Abstract
Melanopsin (OPN4) is a light-sensitive protein that plays a vital role in the regulation of circadian rhythms and other nonvisual functions. Current research on OPN4 has focused on mammals; more evidence is needed from non-mammalian vertebrates to fully assess the significance of the non-visual photosensitization of OPN4 for circadian rhythm regulation. There are species differences in the regulatory mechanisms of OPN4 for vertebrate circadian rhythms, which may be due to the differences in the cutting variants, tissue localization, and photosensitive activation pathway of OPN4. We here summarize the distribution of OPN4 in mammals, birds, and teleost fish, and the classical excitation mode for the non-visual photosensitive function of OPN4 in mammals is discussed. In addition, the role of OPN4-expressing cells in regulating circadian rhythm in different vertebrates is highlighted, and the potential rhythmic regulatory effects of various neuropeptides or neurotransmitters expressed in mammalian OPN4-expressing ganglion cells are summarized among them.
Subject terms: Circadian regulation, Molecular neuroscience
This review looks at melanopsin across vertebrate species and discusses melanopsin-positive cells in the regulation of circadian rhythm.
Introduction
In the vertebrate retina, the sensitivity of dim-light vision is supported by rod photoreceptors, whereas cone photoreceptors mediate color discrimination and high visual acuity at higher light intensities1,2. Compared with visual forming visual pathways, the regulation of non-image forming visual pathways is performed by intrinsically photosensitive retinal ganglion cells (ipRGCs), such as circadian entrainment3, pupillary light reflex4–6, and time-restricted feeding7. Although ipRGCs are less represented in mammalian retina (mice: ~1–5%, human: ~0.4–1.5%)8–12, melanopsin (OPN4), as an opsin, gives it powerful non-image forming function13,14.
OPN4 is a G protein-coupled receptor initially identified in the dermal melanocytes of Xenopus laevis. It includes an extracellular amino-terminal and seven transmembrane domains with high homology to invertebrate opsins15. The OPN4 gene has been detected in most vertebrates and analyzed in two lineages, xenopus (OPN4x) and mammalian (OPN4m) orthologs16. It was also found that there are two OPN4m splice variants in mice and humans, the short (OPN4-S) and long (OPN4-L) isoforms, which differ mainly in the number of phosphorylatable serines and threonines in the C-terminus, which may lead to differences in the inactivation dynamics of OPN4 in different species16,17. Regarding photosensitivity, the λmax (peak sensitivity) of OPN4 was 480 nm as measured directly in light response to ipRGCs, confirmed by mouse models lacking rods and cones13,18,19. Notably, the peak sensitivity of OPN4 shows some minor differences in many studies depending on differences in the detection methods, technology, or species20,21.
Here, we collate the distribution of OPN4 in mammals, birds, and teleost fish based on published evidence. We then highlight the mechanisms by which the non-visual photosensitization of OPN4 mediates in vertebrate circadian rhythm regulation. Taking the photosensitive activation of OPN4 as a starting point, our review focuses on the mechanism of OPN4-mediated photoentrainment action in circadian rhythm regulation in vertebrates. Admittedly, the other OPN4 activations of G-protein coexist and have been summarized in recent relevant reviews22–24. In this article, the Gq/11 pathway in the OPN4-mediated phototransduction was mainly described due to its widespread presence in vertebrate ipRGCs25–28. In addition, OPN4-mediated light entrainment impacts melatonin secretion in the vertebrate retina and pineal gland. This leads to more diverse rhythmic regulatory pathways in non-mammalian vertebrates than mammals.
Distribution of OPN4 in vertebrates
Mammalian
Mammalian OPN4 is derived from a single OPN4 gene with two splice variants in ipRGCs, which has been localized and accurately classified by much evidence. OPN4 is also distributed in mammalian peripheral tissues (Table 1), but its functions remain to be further investigated. Therefore, OPN4 in mammalian ipRGCs will be discussed first.
Table 1.
Photosensitivity of vertebrate OPN4 in peripheral organs.
| Taxa | Species | Location | Related effects | References |
|---|---|---|---|---|
| Amphibia | Xenopus laevis | Melanocyte | Skin pigmentation | Provencio et al.15 |
| Reptile | Hydrophiinae | Skin | Tail phototaxis | Crowe-Riddell et al.27 |
| Mammal | Mouse | Aortas, pulmonary arteries, airway smooth muscle | Light-dependent relaxation | Sikka et al.172; Barreto et al.173; Yim et al.174 |
| Mammal | Mouse | Melanocytes | Pigmentation | de Assis et al.175 |
| Mammal | Human | Mesenchymal stem cells | Angiogenesis | Yang et al.176 |
| Mammal | Human | Subcutaneous white adipose tissue | Lipolysis of lipid droplets | Ondrusova et al.177 |
The OPN4-expressing ipRGCs were previously thought to be a homogeneous cell population with sparsely branched dendritic trees on the outermost layer of the inner plexiform layer in mammals8. Subsequently, the expression of OPN4 in M1-M6 ipRGCs in the mouse retina has been identified12,29. This OPN4 in ipRGCs with various morphological and physiological characteristics can provide complete light-dark discrimination and partial vision in rodless/coneless (rd/rd cl) mice30,31. Among these subtypes of ipRGCs, M1 expressed the highest content of OPN4, and it mainly exerts OPN4-induced photoentrainment32–34. Correspondingly, the suprachiasmatic nucleus (SCN) is innervated primarily by M1-subtype ipRGCs (~80%), and OPN4 in M1-subtype ipRGCs significantly regulates rhythmic regulation in mammals35,36.
Birds
Mammals lost OPN4x during evolution and chromosomal re-arrangements37,38, which accompanied mammal adaptation to the nocturnal niche39,40. In a bird’s retina, two lineages for OPN4 are expressed41. OPN4m is stably expressed in the retinal ganglion cells (RGCs) during the development of birds, whereas OPN4x was limited to the forming RGCs at embryonic 8 (E8), but mainly expressed in PROX1-positive horizontal cells (HCs) at E1542. These OPN4-expressing horizontal cell precursors continue to express OPN4x after migrating and developing into horizontal cells43.
In contrast to mammals, the distribution of OPN4 in birds is no longer concentrated in the retina (Fig. 1)44. Bird pinealocytes are directly photosensitive45, and the reconstitution of the recombinant proteins with 11-cis-retinal demonstrated that it expresses two lineages of melanopsins46. The transcriptional levels of OPN4 in the pineal gland showed a more robust diurnal feature than that in the retina and were significantly increased at night41. Although avian pinealocytes possess both OPN4m and OPN4x (also called OPN4-1 and OPN4-2 in chickens), their distribution is not cell-specific. It may activate different types of G proteins to perform light-sensing functions47. In addition, multiple nuclei composing deep brain photoreceptors in birds also express OPN4 (Fig. 1), including the lateral septal organ, premammillaris nucleus, paraventricular nucleus (PVN), and paraventricular organ48. OPN4-positive dopaminergic neurons in these nuclei can respond to daytime length49.
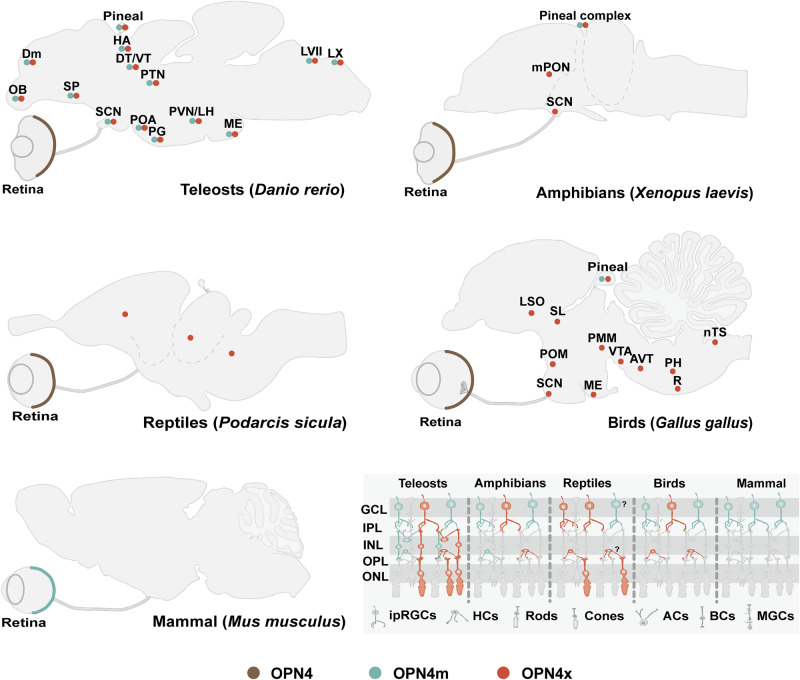
Fig. 1. Distribution of OPN4 in teleost fish, amphibian, reptiles, birds, and mammals on generalized sagittal sections.
