Abstract
Swyer syndrome is a rare form of primary amenorrhea resulting from gonadal dysgenesis. It is characterized by the presence of a female phenotype with a 46, XY karyotype. In our case, CT scans revealed the absence of the uterus and bilateral ovaries of the 16-year-old female patient. Calcific nodules were found in both inguinal areas, which were suspected to be calcified atrophic testes. A chromosomal study confirmed the diagnosis of Swyer syndrome. Herein, we report a rare case of Swyer syndrome.
Keywords: Swyer Syndrome; Computed Tomography, X-Ray; Karyotype
Abstract
Swyer 증후군은 생식샘 발달에 영향을 미치는 희귀 유전질환으로 원발성 무월경의 드문 원인이다. 이 증후군은 46, XY 핵형을 가지나 표현형은 여성으로 나타나는 특징을 보인다. 이번 증례는 원발성 무월경을 주소로 내원한 16세 여성으로 컴퓨터단층촬영에서 자궁과 양측 난소가 관찰되지 않으며 양쪽 사타구니 부위에서 위축된 고환으로 보이는 석회화 결절이 관찰되었다. 염색체 연구에서 46, XY로 Swyer 증후군으로 확진되었다. 이에 저자들은 원발성 무월경의 드문 원인으로 Swyer 증후군을 보고하고자 한다.
INTRODUCTION
Swyer syndrome is a rare form of primary amenorrhea resulting from gonadal dysgenesis. It was first described by Gim Swyer in 1955. The syndrome is characterized by the presence of a female phenotype with a 46, XY karyotype. The gonads are either absent or streak gonads, and the uterus and fallopian tubes are usually absent or hypoplastic (1). Patients with Swyer syndrome have an increased risk of developing gonadal tumors, particularly gonadoblastoma, and may experience a delay in the onset of puberty (2). We report a case of Swyer syndrome in a 16-year-old female who presented with primary amenorrhea.
CASE REPORT
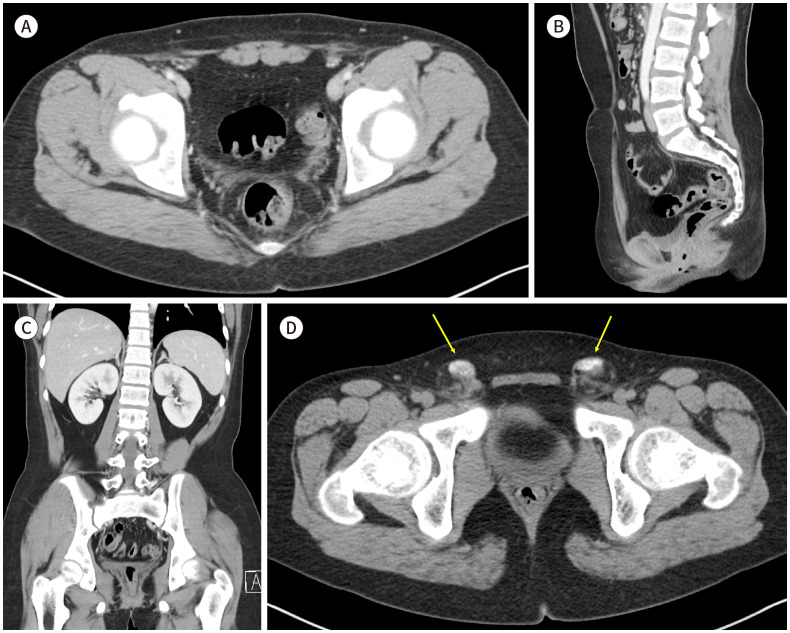
A 16-year-old female visited our hospital with a chief complaint of primary amenorrhea. She had no significant medical history, and her family history was unremarkable. Physical examination revealed normal breast development and normal female external genitalia but scanty pubic and axillary hair. A gynecological ultrasonography examination of the internal genitalia revealed the absence of the uterus, both fallopian tubes, and both ovaries. A CT scan was performed to further evaluate the pelvic region. The CT scan showed the absence of the uterus and bilateral ovaries (Fig. 1A-C). Additionally, calcific nodules were observed in both inguinal areas, which were suspected to be calcified atrophic testes (Fig. 1D). A hormonal study was conducted, which revealed showed Testosterone: 0.03 ng/mL (0.05–0.4), estradiol: 6.69 pg/mL (11.3–43.2), luteinizing hormone (LH): 44.4 mIU/mL (1.7–8.6), follicle-stimulating hormone: 30.7 mIU/mL (1.5–12.4). The hormonal study represented primary hypogonadism and a chromosomal study revealed a male karyotype with sex chromosome XY. Based on these findings the patient was diagnosed with Swyer syndrome. Following diagnosis, the patient was referred to a general surgeon for gonadectomy due to the presence of inguinal nodules, which posed a risk for developing gonadal tumors. The patient was referred to an endocrinology department for consideration of hormonal replacement therapy. Finally, the patient was referred to a psychiatry department to provide emotional stability, determine social sex, and psychiatric treatment.
Fig. 1. A 16-year-old female dignosed with Swyer syndrome.
