Abstract
Catecholamine-enhancing psychostimulants, such as methylphenidate have long been argued to undermine creative thinking. However, prior evidence for this is weak or contradictory, stemming from studies with small sample sizes that do not consider the well-established large variability in psychostimulant effects across different individuals and task demands. We aimed to definitively establish the link between psychostimulants and creative thinking by measuring effects of methylphenidate in 90 healthy participants on distinct creative tasks that measure convergent and divergent thinking, as a function of individuals’ baseline dopamine synthesis capacity, indexed with 18F-FDOPA PET imaging. In a double-blind, within-subject design, participants were administered methylphenidate, placebo or selective D2 receptor antagonist sulpiride. The results showed that striatal dopamine synthesis capacity and/or methylphenidate administration did not affect divergent and convergent thinking. However, exploratory analysis demonstrated a baseline dopamine-dependent effect of methylphenidate on a measure of response divergence, a creativity measure that measures response variability. Response divergence was reduced by methylphenidate in participants with low dopamine synthesis capacity but enhanced in those with high dopamine synthesis capacity. No evidence of any effect of sulpiride was found. These results show that methylphenidate can undermine certain forms of divergent creativity but only in individuals with low baseline dopamine levels.
Subject terms: Cognitive control, Human behaviour
Introduction
Brain dopamine has long been implicated in various cognitive functions and dysfunction. It is implicated in many psychiatric and neurological disorders, including attention deficit hyperactivity disorder (ADHD), major depression, Parkinson’s disease (PD), autism spectrum disorder, schizophrenia, stress, and fatigue. Such disorders and states are accompanied by cognitive deficits that can be treated with dopaminergic drugs. The cognitive-enhancing drug that is most commonly used today, particularly for ADHD, is methylphenidate (MPH), a psychostimulant that increases dopamine and noradrenaline transmission by blocking their transporters. Moreover, psychostimulants are increasingly being used for cognitive enhancement, such as in smart pills, by healthy people [1]. Estimates of the proportion of healthy students using drugs such as MPH off-label range from 4% to 16% [2].
However, smart drugs do not help everyone in all contexts. Dopaminergic drug effects vary greatly across individuals and across different tasks, with some individuals and tasks being impaired by dopaminergic drugs [3]. Some have suggested that drugs, such as MPH, can impair creative thinking by minimizing distractibility, out-of-the-box thinking, and mind-wandering [4]. However, the evidence for such side effects on creative thinking is contradictory [5, 6].
The objective of the present study was to identify the cognitive and neurochemical mechanisms of the beneficial and detrimental effects of MPH on creativity, where we define creativity as the generation of novel and useful ideas [7]. There is a long history of theorizing and experimental work suggesting a link between dopamine and creative innovation and drive [8–12]. Based on a recent proposal by Boot et al. [9], we tested the following hypothesis: The effects of dopaminergic drugs such as MPH on creative performance depend on two factors: (i) task demands for convergent versus divergent thinking (cognitive stability versus flexibility) and (ii) individual differences in baseline dopamine levels. Specifically, we tested the prediction that creative tasks requiring cognitive flexibility (i.e., divergent thinking) are more vulnerable to the detrimental effects of MPH than creative tasks requiring cognitive stability, that is, convergent thinking, which should be improved by MPH. Moreover, we predicted that such a shift in performance towards cognitive stability away from flexibility should be greatest in individuals with the lowest baseline levels of dopamine synthesis capacity.
This hypothesis is grounded in work on dopamine’s role in human cognitive control and working memory demonstrating that dopamine’s effects vary greatly across different cognitive task demands [3, 13–18]. Specifically, the current study was motivated directly by a study demonstrating the beneficial effects of MPH on stable, distractor-resistant working memory, but detrimental effects on a well-matched task requiring flexible working memory updating [19]. This finding is consistent with the proposal that adaptive cognition depends on a dynamic equilibrium between distractor-resistant goal stabilization, implicating optimal levels of dopamine in the prefrontal cortex [20–22] and goal flexibility or working memory updating [23], implicating optimal levels of dopamine in the striatum [3, 24–27] (but see [26]). In line with the predominantly frontal mechanism of action of MPH [27, 28] and the enhancing effects of MPH in ADHD, Fallon et al. [17] observed that these contrasting effects of MPH on flexible and stable working memory were accompanied by contrasting effects on BOLD signals in the prefrontal cortex.
The question of whether the detrimental effects of MPH on the flexible updating (versus the stabilizing) processes of working memory generalize to creativity tasks requiring divergent (versus convergent) thinking was based on the proposal that there are two pathways to creativity [29]: the flexibility pathway and the persistence pathway, where flexibility is defined as the ease with which people switch attention between different task approaches/categories, and persistence is defined as focused task-oriented attention. This led Boot and colleagues [9] to propose that striatal dopamine might promote flexible thinking and the original ideation required for divergent thinking at the expense of convergent thinking, whereas prefrontal dopamine might promote the persistence of attention required for convergent thinking while undermining divergent thinking. Here, we address the question of whether MPH elicits this latter bias, shifting the system toward more convergent thinking at the cost of divergent thinking. This hypothesis concurs with the detrimental effects of MPH on flexible working memory updating [17, 27] and is generally in line with anecdotal evidence that MPH can undermine creativity in ADHD [30, 31] while enhancing focusing and distractor resistance.
MPH acts not only in the prefrontal cortex [27, 32], but also in the striatum [33]. To disentangle this alternative striatal dopamine account for the effects of MPH, we compared the effects of MPH with those of a relatively low dose (400 mg) of the D2 receptor antagonist sulpiride, which should elicit increases in striatal dopamine release due to predominant presynaptic D2 autoreceptor binding [34, 35]. In contrast to MPH, we anticipated that sulpiride would have the opposite effect, boosting divergent thinking but undermining convergent thinking, particularly in participants with low baseline levels of dopamine. This concurs with findings from studies with patients with PD, which is characterized by severe dopamine depletion in the striatum (but relatively intact dopamine levels in the prefrontal cortex, at least in the earliest stages of the disease [36–41]). Thus, patients with PD have been shown to exhibit impaired creative flexibility on a standardized creativity test (Torrance Test of Creative Thinking) [42], which was remediated by common antiparkinsonian dopaminergic medication [43]. Moreover, a recent drug intervention study demonstrated that PD patients on dopaminergic medication generated more divergent responses on a novel quantitative option generation task despite the reduced uniqueness of each response than PD patients off their medication [12].
In addition to investigating drug effects as a function of task demands, we stratified drug effects by baseline levels of striatal dopamine synthesis capacity, as measured using 18F-FDOPA positron emission tomography (PET) imaging. This was motivated by accumulating evidence that dopaminergic drug effects in the healthy population can be isolated only when individual differences in baseline dopamine levels are considered [3]. In a recent study, we found that the enhancing effects of MPH on cognitive control were the greatest in participants with the lowest baseline levels of dopamine [44]. Moreover, evidence indicates that MPH is more effective in enhancing cognition in people with ADHD than in those without ADHD, which has been suggested to be accompanied by low dopamine synthesis capacity [45]. This baseline dependency might account for the failure of a previous study to uncover any effects of MPH on divergent creativity across a group of 48 healthy volunteers for whom measurements of dopamine synthesis capacity were not available [5]. Here, we tested the prediction that the undermining effects of MPH on divergent (versus convergent) creativity would be observed only in those with the lowest baseline levels of dopamine synthesis capacity.
To this end, a large sample of 100 healthy volunteers was tested on a classic test of divergent verbal creativity, the Alternate Uses Task (AUT), a classic test of convergent verbal creativity, the Remote Associates Task (RAT), and a novel task that simultaneously provided separate measures of divergent and convergent verbal creativity: the Alternate Names Task (ANT) [9]. All tasks were completed in three sessions: once after MPH, once after sulpiride, and once after placebo. In a separate session, the participants underwent 18F-FDOPA PET. In summary, divergent creativity scores on the AUT and ANT were predicted to be impaired, whereas convergent creativity scores on the RAT and ANT were predicted to be enhanced by MPH in participants with low dopamine synthesis capacity. Conversely, divergent creativity was predicted to be enhanced and convergent creativity was impaired by sulpiride in participants with low dopamine synthesis capacity.
