. The Cryptosporidium small subunit (18S) rRNA gene was amplified with the oligonucleotide primers CRP-DIAG1 forward (5-TTCTAGAGCTAATACATGCG-30) and CRP-DIAG1 reverse (50-CATTTCCTTCGAAACAGGA-30). The PCR assay was performed in total volume of 25 µl including 1 µl of each primer of (20 pmol), 12.5 µl of PCR Master Mix (EmeraldAmp Max, Takara, Japan), 5.5 µl of water, and 5 µl of DNA template. The PCR Conditions was performed as described by Paul et al.34.
For the secondary/nested PCR, 1 µl of the primary PCR products was utilized as a template and amplified using the primers CRP-DIAG2 forward (50-GGAAGGGTTATTTATTAGATAAAG-30) and CRP-DIAG2 reverse (50-AAGGAGTAAGGAACAACCTCCA-30). The reaction mixture was initially denaturated at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for one min, annealing at 57 °C for 1 min, elongation at 72 °C for 1 min, and final elongation at 72 °C for 10 min as described by Paul et al.34. The amplified products were identified using 1.5% agarose gel electrophoresis and ethidium bromide staining.
Data analysis
The statistical SPSS software programme, Version 24.0 (IBM, USA), was used for all data analyses. To ascertain the relationship between predicted risk variables and the occurrence of Cryptosporidium infection, the univariate logistic regression approach was applied. When the P value is less than 0.05, the findings are considered statistically significant. A multivariable analysis comprised factors that were significantly (P < 0.05) related to the outcome variable in the univariable analysis20,35–40. A test for multicollinearity was also conducted to determine confounding factors and assess the fit of the multivariate model.
Results
Cryptosporidium oocysts were detected in 81 (18.84%) of the 430 examined calves and the highest prevalence rate (24.67%) was observed in Kafr ElSheikh governorate, but the lowest rate (14.29%) found in Qalyubia governorate, Fig. 1. PCR analysis of all positive samples with microscopic examination revealed predicted bands for Cryptosporidium spp. at 1,325 (Fig. 3) in primary PCR which confirm presence of C. parvum in all samples in secondary PCR at 835 bp (Fig. 4).
Figure 3.
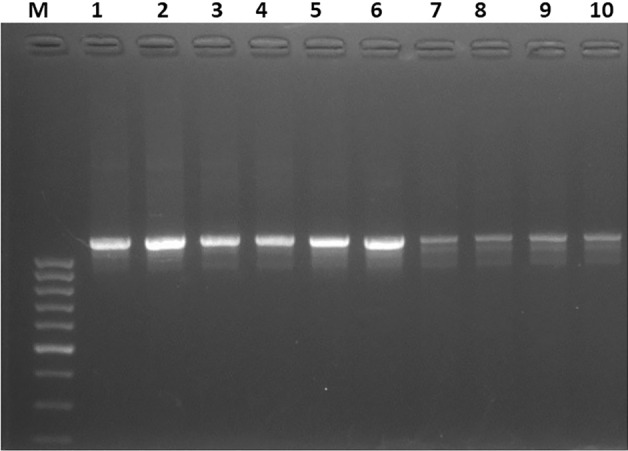
Identification of Cryptosporidium spp. using PCR assay targeting 18S rRNA. Lane M: ladder (100 bp) and lane 1–10 positive samples with detected band at 1325bp.
Figure 4.
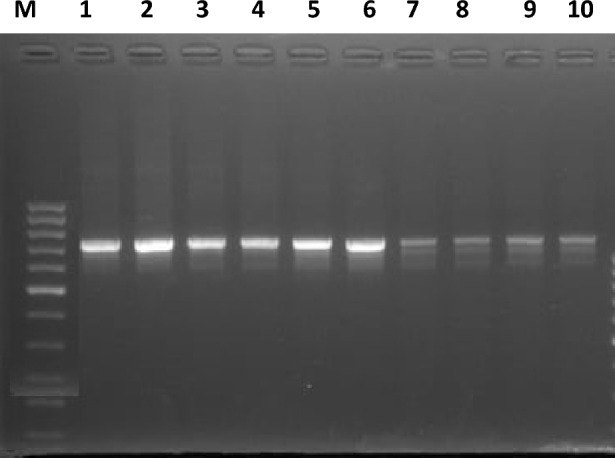
Identification of Cryptosporidium parvum using PCR assay targeting 18S rRNA. Lane M: ladder (100 bp) and lane 1–10 positive samples for C. parvum with detected band at 835 bp.
