Summary
Background
Malaria in early pregnancy is a risk factor for preterm birth and is associated with sustained inflammation and dysregulated angiogenesis across gestation. This study investigated whether malaria is associated with increased gut leak and whether this contributes to systemic inflammation, altered angiogenesis, and preterm birth.
Methods
We quantified plasma concentrations of gut leak markers, soluble CD14 (sCD14) and lipopolysaccharide binding protein (LBP) from 1339 HIV-negative pregnant Malawians at <24 weeks gestational age. We assessed the relationship of sCD14 and LBP concentrations with markers of inflammation, angiogenesis, and L-arginine bioavailability and compared them between participants with and without malaria, and with and without preterm birth.
Findings
Plasma concentrations of sCD14 and LBP were significantly higher in participants with malaria and were associated with parasite burden (p < 0.0001, both analyses and analytes). The odds ratio for preterm birth associated with one log sCD14 was 2.67 (1.33 to 5.35, p = 0.006) and 1.63 (1.07–2.47, p = 0.023) for LBP. Both gut leak analytes were positively associated with increases in proinflammatory cytokines CRP, sTNFR2, IL18-BP, CHI3L1 and Angptl3 (p < 0.05, all analytes) and sCD14 was significantly associated with angiogenic proteins Angpt-2, sENG and the sFLT:PlGF ratio (p < 0.05, all analytes). sCD14 was negatively associated with L-arginine bioavailability (p < 0.001).
Interpretation
Malaria in early pregnancy is associated with intestinal barrier dysfunction, which is linked to an increased risk of preterm birth.
Funding
Open Philanthropy, Canadian Institutes of Health Research, Canada Research Chair program, European and Developing Countries Clinical Trials Partnership, Bill & Melinda Gates Foundation.
Keywords: Malaria in pregnancy; Preterm birth; Gut leak; Intestinal barrier disruption; sCD14; LBP; Inflammation; L-arginine, Plasmodium falciparum
Research in context.
Evidence before this study
We searched PubMed for original articles using the search terms “malaria” OR “plasmodium” OR “falciparum” AND “gut leak” OR “intestinal barrier” OR “gut barrier” OR “intestinal permeability” OR “gut permeability” OR “microbial translocation” AND “pregnancy” OR “preterm birth” OR “preterm labour” OR “prematurity”. No language or time restrictions were used. Findings were restricted to primary research in humans reporting circulating plasma biomarkers of gut leak during pregnancy. From these articles, we performed secondary searches of their references.
The results of our search found no published studies of gut leak in malaria in pregnancy. When we excluded the malaria criteria, we identified seven studies of microbial translocation in pregnancy; six of these were in HIV-positive populations, and one in women with depression. Five of the studies were from the United States or Europe (combined N = 208); one from Malawi (N = 149) and one from India (N = 107). Five of the studies were case–control design, one was a nested single site cohort within a larger multi-site study, and one prospective observational cohort of HIV-positive pregnant women (N = 149). Two of the studies reported pregnancy outcomes: Shivakoti et al. reported an increased odds ratio of preterm birth associated with markers of gut leak, and Baroncelli et al. reported an inverse association between sCD14 and birth weight.
Added value of this study
To our knowledge, this is the first study to implicate gut leak in the outcomes of pregnancies complicated by malaria. Malaria threatens over 200 million pregnancies globally every year and contributes substantially to adverse birth outcomes and neonatal mortality. Our study was conducted in a large, prospective clinical trial (n = 1339) situated in a Malawian population that carries a high burden of malaria in pregnancy and its sequelae. Our findings show that plasma concentrations of the gut leak markers, sCD14 and LBP, are increased in pregnant women with malaria, and confer an increased risk of preterm birth. Malaria is known to be an important risk factor for preterm birth, and our mediation analysis demonstrates that approximately 10% of this effect can be attributed to gut leak. Gut leak was associated with perturbations in plasma inflammatory cytokines and angiogenic mediator concentrations, both of which play critical roles in placental development and function. Importantly, participants in our study were at high risk of malnutrition, which together with malaria and pregnancy, contributes to deficiency in bioavailable L-arginine, an essential amino acid for placental vascular development and function. Our analysis demonstrates that low bioavailable L-arginine is associated with increased gut leak, and implicates gut leak as a potential mechanistic link between malnutrition, malaria, systemic inflammation and adverse pregnancy outcomes.
Implications of all the available evidence
Attempts to prevent malaria in pregnancy or mitigate its consequences have not yet eliminated the risk of adverse birth outcomes faced by pregnant women in malaria-endemic regions. Understanding the pathways by which malaria compromises pregnancy outcomes is essential for developing safe, effective, affordable, and culturally appropriate interventions to improve the health of pregnant women and their children. Our study identifies gut leak as pathway by which malaria may disrupt normal inflammatory and angiogenic processes required for healthy pregnancy outcomes and identifies a potential role for nutritional supplementation with L-arginine, or its precursor L-citrulline, to improve pregnancy outcomes in women at risk of malaria.
