Abstract
Parts of katG and rpoB from 27 Russian Mycobacterium tuberculosis isolates were sequenced to detect mutations causing resistance to isoniazid (INH) and rifampin (RMP), respectively. All 24 INH-resistant isolates had a mutated katG, and 22 of them (91.7%) carried a mutation coding for a Ser315Thr shift. An rpoB mutation was noted for each of the 21 RMP-resistant isolates, with Ser531Leu being the most prevalent change encoded. Only two isolates had identical IS6110 fingerprints.
During the present decade, notification rates of tuberculosis and resistance rates have grown substantially in Russia. In 1995, the incidence of tuberculosis in the Russian Federation was 66 per 100,000, which when compared to the notification rate of 34 per 100,000 in 1991 reflects an annual increase rate of 14.2% (12, 13). In a recent survey in northwestern Russia, initial drug resistance to at least one antituberculous drug rose from 17.0 to 24.0% from 1991 through 1994, and initial multidrug resistance (i.e., resistance to at least isoniazid [INH] and rifampin [RMP]) was shown in 5.1% of the tuberculosis cases. Acquired resistance already existed in northwestern Russia 10 years ago, but since then, the resistance pattern has gradually shifted toward multidrug resistance (18).
The genetic basis of INH resistance in M. tuberculosis has been attributed to at least two different genes. Deletion and mutations of the katG gene encoding catalase-peroxidase have been shown to cause resistance to INH (21). katG converts INH to an active form, which affects mycobacterial proteins (such as InhA) that are required for the synthesis of mycolic acids. Mutations of the inhA operon have been associated with INH-resistant M. tuberculosis isolates (1, 10, 11). Of the two genes, however, katG seems to be more frequently altered (11).
Resistance to RMP is based on alterations in the rpoB gene (15). The most frequently encountered rpoB mutations in the RMP-resistant isolates are concentrated on a short, 69-bp region of the gene (5, 6, 19), and consequently it has been possible to develop targeted gene technology applications for diagnosis of RMP-resistant tuberculosis (2, 3, 19).
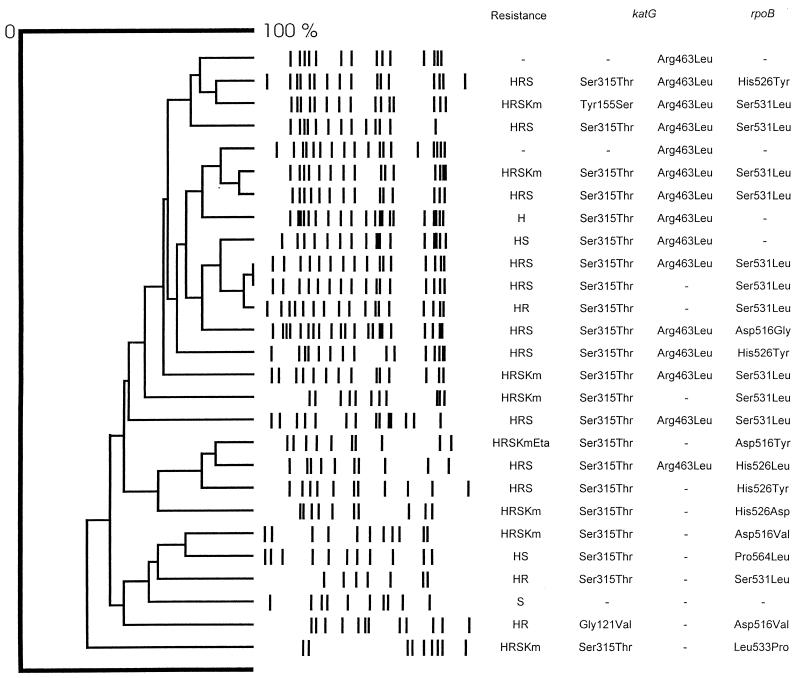
This study was based on 27 clinically unrelated M. tuberculosis isolates recovered from the St. Petersburg area in Russia from November 1993 through March 1995. Löwenstein-Jensen medium was used for the cultivation of the isolates, and susceptibility testing was done by the proportion method on 7H10 agar (Sensi-Disk; BBL, Becton Dickinson Microbiology Systems, Cockeysville, Md.). Isolates were considered resistant when bacterial growth occurred at a concentration of 1.0 μg of INH per ml and 5 μg of RMP per ml. Twenty-one isolates were resistant to INH and RMP, another three showed resistance to INH only, and the remaining three were susceptible to both drugs. Resistance to streptomycin, ethionamide, and kanamycin was also determined (Fig. 1). All the isolates were subjected to the IS6110 restriction fragment length polymorphism (RFLP) analysis according to the standard protocol (17).