By using univariate logistic regression analysis, five variables had a substantial impact on the prevalence of Cryptosporidium infection in calves. The prevalence of the disease was similar between the sex (P = 0.764), between breeds (P = 0.076), across geographic regions (P = 0.064), and in terms of body condition (P = 0.785), Table 1.
Table 1.
The prevalence of Cryptosporidium infection in calves in relation to different factors.
| Factors | No of examined animals | No of positive | No of negative | % of positive | 95% CI | Statistic |
|---|---|---|---|---|---|---|
| Locality | ||||||
| Kafr ElSheikh | 150 | 37 | 113 | 24.67 | 18.46–32.14 |
χ2 = 5.494 df = 2 P = 0.064 |
| Qalyubia | 140 | 20 | 120 | 14.29 | 9.45–21.04 | |
| Gharbia | 140 | 24 | 116 | 17.14 | 11.8–24.24 | |
| Breed | ||||||
| Holstein | 170 | 25 | 145 | 14.71 | 10.17–20.81 |
χ2 = 3.139 df = 1 P = 0.076 |
| Mixed-breed | 260 | 56 | 204 | 21.54 | 16.98–26.93 | |
| Sex | ||||||
| Male | 190 | 37 | 153 | 19.47 | 14.47–25.68 |
χ2 = 0.090 df = 1 P = 0.764 |
| Female | 240 | 44 | 196 | 18.33 | 13.95–23.71 | |
| Age (month) | ||||||
| < 2 | 100 | 13 | 87 | 13.00 | 7.76–20.98 |
χ2 = 7.905 df = 2 P = 0.011* |
| 2–6 | 150 | 23 | 127 | 15.33 | 10.44–21.95 | |
| > 6 | 180 | 45 | 135 | 25.00 | 19.24–31.8 | |
| Body condition score | ||||||
| Poor | 180 | 35 | 145 | 19.44 | 14.32–25.83 |
χ2 = 0.075 df = 1 P = 0.785 |
| Good | 250 | 46 | 204 | 18.40 | 14.09–23.67 | |
| Feed source | ||||||
| Pasture | 150 | 18 | 132 | 12.00 | 7.73–18.17 |
χ2 = 7.043 df = 1 P = 0.008* |
| Pasture and milk | 280 | 63 | 217 | 22.50 | 18–27.74 | |
| Hygienic status | ||||||
| Good | 170 | 22 | 148 | 12.94 | 8.7–18.82 |
χ2 = 6.393 df = 1 P = 0.011* |
| Poor | 260 | 59 | 201 | 22.69 | 18.02–28.16 | |
| Presence of diarrhea | ||||||
| Yes | 320 | 70 | 250 | 21.88 | 17.7–27.63 |
χ2 = 7.550 df = 1 P = 0.006* |
| No | 110 | 11 | 99 | 10.00 | 5.68–17.02 | |
| Contact with domestic animals | ||||||
| Yes | 190 | 48 | 142 | 25.26 | 19.61–31.89 |
χ2 = 9.194 df = 1 P = 0.002* |
| No | 240 | 33 | 207 | 13.75 | 9.96–18.68 | |
| Total | 430 | 81 | 349 | 18.84 | 15.43–22.81 | |
*The result considered significant if P < 0.05.
The prevalence in calves older than six months was substantially (P = 0.011) greater than in calves younger than six months. In addition, the Cryptosporidium infection increased significantly in calves living in poor hygienic condition (22.69%, 95%CI 18.02–28.16) compared to calves living in good condition status (12.94%, 95%CI 8.7–18.82), and it was significantly higher in calves feeding on pasture and milk (22.5%, 95%CI 18–27.74) than in calves feeding on pastures only (12%, 95%CI 7.73–18.17), Table 1. Additionally, compared to non-diarrheic calves, diarrheic calves had a considerably higher prevalence of Cryptosporidium (21.88%, 95% CI 17.7–27.63, P = 0.006), and calves that had contact with other domestic animals had a significantly higher prevalence (25.26%, 95% CI 19.61–31.89, P = 0.002), Table 1.