In teleost fish and birds, two orthologs of OPN4 are distributed in the retina, brain, and pineal gland44,61,64,166–168. For teleosts, amphibians, and reptiles, splice variants of OPN4 are classified as OPN4m and OPN4x. In the reptile brain, OPN4x expression has been detected in the telencephalon, mesencephalon, and rhombencephalon, but the specific nuclei are still unclear. It is important to note that the available evidence does not determine the cell type of OPN4x in the inner nuclear layer or whether OPN4m is present in RGCs in reptiles. Mammalian OPN4 is mainly expressed in the retina, which integrates more complex photosensitive functions and widely projects to different brain regions through different subtypes of ipRGCs to regulate various physiological functions14. Brackets indicate representative species. AC amacrine cell, AVT area ventralis of tsai, BC bipolar cell, Dm the medial zone of the dorsal telencephalic region, DT dorsal thalamus, HA habenula, HC horizontal cell, ipRGC intrinsically photosensitive retinal ganglion cell, LH lateral hypothalamic nucleus, LSO lateral septal organ, LVII facial lobe, LX vagal lobe, ME median eminence, MGC Muller glial cell, mPON magnocellular preoptic nucleus, nTS nucleus tractus solitarius, PG preglomerular area, PH plexus of horsley, PMM nucleus premammillaris, POA preoptic area, POM medial preoptic nucleus, PTN posterior tuberal nucleus, PVN periventricular nucleus, R raphe nucleus, SCN suprachiasmatic nucleus, SL nucleus septalis lateralis, SP subpallium, VT ventral thalamus, VTA ventral tegmental area.
Reptiles
The studies on OPN4 in reptiles has mainly focused on lizards, sea snakes, and turtles, but there still needs to be more evidence to locate the expression sites of OPN4 and its isoforms accurately. To date, OPN4m was not detected in sea snakes, while OPN4x was mainly expressed in RGCs and cone cells50. Although OPN4x-positive staining was also observed in the inner nuclear layer50, the cell type could not be determined. In freshwater turtles, OPN4m is highly expressed in the retina, but it is not yet certain whether OPN4m is localized in RGCs51. In extraretinal photoreceptors, OPN4x is also expressed in the lateral eye and brain of ruin lizards but has not been detected in the pineal gland52.
Amphibians
When it was discovered, melanopsin was found in the retina, melanophores, and deep brain photoreceptors of Xenopus laevis15. Both OPN4m and OPN4x have been localized in RGCs, horizontal cells, and pineal complex53,54. OPN4-expressing RGCs have been shown to participate in the melanocyte pigmentation process by producing alpha-melanocyte stimulating hormone in the pituitary gland55. OPN4 in the pineal complex may participate in the change of skin color through the neuroendocrine pathway54. These photosensitive neuroendocrine circuits enable Xenopus to maintain rapid physiological pigmentation change.
Teleost fish
Five splice variants were detected in zebrafish (OPN4.1, OPN4a, OPN4b, OPN4xa, and OPN4xb), which are derived from two melanopsin lineages (OPN4m and OPN4x) to confer overall photosensitivity to the teleost retina and to adapt to the dynamic light environments in the aquatic habitats56. Similar to birds, both lineages of OPN4 are expressed in RGCs, and partial OPN4 splice variants are distributed in horizontal cells57–59, which independently mediates the role of HCs in photosensitive signaling60. In extraretinal tissue, OPN4m was detected in the dorsal thalamus, ventral hypothalamus, and nucleus lateralis tuberis pars lateralis; OPN4x was evident in the SCN and habenular nucleus59. Evidence for functional partitioning suggests that OPN4m mediates the light-seeking behavior in larvae distributed in the preoptic area61, whereas OPN4x regulates circadian rhythms in the SCN62. The zebrafish pineal gland is a photosensitive structure with various opsins, a subpopulation of pinealocytes capable of sensing shorter wavelength light, characterized by the expression of OPN4x63. In addition, two splice variants of OPN4, OPN4.1 and OPN4xb, were detected in the pineal gland, which is responsible for inhibiting melatonin synthesis during the day and maintaining voluntary movements in a state of absolute arousal64.
Overall, current evidence has shown that OPN4 is mainly distributed in the retina of mammals, while it is also widely expressed in the brains of teleost fish, amphibians, reptiles, and birds (Fig. 1).
Light activation of OPN4 in the retina
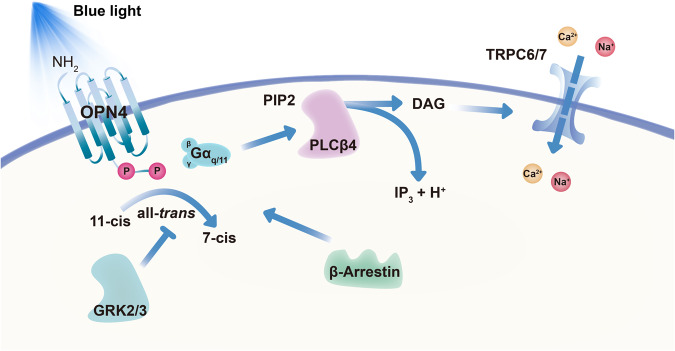
OPN4 is a G protein-coupled receptor with 11-cis retinal as a covalently bound protonated Schiff base (PSB11)65. Under the induction of light, the conformation of 11-cis retinal changed with the transformation of PBS11 to its all-trans isomer, which changed the state of PSB11 to a 7-cis state66–68. In this series of changes, the 11-cis and 7-cis retinal indicate OPN4’s silent state, while the all-trans structure indicates light signaling conversion68. This tristability confers on OPN4 a sustained response to light and a broader spectrum of its own69. Following this reaction, the Gq/11 class of G-proteins will become active and further trigger the activation of phospholipase C-beta 4 (PLCβ4). This leads to the hydrolysis of phosphatidylinositol 4,5-bisphosphate to form inositol triphosphate and diacylglycerol through the transient receptor potential cation channel subfamily C member 6/7 (TrpC6/7) nonselective cation channels in the cell membrane and finally increases the intracellular Ca2+ concentration (Fig. 2)14,21,23. Using calcium ion probes, Sekaran et al. consistently found that OPN4 can specifically respond to a wavelength of 470 nm with a significant increase in Ca2+ concentration in a mouse model lacking cone and rod photoreceptors70. In addition, a recent study demonstrated that internally released Ca2+ marks the opening of the OPN4-mediated light-sensitive pathway71, which is the opposite of the hyperpolarization of rods and cones14. In the opsin photosensitive response termination, OPN4 is subject to C-terminal phosphorylation. Its phosphorylation process preferentially interacts with G protein-coupled receptor, kinase 2/3 (GRK2/3), preventing OPN4-expressing ipRGCs from generating sustained action potentials after light stimulation72–74. Meanwhile, arrestin is also involved in the inhibition and reactivation of the light response of OPN4. When the C-terminus of OPN4 is phosphorylated, it can bind to arrestin. β-arrestin 2 primarily regulates the deactivation of OPN4, whereas β-arrestin 1 initiates regeneration of OPN475,76. The above responses allow ipRGCs to sustain responses under prolonged illumination (Fig. 2).
Fig. 2. Activation and termination of OPN4 in M1-subtype ipRGCs of mammals.
The OPN4-mediated light-sensitive pathways are predominantly triggered by the downstream Gq/11, PLCβ4, and TRPC6/7 cation channels in mammals. Retinaldehyde is covalently bonded to the transmembrane structure in OPN4, and light (especially near 480 nm) can change its conformation from an 11-cis to an all-trans state to a 7-cis state (silent state). It will trigger downstream Gq/11 coupling, causing PLCβ4 to break down PIP2 into DAG and IP3, where DAG activates the opening of the TRPC6/7 cation channels. The activated C-terminus of OPN4 is phosphorylated in response to GRK2/3, resulting in inactivation. This process may also involve β-Arrestin 2. In addition, β-Arrestin 1 leads to the isomer regeneration of OPN4, which serves subsequent light activation. DAG diacylglycerol, Gq/11 G protein subunit alpha q/11, GRK2/3 G protein-coupled receptor kinase 2/3, PLCβ4 phospholipase C-beta 4, PIP2 phosphatidylinositol bisphosphate, IP3 inositol triphosphate, TRPC6/7 transient receptor potential cation channel subfamily C member 6/7.
Due to the complexity of the G protein family and the variation of OPN4 subtypes in different species, the optical signal transduction of the OPN4 pathway is mainly dependent on Gq/11 in M1-subtype ipRGCs in mammalian and partial OPN4-expressing cells in non-mammalian vertebrates25,28,47,77,78. Recent studies have shown that adenylyl cyclase 2 and cAMP mediate the phototransduction of OPN4 in M4-subtype ipRGCs24. Considering the ability of retinal adenosine to influence photosensitive electrophysiological activity in the retina79, the effect of cAMP on OPN4 phototransduction cannot be ignored. Admittedly, OPN4-mediated phototransduction mechanisms have also been implicated in species that involve Gi/o (human, mouse, and amphioxus), Gs (chicken), or Gt (chicken) activity26,47,80.