Introduction
Plasmodium falciparum (P. falciparum) infection during pregnancy increases the risk of severe maternal disease and adverse pregnancy outcomes including pregnancy loss, preterm birth, and babies born small-for-gestational age.1, 2, 3 In regions of Africa with moderate-to high transmission, over 40 million pregnant individuals are at risk of malaria every year and it remains a major contributor to adverse pregnancy outcomes and neonatal death in low- and middle-income countries (LMICs).4 Recent evidence supports the hypothesis that adverse birth outcomes associated with P. falciparum infection in pregnancy result from disruption of inflammatory and angiogenic pathways early in pregnancy, which subsequently impairs placental vascular development and function.5, 6, 7 Plasmodium falciparum infection also results in reduced bioavailable L-arginine,6,8,9 an essential amino acid in pregnancy that contributes to both placental development6,10 and intestinal barrier function through nitric oxide (NO)-mediated mechanisms.11, 12, 13, 14, 15, 16
An evolving body of research implicates intestinal barrier disruption in the pathogenesis of several conditions associated with systemic inflammation, including sepsis and HIV infection.17, 18, 19 Under conditions of increased intestinal permeability, microbial components translocate from the gastrointestinal lumen to the systemic circulation where they trigger innate immune signaling cascades. For example, when the gram-negative bacterial cell wall component lipopolysaccharide (LPS) enters the bloodstream, it complexes with LBP which then binds CD14 to deliver LPS to the TLR4 receptor and activate MyD88-dependent signaling pathways that augment inflammatory cascades.17 While direct quantification of intestinal permeability using physiologic assays is challenging, circulating plasma markers including LBP and CD14 provide surrogate measures of microbial translocation and allow evaluation of large sample cohorts.20,21
This study explored the hypothesis that P. falciparum infection during pregnancy disrupts intestinal barrier integrity, contributing to systemic inflammation and adverse pregnancy outcomes including preterm birth. We further posited that malaria-induced gut leak is associated with low bioavailable L-arginine, an amino acid that contributes to intestinal barrier integrity. To test these hypotheses, we quantified established markers of gut leak17 in HIV-negative pregnant Malawian women who were at risk of P. falciparum malaria. We compared the concentrations of soluble CD14 (sCD14) and LBP between participants with and without malaria and assessed their relationships in early gestation (<24 weeks) with preterm birth. We also assessed the relationship between gut leak and inflammation, angiogenesis, and L-arginine bioavailability.
Methods
Study population & trial design
This study is a secondary analysis of a prospective randomized controlled trial (RCT) described by Madanitsa et al.22 Briefly, the parent RCT was a two-arm trial enrolling pregnant individuals in Malawi from 21 July 2011 to 18 March 2013 who were randomized to either intermittent preventative treatment in pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP) or intermittent screening and treatment in pregnancy (ISTp) with dihydroartemisinin-piperquine (DP). Participants were eligible for the parent RCT if they did not have HIV infection, had singleton gestation, hemoglobin >70 g/L, had not previously been treated with IPTp-SP, and agreed to deliver in a local health care facility. Pregnancy dating was performed by ultrasound during the second trimester.22 Participants included in the present study were those enrolled in the parent RCT between gestational ages of 13–23 weeks, inclusive; with a P. falciparum PCR result from enrolment blood sample collection; a documented pregnancy outcome; and enrolment plasma samples available for analysis (Fig. 1).
Fig. 1.
Participant and Study Flow Chart. The current study is a secondary analysis of a randomized controlled trial (RCT) cohort. This parent RCT enrolled 1873 participants, of which 1339 met the inclusion criteria for the current study. For the current study, the inclusion criteria were gestational age <24 weeks at enrolment, valid P. falciparum PCR result at enrolment, documented pregnancy outcome, and sufficient volume of stored plasma available for analysis. The parent RCT also included a randomly selected subset of 384 primigravid participants, from whom plasma concentrations of L-arginine and asymmetric dimethyl arginine were previously quantified. Of these, 331 met the inclusion criteria for the current study. RCT: randomized controlled trial. NO: nitric oxide.
This study is reported according to the Strengthening Reporting of Observational Studies in Epidemiology (STROBE) guideline (Supplementary Table S1). All assessments and analyses were the same across groups. Individuals performing the biomarker assays were blinded to the participants’ malaria status and pregnancy outcomes.
Study definitions
Preterm birth was defined as birth <37 weeks gestational age based on second trimester ultrasound dating22 and was coded as a dichotomous outcome. Malaria PCR status was established by real-time PCR of dried blood spots collected at enrollment and study visits at gestational ages between 28 and 33 weeks, and 34–36 weeks, using probes that targeted both the parasite gene pfldh and the human gene beta-tubulin as an internal control.5 Participants with positive malaria PCR status were further characterized as having either patent or sub-patent malaria. Patent infection was defined as a positive result on either blood smear microscopy and/or malaria rapid diagnostic test (RDT); sub-patent infection was defined as positive by PCR only. Socioeconomic status was defined in tertiles by principal component analysis based on a survey of household characteristics and assets22 and was maintained as a continuous variable when used for adjustment of multivariable models. Body Mass Index (BMI) was calculated at enrolment as weight in kilograms divided by height in meters squared.