Table 2 showed the results of multivariate logistic regression analysis on significant factors (P < 0.05) in univariate analysis, which were age, feed source, sanitary state, presence of diarrhea, and contact with other domestic animals. The prevalence of Cryptosporidium infection increased significantly with age, older calves were two times (OR 1.96, 95%CI 0.97–3.94) more likely to contract the Cryptosporidium infection as compared to young calves. Farms had poor hygiene condition and pasture and milk as source of feeding increased the risk of Cryptosporidium infection by two folds (OR 2.25, 95%CI 1.28–3.94) and (OR 2.07, 95%CI 1.15–3.72), respectively. Animals with diarrhea were 2.47 times (OR 2.47, 95%CI 1.23–4.96) more likely to acquire Cryptosporidium infection than normal calves. Moreover, the risk of Cryptosporidium infection increased two times (OR 2.08, 95%CI 1.24–3.50) more among calves in contact with domestic animals than other.
Table 2.
Risk factors associated with Cryptosporidium prevalence in calves.
| Variable | B | S.E | OR | 95% CI for OR | P value |
|---|---|---|---|---|---|
| Age | |||||
| 2–6 | 0.178 | 0.385 | 1.19 | 0.56–2.54 | 0.645 |
| > 6 | 0.672 | 0.356 | 1.96 | 0.97–3.94 | 0.039 |
| Feed source | |||||
| Pasture and milk | 0.727 | 0.300 | 2.07 | 1.15–3.72 | 0.015 |
| Hygienic status | |||||
| Poor | 0.810 | 0.287 | 2.25 | 1.28–3.94 | 0.005 |
| Presence of diarrhea | |||||
| Yes | 0.906 | 0.355 | 2.47 | 1.23–4.96 | 0.011 |
| Contact with domestic animals | |||||
| Yes | 0.732 | 0.265 | 2.08 | 1.24–3.50 | 0.006 |
B Logistic regression coefficient, SE Standard error, OR Odds ratio, CI Confidence interval.
Discussion
Cryptosporidiosis in animals is considered a zoonotic risk to humans, due to the release of large numbers of resistant oocysts, which contaminate surface water. The Veterinary researchers were interested in cryptosporidiosis because, in addition to its zoonotic significance, it may develop into a dangerous, difficult-to-control disease in many farm animals and cause large economic losses. The present study aimed to evaluate the prevalence of Cryptosporidium infection and asses the associated risk factors in calves.
In the present study, the total Cryptosporidium prevalence in calves was found to be 18.84% (81/430). This corresponds to the findings of Ayele et al.41, who reported an infection rate of 18.6% in dairy calves in northwest Ethiopia. In addition, the prevalence rate in this study is consistent with the previously reported rate (19.2%) for bovine calves in Ismailia governorates in Egypt30. Similar prevalence rate (17.9%) was found in dairy calves from France42. This study's prevalence rate for Cryptosporidium infection was lower than the reported rates in eastern Ethiopia 27.8% by Regassa et al.43, USA 35.5% by Santın et al.44, Vietnam 44.3% by Nguyen et al.45 and in UK 27.9% by Brook et al.46 but higher than 7.8% in, 13.6%, and 15.8% which reported by Wegayehu et al.47, Ayana and Alemu48, and Wegayehu et al.49 in Ethiopia, respectively.
Furthermore, the detectable species in examined calves was C. parvum which come in accordance with the findings of Singh et al.50 who reported 79.41% of young dairy calves in Punjab infected by C. parvum. Also, other studies reported the more prevalent Cryptosporidium species in calves in Ethiopia and Egypt is C. parvum with prevalence rate of 18.6% and 32.2%, respectively30,51.
The differences in overall Cryptosporidium prevalence between studies could be attributed to differences in ecology, research design, seasons, management system, age, herd size, and laboratory tests used23,28,46,48,52–58. The diagnostic procedures used in this survey are less sensitive and can produce misleading negative results. This could potentially be the explanation for report variation59.
The sex had no effect on the prevalence of Cryptosporidium infection in the current study, which come in agreement with previous findings of Paul et al.34. In contrast, other studies reported significant role for sex on prevalence of Cryptosporidium in calves32,60.
The significant effect of age on Cryptosporidium prevalence in calve in this study was consistent with previous findings of Wegayehu et al.49in Ethiopia, they found higher prevalence in calves older than 3 months and stated infection was age related and 92.1% were infected with C. andrsoni which infect older age calves. In contrast, Geurden et al.61, Ayele et al.41 and Venu et al.59 stated that infection rate decreased with the increase of age. Similarly, the effect of age on prevalence of Cryptosporidium infection in calve was observed in other studies16,41. This might be due to lower tolerance of young calves as a result of immature immune system. Calves under four months of age are more susceptible to contracting Cryptosporidium infection46. This findings is consistent with the findings of Kváč et al.62, who observed that infection resistance can evolve with age due to immunological development over time.