Materials and methods
Participants, design, and procedure
This study was part of a larger within-subject, double-blind, placebo-controlled, cross-over design pharmaco-fMRI-[18F]-fluoro-dopa (18F-FDOPA) PET study that investigated the effects of MPH (MPH) and sulpiride (SUL) on a series of tasks [46]. Effects of 18F-FDOPA and/or dopaminergic drug administration on other metrics have been published previously [44, 47–51]. The study sample consisted of 100 healthy participants. All participants were between 18-45 years old (mean = 23, SD = 5.04, range: 18 - 43), right-handed and native-Dutch speakers. The gender balance was kept equal across the sample. All participants provided written informed consent to take part in the study, which was approved by the regional research ethics committee (“Commissie Mensgebonden Onderzoek”, CMO region Arnhem-Nijmegen, The Netherlands: protocol NL57538.091.16). Participants were recruited through advertisements and were financially compensated for their participation. Participants were excluded based on whether they met any of the exclusion criteria to ensure they had no relevant medical history, psychiatric symptoms, no diagnosis (or history) of relevant psychiatric, neurological, endocrine, or neuroendocrine treatment, history of over the counter medication within the last two months or prescribed medication within the last month prior to the study; regular use of corticosteroids; habitual smoking; diabetes; abnormal hearing or uncorrected vision; glaucoma; irregular sleep/wake rhythm; possible pregnancy and no appropriate contraception and had to agree to abstain from alcohol and smoking 24 hours, and psychotropic medication and recreational drugs 72 hours before each session (see [46] for a comprehensive list of inclusion/exclusion criteria). Six participants dropped out during the study, resulting in 94 participants. For one participant, the task stimuli were not randomized owing to technical difficulties. Three participants did not complete all the creativity tasks and hence were removed from further data analyses. The remaining 90 participants completed all three drug sessions and underwent the PET scan procedures.
The study consisted of five sessions, with an interval of at least one week between each session. The first study day served as an intake on which participants were screened for inclusion criteria, underwent an anatomical MR scan, completed two working memory tests, a cryztalized intelligence test and a recording of spontaneous eye blink rate.
In the next three pharmacological sessions, participants received an oral administration of 20 mg of MPH, 400 mg of SUL, or a placebo. MPH plasma concentrations peak after 2 hours [52] and sulpiride plasma concentrations peak after 3 hours [53]. The timing of drug administration was optimized for the cognitive effort and reversal learning tasks, the results of which are reported elsewhere [44, 47, 48]. To account for the difference in the peak times of methylphenidate and sulpiride and not break blindness to drug status, we used a double-dummy design. Participants received (supervised) two identical capsules each day, one approximately 290 minutes before the creativity tasks and the other 90 minutes after the first one. One of the capsules was a placebo and the other contained the drug (or another placebo in the placebo session). All creativity tasks were administered outside of the scanner after the effort, learning and motivation tasks (reported elsewhere, see complete overview of tasks here 46). The order of drug administration was randomized across participants (see SI Table 1 for the number of participants who received MPH, SUL, and PBO in each session). Blood pressure, heart rate, medical symptoms, and mood measures were monitored three times per session: before the start of the task battery, 20 min after the intake of the second capsule, and after the task battery [46].
During the fifth session, participants underwent 18F-FDOPA PET to quantify their dopamine synthesis capacity. The dynamic PET scan involved a bolus injection of approximately 185 MBq 18F-FDOPA into the antecubital vein. The mean time difference between the PET session and the MPH session was 50.89 days, standard error of mean = 2.86 days. Fifty minutes before the PET scan started, participants received 150 mg of carbidopa and 400 mg of entacapone to minimize peripheral metabolism of 18F-FDOPA by peripheral decarboxylase and catechol-O-methyltransferase (COMT), respectively, thereby increasing the signal-to-noise ratio in the brain [54–57]. Dopamine synthesis capacity was computed per voxel as the 18F-FDOPA influx constant per minute (Ki) relative to the cerebellar gray matter reference region using Gjedde–Patlak graphical analysis of the PET frames from the 24th to 89th minute [34]. Next, Ki values were extracted and averaged from three striatal regions of interest (ROIs)—ventral striatum, putamen, and caudate nucleus—defined using masks derived from an independent functional connectivity analysis of the striatum ([58], see SI Figure 1 for the ROI masks) and exactly the same as reported by Westbrook et al. [44]. The correlation between Ki measures are reported in SI Figure 2. Further details of the PET methods can be found in refs. [44, 47–49].
For each drug session, all participants performed the same three creativity tasks that were also administered in our previous study [5]: Alternative Uses Task (AUT) to assess divergent creative thinking [59], Remote Associates Task (RAT) to assess convergent creative thinking [51], and Alternative Names Task (ANT) to jointly assess divergent and convergent creative thinking [9]. The order of the three creativity tasks was held constant within individuals across sessions, but counterbalanced across participants (see SI Tables 1–3).
Creativity tasks
AUT for assessing divergent thinking
To assess the effects of dopamine synthesis capacity and dopaminergic drug administration on divergent thinking, we used the AUT [59]. The AUT measures divergent thinking. In three separate 2-min trials per session, participants were asked to generate many original ways to use a common object. Three sets of three objects (Set 1: brick, newspaper, fork; Set 2: bottle, towel, paperclip; Set 3: cord, tin can, book) were matched in terms of the (variance in) flexibility and originality of ideas generated in previous studies [5, 9]. Within each set, the object names were presented in Dutch in randomized order. In each experimental session, participants completed a different set. The order of the three sets was counterbalanced across participants (see SI Table 4). One trained coder (MB), blinded to the drug conditions, scored the participants’ ideas in terms of fluency (the number of generated ideas), flexibility (the number of different conceptual categories to which the ideas belonged), and originality (the extent to which an idea is mentioned infrequently and deviates from the common use of an object). For flexibility, ideas were categorized into conceptual categories. For example, for the object “brick”, the idea “to pave the street” was coded in the category “building something”, whereas the idea “use as a domino tile” was coded in the category “to play with”. The coder also rated each idea for originality and the extent to which it was unusual and uncommon (1 = not original at all, to 5 = very original). For each session, originality ratings were averaged across all ideas to correct for differences in fluency. Before scoring, the coder had extensive training and achieved excellent inter-rater reliability for both flexibility (Cohen’s κ > .96, ps < .001) and originality (ICCs > .86, ps < .001) for the AUT training sets. Across sessions, participants generated an average of 8.63 ideas (SD = 3.41) in nine categories with an average originality of 2.15 (SD = 0.23), which is comparable to other studies [5, 9]. To control for type 1 error in analyses, each sessions’ fluency, flexibility and originality scores were z-transformed and averaged across the presented objects in that session to derive a composite divergent thinking score [5], which was used as the main outcome measure of this task, as was the case in our prior study on MPH effects on creativity [9].
RAT for assessing convergent thinking
To assess the effects of striatal dopamine synthesis capacity and dopaminergic drug administration on convergent thinking, we used RAT [51]. The RAT assesses convergent thinking. In this task, participants were asked to come up with one word associated with each of the presented words. In each session, participants received 10 questions in which they were given three words (e.g., jar, stain, blue) and were asked to generate a word that was associated with all of them (i.e., ink). The difficulty of the questions was similar across sessions, based on previous datasets (not presented here). This task was self-paced, and the participants could skip an item. On average, participants skipped 1.23 items (SD = 1.72) across sessions and there was no significant difference in the number of skipped items between sessions (β = 0.04, SE = 0.10, t(192.00) = 0.71, p = 0.55). As in our previous study on MPH effects on creativity [9], the number of correctly solved items was the measure of convergent thinking and was used as the main outcome measure of this task. On average, the participants solved 4.96 items (SD = 1.61) across sessions.