Laboratory assessments
Quantification of plasma gut leak markers
EDTA plasma concentrations of gut leak markers were assessed by custom Luminex assay (sCD14, LBP, 1:400 dilution) according to the manufacturers’ instructions. Luminex data was collected using xPONENT version 4.2 software on a Luminex MagPix machine (Luminex, Toronto, Canada). Outputs greater than the top standard concentration were adjusted to the top standard value. Outputs less than the lowest standard dilution concentration were adjusted to one-third the value of lowest standard (see Supplementary Table S2).
Quantification of plasma inflammatory and angiogenic mediators
Protein mediators of inflammation and angiogenesis were previously selected and quantified in EDTA plasma as described5,7 using Luminex multiplex assays (R&D Systems, Minneapolis, MN): custom kit [soluble Endoglin (sENG), Placental Growth Factor (PlGF), soluble fms-like Tyrosine Kinase-1 (sFlt-1/soluble VEGFR1)]; Kit #LXSAHM-5 [soluble Intracellular Adhesion Molecule-1 (sICAM-1), Angiopoietin-like 3 (Angptl3), Chitinase 3-like protein (CHI3L1), and soluble Tumor Necrosis Factor receptor II (sTNFR2)]; and custom kit #LXSAHM-01 [angiopoietin-1 (Angpt-1) and angiopoietin-2 (Angpt-2)]. C-Reactive Protein (CRP) and Interleukin-18 binding protein (IL18-BP) were quantified by ELISA (DuoSet ELISA kits).
Quantification of bioavailable L-arginine
Concentrations of L-arginine, asymmetric dimethyl arginine (ADMA), and symmetric dimethyl arginine (SDMA) were previously quantified from a randomly selected subset of primigravid ISTp trial participants using high-pressure liquid chromatography electrospray tandem mass spectrometry as reported.6 L-arginine bioavailability was quantified as the ratio of L-arginine concentration to ADMA concentration, as previously established.23
Statistical analyses
Statistical analyses were conducted using GraphPad Prism 9 and IBM SPSS Statistics 28. The statistical analysis plan described below was outlined before the analysis and was based on similar approaches previously used by our group and others5, 6, 7,24 to analyze comparable data sets.
Comparisons of medians for continuous variables were conducted by Mann–Whitney U-tests or Kruskal–Wallis tests with Dunn's multiple comparisons test. For linear and binomial logistic regression analyses, the concentrations of sCD14, LBP, and markers of inflammation and angiogenesis were transformed using the base 10 logarithm to stabilize variance. In the linear regression models, there was homoscedasticity and normal distribution of residuals. In the logistic regression models, linearity of log10-transformed concentrations of continuous independent variables with respect to the logit of the dependent variable was confirmed by the Box-Tidwell procedure; Bonferroni correction for significance was applied in the multivariable analyses. Multivariable linear and logistic regression models included the following covariates: maternal age, gestational age at enrolment, BMI, and socioeconomic status. Models of the preterm birth or gestational age at delivery outcomes also included systolic blood pressure at enrolment and ISTp trial treatment arm in the multivariable analyses. In the multivariable analyses, (i) gravidity and parity were not included as there was significant co-linearity with maternal age [Pearson R = 0.84 (95% C.I. 0.82–0.85), p < 0.001; Pearson R = 0.75 (0.72–0.78), p < 0.001; respectively]; (ii) education attainment was not included as there was significant co-linearity with socioeconomic status [Pearson's R = 0.36 (0.31–0.41), p < 0.001]; (iii) middle upper arm circumference (MUAC) was not included due to significant collinearity with BMI [Pearson's R = 0.66 (0.63–0.69), p < 0.001] (Supplementary Table S3). Mediation analyses were performed using the PROCESS v4.0 (Andrew F. Hayes, 2021) macro for SPSS 28. Missing data were excluded from the analysis and the percentage of missing data is reported in Supplementary Table S4.
Ethics
This study was approved by the Malawian College of Medicine Research and Ethics Committee (COMREC reference number: P.08/13/1477), the Liverpool School of Tropical Medicine (IREC number: RETH000693), and the University Health Network Institutional Review Board (UHN REB #13-6741-AE). Written informed consent was obtained from all study participants. The parent trial was registered with the Pan African Clinical Trials Registry, PACTR201103000280319; ISRCTN Registry ISRCTN69800930.
Role of the funders
The funders of the trial had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Results
Clinical characteristics and malaria status
The parent RCT enrolled 1873 pregnant participants.22 1339 met the inclusion criteria for the present study (Fig. 1). Demographic and outcome characteristics did not differ between parent and derived cohorts except for a higher proportion of participants allocated to the treatment arm of the parent RCT (51.2% vs 44.2%, respectively, p = 0.012), a higher gestational age at enrolment (20.14 weeks [C.I. 18.29, 21.86] vs 19.64 weeks [C.I. 18.00, 21.14], respectively, p = 0.004), and differences in education status among those included in the present study compared to those not meeting inclusion criteria (Supplementary Table S5). There were no significant differences in the rates of P. falciparum infection or the preterm birth outcome between participants of the two RCT study arms,22 thus data from all participants were combined in the current study analysis. Participants included in the current study had a median age of 21 years (interquartile range [IQR] 18, 26) and 450 (33.7%) were primigravid (Table 1). The samples analyzed for concentrations of gut leak markers were obtained at enrolment before the initiation of any parent RCT study interventions. There were no differences in concentrations of sCD14 or LBP between participants of the two RCT study arms who were included in the current study (sCD14: 906.951 ng/mL [IQR 751.256, 1163.789] IPTp-SP arm vs 936.970 ng/mL [IQR 735.403, 1178.650] ISTp-DP arm, p = 0.912. LBP: 4.036 ng/mL [IQR 3.902, 4.176] IPTp-SP arm vs 4.032 [IQR 3.889, 4.179] ISTp-DP arm, p = 0.872; Mann–Whitney U tests).