Additionally, a significant correlation was found between the hygienic condition of the farm and the occurrence of Cryptosporidium infection in calves. The current result is confirmed by the findings of Abebe et al.63, who found a significant association between Cryptosporidium infection and the hygiene status of the farms. In addition, a similar results were reported by El-Khodery and Osman24 and Castro-Hermida et al.64, they confirmed that poor hygiene enhances the infection rate and dissemination of Cryptosporidium species in animals. This could be attributed to the fact that muddy or dirty farm could probably establish a favourable condition for the presence or existence of Cryptosporidium oocysts in the surround environment of animals. This can be increasing the risk of contamination of food and water, hence increase the risk of Cryptosporidium infection in calves41,52,65–70.
The present findings are directly in line with previous findings of Ayele et al.41, who observed that the prevalence of Cryptosporidium increased significantly among calves feeding on pasture and milk. This may be due to pasture being contaminated with infectious oocysts, and switching between pasture and milk may produce digestive disturbances that increase the prevalence of cryptosporidiosis.
Cryptosporidiosis causes sever watery diarrhea in calves. The findings of the present study revealed strong association between presence of diarrhea and prevalence of Cryptosporidium in calves. This was explained by the fact that diarrheal animals had a higher rate of oocyst shedding than calves with regular faeces. This is consistent with those has been found in previous studies of El-Khodery and Osman24 and Abebe et al.63. This could be as a result of the infection causing villous atrophy and crypt hyperplasia, which reduces the intestine's surface area available for absorption71. Consequently, interfere with absorption of water, glucose and sodium leading to diarrhea72. Additionally, the parasite may be able to decrease the activity of the enzyme disaccharidase, which would reduce the amount of sugars broken down. This would lead to bacterial growth, the production of volatile fatty acids, and changes in osmotic pressure, which would then cause severe watery diarrhea73.
Different animal species and human are susceptible to Cryptosporidium infection and the infection can be transmitted by direct or indirect routes through fecal–oral route32. Consequently, mixing different animals species could facilitate the transmission of the disease42. Similarly, Mohammed et al.74 observed that keeping animals separately or avoiding close contact with animals of various species can reduce the risk of Cryptosporidium infection.
Conclusion
The prevalence of Cryptosporidium infection was widely spreading among calves in the studied governorates with rate of 18.84%. Based on the present findings, age, feed source, farm hygiene, occurrence of diarrhea, and interaction with various domestic animals were all risk factors for Cryptosporidium infection. It is essential to raise awareness of risk factors, sources of infection, and modes of transmission to control and reduce the disease transmission between animals and human. Further molecular researches in different areas of the country are recommended to determine species allocation and the disease's national impact.
Supplementary Information
Acknowledgements
The authors would like to acknowledge the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia for the financial support of this research through the Grant Number 3779.
Author contributions
Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing-original draft preparation, A.S., O.A.A., H.S.G., M.M., M.S. and A.A.; writing-review and editing, A.S., M.M., O.A.A., H.S.G., M.S. and A.A.; project administration, M.M.; funding acquisition, A.S., M.M., O.A.A., H.S.G., M.S. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported through the Annual Funding track by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant Number 3779).
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohamed Marzok, Email: mmarzok@kfu.edu.sa.
Abdelfattah Selim, Email: Abdelfattah.selim@fvtm.bu.edu.eg.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-44434-7.