Photosensitive regulation of circadian rhythms by OPN4
OPN4-induced non-visual photosensitive signals can target numerous nuclei, including the SCN, intergeniculate leaflet, ventral lateral geniculate nucleus, and olivary pretectal nucleus (OPN)81. The SCN is the center for orchestrating mammalian circadian rhythms, while the OPN is essential for regulating the pupillary light reflex82,83. The synaptic structures at the ends of these tracts specialize in different nucleus regions, leading to differences in threshold sensitivity, speed, and accuracy of visual responses in these nuclei84. As we mentioned above, M1-subtype ipRGCs form the core of non-visual photosensitivity. According to the molecularly defined Brn3b transcription factor expression, the M1 subtype consists of two distinct subpopulations. The majority of projections from M1 ipRGCs to the thalamus and midbrain are Brn3b-positive M1-subtype ipRGCs85, which regulate OPN4-dependent pupillary light reflexes and light-induced acute body temperature changes83,86,87. The SCN is innervated by Brn3b-negative M1-type ipRGCs83, and it is here that the SCN orchestrates multiple oscillators with a duration of almost 24 h88,89. Therefore, the non-visual function of OPN4 contributes to controlling central rhythms in mammals via Brn3b-negative M1-subtype ipRGCs projections in the SCN region.
Unlike mammals, chick retinal ganglion cells were classified into six subgroups according to their somal and dendritic characteristics (subgroups Ic, Is, IIc, IIs, IIIs, and IVc)90,91. The subgroups IIs and IIIs had a more significant proportion of thalamic projection92. Identifying the function of RGCs from chickens is still challenging, despite similarities in RGC projection pathways to the brain between birds and mammals. Brn3b molecular markers commonly used in mammals may not be suitable for birds. All types of Brn3 factors (Brn3a, Brn3b, and Brn3c) can promote the differentiation of chick RGCs and are not mainly regulated by Brn3b as in mammals77. Furthermore, species differences make it challenging to directly administer current antibodies and viral vectors to birds’ retinas or central nervous systems. Nevertheless, studying retinal ganglion cell subtypes in chicks may be more effectively accomplished using in vivo transfection or electroporation transfection93–95. For non-mammalian vertebrates, the pineal gland of birds and teleost fish has rhythmic pacing functions and is involved in constituting the multi-oscillatory circadian timing system96,97. The photosensitization of photoreceptors in the retina by OPN4 may have limited effects on circadian rhythms in these species. Therefore, when discussing OPN4-mediated non-visual photosensitive functions, the pineal gland of non-mammalian vertebrates will also be emphasized.
It should be noted that cones and rods can affect not just the local biological clock of the retina98,99, but also the master clock of the SCN100. During development, ipRGCs form functional connections with the cone/rod system in the inner reticular layer, allowing them to serve as relays to transmit collected rod and cone information to the brain while retaining their intrinsic photosensitivity30,101. Photoentrainment induced by rods can influence the master clock via cone circuits, which may complement the function of photoentrainment in ipRGCs in dim light102. Accordingly, the light power required to activate OPN4 (>1 μW) under in vitro conditions is higher than conventional retinoids (~0.2 μW)103. At the same time, ultraviolet (λmax 365 nm) and green (λmax 505 nm) sensitive cone cells are also able to indirectly influence the electrophysiological activity of the neurons in the SCN via ipRGCs, contributing to photoentrainment100,104. Additionally, harmonizing the photosensitive signals from the cones, rods, and ipRGCs also plays a crucial role in ensuring the pupillary light reflex functions properly105. Thus, the influence of cones and rods on circadian rhythm regulation should not be undervalued.
Contribution of OPN4 to mammalian circadian rhythms
Exposure to monochromatic blue light (460 nm) can suppress human melatonin levels and interfere with resetting circadian rhythm106,107. As part of this regulation, the photosensitive signal of OPN4 is first transmitted to the SCN through the retino-hypothalamic tract (RHT), followed by the paraventricular nucleus and the intermediolateral nucleus via the polysynaptic circuit distributed in the SCN region, and finally to the release of melatonin innervated by the sympathetic nerve in the superior cervical ganglion (SCG)108,109. In addition to this approach, OPN4-positive ipRGCs can rely on self-synthesized neurotransmitters and neuropeptides to more directly and rapidly affect the SCN master clock.
Retinal glutamatergic signals are responsible for transmitting external light information to the SCN, and binocular enucleation induced a significant decrease in vesicular glutamate transporter 2 (Vglut2) immunoreactivity in the ventrolateral part of the SCN110. The experiments in OPN4Cre/+::Vglut2flox/flox transgenic mice proved that the glutamate transmission from ipRGCs is necessary for light to entrain circadian rhythms in dim light111. Regarding synaptic connections, glutamatergic ipRGCs have neural projections with many photosensitive neurons in the SCN. Many Vglut2-immunoreactive axons were observed to be in synaptic contact with vasoactive intestinal peptide (VIP)- and gamma-aminobutyric acid (GABA)-positive neurons112. ipRGCs have direct synaptic connections with arginine vasopressin (AVP) neurons in the dorsal SCN113. Glutamatergic signaling primarily controls the expression of clock genes concerning the regulation of the SCN master clock. The glutamatergic activation of the N-methyl-d-aspartic acid (NMDA) receptor leads to an influx of extracellular Ca2+, followed by Ca2+/calmodulin-dependent kinase II and nitric oxide synthase activation114,115. Then, the increased nitric oxide levels activate ryanodine receptors (RyRs) in the intracellular endoplasmic reticulum116. Finally, intracellular Ca2+ is released by activated RyR, leads to phosphorylation of cAMP response element-binding (CREB) protein, and regulates transcription of period and cryptochrome by CLOCK and BMAL117. During the maintenance of the circadian rhythm, the transcription factor CREB can integrate photosensitive information and mediate the reset of the circadian rhythm118. It is undeniable that the strength of this OPN4-mediated glutamatergic signaling is different in species with diurnal activity patterns, which is also reflected in their nonidentical phase-response curves (PRC). The projection of ipRGCs-SCN in the Nile rat (Arvicanthis niloticus) is comparable to that of the Syrian hamsters119. However, there are differences in sensitivity to phase movement between the two species on the NMDA-induced PRC120,121, which is also reflected in the strong resistance of Arvicanthis niloticus to NMDA122.
ipRGCs also express a peptide neurotransmitter called pituitary adenylate cyclase-activating peptide (PACAP) and colocalize with glutamate at the terminals of RHT in the SCN123,124. Previous studies have shown that adding PACAP to SCN slices in wild mice at circadian time (CT) 6 can advance the peak of the SCN activity rhythm in this and subsequent circadian rhythms125. However, the phase and amplitude of the neuronal firing rhythm do not change in Adcyap1 (adenylate cyclase activating polypeptide 1, encoding PACAP) knockout mice at CT6 and CT7 in the SCN126. In addition, light stimulation in the early night (CT15) delayed the phase, while light stimulation in the late night (CT21) advanced the phase127. Consistently, the influence of PACAP on the circadian rhythm depends on glutamate in the late night (phase advance), and the independent regulation of circadian rhythms by glutamate occurs in the early night (phase delay)126,128,129. The time-dependence phase shift at night may be due to PACAP and glutamate acting on different SCN neuronal subpopulations. Compared to glutamate, the positive signals for PACAP were mainly distributed in the dorsomedial SCN and a small amount in the central/ventral SCN126,130. Using c-Fos to mark neuronal activity, neurons with significant light responses during the subjective daytime were distributed in the dorsal SCN, and light did not affect the rhythm phase of mice131. This phenomenon is consistent with the evidence that circadian rhythms are not altered in Adcyap1 knockout mice. Regarding regulatory mechanisms, PACAP has a regulatory effect on glutamatergic calcium signaling and has a different time window from glutamate in regulating CREB phosphorylation132,133. PACAP can regulate circadian rhythm by differentially regulating mitogen- and stress-activated protein kinase 1 phosphorylation downstream of p42/44 mitogen-activated protein kinase between day and night134. Therefore, in terms of the autonomous rhythm of SCN neurons, PACAP may be a supplementary factor to OPN4-mediated SCN mastering circadian clock rhythms in response to the risk of potential circadian imbalance underlying Vglut2 deficiency when glutamate stimulation alone is insufficient.
Most OPN4-containing cells also expressed vasopressin (VP), which has glutamatergic nerve fibers projecting to the non-visual nuclei of the brain, and the application of VP receptor antagonist decreases the response of SCN neurons to photic entrainment of the RHT135. Additionally, vasopressinergic axons can affect the activity of ventral SCN cells in a VP-dependent manner136. Applying the antagonists of vasopressin V1a and V1b receptors to the SCN can promote (near instantaneous) re-entrainment to the new light/dark cycle137. Current evidence suggests that VP+ ipRGCs have synaptic co-localization with gastrin releasing peptide (GRP)- and VIP-positive neurons, but VP+ ipRGCs are not directly connected in AVP neurons135. According to single-nucleus RNA sequencing assays, all AVP clusters expressed glutamate receptor subunits with minimal expression of GABA receptors. However, some of their AVP nonlight-responsive clusters could express VIP receptor type 2138. Therefore, we speculate that photosensitive AVP, GRP, and VIP neurons may be downstream neurons of ipRGCs when OPN4-expressing ipRGCs secrete both glutamate and VP.