FIG. 1.
IS6110 RFLP patterns, drug resistance data, and katG- and rpoB-encoded mutations in 27 M. tuberculosis isolates from the St. Petersburg area. Drug resistance abbreviations: H, INH; R, RMP; Km, kanamycin; Eta, ethionamide.
DNA extracted for the RFLP study was used as a template for PCR. For katG studies, the segment of DNA covering the area coding for amino acids Ser315 and Arg463 was amplified with primers described elsewhere (9). The complete katG was sequenced from those isolates, which did not have any mutation in the initially sequenced region of the gene. A 357-bp region of rpoB was amplified with an upstream primer, RP1 (5′-GGAGCGGATGACCACCCA), and a downstream primer, RB2 (5′-GCGGTACGGCGTTTCGATGAA). The primers were also synthesized as biotinylated versions, in which a biotin amidite group (Biodite; Pharmacia Biotech, Espoo, Finland) was added to the oligonucleotide at the 5′ end. The amplification was carried out with a thermal cycler (GeneAmp PCR System 2400; Perkin-Elmer, Foster City, Calif.). A total of 40 cycles of denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and synthesis at 72°C for 45 s were carried out, with a final extension of PCR products at 72°C for 7 min. The biotinylated PCR products were rendered single-stranded with streptavidin-coated Dynabeads as described in the manufacturer’s instructions (Dynabeads M-280 streptavidin; Dynal AS, Oslo, Norway).
The DNA sequence of both strands was determined with the ABI PRISM dye terminator with cycle sequencing ready reaction kit with AmpliTaq FS DNA polymerase and the 373-18 DNA sequencer (Applied Biosystems, Inc., Foster City, Calif.). The data were assembled and edited by using SeqEd v. 1.0.3 software (Applied Biosystems), and the sequences were compared with the published sequences in the GenBank database.
Twenty-seven M. tuberculosis isolates were investigated by sequencing and RFLP to evaluate drug resistance mechanisms and to assess possible genetic similarities between the isolates. Twenty-six different IS6110 RFLP patterns were obtained. Two isolates produced identical fingerprints, yet their mutational patterns differed from each other slightly (Fig. 1).
Each INH-resistant isolate had a mutated katG gene (Fig. 1). The sequencing of this part of katG revealed the mutation coding for the Ser315Thr substitution in 22 of the 24 (91.7%) INH-resistant isolates. Every Ser-to-Thr change at position 315 originated from an AGC-to-ACC substitution. Twelve of the 22 isolates contained substitutions at both positions 315 and 463. The two remaining INH-resistant isolates that did not contain the Ser315Thr change had point mutations resulting in either Gln88Arg or Tyr155Ser amino acid shifts. Two INH-susceptible isolates carried the mutation coding for an amino acid substitution at position 463, and one susceptible isolate had a wild-type katG gene.
The sequenced part of rpoB included the 69-bp region, which is strongly associated with RMP resistance. Each of the 21 RMP-resistant isolates had a mutation in this region, the most prevalent mutations coding for Ser531Leu (11 isolates) and His526Tyr (3 isolates). One isolate had a point mutation which changed Pro564 to Leu. This isolate was susceptible to 5 μg of RMP per ml but resistant to a 1-μg/ml concentration.
This study shows that the katG mutation coding for the Ser315Thr substitution was extremely prevalent among clinically unrelated M. tuberculosis isolates from the St. Petersburg area, although the isolates were largely heterogeneous in their IS6110 RFLP patterns. Of the INH-resistant isolates, 91.7% carried a katG mutation leading to the Ser315Thr change. In recent studies, this amino acid substitution has been detected in 44 to 64% of INH-resistant isolates (4, 11). The Ser315Thr change has been confirmed to cause INH resistance in site-directed mutagenesis studies, yet 30 to 40% of the proper function of the catalase-peroxidase remains (14). Recently, Li and coworkers reported that katG mutations, which result in low-level enzymatic activities, cause attenuated virulence of M. tuberculosis in mice and guinea pigs (8). However, the enrichment of the mutation leading to the Ser315Thr substitution among heterogeneous INH-resistant bacteria supports the advantageousness of the change for M. tuberculosis.