Table 1.
Participant characteristics.
| Linear regression analysis |
|||||
|---|---|---|---|---|---|
| n | Malaria positive at enrolment (n = 595) | Malaria negative at enrolment (n = 744) | p | ||
| Participant characteristics | |||||
| Age at enrolment, years | 1336 | 21.0 [18, 26] | 20 [19, 27] | 22 [18, 24] | <0.0001 |
| Primigravidae, n (%) | 1336 | 450 (33.7) | 264 (44.4) | 186 (25.1) | <0.0001 |
| Gestational age at enrolment, weeks | 1339 | 21.1 [18.3, 21.9] | 20.1 [18.3, 23.9] | 20.1 [18.1, 23.9] | 0.8725 |
| Height, cm | 1336 | 154 [150, 175] | 153 [150, 156] | 154 [150, 158] | 0.0343 |
| Weight, kg | 1336 | 54.1 [50.0, 59.0] | 53.0 [49.2, 57.8] | 55.0 [50.4, 60.0] | <0.0001 |
| BMI, kg/m2 | 1336 | 22.8 [21.2, 24.7] | 22.5 [20.9, 24.3] | 23.0 [21.5, 25.0] | 0.0002 |
| MUAC, cm | 1308 | 24.0 [23.0, 25.7] | 24.0 [23.0, 25.4] | 24.4 [23.0, 37.8] | <0.0001 |
| Systolic blood pressure at enrolment, mmHg | 1309 | 111 [105, 119] | 111 [105, 119] | 111 [104, 119] | 0.6590 |
| Diastolic blood pressure at enrolment, mmHg | 1309 | 66.0 [61.0, 72.0] | 66.0 [60.0, 72.0] | 67.0 [61.0, 72.0] | 0.4446 |
| Hemoglobin at enrolment, g/dL | 1326 | 11.2 [10.1, 12.1] | 10.6 [9.60, 11.7] | 11.5 [10.7, 12.4] | <0.0001 |
| Socioeconomic status (tertile) | 1333 | <0.0001 | |||
| 1, n (%) | 449 (33.7) | 227 (38.3) | 222 (30.0) | ||
| 2, n (%) | 449 (33.7) | 243 (41.0) | 243 (32.8) | ||
| 3, n (%) | 435 (32.6) | 160 (27.0) | 275 (37.2) | ||
| Education status (tertile) | 1333 | 0.4415 | |||
| 1 | 387 (29.0) | 167 (28.2) | 220 (29.7) | ||
| 2 | 734 (55.1) | 348 (58.7) | 386 (52.2) | ||
| 3 | 212 (15.9) | 78 (13.2) | 134 (18.1) | ||
| Parent RCT treatment arm, IPTp; n (%) | 1336 | 685 (51.3) | 379 (48.4) | 363 (48.9) | 0.8739 |
| Malaria at enrolment | |||||
| PCR positive, n (%) | 1339 | 595 (44.4) | |||
| Patent (RDT and/or microscopy positive), n (%) | 1339 | 318 (23.7) | |||
| Pregnancy and birth outcomes | |||||
| Gestational age at delivery, weeks | 1339 | 38.6 [37.1, 39.6] | 38.4 [37.0, 39.4] | 38.7 [37.3, 39.7] | 0.0013 |
| Birth weight, g | 1293 | 3000 [2652, 4500] | 2900 [2600, 3100] | 3000 [2700, 3200] | <0.0001 |
| Infant sex (female) | 1336 | 665 (49.8) | 366 (49.4) | 299 (50.4) | 0.7008 |
| APO, n (%) | 1339 | 424 (31.7) | 205 (34.4) | 219 (29.4) | 0.0499 |
| PTB, n (%) | 1312 | 275 (21.0) | 142 (24.2) | 133 (18.3) | 0.0082 |
| LBW, n (%) | 1296 | 132 (10.2) | 75 (13.0) | 57 (7.9) | 0.0024 |
| SGA, n (%) | 1296 | 136 (10.5) | 63 (11.0) | 73 (10.1) | 0.6276 |
| Miscarriage, n (%) | 1339 | 7 (0.5) | 2 (0.3) | 5 (0.7) | 0.3975 |
| Stillbirth, n (%) | 1332 | 20 (1.5) | 8 (1.3) | 12 (1.6) | 0.6823 |
| Arginine pathway at enrolment | (n = 185) | (n = 146) | |||
| L-arginine, umol/L | 331 | 27.0 [20.7, 38.6] | 26.8 [20.8, 35.5] | 27.4 [20.3, 42.8] | 0.2817 |
| ADMA, umol/L | 331 | 0.429 [0.382, 0.480] | 0.447 [0.402, 0.502] | 0.403 [0.360, 0.455] | <0.0001 |
| SDMA, umol/L | 331 | 0.358 [0.318, 0.403] | 0.368 [0.332, 0.419] | 0.345 [0.312, 0.393] | 0.0025 |
| L-arginine/ADMA ratio | 331 | 62.4 [45.6, 87.0] | 58.8 [43.5, 79.1] | 69.9 [49.3, 98.4] | 0.0039 |
Data are presented as medians [interquartile range] or n (%), unless otherwise specified. Comparisons with significant differences (p < 0.05) are bolded.