The effect of GABA on the master clock can be excitatory or inhibitory in different contexts, but there is no doubt about its importance139,140. The light information transmitted by ipRGCs can induce oscillations in the GABAergic system in the SCN. This may be because GABAB receptors are highly localized ventral to the SCN and are closely related to the signal afferents and the terminal synaptic remodeling of RGCs141. Studies in hamsters have shown that the antagonism of either GABAA (ionotropic) or GABAB receptors (metabotropic) in the SCN significantly increases the phase-shifting effects of light induction before a light pulse is provided in the early night rather than in the late night, suggesting that the inhibition of phase shift by extracellular GABA occurs mainly in the early night142,143. These data proved that changes in GABA in the SCN region are synchronized with non-visual light signals in the RGCs. Through GABAergic signaling, OPN4-expressing ipRGCs can also preserve circadian stability. Some ipRGCs co-expressing Gad2 and OPN4 can transmit GABAergic signals to the SCN to inhibit excessive light entrainment, and neurons receiving these GABAergic signals contain some VIP neuronal subsets144. Correspondingly, VIP neurons maintain the regular operation of the circadian rhythm, and inhibiting VIP neurons leads to increased phase shift145.
Therefore, the optical signal mediated by OPN4 in ipRGCs is transmitted to the SCN via RHT. Light entrainment is mainly determined by glutamatergic transmitters and supplemented by multiple neuropeptides in the SCN to adjust the phase shift and intensity (Fig. 3). Simultaneously, GABAergic neurotransmitters may act as inhibitors in this terminal region. These inputs may prevent unnecessary adjustments of the master circadian clock in the SCN by external environmental light. Notably, the expression time of neuropeptides does not match the timing of the phase shift caused by it (such as PACAP)126. Considering that neuropeptides need to undergo an extended length of RHT (mice: ~10 mm; rat: >20 mm) after they are synthesized from the cell body to the SCN region, they are transported only about ~140 mm per day along axons84,146. Therefore, when researching circadian rhythms, it would be interesting to look into the rate of transmission in the RHT and the rhythm of these neuropeptides’ expression in the retina.
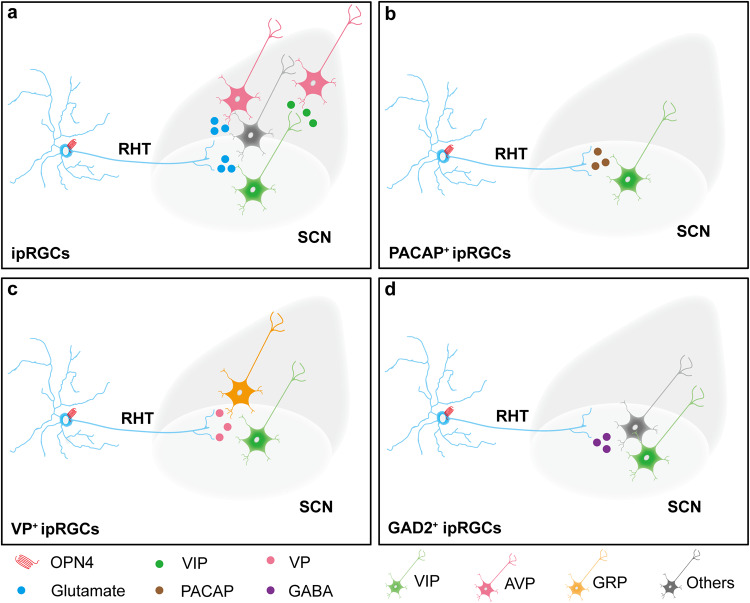
Fig. 3. The light entrainment of OPN4 on circadian rhythms may involve multiple neural projection pathways, including neurotransmitters or neuropeptides.
ipRGCs are a class of retinal ganglion cells that express OPN4 (red) and can transmit OPN4-mediated photosensitive signals to the SCN via RHT projections. a Vglut2, but not Vglut1, packages glutamate (solid blue circles) into synaptic vesicles in these axons. These ipRGCs axons mainly make synaptic contacts with VIP neurons (green), AVP (pink), and other light-responsive neurons (gray) in the SCN169,170. The VIP neurons form part of the SCN core region and may communicate with AVP neurons via VIP receptor type 2. b Some ipRGC axons can also release PACAP (solid brown circles) to regulate VIP neurons via VPAC2 and PAC1 receptors171. In addition, some OPN4-expressing ipRGCs also expressed VP (solid pink circles). c The axons of these ipRGCs are glutamatergic and VP-positive, and light stimulation can affect their secretion of VP. VP+ ipRGCs showed synaptic co-localization with GRP (yellow) and VIP neurons, but VP+ ipRGCs were not directly connected to AVP neurons135. d Some ipRGCs expressed GAD2 and could transmit GABA (solid purple circles) to the SCN regions. These GABAergic signals can excite or inhibit some SCN neurons, including VIP neurons, and maintain the homeostasis of the central rhythms144. AVP arginine vasopressin, GAD2 glutamic acid decarboxylase 2, GABA γ-aminobutyric acid, GRP gastrin releasing peptide, ipRGC intrinsically photosensitive retinal ganglion cell, RHT retinal hypothalamic tract, SCN supra-chiasmatic nucleus, VIP vasoactive intestinal peptide, VP vasopressin, PACAP pituitary adenylate cyclase-activating peptide.
Contribution of OPN4 to circadian rhythms in birds
The central biological clock system of the bird is formed by the hypothalamus, retina, and pineal gland147. The non-visual photosensitization in GUCY1* chickens, a null mutation chicken model that causes blindness at hatching, is more complex than that in rd/rd cl mice. When blocking the input of light signals from the head, blocking the perception of light by opsins in the SCN and pineal gland directly reduces the effect of light drive on circadian rhythms6,148. On this basis, GUCY1* chickens showed a feeding rhythm disruption after enucleation6. This finding implies that the retina plays an essential role in maintaining the circadian rhythm in chickens. Additionally, when only hypothalamic photoreceptors were retained, GUCY1* chickens were still able to maintain a brief circadian phase shift to adapt to the next light-dark cycle, indicating that photoreceptors from the hypothalamus may play a role in light regulation of circadian feeding behavior148. Although the evidence presented above does not exclude the possibility that other opsins have non-visual effects, the unique modulation of eating rhythm in GUCY1* chicken is particular to the light with wavelengths near the maximum absorption peak of OPN4. Another noteworthy example under light stimulation is that the pupillary light responses of chickens follow a circadian rhythm comparable to that of mammals149. It has been observed that GUCY1* chickens can maintain the circadian rhythm of pupillary light responses and reach maximum sensitivity at 480 nm150. Considering the light-absorbing properties of OPN4 and the distinct nonvisual photosensitivity function, the above findings strongly indicate that chicken retinal OPN4 regulates circadian rhythm. Notably, the photosensitivity function of OPN4 in the chicken retina may be more complex than is currently known. Chicken horizontal cells express OPN4x, which controls the release of GABA and regulates the membrane potential of photoreceptors following photosensitive activation43. Although this function is oriented more towards vision modulation, it cannot be excluded that OPN4x-expressing HCs may also affect the non-visual photosensitivity of OPN4-expressing retinal ganglion cells (RGCs) through signaling crosstalk.
The pineal gland of birds shows a robust melatonin secretion rhythm in vivo and in vitro. Monochromatic blue light (480 nm) can advance the phase of the rhythm-negative regulatory genes and inhibit the mRNA levels of Cry1 and Aanat (a key enzyme in melatonin synthesis) in the pineal gland, both in vivo and in vitro151,152. Since the specific membrane receptors for melatonin are distributed in the SCN region, melatonin can act directly on the SCN in an endocrine form to regulate the clock rhythms in the SCN153. Compared with the pineal gland, the chicken retina is a relatively independent organ in the circadian rhythm, and pinealectomy does not alter the circadian oscillations in the retina154. The main effect of monochromatic blue light on the retinal circadian clock is to delay the phase of OPN4 rather than the phase shifts of clock genes or the mRNA levels of Aanat147. The SCN is the primary retinorecipient hypothalamic structure in birds155. When OPN4 in the chicken retina is excited by light, its non-visual light signals are mainly transmitted to the SCN. Then, the axons emitted from the SCN regulate downstream nuclei, such as the PVN and the infundibular nucleus (similar to the mammalian arcuate nucleus)156. It has been demonstrated that the hypothalamic appetite-related genes show a circadian rhythm157. Is it possible that ipRGCs expressing OPN4 might indirectly regulate the appetite of broilers through their neural projections to the SCN? Further investigation of the relationship between the non-visual photosensitive function of OPN4 and the feeding rhythm will help answer this question.
Contribution of OPN4 to circadian rhythms in teleost fish
Although the light-sensing mechanism of an extraretinal photoreceptor is unclear, it may represent the most basic approach to light-sensing44,158. The eye and pineal gland are the main central clock structures for zebrafish, which conduct autonomous oscillations, photoreception, and melatonin production159. The eomesa-expressing RGCs and pineal gland in zebrafish both express Opn4.1 and Opn4xb160,161. When knocked out the Opn4.1 and Opn4xb in the zebrafish, genes involved in phototransduction and tryptophan metabolism were significantly altered, resulting in increasing melatonin synthesis64. Meanwhile, by affecting the synaptic plasticity in hypothalamic neurons or directly acting on melatonin receptors distributed in the hypothalamic SCN, the light signals can control the circadian rhythms through OPN4 in zebrafish162–165. Therefore, OPN4 expressed in the retina and central nervous system constructs photosensitive sensing in zebrafish and regulates circadian rhythms through melatonin.