References
- 1.Wang R, et al. Characteristics of Cryptosporidium transmission in preweaned dairy cattle in Henan, China. J. Clin. Microbiol. 2011;49:1077–1082. doi: 10.1128/JCM.02194-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Díaz P, et al. The age-related Cryptosporidium species distribution in asymptomatic cattle from North-Western Spain. Animals. 2021;11:256. doi: 10.3390/ani11020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broglia A, Reckinger S, Cacció S, Nöckler K. Distribution of Cryptosporidium parvum subtypes in calves in Germany. Vet. Parasitol. 2008;154:8–13. doi: 10.1016/j.vetpar.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Harp JA, Harmsen AG. Cryptosporidium parvum infection in gene-targeted B cell-deficient mice. J. Parasitol. 2003;89:391–393. doi: 10.1645/0022-3395(2003)089[0391:CPIIGB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Shafieyan H, Alborzi A, Ahmadinejad H, Tabandeh M. Prevalence of Cryptosporidium species in ruminants of Lorestan province, Iran. J. Parasit. Dis. 2014;12:63–94. doi: 10.1007/s12639-014-0642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalmers RM, Davies AP. Minireview: Clinical cryptosporidiosis. Exp. Parasitol. 2010;124:138–146. doi: 10.1016/j.exppara.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Ryan U, Fayer R, Xiao L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology. 2014;141:1667–1685. doi: 10.1017/S0031182014001085. [DOI] [PubMed] [Google Scholar]
- 8.Dumaine JE, Tandel J, Striepen B. Cryptosporidium parvum . Trends Parasitol. 2020;36:485–486. doi: 10.1016/j.pt.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Snelling WJ, et al. Cryptosporidiosis in developing countries. J. Infect. Dev. Ctries. 2007;1:242–256. doi: 10.3855/jidc.360. [DOI] [PubMed] [Google Scholar]
- 10.Goma F, et al. The prevalence and molecular characterisation of Cryptosporidium spp. in small ruminants in Zambia. Small Rumin. Res. 2007;72:77–80. doi: 10.1016/j.smallrumres.2006.08.010. [DOI] [Google Scholar]
- 11.de Graaf DC, Vanopdenbosch E, Ortega-Mora LM, Abbassi H, Peeters JE. A review of the importance of cryptosporidiosis in farm animals. Int. J. Parasitol. 1999;29:1269–1287. doi: 10.1016/S0020-7519(99)00076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trotz-Williams LA, et al. Calf-level risk factors for neonatal diarrhea and shedding of Cryptosporidium parvum in Ontario dairy calves. Prev. Vet. Med. 2007;82:12–28. doi: 10.1016/j.prevetmed.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCluskey B, Greiner E, Donovan G. Patterns of Cryptosporidium oocyst shedding in calves and a comparison of two diagnostic methods. Vet. Parasitol. 1995;60:185–190. doi: 10.1016/0304-4017(95)00790-4. [DOI] [PubMed] [Google Scholar]
- 14.Nasir A, Avais M, Khan M, Ahmad N. Prevalence of Cryptosporidium parvum infection in Lahore (Pakistan) and its association with diarrhea in dairy calves. Int. J. Agric. Biol. 2009;11:221–224. [Google Scholar]
- 15.Azami M. Prevalence of Cryptosporidium infection in cattle in Isfahan, Iran. J. Eukaryot. Microbiol. 2007;54:100–102. doi: 10.1111/j.1550-7408.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- 16.Joute J, Gill J, Singh B. Prevalence and molecular epidemiology of Cryptosporidium parvum in dairy calves in Punjab (India) J. Parasit. Dis. 2016;40:745–749. doi: 10.1007/s12639-014-0571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogendo, A. et al. Cryptosporidium infection in calves and the environment in Asembo, Western Kenya: 2015. Pan Afr. Med. J.28, 9 (2017). [DOI] [PMC free article] [PubMed]
- 18.Morgan FU, et al. Comparison of PCR and microscopy for detection of Cryptosporidium parvum in human fecal specimens: clinical trial. J. Clin. Microbiol. 1998;36:995–998. doi: 10.1128/JCM.36.4.995-998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster K, Smith H, Giles M, Dawson L, Robertson L. Detection of Cryptosporidium parvum oocysts in faeces: Comparison of conventional coproscopical methods and the polymerase chain reaction. Vet. Parasitol. 1996;61:5–13. doi: 10.1016/0304-4017(95)00811-X. [DOI] [PubMed] [Google Scholar]
- 20.Selim A, Abdelhady A. Neosporosis among Egyptian camels and its associated risk factors. Trop. Anim. Health Prod. 2020;52:3381–3385. doi: 10.1007/s11250-020-02370-y. [DOI] [PubMed] [Google Scholar]