BMI, body mass index; MUAC, middle upper arm circumference; ADMA asymmetric dimethylarginine; SDMA, symmetric dimethyl arginine; APO, adverse pregnancy outcome; PTB, preterm birth; LBW, low birth weight. (GraphPad, Prism). Percentages reported for bivariate analyses were corrected for missing values in the denominator.
In the current study cohort, a total of 44.4% (595/1339) of participants had positive malaria PCR status at enrolment, and of these 53.4% (318/595) had patent infections. Participants who were positive for malaria by PCR at enrolment differed significantly from malaria negative participants by age, primigravidity, enrolment weight, body mass index (BMI), MUAC, hemoglobin concentration, and socioeconomic status (Table 1). Malaria at enrolment was associated with lower gestational age at delivery, an increased rate of preterm birth, and lower birth weight (Table 1). Positive malaria PCR status at enrolment was also associated with decreased L-arginine bioavailability (indicated as the ratio of L-arginine to ADMA concentrations), and increased ADMA, and SDMA concentrations at enrolment (Table 1), consistent with those previously reported.6
Plasmodium falciparum infection during pregnancy is associated with elevated circulating markers of intestinal barrier dysfunction
The median concentrations of plasma sCD14 and LBP were higher among pregnant participants who tested positive for P. falciparum by PCR than those who tested negative (Fig. 2, Supplementary Table S6). There were significant, stepwise increases in median concentrations of both sCD14 and LBP between participants who had subpatent malaria and patent malaria (Fig. 2, Supplementary Table S6).
Fig. 2.
Markers of intestinal barrier dysfunction are associated with P. falciparum infection in early pregnancy. Plasma concentration distributions of sCD14 (left panel) and LBP (right panel), and malaria PCR and infection status were assessed from each participant at gestational age <24 weeks. CD14 and LBP were quantified from plasma using Luminex. P. falciparum PCR status is defined as either negative (yellow) or positive (pink). Comparisons made by Mann–Whitney U tests (A). P. falciparum infection status is defined as either uninfected (PCR negative; yellow), subpatent infection (PCR positive, RDT and microscopy negative; light pink), or patent infection (PCR positive, and RDT and/or microscopy positive; dark pink). Comparisons made by Kruskal–Wallis tests with Dunn's multiple comparisons tests (B). sCD14, soluble CD14; LBP, lipopolysaccharide binding protein; RDT, rapid diagnostic test.
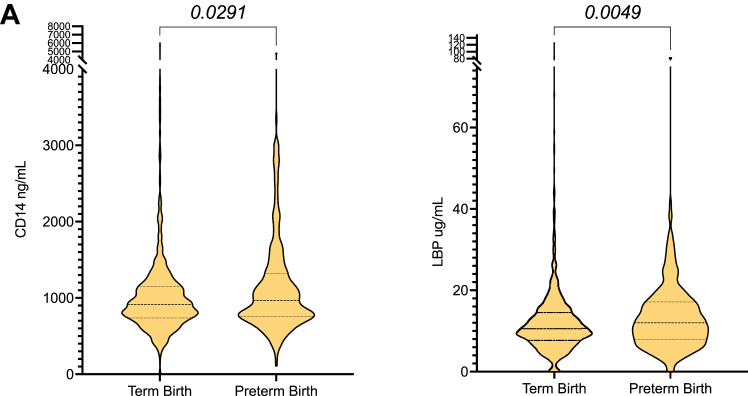
Intestinal barrier dysfunction before 24 weeks gestation is associated with preterm birth
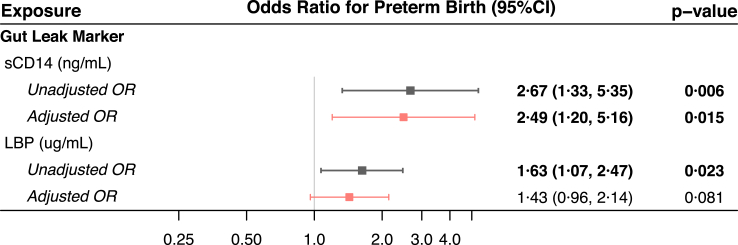
Median plasma concentrations of sCD14 and LBP were significantly higher in participants who subsequently delivered preterm than in those who delivered at term (Fig. 3, Supplementary Table S7). The odds ratios for preterm birth associated with a one-log increase in sCD14 concentration were 2.67 (95% confidence interval 1.33–5.35, p = 0.006) and 2.49 (1.20–5.16, p = 0.015) in the unadjusted and adjusted analyses, respectively. These were 1.63 (1.07–2.47, p = 0.023) and 1.43 (0.96–2.14, p = 0.081), respectively, for a one-log increase in LBP concentration (Fig. 4).