Conclusions
OPN4 is a member of the G protein-coupled receptor family. Mammalian OPN4-expressing ipRGCs also express a variety of neurotransmitters and neuropeptides, which together with OPN4 regulate circadian rhythms. In contrast to mammals, teleost fish and birds have a more complicated system for controlling their circadian rhythms, and OPN4, which is expressed in the retina, brain, and pineal gland, is crucial for photosensitivity.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31972632, 31873000, and 31572474) and the Natural Science Foundation of Beijing Municipality (6192012).
Author contributions
Conceptualization, J.C., D.P.; writing—original draft, D.P.; supervision, Z.W., Y.C.; project administration, J.C., Y.C. All authors have read and agreed to the published version of the manuscript.
Peer review
Peer review information
Communications Biology thanks Mario Guido and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Manuel Breuer. A peer review file is available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-023-05432-7.
References
- 1.Yokoyama S. Evolution of dim-light and color vision pigments. Annu. Rev. Genomics Hum. Genet. 2008;9:259–282. doi: 10.1146/annurev.genom.9.081307.164228. [DOI] [PubMed] [Google Scholar]
- 2.Musilova Z, Salzburger W, Cortesi F. The visual opsin gene repertoires of teleost fishes: evolution, ecology, and function. Annu. Rev. Cell Dev. Biol. 2021;37:441–468. doi: 10.1146/annurev-cellbio-120219-024915. [DOI] [PubMed] [Google Scholar]
- 3.Tu DC, et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Douglas RH. The pupillary light responses of animals; a review of their distribution, dynamics, mechanisms and functions. Prog. Retin. Eye Res. 2018;66:17–48. doi: 10.1016/j.preteyeres.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Lucas RJ, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 6.Valdez DJ, et al. A nonmammalian vertebrate model of blindness reveals functional photoreceptors in the inner retina. FASEB J. 2009;23:1186–1195. doi: 10.1096/fj.08-117085. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez DC, et al. Retinal innervation tunes circuits that drive nonphotic entrainment to food. Nature. 2020;581:194–198. doi: 10.1038/s41586-020-2204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannibal J, et al. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest. Ophthalmol. Vis. Sci. 2004;45:4202–4209. doi: 10.1167/iovs.04-0313. [DOI] [PubMed] [Google Scholar]
- 10.Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 11.Sand A, Schmidt TM, Kofuji P. Diverse types of ganglion cell photoreceptors in the mammalian retina. Prog. Retin. Eye Res. 2012;31:287–302. doi: 10.1016/j.preteyeres.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mure LS. Intrinsically photosensitive retinal ganglion cells of the human retina. Front. Neurol. 2021;12:636330. doi: 10.3389/fneur.2021.636330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 14.Do MTH. Melanopsin and the intrinsically photosensitive retinal ganglion cells: biophysics to behavior. Neuron. 2019;104:205–226. doi: 10.1016/j.neuron.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl Acad. Sci. USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirzad-Wasei N, DeGrip WJ. Heterologous expression of melanopsin: present, problems and prospects. Prog. Retin. Eye Res. 2016;52:1–21. doi: 10.1016/j.preteyeres.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Upton BA, et al. Evolutionary constraint on visual and nonvisual mammalian opsins. J. Biol. Rhythms. 2021;36:109–126. doi: 10.1177/0748730421999870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 19.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hankins MW, Peirson SN, Foster RG. Melanopsin: an exciting photopigment. Trends Neurosci. 2008;31:27–36. doi: 10.1016/j.tins.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Hughes S, Hankins MW, Foster RG, Peirson SN. Melanopsin phototransduction: slowly emerging from the dark. Prog. Brain Res. 2012;199:19–40. doi: 10.1016/B978-0-444-59427-3.00002-2. [DOI] [PubMed] [Google Scholar]
- 22.Leung NY, Montell C. Unconventional roles of opsins. Annu. Rev. Cell Dev. Biol. 2017;33:241–264. doi: 10.1146/annurev-cellbio-100616-060432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stachurska A, Sarna T. Regulation of melanopsin signaling: key interactions of the nonvisual photopigment. Photochem. Photobiol. 2019;95:83–94. doi: 10.1111/php.12995. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Li G, Jiang Z, Yau KW. Unusual phototransduction via cross-motif signaling from G(q) to adenylyl cyclase in intrinsically photosensitive retinalganglion cells. Proc. Natl Acad. Sci. USA. 2023;120:e2216599120. doi: 10.1073/pnas.2216599120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue T, et al. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479:67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax approximately 479 nm) supporting activation of Gq/11 and Gi/o signalling cascades. Proc. Biol. Sci. 2013;280:20122987. doi: 10.1098/rspb.2012.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowe-Riddell JM, et al. Phototactic tails: evolution and molecular basis of a novel sensory trait in sea snakes. Mol. Ecol. 2019;28:2013–2028. doi: 10.1111/mec.15022. [DOI] [PubMed] [Google Scholar]
- 28.Ramos BC, Moraes MN, Poletini MO, Lima LH, Castrucci AM. From blue light to clock genes in zebrafish ZEM-2S cells. PLoS ONE. 2014;9:e106252. doi: 10.1371/journal.pone.0106252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sondereker KB, Stabio ME, Renna JM. Crosstalk: the diversity of melanopsin ganglion cell types has begun to challenge the canonical divide between image-forming and non-image-forming vision. J. Comp. Neurol. 2020;528:2044–2067. doi: 10.1002/cne.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J. Neurophysiol. 2008;100:371–384. doi: 10.1152/jn.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ecker JL, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 33.Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J. Comp. Neurol. 2010;518:2405–2422. doi: 10.1002/cne.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes S, et al. Signalling by melanopsin (OPN4) expressing photosensitive retinal ganglion cells. Eye. 2016;30:247–254. doi: 10.1038/eye.2015.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur. J. Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Duc D, Schoneberg T. Adaptation to nocturnality - learning from avian genomes. Bioessays. 2016;38:694–703. doi: 10.1002/bies.201600006. [DOI] [PubMed] [Google Scholar]
- 38.Bellingham J, et al. Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 2006;4:e254. doi: 10.1371/journal.pbio.0040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaas JH, Qi H-X, Stepniewska I. Escaping the nocturnal bottleneck, and the evolution of the dorsal and ventral streams of visual processing in primates. Philos. Trans. R. Soc. B Biol. Sci. 2021;377:20210293. doi: 10.1098/rstb.2021.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borges R, et al. Adaptive genomic evolution of opsins reveals that early mammals flourished in nocturnal environments. BMC Genomics. 2018;19:121. doi: 10.1186/s12864-017-4417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey MJ, Cassone VM. Melanopsin expression in the chick retina and pineal gland. Brain Res. Mol. Brain Res. 2005;134:345–348. doi: 10.1016/j.molbrainres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Verra DM, Contin MA, Hicks D, Guido ME. Early onset and differential temporospatial expression of melanopsin isoforms in the developing chicken retina. Invest. Ophthalmol. Vis. Sci. 2011;52:5111–5120. doi: 10.1167/iovs.11-75301. [DOI] [PubMed] [Google Scholar]
- 43.Morera LP, Diaz NM, Guido ME. Horizontal cells expressing melanopsin x are novel photoreceptors in the avian inner retina. Proc. Natl Acad. Sci. USA. 2016;113:13215–13220. doi: 10.1073/pnas.1608901113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez JH, Tolla E, Dunn IC, Meddle SL, Stevenson TJ. A comparative perspective on extra-retinal photoreception. Trends Endocrinol. Metab. 2019;30:39–53. doi: 10.1016/j.tem.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Natesan A, Geetha L, Zatz M. Rhythm and soul in the avian pineal. Cell Tissue Res. 2002;309:35–45. doi: 10.1007/s00441-002-0571-6. [DOI] [PubMed] [Google Scholar]
- 46.Torii M, et al. Two isoforms of chicken melanopsins show blue light sensitivity. FEBS Lett. 2007;581:5327–5331. doi: 10.1016/j.febslet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 47.Torii M, Kojima D, Nishimura A, Itoh H, Fukada Y. Light-dependent activation of G proteins by two isoforms of chicken melanopsins. Photochem. Photobiol. Sci. 2015;14:1991–1997. doi: 10.1039/c5pp00153f. [DOI] [PubMed] [Google Scholar]