Since 91.7% of the INH-resistant isolates of the study had the katG mutation encoding the Ser315Thr substitution that definitely caused resistance, it is highly unlikely that mutations in other genes, such as the inhA operon, could significantly contribute to the resistance. The high prevalence of the single amino acid shift may help to develop a rapid screening method for the detection of INH-resistant M. tuberculosis isolates in the St. Petersburg area, for which the process is under way.
The Arg463Leu shift in KatG was noted in 15 isolates, two of which were susceptible to 1.0 μg of INH per ml. Rouse et al. reported a MIC of 1.0 μg/ml for an isolate subjected to mutagenesis causing the Arg463-to-Leu change (14). One of the susceptible isolates with the shift in our study was resistant to 0.4 μg of INH per ml, while the other was fully susceptible, which partly agrees with the finding of Rouse et al. Hence, our results support the earlier ones that the Arg463Leu substitution in KatG cannot be regarded as a significant marker for INH resistance in M. tuberculosis (7).
The array of rpoB mutations was similar to those published in other reports, with Ser531Leu and His526Tyr being the most common substitutions encoded (5, 15, 16, 19). This finding further strengthens the assumption that the mutations of rpoB are globally responsible for resistance to RMP. Therefore, it is evident that the new rapid methods for the detection of mutations in rpoB would be valuable adjuncts in the repertoire of laboratory methods in the survey area.
Although clonal spread was not markedly noted among these isolates in the St. Petersburg area, it is hazardous to draw any general implications concerning the transmission of drug-resistant tuberculosis. A thorough evaluation of the clonality of M. tuberculosis isolates in the study area would demand a greater number of isolates and the use of an additional typing method, since IS6110 RFLP patterns change at a relatively rapid rate (20).
To conclude, in our isolates from the St. Petersburg area, a significant predominance of the Ser315Thr mutation encoded by the katG sequence among heterogeneous INH-resistant M. tuberculosis isolates was noted. The results further imply that it would be useful to develop a rapid assay for detecting the mutations that cause the shift at position 315 and that lead to INH resistance.
Acknowledgments
This study was supported by the Finnish Anti-Tuberculosis Association Foundation and the Finnish National Research and Development Centre for Welfare and Health/HEDEC infectious diseases project in St. Petersburg, Russia.
REFERENCES
- 1.Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um K S, Wilson T, Collins D, de Lisle G, Jacobs W R. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263:227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 2.De Beenhouwer H, Lhiang Z, Jannes G, Mijs W, Machtelinckx L, Rossau R, Traore H, Portaels F. Rapid detection of rifampicin resistance in sputum and biopsy specimens from tuberculosis patients by PCR and line probe assay. Tubercle Lung Dis. 1995;76:425–430. doi: 10.1016/0962-8479(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 3.Felmlee T A, Liu Q, Whelen C, Williams D, Sommer S S, Persing D H. Genotypic detection of Mycobacterium tuberculosisrifampin resistance: comparison of single-strand conformation polymorphism and dideoxy fingerprinting. J Clin Microbiol. 1995;33:1617–1623. doi: 10.1128/jcm.33.6.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas W H, Schilke K, Brand J, Amthor B, Weyer K, Fourie P B, Bretzel G, Sticht-Groh V, Bremer H J. Molecular analysis of katG gene mutations in strains of Mycobacterium tuberculosiscomplex from Africa. Antimicrob Agents Chemother. 1997;41:1601–1603. doi: 10.1128/aac.41.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapur V, Li L-L, Iordanescu S, Hamrick M R, Wanger A, Kreiswirth B N, Musser J M. Characterization by automated DNA sequencing of mutations in the gene (rpoB) encoding the RNA polymerase β subunit in rifampin-resistant Mycobacterium tuberculosisstrains from New York City and Texas. J Clin Microbiol. 