Fig. 3.
Markers of intestinal barrier dysfunction in early pregnancy are associated with preterm birth. (A) Plasma concentration distributions of sCD14 (left column) and LBP (right column) measured by luminex in pregnant participants at gestational age <24 weeks who delivered at term vs those who delivered preterm. Statistical analysis by Mann–Whitney U tests. sCD14: soluble CD14; LBP: lipopolysaccharide binding protein; IQR: interquartile range.
Fig. 4.
sCD14 and LBP concentrations are associated with preterm birth. Unadjusted (black) and adjusted (pink) odds ratios (squares) with 95% confidence intervals from binary logistic regression models for the associations between log-transformed concentrations of sCD14 and LBP at enrolment and preterm birth. Adjusted models include enrolment log-transformed biomarker concentration, malaria PCR status, gestational age, maternal age, BMI, systolic blood pressure, socioeconomic status. Statistically significant models (p < 0.05) are bolded. sCD14: soluble CD14; LBP: lipopolysaccharide binding protein; OR: odds ratio; CI: confidence interval.
Soluble CD14 and LBP were also associated with preterm birth in a subgroup of participants who did not have evidence of P. falciparum infection during pregnancy (negative PCR at all timepoints, n = 437). In this subset, the odds ratios for preterm birth associated with a one-log increase in sCD14 concentration were 3.60 (0.99–13.07, p = 0.052) and 3.97 (1.04–15.15, p = 0.044) in the unadjusted and adjusted analyses, respectively, and 2.87 (1.05–7.84, p = 0.039) and 3.30 (1.08–10.04, p = 0.036) for a one-log increase in LBP concentration (Supplementary Figure S1).
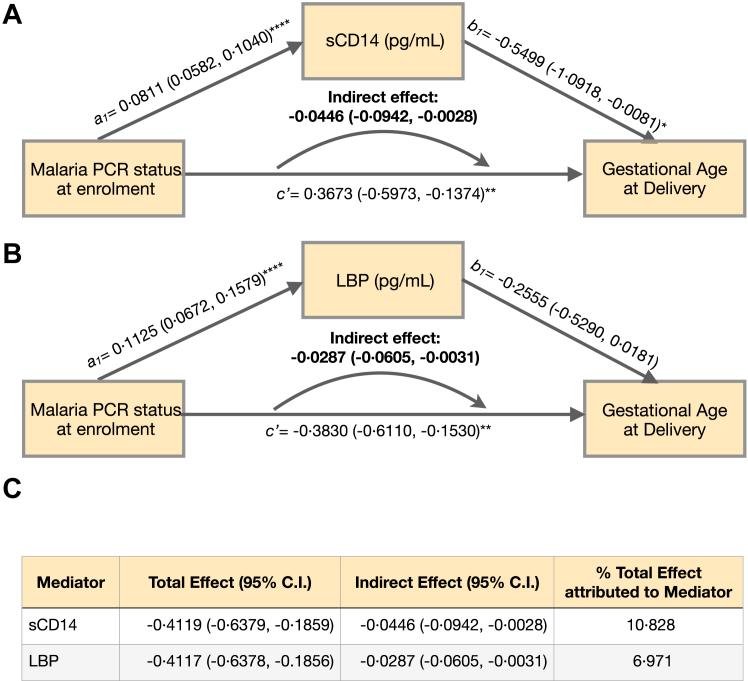
Intestinal barrier dysfunction mediates the effect of malaria on gestational age at delivery
Using data from this cohort, we previously reported that P. falciparum infection at gestational age <24 weeks significantly increased the risk of preterm birth outcomes.5 Here we conducted mediation analyses to assess whether the effect of malaria on gestational age at delivery is mediated by intestinal barrier dysfunction. Path diagrams and regression estimates for each path component are presented in Fig. 5. Regression analyses demonstrated that P. falciparum PCR status at enrolment was significantly associated with the concentrations of sCD14 (a1: 0.0811 (0.0582–0.1040, p < 0.0001); Fig. 5A) and LBP (a1: 0.1125 (0.0672–0.1579), p < 0.0001); Fig. 5B). Each one unit increase in the concentration of sCD14 was negatively associated with gestational age at delivery (sCD14 b1: −0.05499 (−1.0918 to −0.0081, p = 0.0446); Fig. 5A). Increases in LBP displayed a trend towards a negative association with gestational age at delivery, but this did not achieve statistical significance (LBP b1: −0.2555 (−0.5290 to 0.0181, p = 0.0627); Fig. 5B). sCD14 concentrations accounted for 10.83% of the total effect of malaria at enrolment on gestational age at delivery (Fig. 5A, C), and LBP concentrations at enrolment accounted for 6.97% of the total effect (Fig. 5B, C).