- 48.Kuenzel WJ, Kang SW, Zhou ZJ. Exploring avian deep-brain photoreceptors and their role in activating the neuroendocrine regulation of gonadal development. Poult. Sci. 2015;94:786–798. doi: 10.3382/ps.2014-04370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang SW, Thayananuphat A, Bakken T, El Halawani ME. Dopamine-melatonin neurons in the avian hypothalamus controlling seasonal reproduction. Neuroscience. 2007;150:223–233. doi: 10.1016/j.neuroscience.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 50.Hauzman E, Kalava V, Bonci DMO, Ventura DF. Characterization of the melanopsin gene (Opn4x) of diurnal and nocturnal snakes. BMC Evol. Biol. 2019;19:174. doi: 10.1186/s12862-019-1500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dearworth JR, Jr., et al. A mammalian melanopsin in the retina of a fresh water turtle, the red-eared slider (Trachemys scripta elegans) Vis. Res. 2011;51:288–295. doi: 10.1016/j.visres.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 52.Frigato E, Vallone D, Bertolucci C, Foulkes NS. Isolation and characterization of melanopsin and pinopsin expression within photoreceptive sites of reptiles. Naturwissenschaften. 2006;93:379–385. doi: 10.1007/s00114-006-0119-9. [DOI] [PubMed] [Google Scholar]
- 53.Bertolesi GE, Hehr CL, McFarlane S. Wiring the retinal circuits activated by light during early development. Neural Dev. 2014;9:3. doi: 10.1186/1749-8104-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertolesi GE, et al. The regulation of skin pigmentation in response to environmental light by pineal Type II opsins and skin melanophore melatonin receptors. J. Photochem. Photobiol. B. 2020;212:112024. doi: 10.1016/j.jphotobiol.2020.112024. [DOI] [PubMed] [Google Scholar]
- 55.Bertolesi GE, Hehr CL, McFarlane S. Melanopsin photoreception in the eye regulates light-induced skin colour changes through the production of alpha-MSH in the pituitary gland. Pigment Cell Melanoma Res. 2015;28:559–571. doi: 10.1111/pcmr.12387. [DOI] [PubMed] [Google Scholar]
- 56.Davies WI, et al. Functional diversity of melanopsins and their global expression in the teleost retina. Cell. Mol. Life Sci. 2011;68:4115–4132. doi: 10.1007/s00018-011-0785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drivenes O, et al. Isolation and characterization of two teleost melanopsin genes and their differential expression within the inner retina and brain. J. Comp. Neurol. 2003;456:84–93. doi: 10.1002/cne.10523. [DOI] [PubMed] [Google Scholar]
- 58.Jenkins A, et al. VA opsin, melanopsin, and an inherent light response within retinal interneurons. Curr. Biol. 2003;13:1269–1278. doi: 10.1016/s0960-9822(03)00509-8. [DOI] [PubMed] [Google Scholar]
- 59.Sandbakken M, Ebbesson L, Stefansson S, Helvik JV. Isolation and characterization of melanopsin photoreceptors of Atlantic salmon (Salmo salar) J. Comp. Neurol. 2012;520:3727–3744. doi: 10.1002/cne.23125. [DOI] [PubMed] [Google Scholar]
- 60.Cheng N, Tsunenari T, Yau KW. Intrinsic light response of retinal horizontal cells of teleosts. Nature. 2009;460:899–903. doi: 10.1038/nature08175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandes AM, et al. Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr. Biol. 2012;22:2042–2047. doi: 10.1016/j.cub.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steindal IAF, Whitmore D. Zebrafish circadian clock entrainment and the importance of broad spectral light sensitivity. Front. Physiol. 2020;11:1002. doi: 10.3389/fphys.2020.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sapede D, Chaigne C, Blader P, Cau E. Functional heterogeneity in the pineal projection neurons of zebrafish. Mol. Cell. Neurosci. 2020;103:103468. doi: 10.1016/j.mcn.2020.103468. [DOI] [PubMed] [Google Scholar]
- 64.Dekens MPS, Fontinha BM, Gallach M, Pflugler S, Tessmar-Raible K. Melanopsin elevates locomotor activity during the wake state of the diurnal zebrafish. EMBO Rep. 2022;23:e51528. doi: 10.15252/embr.202051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rinaldi S, Melaccio F, Gozem S, Fanelli F, Olivucci M. Comparison of the isomerization mechanisms of human melanopsin and invertebrate and vertebrate rhodopsins. Proc. Natl Acad. Sci. USA. 2014;111:1714–1719. doi: 10.1073/pnas.1309508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflug. Arch. 2007;454:849–855. doi: 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- 67.Lok C. Vision science: seeing without seeing. Nature. 2011;469:284–285. doi: 10.1038/469284a. [DOI] [PubMed] [Google Scholar]
- 68.Emanuel AJ, Do MT. Melanopsin tristability for sustained and broadband phototransduction. Neuron. 2015;85:1043–1055. doi: 10.1016/j.neuron.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emanuel, A. J. & Do, M. T. H. The multistable melanopsins of mammals. Front. Ophthalmol.10.3389/fopht.2023.1174255 (2023). [DOI] [PMC free article] [PubMed]
- 70.Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr. Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- 71.Peinado G, Osorno T, Gomez Mdel P, Nasi E. Calcium activates the light-dependent conductance in melanopsin-expressing photoreceptors of amphioxus. Proc. Natl Acad. Sci. USA. 2015;112:7845–7850. doi: 10.1073/pnas.1420265112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Somasundaram P, et al. C-terminal phosphorylation regulates the kinetics of a subset of melanopsin-mediated behaviors in mice. Proc. Natl Acad. Sci. USA. 2017;114:2741–2746. doi: 10.1073/pnas.1611893114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blasic JR, Jr., Lane Brown R, Robinson PR. Light-dependent phosphorylation of the carboxy tail of mouse melanopsin. Cell. Mol. Life Sci. 2012;69:1551–1562. doi: 10.1007/s00018-011-0891-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blasic JR, Jr., et al. Identification of critical phosphorylation sites on the carboxy tail of melanopsin. Biochemistry. 2014;53:2644–2649. doi: 10.1021/bi401724r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cameron EG, Robinson PR. β-Arrestin-dependent deactivation of mouse melanopsin. PLoS ONE. 2014;9:e113138. doi: 10.1371/journal.pone.0113138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mure LS, et al. Sustained melanopsin photoresponse is supported by specific roles of β-arrestin 1 and 2 in deactivation and regeneration of photopigment. Cell Rep. 2018;25:2497.e4–2509.e4. doi: 10.1016/j.celrep.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Contin MA, et al. Light activation of the phosphoinositide cycle in intrinsically photosensitive chicken retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2010;51:5491–5498. doi: 10.1167/iovs.10-5643. [DOI] [PubMed] [Google Scholar]
- 78.Krzysztynska-Kuleta OI, Olchawa MM, Sarna TJ. Melanopsin signaling pathway in HEK293 cell line with stable expression of human melanopsin: possible participation of phospholipase C beta 4 and diacylglycerol. Photochem. Photobiol. 2021;97:1136–1144. doi: 10.1111/php.13453. [DOI] [PubMed] [Google Scholar]
- 79.Losenkova K, et al. CD73 controls ocular adenosine levels and protects retina from light-induced phototoxicity. Cell. Mol. Life Sci. 2022;79:152. doi: 10.1007/s00018-022-04187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Y, et al. Synergistically acting agonists and antagonists of G protein-coupled receptors prevent photoreceptor cell degeneration. Sci. Signal. 2016;9:ra74. doi: 10.1126/scisignal.aag0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hattar S, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim KY, et al. Synaptic specializations of melanopsin-retinal ganglion cells in multiple brain regions revealed by genetic label for light and electron microscopy. Cell Rep. 2019;29:628.e6–644.e6. doi: 10.1016/j.celrep.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li JY, Schmidt TM. Divergent projection patterns of M1 ipRGC subtypes. J. Comp. Neurol. 2018;526:2010–2018. doi: 10.1002/cne.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gamlin PD. The pretectum: connections and oculomotor-related roles. Prog. Brain Res. 2006;151:379–405. doi: 10.1016/S0079-6123(05)51012-4. [DOI] [PubMed] [Google Scholar]
- 87.Rupp AC, et al. Distinct ipRGC subpopulations mediate light’s acute and circadian effects on body temperature and sleep. Elife. 2019;8:e44358. doi: 10.7554/eLife.44358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 89.Mohawk JA, Takahashi JS. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci. 2011;34:349–358. doi: 10.1016/j.tins.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, Naito J. Morphological classification of ganglion cells in the central retina of chicks. J. Vet. Med. Sci. 1999;61:537–542. doi: 10.1292/jvms.61.537. [DOI] [PubMed] [Google Scholar]
- 91.Naito J, Chen Y. Morphologic analysis and classification of ganglion cells of the chick retina by intracellular injection of Lucifer Yellow and retrograde labeling with DiI. J. Comp. Neurol. 2004;469:360–376. doi: 10.1002/cne.11010. [DOI] [PubMed] [Google Scholar]
- 92.Chen Y, Naito J. Morphological properties of chick retinal ganglion cells in relation to their central projections. J. Comp. Neurol. 2009;514:117–130. doi: 10.1002/cne.21995. [DOI] [PubMed] [Google Scholar]