- 21.Selim A, Ali A-F, Ramadan E. Prevalence and molecular epidemiology of Johne’s disease in Egyptian cattle. Acta Tropica. 2019;195:1–5. doi: 10.1016/j.actatropica.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Salem G, el-Assaly T. The role of wild rats as a reservoir of some internal parasites in Qalyobia governorate. J. Egypt. Soc. Parasitol. 1999;29:495–503. [PubMed] [Google Scholar]
- 23.Amer S, et al. Cryptosporidium genotypes and subtypes in dairy calves in Egypt. Vet. Parasitol. 2010;169:382–386. doi: 10.1016/j.vetpar.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 24.El-Khodery SA, Osman SA. Cryptosporidiosis in buffalo calves (Bubalus bubalis): prevalence and potential risk factors. Trop. Anim. Health Prod. 2008;40:419–426. doi: 10.1007/s11250-007-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elhaig MM, Selim A, Mandour AS, Schulz C, Hoffmann B. Prevalence and molecular characterization of peste des petits ruminants virus from Ismailia and Suez, Northeastern Egypt, 2014–2016. Small Rumin. Res. 2018;169:94–98. doi: 10.1016/j.smallrumres.2018.07.001. [DOI] [Google Scholar]
- 26.Reisberg K, Selim AM, Gaede W. Simultaneous detection of Chlamydia spp., Coxiella burnetii, and Neospora caninum in abortion material of ruminants by multiplex real-time polymerase chain reaction. J. Vet. Diagn. Investig. 2013;25:614–619. doi: 10.1177/1040638713497483. [DOI] [PubMed] [Google Scholar]
- 27.Selim A, Abdelhady A, Alahadeb J. Prevalence and first molecular characterization of Ehrlichia canis in Egyptian dogs. Pak. Vet. J. 2020;41:117–121. doi: 10.29261/pakvetj/2020.061. [DOI] [Google Scholar]
- 28.Selim A, Yang E, Rousset E, Thiéry R, Sidi-Boumedine K. Characterization of Coxiella burnetii strains from ruminants in a Galleria mellonella host-based model. New Microbes New Infect. 2018;24:8–13. doi: 10.1016/j.nmni.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selim A, Elhaig MM, Taha SA, Nasr EA. Antibacterial activity of silver nanoparticles against field and reference strains of Mycobacterium tuberculosis, Mycobacterium bovis and multiple-drug-resistant tuberculosis strains. Revue scientifique et technique (International Office of Epizootics) 2018;37:823–830. doi: 10.20506/rst.37.3.2888. [DOI] [PubMed] [Google Scholar]
- 30.Helmy YA, Krücken J, Nöckler K, von Samson-Himmelstjerna G, Zessin K-H. Molecular epidemiology of Cryptosporidium in livestock animals and humans in the Ismailia province of Egypt. Vet. Parasitol. 2013;193:15–24. doi: 10.1016/j.vetpar.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Thrusfield M. Veterinary epidemiology. Wiley; 2018. [Google Scholar]
- 32.Maurya PS, et al. Prevalence and risk factors associated with Cryptosporidium spp. infection in young domestic livestock in India. Trop. Anim. Health Prod. 2013;45:941–946. doi: 10.1007/s11250-012-0311-1. [DOI] [PubMed] [Google Scholar]
- 33.Anderson B. Enteritis caused by Cryptosporidium in calves. Vet. Med. Small Anim. Clin. 1981;76:865–868. [PubMed] [Google Scholar]
- 34.Paul S, et al. Prevalence and molecular characterization of bovine Cryptosporidium isolates in India. Vet. Parasitol. 2008;153:143–146. doi: 10.1016/j.vetpar.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 35.Selim A, Abdelhady A. The first detection of anti-West Nile virus antibody in domestic ruminants in Egypt. Trop. Anim. Health Prod. 2020;52:3147–3151. doi: 10.1007/s11250-020-02339-x. [DOI] [PubMed] [Google Scholar]
- 36.Selim A, et al. Prevalence and animal level risk factors associated with Trypanosoma evansi infection in dromedary camels. Sci. Rep. 2022;12:8933. doi: 10.1038/s41598-022-12817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selim AM, Elhaig MM, Moawed SA, El-Nahas E. Modeling the potential risk factors of bovine viral diarrhea prevalence in Egypt using univariable and multivariable logistic regression analyses. Vet. World. 2018;11:259. doi: 10.14202/vetworld.2018.259-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selim A, Attia KA, Alsubki RA, Kimiko I, Sayed-Ahmed MZ. Cross-sectional survey on Mycobacterium avium subsp. paratuberculosis in Dromedary Camels: Seroprevalence and risk factors. Acta Trop. 2022;226:10626. doi: 10.1016/j.actatropica.2021.106261. [DOI] [PubMed] [Google Scholar]