Fig. 5.
Mediation analyses of P. falciparum infection on gestational age at delivery through markers of intestinal barrier dysfunction. Panel A + B: Path diagrams for mediation analyses. Regression coefficients a1, b1, and c’ with 95% confidence intervals for each step in the mediation analyses independently modelling the effect of log-transformed sCD14 concentrations (A) (n = 1286) and log-transformed LBP concentrations (B) (n = 1285) as mediators of the effect of P. falciparum infection status (PCR positivity) at enrolment on gestational age at delivery (outcome). Indirect (mediation) effects were calculated as the products of coefficients (a1 x b1) with bootstrapped confidence intervals. c’ represents the direct effect of P. falciparum infection at enrolment on gestational age at delivery after controlling for CD14 (A) or LBP (B) as mediators. Maternal age, gestational age at enrolment, body mass index, socioeconomic status, and systolic blood pressure at enrolment were included as covariates. n = 1285 ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 for regression coefficients a1, b1, and c’. Panel C: Percentage of the total effect of gut-leak marker concentration on gestational age at delivery is calculated as Indirect Effect/Total Effect x 100.
Intestinal barrier dysfunction is associated with elevated inflammatory pathway markers in early pregnancy (gestational age <24 weeks)
We previously quantified concentrations of inflammatory and angiogenic protein mediators from study enrolment samples (gestational age <24 weeks) and reported their association with preterm birth.5 In this study, we assessed the association between the concentrations of these markers and markers of intestinal barrier dysfunction using linear regression modelling. In regression models, both sCD14 and LBP concentrations were significantly associated with increases in inflammatory markers CRP, sTNFR2, IL18-BP, CHI3L1 and Angptl3, though Angptl3 did not retain significance with sCD14 in the multivariate model. In simple linear regression models, sCD14 was significantly associated with increases in the angiogenic proteins Angpt-2, sENG, and the sFLT:PlGF ratio (Table 2).
Table 2.
Markers of intestinal barrier dysfunction are associated with increased inflammatory and angiogenic markers in early pregnancy.
 |
Linear regression models of the association between log-transformed concentrations of sCD14 and LBP (predictor variables), and log-transformed concentrations of inflammatory and angiogenic mediators known to contribute to adverse pregnancy outcomes (outcome variables). All variables were quantified from enrolment plasma samples at gestational age <24 weeks. Data are presented as results of both simple linear regression analyses (unadjusted) and multivariable linear regression analyses adjusted for enrolment P. falciparum PCR status, maternal age, gestational age, BMI, systolic blood pressure, and socioeconomic status. Statistically significant relationships (p < 0.05) are bolded. Cell shade indicates the magnitude of the β coefficient for statistically significant relationships according to the scale (right).
CRP: C-Reactive Protein; sTNFR2: soluble Tumor Necrosis Factor receptor 2; IL18-BP: Interleukin-18 binding protein; CHI3L1: Chitinase 3-like protein; Angptl3: Angtiopoietin-like 3; Angpt-1: Angiopoietin-1; Angpt-2: Angiopoietin-2; sENG: soluble Endoglin; ICAM-1: soluble Intracellular Adhesion Molecule-1 (sICAM-1); sFlt-1: soluble fms-like Tyrosine Kinase-1; PlGF: Placental Growth Factor.
sCD14, a marker of intestinal barrier dysfunction, is associated with low L-arginine bioavailability
We previously quantified components of the L-arginine/NO biosynthetic pathway from a randomly selected subset of 384 primigravid participants of the parent RCT6; 331 of them met the inclusion criteria for the current study (Fig. 1). In this subset, concentrations of the ADMA and SDMA (competitive inhibitors that reduce the availability of L-arginine for NO biogenesis25) were positively associated with sCD14 concentrations in simple linear regression models; however only SDMA remained significant in the multivariable model (Table 3). The L-arginine concentration and L-arginine:ADMA ratio (indicative of bioavailable L-arginine) were negatively associated with the sCD14 concentration (Table 3). The ADMA concentration was significantly associated with LBP concentration in the unadjusted linear regression model, but this was not significant in the multivariable model. None of the other elements of the L-arginine biosynthetic pathway were significantly associated with LBP (Table 3).
Table 3.
Decreased bioavailable L-arginine is associated with markers of intestinal barrier dysfunction.
 |
Linear regression models of the association between log-transformed concentrations of components of the L-arginine bioavailability axis (L-arginine, ADMA, SDMA, and L-arginine:ADMA ratio; predictor variables) and log-transformed concentrations of sCD14 and LBP (outcome variables). All variables were quantified from enrolment plasma samples at gestational age <24 weeks. n = 331. Data are presented as results of both simple linear regression analyses (unadjusted) and multivariable linear regression analyses adjusted for enrolment P. falciparum PCR status, maternal age, gestational age, BMI, and socioeconomic status. Statistically significant relationships (p < 0.05) are bolded. Cell shade indicates the magnitude of the β coefficient for statistically significant relationships according to the scale (right). sCD14: soluble CD14; LBP: lipopolysaccharide binding protein; ADMA: asymmetric dimethyl arginine; SDMA: symmetric dimethyl arginine; C.I.: confidence interval.