ANT for assessing divergent and convergent thinking
This task was developed to investigate convergent and divergent thinking within the context of the same task instructions, thus allowing direct comparison between the two in a way that is better controlled for incidental state changes or other task-specific factors of no interest, compared with the AUT and RAT [9]. During this task, participants were asked to generate as many new names as possible for items in a category (e.g., martial arts, pizzas, planets) within one minute. Three sets of five categories (Set 1: martial arts, pasta, dances, software companies, and pain killers; Set 2: cleaning products, pizzas, sexually transmitted diseases, Brazilian music styles, IKEA products; Set 3: statistical tests, cocktails, flowers, planets, and Indonesian dishes) were matched in terms of (variance in) rule divergence, rule convergence, and number of names generated in previous studies ([9], data not presented here). Within each set, category names were presented in Dutch in a randomized order. In each experimental session, participants completed a different set. The order of the three sets was counterbalanced across participants (see SI Table 5). For each category, participants were provided with three example names, e.g., “fussilini,” “krapi” and “falucci” for the category “pasta”. These exemplar names all ended with the same, common letter to cue a certain rule. Although participants were not instructed to either follow or deviate from this rule, their responses usually converged with the rule. Number of responses that converged with the cued rule (total number of items ending with the same letter as the exemplar names, e.g., “rollicci” for the category “pasta”) were scored as rule convergent thinking. Number of responses that deviated from the rule (total number of items ending with a different letter than the exemplar names, e.g., “foma” or “fallopa” for the category “pasta”) was scored as rule divergent thinking. Rule convergent and rule divergent thinking scores were used as the main outcome measures of this task, as was the case in our previous study on MPH effects on creativity [9]. In addition to those primary metrics used previously, we also explored a secondary metric of divergent thinking, namely the total number of different name endings (see [5, 9, 21]). We refer to this metric as response divergence (e.g., the response divergence score for “foma,” “rollicci,” “foms,” and “metucini” for the category “pasta” would be “3”). Across sessions, participants generated 22.85 (SD = 15.36) rule convergent ideas, 10.36 (SD = 7.94) rule divergent ideas, and 7.79 (SD = 2.94) divergent responses. The relationship between all creativity task measures can be found in SI Figure 3.
Subjective measures of individual creative ability
To assess the ecological validity of our task measures of creativity, we also obtained subjective reports of individual creative ability using the Kaufman Domains of Creativity scale (K-DOCS [60]). This scale consists of a summary score (M = 2.99, SD = 0.44) and five sub-domain scores. Participants report on a Likert scale whether they are much less (1) or more creative (5) on 50 items loaded onto five subdomains of creativity (everyday creativity, artistic creativity, performance creativity, scholarly creativity, and scientific creativity).
Data analysis
As in our previous study on the effects of MPH on creativity, the primary analyses focused on the effects of MPH on rule divergent and rule convergent creativity scores from the ANT. We complemented these results by additionally analyzing the convergent RAT and divergent AUT results. Next, inspired by the results of Ang et al. [12] who revealed effects of dopamine receptor stimulation with the receptor agonist cabergoline on a measure of (visual) response divergence, we explored the effect of MPH on response divergence, as scored in the ANT. Next, we tested the effects of sulpiride on the same outcome variables.
Task scores were analyzed with mixed-effects models (R statistical software, lme4 package), all of which included session number as a nuisance variable (see SI Table 6 for our rationale to include session as a nuisance variable) using the following model notation:
where i is the number of observations and j corresponds to subject ID. Ki stands for dopamine synthesis capacity. To assess drug effects, each drug condition (MPH and sulpiride) was factorized in relation to placebo and tested in separate models. Given the lack of an a priori hypothesis regarding the specific striatal locus of a putative baseline dopamine effect, we computed the main effects of dopamine synthesis capacity from all three striatal regions of interest (caudate nucleus, putamen, ventral striatum (VS); z-scored) and their interaction with drug effects in three separate statistical models. To account for multiple comparisons within a creativity construct and Ki definitions, an alpha of 0.05 was adjusted using False Discovery Rate (FDR) [61] correction across nine comparisons (three ANT metrics: rule convergent thinking, rule divergent thinking, and response divergence × 3 regions of interest separately for each effect (i.e., Session, MPH, Ki, MPH x Ki). In order to control the FDR at a pre-specified level of significance denoted by δ, we followed the method of ordering the unadjusted p-values (p(1) ≤ p(2) ≤ … ≤ p(m)) and identified the test with the highest rank (j) for which the corresponding p-value (pj) satisfied the condition p(j) ≤ (j/m) x δ. Subsequently, we declared the tests of rank 1, 2, …, j as significant.
We also complemented some of our mixed-effects analysis results using BayesFactor library in R, which computes the Bayesian Information Criterion (BIC). The BIC is a model selection criterion that balances the goodness of fit of a model with the complexity of the model. The BIC is defined as BIC = -2log(L) + klog(n), where L is the likelihood of the data given the model, k is the number of model parameters, and n is the sample size. The BIC is used to compare two or more candidate models and select the model with the lowest BIC value as the preferred model. To assess the strength of evidence for the full model, we computed two BIC values, one for the null hypothesis and one for the alternative hypothesis. Next, we calculated the Bayes factor of the alternative hypothesis over the null hypothesis. The Bayes factor is the ratio of the marginal likelihood of the data under the alternative hypothesis to that under the null hypothesis, and it provides a measure of the strength of evidence in favor of the alternative hypothesis over the null hypothesis. The conversion of BIC values to Bayes factors is done using the formula BF = exp((null - alternative) / 2), where null and alternative are the BIC values for the null and alternative hypotheses, respectively. Bayes Factors that are over 1 are considered evidence for the alternative hypothesis, and those that are below 1 are considered evidence for the null hypothesis. A Bayes Factor of 1 indicates no evidence of a difference between hypotheses [62, 63].
The ecological validity of the ANT was assessed by regressing relevant ANT scores onto self-reported creativity scores, as well as on the established RAT and AUT scores.
Results
To assess the effects of MPH and dopamine synthesis capacity on our primary creativity measures, we ran a mixed-effects analysis with convergent and divergent thinking as dependent variables, drug and dopamine synthesis capacity as predictors, and session number as a nuisance variable. Contrary to our expectations, there was no evidence of the effects of MPH, sulpiride, or dopamine synthesis capacity on our primary indices of divergent or convergent thinking from the ANT (Tables 1–2; SI Figures 4-5). We complemented these findings by testing the effects of MPH on the AUT divergent and RAT convergent thinking scores. There was no significant effect of MPH, sulpiride, or dopamine synthesis capacity on AUT and RAT measures, paralleling the findings of ANT (Tables 1 and 2; SI Figs. 6–10). There were also no significant effects of dopamine synthesis capacity on any of the creativity indices measured during the placebo session (SI Table 7).
Table 1.
Reports the main and interactive effects for all three (striatal region) models for convergent thinking. Ki stands for dopamine synthesis capacity.
| Model results for convergent thinking | |||
|---|---|---|---|
| Ki Definition | Model terms | ANT convergent | RAT convergent |
| Caudate | Session | β = 2.01, SE = 0.83, t (98.51) = 2.43, p = 0.02 | β = 0.22, SE = 0.14, t (125.40) = 1.58, p = 0.12 |
| MPH | β = 0.53, SE = 0.61, t (90.03) = 0.86, p = 0.39 | β = 0.04, SE = 0.11, t (90.09) = 0.41, p = 0.69 | |
| Ki | β = −0.21, SE = 1.52, t (90.04) = −0.14, p = 0.89 | β = −0.11, SE = 0.13, t (90.10) = −0.85, p = 0.40 | |
| MPH x Ki | β = 0.75, SE = 0.61, t (90.01) = 1.22, p = 0.23 | β = −0.05, SE = 0.11, t (90.01) = −0.46, p = 0.65 | |
| Putamen | Session | β = 1.93, SE = 0.85, t (98.93) = 2.29, p = 0.02 | β = 0.23, SE = 0.14, t (126.71) = 1.61, p = 0.11 |
| MPH | β = 0.53, SE = 0.62, t (90.04) = 0.87, p = 0.39 | β = 0.04, SE = 0.11, t (90.01) = 0.40, p = 0.69 | |
| Ki | β = −0.26, SE = 1.52, t (90.03) = −0.17, p = 0.87 | β = −0.05, SE = 0.13, t (90.01) = −0.39, p = 0.70 | |
| MPH x Ki | β = 0.04, SE = 0.63, t (90.25) = 0.08, p = 0.95 | β = −0.01, SE = 0.11, t (90.79) = −0.05, p = 1.00 | |
| VS | Session | β = 2.18, SE = 0.84, t (98.80) = 2.61, p = 0.01 | β = 0.23, SE = 0.14, t (125.81) = 1.63, p = 0.11 |
| MPH | β = 0.52, SE = 0.61, t (90.06) = 0.85, p = 0.40 | β = 0.04, SE = 0.11, t (90.12) = 0.40, p = 0.69 | |
| Ki | β = 2.04, SE = 1.50, t (90.13) = 1.36, p = 0.18 | β = −0.02, SE = 0.13, t (90.37) = −0.18, p = 0.86 | |
| MPH x Ki | β = 0.89, SE = 0.62, t (90.25) = 1.43, p = 0.16 | β = 0.01, SE = 0.11, t (90.74) = 0.05, p = 0.96 | |
Reported values refer to standardized model coefficients (β) for fixed effects. SE Standard Error. p = uncorrected p value. The effect of session on ANT convergent thinking (greater number of convergent ideas across sessions) did not survive multiple comparison corrections.