- 39.Selim A, Khater H, Almohammed HI. A recent update about seroprevalence of ovine neosporosis in Northern Egypt and its associated risk factors. Sci. Rep. 2021;11:14043. doi: 10.1038/s41598-021-93596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selim A, Megahed A, Kandeel S, Alouffi A, Almutairi MM. West Nile virus seroprevalence and associated risk factors among horses in Egypt. Sci. Rep. 2021;11:20932. doi: 10.1038/s41598-021-00449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayele A, Seyoum Z, Leta S. Cryptosporidium infection in bovine calves: Prevalence and potential risk factors in northwest Ethiopia. BMC Res. Notes. 2018;11:1–6. doi: 10.1186/s13104-018-3219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefay D, Naciri M, Poirier P, Chermette R. Prevalence of Cryptosporidium infection in calves in France. Vet. Parasitol. 2000;89:1–9. doi: 10.1016/S0304-4017(99)00230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regassa A, et al. Cryptosporidium in calves, lambs and kids at Haramaya, eastern Ethiopia. Ethiop. Vet. J. 2013;17:81–94. doi: 10.4314/evj.v17i1.7. [DOI] [Google Scholar]
- 44.Santın M, et al. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet. Parasitol. 2004;122:103–117. doi: 10.1016/j.vetpar.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen ST, et al. Prevalence and first genetic identification of Cryptosporidium spp. in cattle in central Viet Nam. Vet. Parasitol. 2007;150:357–361. doi: 10.1016/j.vetpar.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Brook E, Hart CA, French N, Christley R. Prevalence and risk factors for Cryptosporidium spp. infection in young calves. Vet. Parasitol. 2008;152:46–52. doi: 10.1016/j.vetpar.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Wegayehu T, Adamu H, Petros B. Prevalence of Giardia duodenalis and Cryptosporidium species infections among children and cattle in North Shewa Zone, Ethiopia. BMC Infect. Dis. 2013;13:1–7. doi: 10.1186/1471-2334-13-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ayana D, Alemu B. Cryptosporidiosis in calves, lambs and goat kids in Bishoftu, Oromia regional state, Ethiopia. Afr. J. Basic Appl. Sci. 2015;7:233–239. [Google Scholar]
- 49.Wegayehu T, et al. Prevalence and genetic characterization of Cryptosporidium species in dairy calves in Central Ethiopia. PLoS ONE. 2016;11:e0154647. doi: 10.1371/journal.pone.0154647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh BB, et al. Prevalence of Cryptosporidium parvum infection in Punjab (India) and its association with diarrhea in neonatal dairy calves. Vet. Parasitol. 2006;140:162–165. doi: 10.1016/j.vetpar.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 51.Manyazewal A, et al. Prevalence, risk factors and molecular characterization of Cryptosporidium infection in cattle in Addis Ababa and its environs, Ethiopia. Vet. Parasitol. Region. Stud. Rep. 2018;13:79–84. doi: 10.1016/j.vprsr.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selim A, Manaa E, Khater H. Seroprevalence and risk factors for lumpy skin disease in cattle in Northern Egypt. Trop. Anim. Health Prod. 2021;53:350. doi: 10.1007/s11250-021-02786-0. [DOI] [PubMed] [Google Scholar]
- 53.Selim A, Manaa E, Khater H. Molecular characterization and phylogenetic analysis of lumpy skin disease in Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2021;79:101699. doi: 10.1016/j.cimid.2021.101699. [DOI] [PubMed] [Google Scholar]
- 54.Selim A, Manaa EA, Alanazi AD, Alyousif MS. Seroprevalence, risk factors and molecular identification of bovine leukemia virus in Egyptian cattle. Animals. 2021;11:319. doi: 10.3390/ani11020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selim A, Manaa EA, Waheed RM, Alanazi AD. Seroprevalence, associated risk factors analysis and first molecular characterization of chlamydia abortus among Egyptian sheep. Comp. Immunol. Microbiol. Infect. Dis. 2021;74:101600. doi: 10.1016/j.cimid.2020.101600. [DOI] [PubMed] [Google Scholar]
- 56.Selim A, Megahed AA, Kandeel S, Abdelhady A. Risk factor analysis of bovine leukemia virus infection in dairy cattle in Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2020;72:101517. doi: 10.1016/j.cimid.2020.101517. [DOI] [PubMed] [Google Scholar]
- 57.Selim, A., Radwan, A., Arnaout, F. & Khater, H. The recent update of the situation of West Nile Fever among equids in Egypt after three decades of missing information. Pak. Vet. J.40, 390–393 (2020).