- 93.Vergara MN, Gutierrez C, O’Brien DR, Canto-Soler MV. Ex vivo electroporation of retinal cells: a novel, high efficiency method for functional studies in primary retinal cultures. Exp. Eye Res. 2013;109:40–50. doi: 10.1016/j.exer.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H, Yang C, Yusoff NM, Yahaya BH, Lin J. Direction of commissural axon projections in different regions of the spinal cord during chicken embryonic development. Neuroscience. 2017;358:269–276. doi: 10.1016/j.neuroscience.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 95.Guo Y, et al. Intravitreal injection of mitochondrial DNA induces cell damage and retinal dysfunction in rats. Biol. Res. 2022;55:22. doi: 10.1186/s40659-022-00390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trivedi AK, Malik S, Rani S, Kumar V. Pinealectomy abolishes circadian behavior and interferes with circadian clock gene oscillations in brain and liver but not retina in a migratory songbird. Physiol. Behav. 2016;156:156–163. doi: 10.1016/j.physbeh.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 97.Lee S, Nam HG, Kim Y. The core circadian component, Bmal1, is maintained in the pineal gland of old killifish brain. iScience. 2021;24:101905. doi: 10.1016/j.isci.2020.101905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taghert P, et al. Rods contribute to the light-induced phase shift of the retinal clock in mammals. PLoS Biol. 2019;17:e2006211. doi: 10.1371/journal.pbio.2006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhoi JD, Goel M, Ribelayga CP, Mangel SC. Circadian clock organization in the retina: from clock components to rod and cone pathways and visual function. Prog. Retin. Eye Res. 2023;94:101119. doi: 10.1016/j.preteyeres.2022.101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Diepen HC, et al. Distinct contribution of cone photoreceptor subtypes to the mammalian biological clock. Proc. Natl Acad. Sci. USA. 2021;118:e2024500118. doi: 10.1073/pnas.2024500118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J. Neurosci. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Altimus CM, et al. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat. Neurosci. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ratnayake K, Payton JL, Lakmal OH, Karunarathne A. Blue light excited retinal intercepts cellular signaling. Sci. Rep. 2018;8:10207. doi: 10.1038/s41598-018-28254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Oosterhout F, et al. Ultraviolet light provides a major input to non-image-forming light detection in mice. Curr. Biol. 2012;22:1397–1402. doi: 10.1016/j.cub.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keenan WT, et al. A visual circuit uses complementary mechanisms to support transient and sustained pupil constriction. Elife. 2016;5:e15392. doi: 10.7554/eLife.15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 107.Brainard GC, et al. Sensitivity of the human circadian system to short-wavelength (420-nm) light. J. Biol. Rhythms. 2008;23:379–386. doi: 10.1177/0748730408323089. [DOI] [PubMed] [Google Scholar]
- 108.Berson D. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 109.Wahl S, Engelhardt M, Schaupp P, Lappe C, Ivanov IV. The inner clock-blue light sets the human rhythm. J. Biophotonics. 2019;12:e201900102. doi: 10.1002/jbio.201900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fujiyama F, et al. Changes of immunocytochemical localization of vesicular glutamate transporters in the rat visual system after the retinofugal denervation. J. Comp. Neurol. 2003;465:234–249. doi: 10.1002/cne.10848. [DOI] [PubMed] [Google Scholar]
- 111.Gompf HS, Fuller PM, Hattar S, Saper CB, Lu J. Impaired circadian photosensitivity in mice lacking glutamate transmission from retinal melanopsin cells. J. Biol. Rhythms. 2015;30:35–41. doi: 10.1177/0748730414561545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kiss J, Csaki A, Csaba Z, Halasz B. Synaptic contacts of vesicular glutamate transporter 2 fibres on chemically identified neurons of the hypothalamic suprachiasmatic nucleus of the rat. Eur. J. Neurosci. 2008;28:1760–1774. doi: 10.1111/j.1460-9568.2008.06463.x. [DOI] [PubMed] [Google Scholar]
- 113.Fernandez DC, Chang YT, Hattar S, Chen SK. Architecture of retinal projections to the central circadian pacemaker. Proc. Natl Acad. Sci. USA. 2016;113:6047–6052. doi: 10.1073/pnas.1523629113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yokota S, et al. Involvement of calcium-calmodulin protein kinase but not mitogen-activated protein kinase in light-induced phase delays and Per gene expression in the suprachiasmatic nucleus of the hamster. J. Neurochem. 2001;77:618–627. doi: 10.1046/j.1471-4159.2001.00270.x. [DOI] [PubMed] [Google Scholar]
- 115.Agostino PV, Ferreyra GA, Murad AD, Watanabe Y, Golombek DA. Diurnal, circadian and photic regulation of calcium/calmodulin-dependent kinase II and neuronal nitric oxide synthase in the hamster suprachiasmatic nuclei. Neurochem. Int. 2004;44:617–625. doi: 10.1016/j.neuint.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 116.Iyer R, Wang TA, Gillette MU. Circadian gating of neuronal functionality: a basis for iterative metaplasticity. Front. Syst. Neurosci. 2014;8:164. doi: 10.3389/fnsys.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gerstner JR. On the evolution of memory: a time for clocks. Front. Mol. Neurosci. 2012;5:23. doi: 10.3389/fnmol.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McNulty S, Schurov IL, Sloper PJ, Hastings MH. Stimuli which entrain the circadian clock of the neonatal Syrian hamster in vivo regulate the phosphorylation of the transcription factor CREB in the suprachiasmatic nucleus in vitro. Eur. J. Neurosci. 1998;10:1063–1072. doi: 10.1046/j.1460-9568.1998.00114.x. [DOI] [PubMed] [Google Scholar]
- 119.Langel JL, Smale L, Esquiva G, Hannibal J. Central melanopsin projections in the diurnal rodent, Arvicanthis niloticus. Front. Neuroanat. 2015;9:93. doi: 10.3389/fnana.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Novak CM, Albers HE. N-methyl-D-aspartate microinjected into the suprachiasmatic nucleus mimics the phase-shifting effects of light in the diurnal Nile grass rat (Arvicanthis niloticus) Brain Res. 2002;951:255–263. doi: 10.1016/s0006-8993(02)03168-2. [DOI] [PubMed] [Google Scholar]
- 121.Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J. Neurosci. 1999;19:5124–5130. doi: 10.1523/JNEUROSCI.19-12-05124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fogo GM, Shuboni-Mulligan DD, Gall AJ. Melanopsin-containing ipRGCs are resistant to excitotoxic injury and maintain functional non-image forming behaviors after insult in a diurnal rodent model. Neuroscience. 2019;412:105–115. doi: 10.1016/j.neuroscience.2019.05.058. [DOI] [PubMed] [Google Scholar]
- 123.Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J. Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hannibal J, Moller M, Ottersen OP, Fahrenkrug J. PACAP and glutamate are co-stored in the retinohypothalamic tract. J. Comp. Neurol. 2000;418:147–155. [PubMed] [Google Scholar]
- 125.Hannibal J, et al. Pituitary adenylate cyclase-activating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J. Neurosci. 1997;17:2637–2644. doi: 10.1523/JNEUROSCI.17-07-02637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lindberg PT, et al. Pituitary adenylate cyclase-activating peptide (PACAP)-glutamate co-transmission drives circadian phase-advancing responses to intrinsically photosensitive retinal ganglion cell projections by suprachiasmatic nucleus. Front. Neurosci. 2019;13:1281. doi: 10.3389/fnins.2019.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kawaguchi C, et al. Changes in light-induced phase shift of circadian rhythm in mice lacking PACAP. Biochem. Biophys. Res. Commun. 2003;310:169–175. doi: 10.1016/j.bbrc.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 128.Ebling FJ, et al. The role of N-methyl-d-aspartate-type glutamatergic neurotransmission in the photic induction of immediate-early gene expression in the suprachiasmatic nuclei of the Syrian hamster. J. Neuroendocrinol. 1991;3:641–652. doi: 10.1111/j.1365-2826.1991.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 129.Atkins N, Jr., et al. Circadian integration of glutamatergic signals by little SAAS in novel suprachiasmatic circuits. PLoS ONE. 2010;5:e12612. doi: 10.1371/journal.pone.0012612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hannibal J, Georg B, Fahrenkrug J. PAC1- and VPAC2 receptors in light regulated behavior and physiology: studies in single and double mutant mice. PLoS ONE. 2017;12:e0188166. doi: 10.1371/journal.pone.0188166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Duy PQ, et al. Light has diverse spatiotemporal molecular changes in the mouse suprachiasmatic nucleus. J. Biol. Rhythms. 2020;35:576–587. doi: 10.1177/0748730420961214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.von Gall C, et al. CREB in the mouse SCN: a molecular interface coding the phase-adjusting stimuli light, glutamate, PACAP, and melatonin for clockwork access. J. Neurosci. 1998;18:10389–10397. doi: 10.1523/JNEUROSCI.18-24-10389.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kopp MD, Meissl H, Dehghani F, Korf HW. The pituitary adenylate cyclase-activating polypeptide modulates glutamatergic calcium signalling: investigations on rat suprachiasmatic nucleus neurons. J. Neurochem. 2001;79:161–171. doi: 10.1046/j.1471-4159.2001.00553.x. [DOI] [PubMed] [Google Scholar]
- 134.Butcher GQ, Lee B, Cheng HY, Obrietan K. Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism. J. Neurosci. 2005;25:5305–5313. doi: 10.1523/JNEUROSCI.4361-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tsuji T, et al. Vasopressin casts light on the suprachiasmatic nucleus. J. Physiol. 2017;595:3497–3514. doi: 10.1113/JP274025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hume C, Allchorne A, Grinevich V, Leng G, Ludwig M. Effects of optogenetic stimulation of vasopressinergic retinal afferents on suprachiasmatic neurones. J. Neuroendocrinol. 2019;31:e12806. doi: 10.1111/jne.12806. [DOI] [PubMed] [Google Scholar]