Table 2.
Reports the main and interactive effects of model terms for all dopamine synthesis capacity (Ki) definitions for divergent thinking.
| Model results for divergent thinking | |||
|---|---|---|---|
| Ki Definition | Model terms | ANT divergent | AUT divergent |
| Caudate | Session | β = −0.52, SE = 0.52, t (104.02) = −0.99, p = 0.32 | β = 0.01, SE = 0.004, t (97.75) = 0.14, p = 0.89 |
| MPH | β = 0.82, SE = 0.39, t (89.98) = 2.07, p = 0.04 | β = 0.05, SE = 0.03, t (90.04) = 1.85, p = 0.07 | |
| Ki | β = −0.24, SE = 0.76, t (89.98) = −0.32, p = 0.75 | β = 0.02, SE = 0.07, t (90.01) = 0.26, p = 0.80 | |
| MPH x Ki | β = 0.23, SE = 0.39, t (89.94) = 0.59, p = 0.56 | β = 0.05, SE = 0.03, t (90.92) = 1.59, p = 0.12 | |
| Putamen | Session | β = −0.47, SE = 0.53, t (104.40) = −0.88, p = 0.38 | β = −0.01, SE = 0.04, t (98.00) = −0.08, p = 0.94 |
| MPH | β = 0.82, SE = 0.39, t (89.99) = 2.06, p = 0.04 | β = 0.05, SE = 0.03, t (90.03) = 1.83, p = 0.07 | |
| Ki | β = 0.10, SE = 0.76, t (89.97) = 0.13, p = 0.89 | β = 0.03, SE = 0.08, t (90.03) = 0.40, p = 0.69 | |
| MPH x Ki | β = 0.30, SE = 0.40, t (90.31) = 0.74, p = 0.46 | β = 0.01, SE = 0.03, t (90.23) = 0.37, p = 0.71 | |
| VS | Session | β = −0.51, SE = 0.54, t (104.29) = −0.95, p = 0.35 | β = 0.01, SE = 0.03, t (97.65) = 0.36, p = 0.72 |
| MPH | β = 0.82, SE = 0.39, t (89.97) = 2.07, p = 0.04 | β = 0.05, SE = 0.03, t (90.04) = 1.84, p = 0.07 | |
| Ki | β = −0.24, SE = 0.76, t (90.10) = −0314, p = 0.75 | β = 0.05, SE = 0.07, t (90.11) = 0.77, p = 0.44 | |
| MPH x Ki | β = 0.17, SE = 0.40, t (90.27) = 0.42, p = 0.67 | β = 0.05, SE = 0.03, t (90.21) = 1.86, p = 0.07 | |
Reported values refer to standardized model coefficients (β) for fixed effects. SE stands for Standard Error. p = uncorrected p value. The enhancing effect of MPH on AUT divergent thinking did not survive multiple comparison corrections.
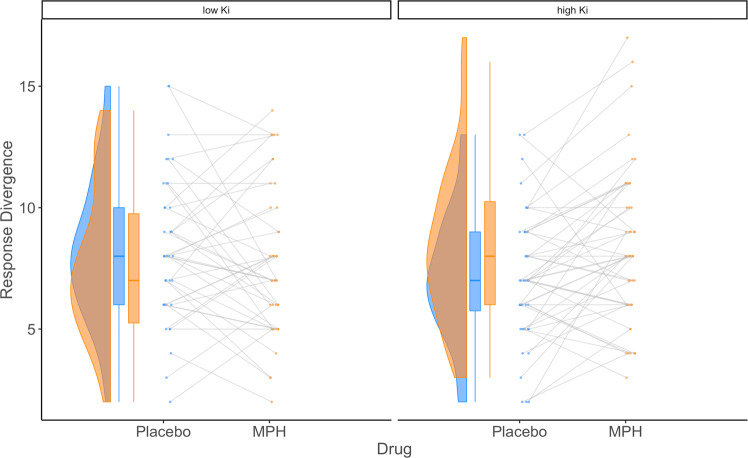
Exploratory analyses of our secondary response divergence metric from the ANT revealed a significant interaction between MPH (vs. placebo) and baseline dopamine synthesis capacity in the putamen (β = 0.46, SE = 0.15, t(90.42) = 3.03, p = 0.003 (Figs. 1 and 2). This interaction survived correction for nine comparisons and was due to MPH reducing response divergence in participants with low baseline dopamine synthesis capacity but increasing it in those with high baseline dopamine levels (Fig. 1). There were no main effects of MPH (β = 0.21, SE = 0.15, t(90.05) = 1.43, p = 0.16) or putamen dopamine synthesis capacity (β = –0.04, SE = 0.27, t(90.03) = –0.15, p = 0.88). There was also no significant effect of dopamine synthesis capacity on response divergence during the placebo session (SI Table 7). Session number had no impact (β = –0.13, SE = 0.20, t(106.80) = –0.63, p = 0.53).
Fig. 1. The effect of MPH on response divergence as a function of dopamine synthesis capacity (Ki) in the putamen.
For illustration purposes, we mean-split the sample into low Putamen Ki and high Ki samples. Compared with placebo, MPH reduced response divergence in participants with low baseline dopamine synthesis capacity but increased it in those with high baseline dopamine synthesis capacity.
Fig. 2. The model estimates for predictors, MPH, dopamine synthesis capacity (Ki) and an interaction of MPH and dopamine synthesis capacity for all three (striatal) models on the exploratory response divergence score of the ANT.
Reported values refer to standardized model coefficients (β) for fixed effects. *significant at uncorrected p < 0.05; **significant at uncorrected p < 0.01. Only the interactive effect of MPH and Ki in the putamen survived multiple comparison correction.
Interaction effects between the drug (MPH vs. placebo) and dopamine synthesis capacity in the caudate nucleus (β = 0.03, SE = 0.15, t(90.03) = 2.22, p = 0.03) did not survive the multiple comparison correction. The caudate nucleus model did not reveal any main effects of session (β = –0.20, SE = 0.20, t(107.10) = –0.99, p = 0.32), MPH (β = 0.22, SE = 0.15, t(90.10) = 1.44, p = 0.15), or caudate dopamine synthesis capacity (β = –0.01, SE = 0.27, t(90.08) = 0.04, p = 0.97). There was no evidence of an interaction between MPH and dopamine synthesis capacity in the ventral striatum (β = 0.27, SE = 0.16, t(90.43) = 1.72, p = 0.09) or the main effects of session (β = –0.18, SE = 0.21, t(107.74) = –0.85, p = 0.40), MPH (β = 0.22, SE = 0.15, t(90.07) = 1.41, p = 0.16), and ventral striatum dopamine synthesis capacity (β = –0.05, SE = 0.27, t(90.21) = –0.20, p = 0.83).
To test the specificity of the effect in various definitions of Ki, we included all three regions of interest and their interactions in a supplementary model. Predictor multicollinearity was assessed by computing variance inflation factors (VIF), which measures the inflation of a regression coefficient due to collinearity between predictors [64, 65]. For example, a VIF above 5 is considered problematic and predictors that yield problematic VIF scores are considered redundant. For this model, VIF index for all predictors was low (VIFMPH = 1.01, VIFVS_Ki = 2.95, VIFPutamen_Ki = 3.85, VIFCaudate_Ki = 2.53, VIFSession = 1.06, VIFVS_Ki*MPH = 2.96, VIFPutamen_Ki*MPH = 3.90, VIFCaudate_Ki*MPH = 2.56). The interaction between dopamine synthesis capacity in the putamen and MPH on response divergence remained significant that included all three striatal regions of interest (putamen, caudate nucleus, and ventral striatum), along with their interactions with MPH (versus placebo) (β = 0.66, SE = 0.29, t(90.10) = 2.27, p = 0.03), while all other main effects and interactions were insignificant (all p > 0.05).