1994;32:1095–1098. doi: 10.1128/jcm.32.4.1095-1098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim B-J, Kim S-Y, Park B H, Lyu M-A, Park I-K, Bai G-H, Kim S-J, Cha C-Y, Kook Y-H. Mutations in the rpoB gene of Mycobacterium tuberculosisthat interfere with PCR-single-strand conformation polymorphism analysis for rifampin susceptibility testing. J Clin Microbiol. 1997;35:492–494. doi: 10.1128/jcm.35.2.492-494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee A S-G, Tang L L-H, Lim I H-K, Ling M-L, Tay L, Wong S-Y. Lack of clinical significance for the common arginine-to-leucine substitution at codon 463 of the katG gene in isoniazid-resistant Mycobacterium tuberculosisin Singapore. J Infect Dis. 1997;176:1125–1126. doi: 10.1086/517320. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Kelley C, Collins F, Rouse D, Morris S. Expression of katG in Mycobacterium tuberculosisis associated with its growth and persistence in mice and guinea pigs. J Infect Dis. 1998;177:1003–1035. doi: 10.1086/515254. [DOI] [PubMed] [Google Scholar]
- 9.Marttila H J, Soini H, Huovinen P, Viljanen M K. katG mutations in isoniazid-resistant Mycobacterium tuberculosisisolates recovered from Finnish patients. Antimicrob Agents Chemother. 1996;40:2187–2189. doi: 10.1128/aac.40.9.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mdluli K, Sherman D R, Hickey M J, Kreiswirth B N, Morris S, Stover K, Barry C E., III Biochemical and genetical data suggest that InhA is not the primary target for activated isoniazid in Mycobacterium tuberculosis. J Infect Dis. 1996;174:1085–1090. doi: 10.1093/infdis/174.5.1085. [DOI] [PubMed] [Google Scholar]
- 11.Musser J M, Kapur V, Williams D L, Kreiswirth B N, van Soolingen D, van Embden J D A. Characterization of the catalase-peroxidase gene (katG) and inhA locus in isoniazid-resistant and -susceptible strains of Mycobacterium tuberculosisby automated DNA sequencing: restricted array of mutations associated with drug resistance. J Infect Dis. 1996;173:196–202. doi: 10.1093/infdis/173.1.196. [DOI] [PubMed] [Google Scholar]
- 12.Perrocheau A, Schwoebel V, Veen J. Surveillance of tuberculosis in the WHO European Region in 1995: results of the feasibility study. Eurosurveillance. 1998;3:2–5. doi: 10.2807/esm.03.01.00110-en. [DOI] [PubMed] [Google Scholar]
- 13.Raviglione M C, Riedel H L, Styblo K, Khomenko A G, Esteves K, Kochi A. Tuberculosis trends in eastern Europe and the former USSR. Tubercle Lung Dis. 1994;75:400–416. doi: 10.1016/0962-8479(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 14.Rouse D A, DeVito J A, Li Z, Byer H, Morris S L. Site-directed mutagenesis of the katG gene of Mycobacterium tuberculosis: effects on catalase-peroxidase activities and isoniazid resistance. Mol Microbiol. 1996;22:583–592. doi: 10.1046/j.1365-2958.1996.00133.x. [DOI] [PubMed] [Google Scholar]
- 15.Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston M J, Matter L, Schopfer K, Bodmer T. Detection of rifampicin-resistant mutations in Mycobacterium tuberculosis. Lancet. 1993;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 16.Telenti A, Honoré N, Bernasconi C, March J, Ortega A, Heym B, Takiff H E, Cole S T. Genotypic assessment of isoniazid and rifampin resistance in Mycobacterium tuberculosis: a blind study at reference laboratory level. J Clin Microbiol. 1997;35:719–723. doi: 10.1128/jcm.35.3.719-723.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P W M, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosisby DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:1987–1995. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viljanen, M. K., B. I. Vyshnevskiy, T. F. Otten, E. Vyshnevskaya, M. Marjamäki, H. Soini, P. J. Laippala, and A. V. Vasilyef. Survey of drug-resistant tuberculosis in northwestern Russia from 1984 through 1994. Eur. J. Clin. Microbiol. Infect. Dis., in press. [DOI] [PubMed]
- 19.Williams D L, Waguespack C, Eisenach K, Crawford J T, Portaels F, Salfinger M, Nolan C M, Abe C, Sticht-Groh V, Gillis T P. Characterization of rifampin resistance in pathogenic mycobacteria. Antimicrob Agents Chemother. 1994;38:2380–2386. doi: 10.1128/aac.38.10.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh R W, de Leon A P, Agasino C B, Hahn J A, Daley C L, Hopewell P C, Small P M. Stability of Mycobacterium tuberculosisDNA genotypes. J Infect Dis. 1998;177:1107–1111. doi: 10.1086/517406. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]