- 137.Yamaguchi Y, et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science. 2013;342:85–90. doi: 10.1126/science.1238599. [DOI] [PubMed] [Google Scholar]
- 138.Xu P, et al. NPAS4 regulates the transcriptional response of the suprachiasmatic nucleus to light and circadian behavior. Neuron. 2021;109:3268.e6–3282.e6. doi: 10.1016/j.neuron.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Myung J, et al. GABA-mediated repulsive coupling between circadian clock neurons in the SCN encodes seasonal time. Proc. Natl Acad. Sci. USA. 2015;112:E3920–E3929. doi: 10.1073/pnas.1421200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ono D, Honma KI, Yanagawa Y, Yamanaka A, Honma S. GABA in the suprachiasmatic nucleus refines circadian output rhythms in mice. Commun. Biol. 2019;2:232. doi: 10.1038/s42003-019-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moldavan MG, Allen CN. GABAB receptor-mediated frequency-dependent and circadian changes in synaptic plasticity modulate retinal input to the suprachiasmatic nucleus. J. Physiol. 2013;591:2475–2490. doi: 10.1113/jphysiol.2012.248047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gillespie CF, Huhman KL, Babagbemi TO, Albers HE. Bicuculline increases and muscimol reduces the phase-delaying effects of light and VIP/PHI/GRP in the suprachiasmatic region. J. Biol. Rhythms. 1996;11:137–144. doi: 10.1177/074873049601100206. [DOI] [PubMed] [Google Scholar]
- 143.Gillespie CF, Mintz EM, Marvel CL, Huhman KL, Albers HE. GABAA and GABAB agonists and antagonists alter the phase-shifting effects of light when microinjected into the suprachiasmatic region. Brain Res. 1997;759:181–189. doi: 10.1016/s0006-8993(97)00235-7. [DOI] [PubMed] [Google Scholar]
- 144.Sonoda T, et al. A noncanonical inhibitory circuit dampens behavioral sensitivity to light. Science. 2020;368:527–531. doi: 10.1126/science.aay3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jones JR, Simon T, Lones L, Herzog ED. SCN VIP neurons are essential for normal light-mediated resetting of the circadian system. J. Neurosci. 2018;38:7986–7995. doi: 10.1523/JNEUROSCI.1322-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol. Rev. 2001;81:1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- 147.Bian J, Wang Z, Dong Y, Cao J, Chen Y. Effect of monochromatic light on the circadian clock of cultured chick retinal tissue. Exp. Eye Res. 2020;194:108008. doi: 10.1016/j.exer.2020.108008. [DOI] [PubMed] [Google Scholar]
- 148.Valdez DJ, Nieto PS, Diaz NM, Garbarino-Pico E, Guido ME. Differential regulation of feeding rhythms through a multiple-photoreceptor system in an avian model of blindness. FASEB J. 2013;27:2702–2712. doi: 10.1096/fj.12-222885. [DOI] [PubMed] [Google Scholar]
- 149.Owens L, et al. Effect of circadian clock gene mutations on nonvisual photoreception in the mouse. Invest. Opthalmol. Vis. Sci. 2012;53:454–460. doi: 10.1167/iovs.11-8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Valdez DJ, Nieto PS, Della Costa NS, Schurrer C, Guido ME. Circadian control of the pupillary light responses in an avian model of blindness, the GUCY1* chickens. Invest. Ophthalmol. Vis. Sci. 2015;56:730–737. doi: 10.1167/iovs.14-15481. [DOI] [PubMed] [Google Scholar]
- 151.Ma S, Wang Z, Cao J, Dong Y, Chen Y. Effect of monochromatic light on circadian rhythm of clock genes in chick pinealocytes. Photochem. Photobiol. 2018;94:1263–1272. doi: 10.1111/php.12963. [DOI] [PubMed] [Google Scholar]
- 152.Jiang N, Wang Z, Cao J, Dong Y, Chen Y. Role of monochromatic light on daily variation of clock gene expression in the pineal gland of chick. J. Photochem. Photobiol. B. 2016;164:57–64. doi: 10.1016/j.jphotobiol.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 153.Zhang L, et al. Melatonin modulates monochromatic light-induced melatonin receptor expression in the hypothalamus of chicks. Acta Histochem. 2017;119:733–739. doi: 10.1016/j.acthis.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 154.Bian J, Wang Z, Dong Y, Cao J, Chen Y. Effect of pinealectomy on the circadian clock of the chick retina under different monochromatic lights. Chronobiol. Int. 2019;36:548–563. doi: 10.1080/07420528.2019.1566740. [DOI] [PubMed] [Google Scholar]
- 155.Cantwell EL, Cassone VM. Chicken suprachiasmatic nuclei: II. Autoradiographic and immunohistochemical analysis. J. Comp. Neurol. 2006;499:442–457. doi: 10.1002/cne.21124. [DOI] [PubMed] [Google Scholar]
- 156.Cantwell EL, Cassone VM. Chicken suprachiasmatic nuclei: I. Efferent and afferent connections. J. Comp. Neurol. 2006;496:97–120. doi: 10.1002/cne.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stutz AM, Staszkiewicz J, Ptitsyn A, Argyropoulos G. Circadian expression of genes regulating food intake. Obesity. 2007;15:607–615. doi: 10.1038/oby.2007.564. [DOI] [PubMed] [Google Scholar]
- 158.Lamb TD, Collin SP, Pugh EN., Jr. Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat. Rev. Neurosci. 2007;8:960–976. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lima-Cabello E, et al. A review of the melatonin functions in zebrafish physiology. J. Pineal Res. 2014;57:1–9. doi: 10.1111/jpi.12149. [DOI] [PubMed] [Google Scholar]
- 160.Kolsch Y, et al. Molecular classification of zebrafish retinal ganglion cells links genes to cell types to behavior. Neuron. 2021;109:645.e9–662.e9. doi: 10.1016/j.neuron.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ziv L, Tovin A, Strasser D, Gothilf Y. Spectral sensitivity of melatonin suppression in the zebrafish pineal gland. Exp. Eye Res. 2007;84:92–99. doi: 10.1016/j.exer.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 162.Appelbaum L, et al. Circadian and homeostatic regulation of structural synaptic plasticity in hypocretin neurons. Neuron. 2010;68:87–98. doi: 10.1016/j.neuron.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Elbaz I, Foulkes NS, Gothilf Y, Appelbaum L. Circadian clocks, rhythmic synaptic plasticity and the sleep-wake cycle in zebrafish. Front. Neural Circuits. 2013;7:9. doi: 10.3389/fncir.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rawashdeh O, de Borsetti NH, Roman G, Cahill GM. Melatonin suppresses nighttime memory formation in zebrafish. Science. 2007;318:1144–1146. doi: 10.1126/science.1148564. [DOI] [PubMed] [Google Scholar]
- 165.Gupta P, Khobragade S, Rajaram S, Shingatgeri V. Assessment of locomotion behavior in adult Zebrafish after acute exposure to different pharmacological reference compounds. Drug Dev. Ther. 2014;5:127. [Google Scholar]
- 166.Kang SW, et al. Melanopsin expression in dopamine-melatonin neurons of the premammillary nucleus of the hypothalamus and seasonal reproduction in birds. Neuroscience. 2010;170:200–213. doi: 10.1016/j.neuroscience.2010.06.082. [DOI] [PubMed] [Google Scholar]
- 167.Kang SW. Central nervous system associated with light perception and physiological responses of birds. Front. Physiol. 2021;12:723454. doi: 10.3389/fphys.2021.723454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chaurasia SS, et al. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J. Neurochem. 2005;92:158–170. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- 169.Kofuji P, et al. Intrinsically photosensitive retinal ganglion cells (iprgcs) are necessary for light entrainment of peripheral clocks. PLoS ONE. 2016;11:e0168651. doi: 10.1371/journal.pone.0168651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mieda M. The central circadian clock of the suprachiasmatic nucleus as an ensemble of multiple oscillatory neurons. Neurosci. Res. 2020;156:24–31. doi: 10.1016/j.neures.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 171.Kalamatianos T, Kallo I, Piggins HD, Coen CW. Expression of VIP and/or PACAP receptor mRNA in peptide synthesizing cells within the suprachiasmatic nucleus of the rat and in its efferent target sites. J. Comp. Neurol. 2004;475:19–35. doi: 10.1002/cne.20168. [DOI] [PubMed] [Google Scholar]
- 172.Sikka G, et al. Melanopsin mediates light-dependent relaxation in blood vessels. Proc. Natl Acad. Sci. USA. 2014;111:17977–17982. doi: 10.1073/pnas.1420258111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Barreto Ortiz S, et al. Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G protein-coupled receptor kinase 2 inhibition. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018;314:L93–L106. doi: 10.1152/ajplung.00091.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yim PD, et al. Airway smooth muscle photorelaxation via opsin receptor activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;316:L82–L93. doi: 10.1152/ajplung.00135.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.de Assis LVM, Moraes MN, Magalhaes-Marques KK, Castrucci AML. Melanopsin and rhodopsin mediate UVA-induced immediate pigment darkening: Unravelling the photosensitive system of the skin. Eur. J. Cell Biol. 2018;97:150–162. doi: 10.1016/j.ejcb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 176.Yang K, et al. Exposure to blue light stimulates the proangiogenic capability of exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2019;10:358. doi: 10.1186/s13287-019-1472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ondrusova K, et al. Subcutaneous white adipocytes express a light sensitive signaling pathway mediated via a melanopsin/TRPC channel axis. Sci. Rep. 2017;7:16332. doi: 10.1038/s41598-017-16689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.