Finally, to assess the effect of sulpiride on response divergence, we used a mixed-effects linear regression model with sulpiride (versus placebo) as a predictor. The results showed no evidence of an effect of sulpiride versus placebo on this exploratory response divergence measure (SI Figs. 6–10). We ran a complementary Bayes Factor analysis, allowing us to quantify the evidence for the absence of an interaction between dopamine synthesis capacity (putamen) and sulpiride on our exploratory response divergence measure. We found strong evidence for the null hypothesis (BF = 0.01), indicating that sulpiride administration had no effect on response divergence.
There was a significant positive relationship between subjective reports of individual creative ability, measured using the Kaufman Domains of Creativity Scale (KDOCS), and ANT response divergence (B = 1.54, SE = 0.53, t(90.00) = 2.90, p < 0.01), AUT divergent thinking (β = 0.47, SE = 0.16, t(89.99) = 2.92, p < 0.01), and ANT divergent thinking (B = 5.14, SE = 1.53, t(90.00) = 3.36, p < 0.001). Conversely, there was no main effect of KDOCS on convergent ANT scores (B = –1.55, SE = 3.36, t(90.00) = –0.46, p = 0.65) or on convergent RAT scores (B = 0.09, SE = 0.25, t(90.00) = 0.35, p = 0.72). These results establish the ecological validity of our indices of divergent thinking, including the response divergence score sensitive to MPH.
Discussion
We investigated whether acute administration of a single oral dose of MPH (20 mg MPH) or sulpiride (400 mg) changed convergent and divergent creative thinking processes in healthy participants as a function of baseline dopamine synthesis capacity. Divergent creativity scores were predicted to be impaired, and convergent creativity scores to be enhanced by MPH, more so in participants with lower dopamine synthesis capacity. Conversely, we predicted divergent creativity to be enhanced and convergent creativity to be impaired by sulpiride in participants with lower dopamine synthesis capacity. Contrary to our expectations, the main outcome measures of convergent and divergent thinking were not significantly affected by MPH, sulpiride, baseline dopamine synthesis capacity, or any interaction. These results demonstrate that the most commonly used metrics of convergent and divergent creativity are insensitive to changes in dopamine [5].
Critically, exploratory analyses did reveal effects of MPH on another metric of divergent creativity, response divergence. Unlike classic divergent thinking metrics, which index the frequency of deviation from a given rule, response divergence indexes the variability in the types of responses generated. Our verbal response divergence metric is remarkably analogous to a metric of visuomotor response divergence derived from an option generation task that was recently demonstrated to be sensitive to administration of the dopamine D2 receptor agonist cabergoline in patients with Parkinson’s disease [12]. Together with this prior study, our results indicate that response divergence is more sensitive to changes in dopamine levels than rule divergence. Notably, subjective creativity scores were captured by both measures of divergent creativity, raising the real-world relevance of the current findings.
The effect of MPH was uncovered only if we considered individual variability in dopamine synthesis capacity. Specifically, we found that MPH impaired response divergence in participants with low dopamine synthesis capacity in the putamen and improved response divergence in participants with high dopamine capacity in the putamen. This finding substantiates the conclusion of Gvirts et al. [66], who revealed in a smaller sample of participants that MPH boosts divergent thinking depending on individual differences in novelty seeking. The present results indicate that such an effect of novelty seeking could reflect the effect of baseline striatal dopamine levels [67].
Our results are consistent with previously reported detrimental effects of MPH on flexible working memory updating in young healthy volunteers [17] and on creative thinking scores in people with ADHD [30, 31], who have been shown to express reduced dopamine synthesis capacity, specifically in the putamen [68]. Several processes might have been altered by MPH to reduce response divergence in people with low dopamine synthesis capacity. For example, MPH might have undermined response divergence by increasing the focus of attention (see [69, 70] showing that MPH increases focused attention), the distractor-resistance of working memory representations [17], or the motivation to exert cognitive effort [44]. It might have also (additionally) acted to undermine divergent creativity by reducing sleepiness [71–73] and the tendency to mind-wander [74], and/or by diminishing the spread of working and/or semantic memory associations, thus facilitating the access to original solutions [75–77]. Dual thinking accounts of creativity indeed highlight that distractibility, drowsiness, and mind-wandering might be necessary for creative scientific problem solving [78]. In this context, it is relevant to note that a subset of participants with low striatal dopamine synthesis capacity reported here have also been shown to exhibit greater smartphone social media use [53], which in turn correlates positively with mind-wandering [79].
The present results suggest that brain catecholamines play an important role in divergent creativity. However, there was no evidence of any significant effects of individual variation in dopamine synthesis capacity on creative thinking scores when participants were tested with placebo (SI Table 7). Thus, while stratification by baseline dopamine synthesis capacity allowed us to uncover a drug effect, the effect of dopamine did not surface as a function of individual differences at baseline. We remain somewhat puzzled by the lack of an effect under placebo, but we suspect that it is more difficult to detect any effects of such stable individual differences in dopamine synthesis capacity, perhaps due to long-term neuroplastic adaptation effects such as a compensatory change in dopamine receptor availability. This hypothesis might be addressed in future studies by conducting complementary PET imaging with a dopamine D2/3 receptor ligand, such as 11C-raclopride, which allows quantification of receptor availability.
One might ask whether one reason for the lack of correlations between dopamine synthesis capacity and the more commonly used metrics of creativity might be the delay between the PET scan and pharmacological test sessions. However, 18F-FDOPA uptake (Ki) represents a relatively stable index of dopamine synthesis capacity, likely capturing a trait-like factor that is insensitive to state-related factors, such as stress or fatigue. Indeed, the test-retest reliability of 18F-FDOPA uptake in striatal regions has been established to be high (intraclass correlation coefficients ranging from 0.68-0.94), even when two scans were separated by two years [80].
While 18F-FDOPA PET imaging is a reliable method for characterizing striatal dopamine synthesis capacity, the direct relationship with endogenous striatal dopamine levels is not clear yet. For example, Ito et al. [81] found a negative relationship between D2 and DA synthesis capacity as measured with 11C-DOPA while Berry and co-workers [82] showed a relationship between striatal dopamine D2/3 binding and dopamine synthesis capacity as indexed with 18F-FMT uptake. On the other hand, Walker et al. [83] found a positive correlation between 18F-FDOPA uptake and dopamine release, as measured by microdialysis, in the 6-OHDA-lesioned rats. It would therefore be of interest to better understand the postulated relationship between endogenous dopamine levels and 18F-FDOPA uptake by performing in the same subjects both 18F-FDOPA PET as well as dopamine D2/3 imaging, combined with an acute alpha-methyl-para-tyrosine (AMPT) challenge [84].
The effect of dopamine synthesis capacity was greatest in the putamen, while the effects in the other part of the dorsal striatum, the caudate nucleus, did not reach significance. There was no evidence of any effect on dopamine synthesis capacity in the ventral striatum. We confirmed the regional specificity of this effect through an additional analysis that included all regions of interest in the same model. Functional heterogeneity within the striatum [85] may underlie this effect. For example, the level of abstraction required by a cognitive flexibility task may recruit distinct portions of the striatum [23, 86–89]. The type of cognitive flexibility required to generate response divergence, as studied here, involves a form of response switching between concrete items, which has been shown to activate and necessitate the putamen [90, 91]. In these prior studies, the activation and necessity of the putamen was not evidenced for a complementary form of switching between abstract rules. In the current study, there was no evidence for an effect of dopamine on rule divergence.
The effects of MPH on response divergence were not accompanied by any effects of sulpiride, either alone or as a function of striatal dopamine synthesis capacity. Given the relatively exclusive impact of sulpiride on dopaminergic transmission in the striatum (and ventral tegmental area) [92], this raises the question of whether the effects of MPH on divergent creativity reflect modulation of dopamine in the prefrontal cortex, a hypothesis that can be tested in future pharmacological neuroimaging studies. In this sample of healthy participants, individual differences in dopamine synthesis capacity, measured here in the striatum, where the signal-to-noise ratio is particularly high, are likely strongly correlated with individual differences in dopamine synthesis capacity in the prefrontal cortex, in which the signal-to-noise ratio is insufficient to measure with 18F-FDOPA PET imaging. MPH might well have undermined divergent creativity by stimulating dopamine receptors in the prefrontal cortex (PFC), thus boosting distractor resistance in working memory representations [93] in those with low baseline dopamine levels in the PFC. In line with the inverted-U-shaped relationship between prefrontal dopamine receptor stimulation and working memory performance [94], MPH might have elicited excessive dopamine receptor stimulation in the PFC in those with high baseline dopamine synthesis capacity, thus reducing working memory focus and releasing divergent creativity.