- 58.Selim AM, Elhaig MM, Gaede W. Development of multiplex real-time PCR assay for the detection of Brucella spp., Leptospira spp. and Campylobacter foetus. Vet. Ital. 2014;50:75. doi: 10.12834/VetIt.222.702.3. [DOI] [PubMed] [Google Scholar]
- 59.Venu R, et al. Factors influencing on prevalence of Cryptosporidium infection in south Indian dairy calves. J. Parasit. Dis. 2013;37:168–172. doi: 10.1007/s12639-012-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhat S, Juyal P, Singla L. Prevalence of cryptosporidiosis in neonatal buffalo calves in Ludhiana district of Punjab, India. Asian J. Anim. Vet. Adv. 2012;7:512–520. doi: 10.3923/ajava.2012.512.520. [DOI] [Google Scholar]
- 61.Geurden T, et al. Prevalence and genotyping of Cryptosporidium in three cattle husbandry systems in Zambia. Vet. Parasitol. 2006;138:217–222. doi: 10.1016/j.vetpar.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Kváč M, Kouba M, Vítovec J. Age-related and housing-dependence of Cryptosporidium infection of calves from dairy and beef herds in South Bohemia, Czech Republic. Vet. Parasitol. 2006;137:202–209. doi: 10.1016/j.vetpar.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 63.Abebe R, Wossene A, Kumsa B. An epidemiological study of Cryptosporidium infection in dairy calves on selected dairy farms of central Ethiopia. Revue de Médecine Véterinaire. 2008;159:107. [Google Scholar]
- 64.Castro-Hermida JA, González-Losada YA, Ares-Mazás E. Prevalence of and risk factors involved in the spread of neonatal bovine cryptosporidiosis in Galicia (NW Spain) Vet. Parasitol. 2002;106:1–10. doi: 10.1016/S0304-4017(02)00036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Selim A, Almohammed H, Abdelhady A, Alouffi A, Alshammari FA. Molecular detection and risk factors for Anaplasma platys infection in dogs from Egypt. Parasites Vectors. 2021;14:429. doi: 10.1186/s13071-021-04943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selim A, Attia K, Ramadan E, Hafez YM, Salman A. Seroprevalence and molecular characterization of Brucella species in naturally infected cattle and sheep. Prev. Vet. Med. 2019;171:104756. doi: 10.1016/j.prevetmed.2019.104756. [DOI] [PubMed] [Google Scholar]
- 67.Selim A, Megahed A, Ben Said M, Alanazi AD, Sayed-Ahmed MZ. Molecular survey and phylogenetic analysis of Babesia vogeli in dogs. Sci. Rep. 2022;12:6988. doi: 10.1038/s41598-022-11079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Selim A, El-Haig M, Galila ES, Gaede W. Direct detection of Mycobacterium avium subsp. Paratuberculosis in bovine milk by multiplex Real-time PCR. Anim. Sci. Pap. Rep. 2013;31:291–302. [Google Scholar]
- 69.Selim A, Khater H. Seroprevalence and risk factors associated with Equine piroplasmosis in North Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2020;73:101549. doi: 10.1016/j.cimid.2020.101549. [DOI] [PubMed] [Google Scholar]
- 70.Selim A, Alanazi AD, Sazmand A, Otranto D. Seroprevalence and associated risk factors for vector-borne pathogens in dogs from Egypt. Parasites Vectors. 2021;14:1–11. doi: 10.1186/s13071-021-04670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foster D, Smith GW. Pathophysiology of diarrhea in calves. Vet. Clin. N. Am. Food Anim. Pract. 2009;25:13–36. doi: 10.1016/j.cvfa.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radostits OM, Gay C, Hinchcliff KW, Constable PD. Veterinary Medicine E-Book: A textbook of the diseases of cattle, horses, sheep, pigs and goats. Elsevier Health Sciences; 2006. [Google Scholar]
- 73.Rickard, L. Veterinary Parasitology. The Practical Veterinarian. Printed in the United States of America (2001).
- 74.Mohammed H, Wade S, Schaaf S. Risk factors associated with Cryptosporidium parvum infection in dairy cattle in southeastern New York State. Vet. Parasitol. 1999;83:1–13. doi: 10.1016/S0304-4017(99)00032-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.