A-C. Axial (A), sagittal (B), and coronal (C) contrast-enhanced CT images show the absence of the uterus and both ovaries in the pelvic cavity.
D. A pre-enhanced axial CT image of a 16-year-old female with Swyer syndrome, displaying calcific nodules of approximately 1.8 cm in size in both inguinal areas (arrows).
Written informed consent for this case report was obtained from the patient’s parents. This study was performed according to the latest ethical principles in the Declaration of Helsinki.
DISCUSSION
Swyer syndrome, also known as pure gonadal dysgenesis, is a rare genetic disorder characterized by the failure of the gonads to develop into testes or ovaries in individuals, resulting in the presence of a female phenotype with a 46, XY karyotype (1). It is usually diagnosed during adolescence or early adulthood, when affected individuals present with primary amenorrhea and a lack of secondary sexual characteristics. The clinical presentation of Swyer syndrome can vary, and in the early stages, affected individuals may not display overt symptoms, leading to delayed diagnosis (2).
To differentiate from Mayer-Rokitansy-Kuster-Hauser syndrome chromosomal analysis is typically used as the gold standard for the diagnosis of Swyer syndrome. In our case, the chromosomal study revealed a male karyotype with sex chromosome XY, which is consistent with a diagnosis of Swyer syndrome. The absence of normal testicular tissue in our patient suggests that the mutation occurred in a gene responsible for testicular development or maintenance, such as SRY or SOX9 (3,4).
The management of Swyer syndrome may require the coordinated efforts of a team of specialists, including pediatricians, endocrinologists, urologists, psychiatrists, and other healthcare professionals. Comprehensive planning is needed to ensure optimal treatment outcomes for affected individuals. Swyer syndrome is treated with estrogen replacement and added progestin within one to two years of continuous estrogen therapy to induce the development of secondary sexual characteristics, a pubertal growth spurt, and optimal bone mineral accumulation. The timing of hormone therapy is critical, and early initiation is recommended to maximize the potential for breast development, pubic hair growth, and other aspects of normal development during puberty. However, there is no evidence of benefit in females without a uterus, the addition of cyclic progesterone (5). In addition, individuals with Swyer syndrome are at an increased risk of developing dysgenesis gonadal malignancy. Regular screening and gonadectomy in early childhood are recommended for preventing gonadal tumors (6). Above all, living with Swyer syndrome can be challenging, as it can affect an individual’s self-image and identity. So counseling and support groups can be beneficial in helping individuals with the condition and manage any associated emotional and psychological issues, such as gender identity and gender role determination (7).
In conclusion, Swyer syndrome should be considered in the differential diagnosis of primary amenorrhea and the absence of visible uterus and ovaries in imaging studies. Chromosomal analysis is necessary for a definitive diagnosis, and early initiation of hormone replacement therapy is crucial for the induction of secondary sexual characteristics and the prevention of osteoporosis. Regular screening for gonadal tumors is recommended due to the increased risk of malignancy in affected individuals. These treatments should be combined with emotional support and psychiatric treatment.
Footnotes
- Data curation, P.S.
- writing—original draft, C.H.G.
- writing—review & editing, C.H.G.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding: None
References
- 1.King TF, Conway GS. Swyer syndrome. Curr Opin Endocrinol Diabetes Obes. 2014;21:504–510. doi: 10.1097/MED.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 2.Michala L, Goswami D, Creighton SM, Conway GS. Swyer syndrome: presentation and outcomes. BJOG. 2008;115:737–741. doi: 10.1111/j.1471-0528.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 3.Elzaiat M, McElreavey K, Bashamboo A. Genetics of 46,XY gonadal dysgenesis. Best Pract Res Clin Endocrinol Metab. 2022;36:101633. doi: 10.1016/j.beem.2022.101633. [DOI] [PubMed] [Google Scholar]
- 4.Berry DP, Herman EK, Carthy TR, Jennings R, Bandi-Kenari N, O’Connor RE, et al. Characterisation of eight cattle with Swyer syndrome by whole-genome sequencing. Anim Genet. 2023;54:93–103. doi: 10.1111/age.13280. [DOI] [PubMed] [Google Scholar]
- 5.Lee PA, Houk CP, Ahmed SF, Hughes IA International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Consensus statement on management of intersex disorders. Pediatrics. 2006;118:e488–e500. doi: 10.1542/peds.2006-0738. [DOI] [PubMed] [Google Scholar]
- 6.Cools M, Drop SL, Wolffenbuttel KP, Oosterhuis JW, Looijenga LH. Germ cell tumors in the intersex gonad: old paths, new directions, moving frontiers. Endocr Rev. 2006;27:468–484. doi: 10.1210/er.2006-0005. [DOI] [PubMed] [Google Scholar]
- 7.Wisniewski AB, Batista RL, Costa EMF, Finlayson C, Sircili MHP, Dénes FT, et al. Management of 46,XY differences/disorders of sex development (DSD) throughout life. Endocr Rev. 2019;40:1547–1572. doi: 10.1210/er.2019-00049. [DOI] [PubMed] [Google Scholar]