While the observation that the effects of MPH depend on baseline dopamine synthesis capacity suggests that they reflect modulation of dopamine, we cannot exclude a role of noradrenaline. MPH blocks both the noradrenaline and the dopamine transporter [95, 96], leading to increases in noradrenaline and dopamine in the PFC [28] and striatum [97]. Thus, MPH might have biased creative thinking by boosting noradrenaline [98] and modulating, for example, the exploration of semantic spaces [99]. Future studies with more selective noradrenaline receptor agents are needed to address this question.
Finally, we cannot exclude the possibility that the observed null effect of sulpiride is due to suboptimal dosing or timing of the creativity tasks in relation to peak drug effects. This is particularly pertinent, given the previously observed effect of cabergoline on response divergence [100]. In the present study, creativity tasks were administered approximately 290 min (±5 h) after drug intake. Given that sulpiride plasma concentrations peak after 3 hours [101], it is possible that sulpiride was no longer maximally active during the performance of the creativity tasks. Indeed, the effects of dopamine D2 receptor agent intake on dopamine release are complex and depend on the dosage and timing of drug administration [102]. For example, low doses are associated with blocking presynaptic D2 receptors and increased PFC dopamine [103, 104], whereas high doses are associated with blocking post-synaptic dopaminergic D2 activity [105, 106]. To exclude the sensitivity of divergent creativity to sulpiride, future studies should observe creative task performance during the peak effects of dopaminergic drug administration and as a function of varying sulpiride doses. In light of the current study’s limitations, there is a need for a validation study employing rigorous methodologies to corroborate the findings, ensuring the robustness and generalizability of the results across different time points, drug doses and task measures.
Supplementary information
Author contributions
CS: data curation, formal analysis, writing – original draft, writing – review & editing, software, visualization; RB: data curation, formal analysis (of the PET data), investigation, software, writing - review & editing. JM: project administration, data curation, investigation, writing - review & editing; LH: investigation, writing - review & editing; DP: investigation, software, writing - review & editing; JB: supervision, writing - review and editing.; R-JV: investigation, writing – review and editing; MB: methodology, writing - review & editing; RC: study conception, study design, overarching supervision of data acquisition, analyses and writing, writing - review & editing.
Funding
This work was supported by a Vici grant to R.C. from the Netherlands Organization for Scientific Research (NWO; Grant No. 453-14-015).
Data availability
All task code is available at https://github.com/zceydas/VICI_Creativity; data is available upon request from the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01615-2.
References
- 1.Sahakian B, Morein-Zamir S. Professor’s little helper. Nature. 2007;450:1157–9. doi: 10.1038/4501157a. [DOI] [PubMed] [Google Scholar]
- 2.Abelman DD. Mitigating risks of students use of study drugs through understanding motivations for use and applying harm reduction theory: a literature review. Harm Reduct J. 2017;14:1–7. doi: 10.1186/s12954-017-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farah MJ, Haimm C, Sankoorikal G, Smith ME, Chatterjee A. When we enhance cognition with Adderall, do we sacrifice creativity? A preliminary study. Psychopharmacology. 2009;202:541–7. doi: 10.1007/s00213-008-1369-3. [DOI] [PubMed] [Google Scholar]
- 5.Baas M, Boot N, van Gaal S, De Dreu CK, Cools R. Methylphenidate does not affect convergent and divergent creative processes in healthy adults. NeuroImage. 2020;205:116279. doi: 10.1016/j.neuroimage.2019.116279. [DOI] [PubMed] [Google Scholar]
- 6.Hoogman M, Stolte M, Baas M, Kroesbergen E. Creativity and ADHD: A review of behavioral studies, the effect of psychostimulants and neural underpinnings. Neurosci Biobehav Rev. 2020;119:66–85. [DOI] [PubMed]
- 7.Runco MA, Jaeger GJ. The standard definition of creativity. Creativity Res J. 2012;24:92–6. [Google Scholar]
- 8.Lhommée E, Batir A, Quesada JL, Ardouin C, Fraix V, Seigneuret E, et al. Dopamine and the biology of creativity: Lessons from Parkinson’s Disease. Front Neurol. 2014;5:55. doi: 10.3389/fneur.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boot N, Baas M, Mühlfeld E, De Dreu CKW, Van Gaal S. Widespread neural oscillations in the delta band dissociate rule convergence from rule divergence during creative idea generation. Neuropsychologia. 2017;104:8–17. doi: 10.1016/j.neuropsychologia.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Flaherty AW. Frontotemporal and dopaminergic control of idea generation and creative drive. J Comp Neurol. 2005;493:147–53. doi: 10.1002/cne.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heilman KM, Nadeau SE, Beversdorf DO. Creative innovation: possible brain mechanisms. Neurocase. 2003;9:369–79. doi: 10.1076/neur.9.5.369.16553. [DOI] [PubMed] [Google Scholar]
- 12.Ang YS, Manohar S, Plant O, Kienast A, Le Heron C, Muhammed K, et al. Dopamine modulates option generation for behavior. Curr Biol. 2018;28:1561–9. doi: 10.1016/j.cub.2018.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cools R. Chemistry of the adaptive mind: lessons from dopamine. Neuron. 2019;104:113–31. doi: 10.1016/j.neuron.2019.09.035. [DOI] [PubMed] [Google Scholar]
- 14.Dreisbach G, Goschke T. How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. J Exp Psychol: Learn, Mem, Cognition. 2004;30:343. doi: 10.1037/0278-7393.30.2.343. [DOI] [PubMed] [Google Scholar]
- 15.Dreisbach G, Fröber K. On how to be flexible (or not): Modulation of the stability-flexibility balance. Curr Dir Psychol Sci. 2019;28:3–9. [Google Scholar]
- 16.Robbins TW. Dopamine and cognition. Curr Opin Neurol. 2003;16:S1–S2. doi: 10.1097/00019052-200312002-00001. [DOI] [PubMed] [Google Scholar]
- 17.Fallon SJ, Mattiesing RM, Muhammed K, Manohar S, Husain M. Fractionating the neurocognitive mechanisms underlying working memory: Independent effects of dopamine and Parkinson’s disease. Cereb Cortex. 2017;27:5727–38. doi: 10.1093/cercor/bhx242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman-Rakic P. Cellular basis of working memory. Neuron. 1995;14:477–85. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 19.Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nat Neurosci. 2000;3:1184–91. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- 20.Furman DJ, Zhang Z, Chatham CH, Good M, Badre D, Hsu M, et al. Augmenting frontal dopamine tone enhances maintenance over gating processes in working memory. J Cogn Neurosci. 2021;33:1753–65. doi: 10.1162/jocn_a_01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Dreu CKW, Nijstad BA, Baas M, Wolsink I, Roskes M. Working memory benefits creative insight, musical improvisation, and original ideation through maintained task-focused attention. Pers Soc Psychol Bull. 2012;38:656–69. doi: 10.1177/0146167211435795. [DOI] [PubMed] [Google Scholar]
- 22.Frank MJ, Loughry B, O’Reilly RC. Interactions between the frontal cortex and basal ganglia in working memory: A computational model. Cogn, Affect, Behav Neurosci. 2001;1:137–60. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- 23.O’Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- 24.Cools R, Lewis SJ, Clark L, Barker RA, Robbins TW. L-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson’s disease. Neuropsychopharmacology. 2007;32:180–9. [DOI] [PubMed]
- 25.Chatham CH, Badre D. Multiple gates on working memory. Curr Opin Behav Sci. 2015;1:23–31. doi: 10.1016/j.cobeha.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zmigrod L, Robbins TW. Dopamine, cognitive flexibility, and IQ: Epistatic catechol-o-MethylTransferase: DRD2 gene–gene interactions modulate mental rigidity. J Cogn Neurosci. 2021;34:153–79. doi: 10.1162/jocn_a_01784. [DOI] [PubMed] [Google Scholar]
- 27.Spencer RC, Klein RM, Berridge CW. Psychostimulants act within the prefrontal cortex to improve cognitive function. Biol Psychiatry. 2012;72:221–7. doi: 10.1016/j.biopsych.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berridge CW, Spencer RC. Differential cognitive actions of norepinephrine a2 and a1 receptor signaling in the prefrontal cortex. Brain Res. 2016;1641:189–96. doi: 10.1016/j.brainres.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nijstad BA, De Dreu CK, Rietzschel EF, Baas M. The dual pathway to creativity model: Creative ideation as a function of flexibility and persistence. Eur Rev Soc Psychol. 2010;21:34–77. [Google Scholar]
- 30.Brinkman WB, Sherman SN, Zmitrovich AR, Visscher MO, Crosby LE, Phelan KJ, et al. In their own words: Adolescent views on ADHD and their evolving role managing medication. Acad Pediat. 2012;12:53–61. doi: 10.1016/j.acap.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovshoff H, Banaschewski T, Buitelaar JK, Carucci S, Coghill D, Danckaerts M, et al. Reports of perceived adverse events of stimulant medication on cognition, motivation, and mood: Qualitative investigation and the generation of items for the medication and cognition rating scale. J Child Adolesc Psychopharmacol. 2016;26:537–47. doi: 10.1089/cap.2015.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dockree PM, Barnes JJ, Matthews N, Dean AJ, Abe R, Nandam LS, et al. The effects of methylphenidate on the neural signatures of sustained attention. Biol psychiatry. 2017;82:687–94. doi: 10.1016/j.biopsych.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ, et al. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002;6(1_suppl):31–43. doi: 10.1177/070674370200601s05. [DOI] [PubMed] [Google Scholar]
- 34.Arnsten AF, Wang M, Paspalas CD. Dopamine’s actions in primate prefrontal cortex: challenges for treating cognitive disorders. Pharmacol Rev. 2015;67:681–96. doi: 10.1124/pr.115.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta MA, Montgomery AJ, Kitamura Y, Grasby PM. Dopamine D2 receptor occupancy levels of acute sulpiride challenges that produce working memory and learning impairments in healthy volunteers. Psychopharmacology. 2008;196:157–65. doi: 10.1007/s00213-007-0947-0. [DOI] [PubMed] [Google Scholar]
- 36.Agid Y, Ruberg M, Javoy-Agid F, Hirsch E, Raisman-Vozari R, Vyas S, et al. Are dopaminergic neurons selectively vulnerable to Parkinson’s disease? Adv Neurol. 1993;60:148–64. [PubMed] [Google Scholar]
- 37.Morrish PK, Sawle GV, Brooks DJ. An [18F] dopa–PET and clinical study of the rate of progression in Parkinson’s disease. Brain. 1996;119:585–91. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- 38.Rakshi J, Uema T, Ito K, Bailey D, Morrish P, Ashburner J, et al. Frontal, midbrain and striatal dopamergic function in early and advanced Parkinson’s disease. A 3D [(18)F]dopa-PET study. Brain. 1999;122:1637–50. doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- 39.Kaasinen V, Nurmi E, Bergman J, Eskola O, Solin O, Sonninen P, et al. Personality traits and brain dopaminergic function in Parkinson’s disease. Proc Natl Acad Sci. 2001;98:13272–7. doi: 10.1073/pnas.231313198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain. 2008;131:1294–302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- 41.Cools R, Miyakawa A, Sheridan M, D’Esposito M. Enhanced frontal function in Parkinson’s disease. Brain. 2010;133:225–33. doi: 10.1093/brain/awp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canesi M, Rusconi ML, Isaias IU, Pezzoli G. Artistic productivity and creative thinking in Parkinson’s disease. Eur J Neurol. 2012;19:468–72. doi: 10.1111/j.1468-1331.2011.03546.x. [DOI] [PubMed] [Google Scholar]
- 43.Inzelberg R. The awakening of artistic creativity and Parkinson’s disease. Behav Neurosci. 2013;127:256. doi: 10.1037/a0031052. [DOI] [PubMed] [Google Scholar]
- 44.Westbrook A, Van Den Bosch R, Määttä JI, Hofmans L, Papadopetraki D, Cools R, et al. Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science. 2020;367:1362–6. doi: 10.1126/science.aaz5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18] fluorodopa positron emission tomographic study. J Neurosci. 1998;18:5901–7. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Määttä JI, van den Bosch R, Papadopetraki D, Hofmans L, Lambregts B, Westbrook A, et al. Predicting effects of methylphenidate and sulpiride on brain and cognition: A pharmaco-fMRI, PET study. Des Descr. 2017. 10.31219/osf.io/d3h8e.
- 47.Hofmans L, Papadopetraki D, van den Bosch R, Määttä JI, Froböse MI, Zandbelt B, et al. Methylphenidate boosts choices of mental labor over leisure depending on striatal dopamine synthesis capacity. Neuropsychopharmacology. 2020;45:2170–9. doi: 10.1038/s41386-020-00834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofmans L, Westbrook A, van den Bosch R, Booij J, Verkes RJ, Cools R. Effects of average reward rate on vigor as a function of individual variation in striatal dopamine. Psychopharmacology. 2022;239:465–78. doi: 10.1007/s00213-021-06017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Bosch R, Lambregts B, Määttä J, Hofmans L, Papadopetraki D, Westbrook A, et al. Striatal dopamine dissociates methylphenidate effects on value-based versus surprise-based reversal learning. Nat Commun. 2022;13:1–15. doi: 10.1038/s41467-022-32679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen P, Geurts DE, Määttä JI, van den Bosch R, Hofmans L, Papadopetraki D, et al. Effect of striatal dopamine on Pavlovian bias. A large [18F]-DOPA PET study. Behav Neurosci. 2023;137:184–95. doi: 10.1037/bne0000547. [DOI] [PubMed] [Google Scholar]
- 51.Mednick S. The associative basis of the creative process. Psycholog Rev. 1962;69:220. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- 52.Swanson J, Gupta S, Lam A, Shoulson I, Lerner M, Modi N, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psych. 2003;60:204–11. doi: 10.1001/archpsyc.60.2.204. [DOI] [PubMed] [Google Scholar]
- 53.Westbrook A, Ghosh A, van den Bosch R, Määttä JI, Hofmans L, Cools R. Striatal dopamine synthesis capacity reflects smartphone social activity. Iscience. 2021;24:102497. doi: 10.1016/j.isci.2021.102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyes BE, Cumming P, Martin WRW, McGeer EG. Determination of plasma [18F]-6-fluorodopa during positron emission tomography: elimination and metabolism in carbidopa treated subjects. Life Sci. 1986;39:2243–52. doi: 10.1016/0024-3205(86)90403-0. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman JM, Melega WP, Hawk TC, Grafton SC, Luxen A, Mahoney DK, et al. The effects of carbidopa administration on 6-[18F] fluoro-L-dopa kinetics in positron emission tomography. J Nucl Med. 1992;33:1472–7. [PubMed] [Google Scholar]
- 56.Ishikawa T, Dhawan V, Kazumata K, Chaly T. Comparative nigrostriatal dopaminergic imaging with iodine-123-betaCIT-FP/SPECT and fluorine-18-FDOPA/PET. J Nucl Med. 1996;37:1760. [PubMed] [Google Scholar]
- 57.Léger G, Gjedde A, Kuwabara H, Guttman M, Cumming P. Effect of catechol‐O‐methyltransferase inhibition on brain uptake of [18F] fluorodopa: Implications for compartmental modelling and clinical usefulness. Synapse. 1998;30:351–61. doi: 10.1002/(SICI)1098-2396(199812)30:4<351::AID-SYN2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 58.Piray P, den Ouden HE, van der Schaaf ME, Toni I, Cools R. Dopaminergic modulation of the functional ventrodorsal architecture of the human striatum. Cereb Cortex. 2017;27:485–95. doi: 10.1093/cercor/bhv243. [DOI] [PubMed] [Google Scholar]
- 59.Guilford JP. The nature of human intelligence. New York, NY: McGraw-Hill 1967.
- 60.Kaufman JC. Counting the muses: development of the Kaufman Domains of Creativity Scale (K-DOCS) Psychol Aesthet, Creativity, Arts. 2012;6:298. [Google Scholar]
- 61.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- 62.Lee MD, Wagenmakers EJ. Bayesian cognitive modeling: A practical course. Cambridge University Press 2014.
- 63.Jeffreys, H Theory of probability (3rd ed.). Oxford, England: Oxford University Press 1961.
- 64.Bruce P, Bruce A. Practical Statistics for Data Scientists. O’Reilly Media (2017).
- 65.Gareth J, Witten D, Hastie T Tibshirani R. An Introduction to Statistical Learning: With Applications in R. Springer Publishing Company, Incorporated 2014.
- 66.Gvirts HZ, Mayseless N, Segev A, Lewis DY, Feffer K, Barnea Y, et al. Novelty-seeking trait predicts the effect of methylphenidate on creativity. J Psychopharmacol. 2017;31:599–605. doi: 10.1177/0269881116667703. [DOI] [PubMed] [Google Scholar]
- 67.Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behav brain Sci. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- 68.Ludolph AG, Kassubek J, Schmeck K, Glaser C, Wunderlich A, Buck AK, et al. Dopaminergic dysfunction in attention deficit hyperactivity disorder (ADHD), differences between pharmacologically treated and never treated young adults: a 3, 4-dihdroxy-6-[18F] fluorophenyl-l-alanine PET study. Neuroimage. 2008;41:718–27. doi: 10.1016/j.neuroimage.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 69.Ter Huurne N, Fallon SJ, van Schouwenburg M, van der Schaaf M, Buitelaar J, Jensen O, et al. Methylphenidate alters selective attention by amplifying salience. Psychopharmacology. 2015;232:4317–23. doi: 10.1007/s00213-015-4059-y. [DOI] [PubMed] [Google Scholar]
- 70.Linssen AM, Sambeth A, Vuurman EF, Riedel WJ. Cognitive effects of methylphenidate in healthy volunteers: a review of single dose studies. Int J Neuropsychopharmacol. 2014;17:961–77. doi: 10.1017/S1461145713001594. [DOI] [PubMed] [Google Scholar]
- 71.Lacaux C, Izabelle C, Sanantonio G, De Villèle L, Frain J, Lubart T, et al. Increased creative thinking in narcolepsy. Brain. 2019;142:1988–99. doi: 10.1093/brain/awz137. [DOI] [PubMed] [Google Scholar]
- 72.Lacaux C, Andrillon A, Bastoul C, Idir Y, Fonteix-Galet A, Arnulf I, et al. Sleep onset is a creative sweet spot. Sci Adv. 2021;7:eabj5866. doi: 10.1126/sciadv.abj5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jarosz AF, Colflesh GJ, Wiley J. Uncorking the muse: Alcohol intoxication facilitates creative problem solving. Conscious Cogn. 2012;21:487–93. doi: 10.1016/j.concog.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 74.Schooler JW, Smallwood J, Christoff K, Handy TC, Reichle ED, Sayette MA. Meta-awareness, perceptual decoupling and the wandering mind. Trends Cogn Sci. 2011;15:319–26. doi: 10.1016/j.tics.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 75.Ansburg PI, Hill K. Creative and analytic thinkers differ in their use of attentional resources. Personal Individ Diff. 2003;34:1141–52. [Google Scholar]
- 76.Finke RA, Ward TB, Smith SM. Creative cognition: Theory, research, and applications. Cambridge, MA: MIT Press. 1992.
- 77.Martindale C. Creativity and connectionism. Creat Cogn Appr. 1995;249:268. [Google Scholar]
- 78.Scheffer M, Bascompte J, Bjordam TK, Carpenter SR, Clarke LB, Folke C, et al. Dual thinking for scientists. Ecol Soc. 2015;20.
- 79.Sumuer E, Kaşıkcı DN. The role of smartphones in college students’ mind-wandering during learning. Comput Educ. 2022;190:104616. [Google Scholar]
- 80.Egerton A, Demjaha A, McGuire P, Mehta MA, Howes OD. The test–retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. Neuroimage. 2010;50:524–31. doi: 10.1016/j.neuroimage.2009.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ito H, Kodaka F, Takahashi H, Takano H, Arakawa R, Shimada H, et al. Relation between presynaptic and postsynaptic dopaminergic functions measured by positron emission tomography: implication of dopaminergic tone. J Neurosci. 2011;31:7886–90. doi: 10.1523/JNEUROSCI.6024-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berry AS, Shah VD, Furman DJ, White RL, III, Baker SL, O’Neil JP, et al. Dopamine synthesis capacity is associated with D2/3 receptor binding but not dopamine release. Neuropsychopharmacology. 2018;43:1201–11. doi: 10.1038/npp.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walker MD, Dinelle K, Kornelsen R, McCormick S, Mah C, Holden JE, et al. In-vivo measurement of LDOPA uptake, dopamine reserve and turnover in the rat brain using [18F] FDOPA PET. J Cereb Blood Flow Metab. 2013;33:59–66. doi: 10.1038/jcbfm.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laruelle M, D’Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, et al. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 1997;17:162–74. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 85.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal–ventral divide of the striatum. Trends Neurosci. 2004;27:468–74. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 86.Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- 87.Roberts AC, Wallis JD. Inhibitory control and affective processing in the prefrontal cortex: neuropsychological studies in the common marmoset. Cereb Cortex. 2000;10:252–62. doi: 10.1093/cercor/10.3.252. [DOI] [PubMed] [Google Scholar]
- 88.Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbitofrontal cortex. J Neurosci. 1999;20:9029–38. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, et al. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex. 2001;11:85–92. doi: 10.1093/cercor/11.1.85. [DOI] [PubMed] [Google Scholar]
- 90.Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–35. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cools R. Dopaminergic modulation of cognitive function – Implication for L-DOPA therapy in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–34. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 92.Grace AA, Moore H, O’Donnell P. The modulation of corticoaccumbens transmission by limbic afferents and dopamine: a model for the pathophysiology of schizophrenia. In Advances in pharmacology (Vol. 42, pp. 721-4). Academic Press 1997. [DOI] [PubMed]
- 93.Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psych. 2008;64:739–49. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 94.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine Dl receptors in prefrontal cortex. Nature. 1995;376:572–5. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 95.Arnsten AF, Scahill L, Findling RL. Alpha-2 adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. J Child Adolesc Psychopharmacol. 2007;17:393–406. doi: 10.1089/cap.2006.0098. [DOI] [PubMed] [Google Scholar]
- 96.Ding YS, Fowler JS, Volkow ND, Dewey SL, Wang GJ, Logan J, et al. Chiral drugs: comparison of the pharmacokinetics of [11C] d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacology. 1997;131:71–8. doi: 10.1007/s002130050267. [DOI] [PubMed] [Google Scholar]
- 97.Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psych. 2005;57:1410–5. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 98.Beversdorf DQ. Neuropsychopharmacological regulation of performance on creativity-related tasks. Curr Opin Behav Sci. 2019;27:55–63. doi: 10.1016/j.cobeha.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beversdorf DQ, Hughes JD, Steinberg BA, Lewis LD, Heilman KM. Noradrenergic modulation of cognitive flexibility in problem solving. Neuroreport. 1999;10:2763–7. doi: 10.1097/00001756-199909090-00012. [DOI] [PubMed] [Google Scholar]
- 100.Musslick S, Cohen JD. Rationalizing constraints on the capacity for cognitive control. Trends Cogn Sci. 2021;25:757–75. doi: 10.1016/j.tics.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Mehta MA, McGowan SW, Lawrence AD, Aitken MR, Montgomery AJ, Grasby PM. Systemic sulpiride modulates striatal blood flow: relationships to spatial working memory and planning. Neuroimage. 2003;20:1982–94. doi: 10.1016/j.neuroimage.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 102.Mueller EM, Makeig S, Stemmler G, Hennig J, Wacker J. Dopamine effects on human error processing depend on catechol-O-methyltransferase VAL158MET genotype. J Neurosci. 2011;31:15818–25. doi: 10.1523/JNEUROSCI.2103-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuroki T, Meltzer HY, Ichikawa J. Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Therapeutics. 1999;288:774–81. [PubMed] [Google Scholar]
- 104.Mereu G, Casu M, Gessa GL. (—)-Sulpiride activates the firing rate and tyrosine hydroxylase activity of dopaminergic neurons in unanesthetized rats. Brain Res. 1983;264:105–10. doi: 10.1016/0006-8993(83)91125-3. [DOI] [PubMed] [Google Scholar]
- 105.Eisenegger C, Naef M, Linssen A, Clark L, Gandamaneni PK, Müller U, et al. Role of dopamine D2 receptors in human reinforcement learning. Neuropsychopharmacology. 2014;39:2366–75. doi: 10.1038/npp.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boschen SL, Andreatini R, Da Cunha C. Activation of postsynaptic D2 dopamine receptors in the rat dorsolateral striatum prevents the amnestic effect of systemically administered neuroleptics. Behav Brain Res. 2015;281:283–9. doi: 10.1016/j.bbr.2014.12.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All task code is available at https://github.com/zceydas/VICI_Creativity; data is available upon request from the authors.