Discussion
In this study, we investigated the contribution of intestinal barrier disruption to preterm birth outcomes in the context of P. falciparum infection during pregnancy. We demonstrated that concentrations of gut leak markers, sCD14 and LBP, at gestational age <24 weeks were positively associated with malaria parasite burden and an increased risk of preterm birth. Both sCD14 and LBP concentrations were associated with markers of systemic inflammation, and sCD14 was also associated with dysregulation of angiogenic mediators critical for placental vascular development. Bioavailable L-arginine was inversely associated with intestinal barrier disruption. This suggests that P. falciparum infection during pregnancy before 24 weeks gestation is associated with intestinal barrier dysfunction, and that this is associated with a cascade of inflammatory and angiogenic responses linked to dysregulation of placental development, and an increased risk of preterm birth.
Accumulating evidence indicates that impaired intestinal barrier function may contribute to the sequelae of malaria. Histopathologically, P. falciparum parasitized erythrocytes sequester within the enteric microvascular circulation26 with the potential to cause local ischemia, malabsorption, and increased gastrointestinal permeability.27,28 Intestinal barrier dysfunction has been linked to the complications of malaria including metabolic acidosis29 and invasive non-typhoidal Salmonella bacteremia.30 In this study, we observed increased concentrations of gut leak markers in participants with P. falciparum infection compared to those who were uninfected. Moreover, the concentrations of both sCD14 and LBP increased with parasite burden.
Malaria in early pregnancy is marked by systemic inflammation and dysregulated angiogenesis that persist across gestation,5 impairing placental vascular development, and contributing to adverse pregnancy outcomes.7 Our findings demonstrate that both sCD14 and LBP are associated with increased concentrations of inflammatory proteins implicated in preterm birth including CRP, sTNFR2, IL-18BP, and CHI3L1.5 sCD14 is also associated with increases in angiogenic mediators Angpt-2, sENG, and the sFLT:PlGF ratio, which are all associated with adverse pregnancy outcomes.7,31,32 Importantly, this study demonstrates that intestinal barrier dysfunction early in pregnancy (gestational age <24 weeks) is significantly associated with preterm birth. Concentrations of both gut leak markers in early pregnancy were higher among participants who ultimately delivered premature infants, and both sCD14 and LBP were associated with increased odds of preterm birth. Mediation analysis attributed approximately 11% and 7% of P. falciparum's effect on gestational age at delivery to the concentrations of sCD14 and LBP prior to 24 weeks gestation, respectively. Collectively, these data implicate P. falciparum–associated gut leak in a novel pathway that contributes to a cascade of inflammatory and angiogenic dysregulation that may culminate in preterm birth.
Similar to these findings with malaria, studies of HIV-positive pregnant women have also demonstrated an association between intestinal barrier dysfunction, inflammation, and preterm birth.24,33 In one study from Spain, markers of microbial translocation were not independently associated with preterm birth in women without HIV infection.33 In contrast, our study of HIV-negative women found that gut leak was associated with preterm birth in participants with and without malaria. Possible explanations for this discordance include the smaller sample size of the previous study (n = 42 HIV negative participants) and differences in demographics, with our study participants being at higher risk for malnutrition and other infections. Taken together, these reports suggest that women living in LMICs with high rates of infection (e.g., HIV, malaria), and malnutrition may be susceptible to intestinal barrier dysfunction and increased risk for preterm birth.
Low bioavailable L-arginine has been identified as a potentially modifiable risk factor for adverse outcomes in pregnancies complicated by malaria.10 During malaria, L-arginine, the immediate precursor to NO biosynthesis, is depleted by several mechanisms including increased destruction by host and parasite arginases, increased consumption by host NO synthase (NOS), and scavenging by cell free hemoglobin following lysis of parasitized red blood cells.34 Moreover, a recent study by Rubach et al. described a diminished supply of L-arginine precursor amino acids, such as L-glutamine and L-citrulline, in the setting of malaria, which may limit the capacity for de novo L-arginine regeneration. During pregnancy, fetal and placental growth demands further reduce maternal L-arginine bioavailability.25 In a survey of >7000 pregnant women, low dietary L-arginine was associated with an increased risk of preterm birth,35 and experimental models have demonstrated that supplemental L-arginine significantly improves placental vascular development and pregnancy outcomes.6 Here, we found that sCD14, a marker of intestinal permeability, was negatively associated with bioavailable L-arginine. L-arginine and NO are involved in maintaining the intestinal barrier via increased mucous and fluid secretion, mitigation of inflammatory responses, molecular regulation of intestinal tight and adherens junctions, and mucosal healing after ischemia.15 Preclinical models have demonstrated that arginine supplementation ameliorates malaria-induced intestinal permeability.8 Further research into the clinical use of antenatal L-arginine supplementation to reduce gut leak, and improve birth outcomes in pregnancies complicated by P. falciparum malaria is required.
There were no significant relationships between the components of the L-arginine axis and LBP. While sCD14 and LBP play complementary roles in the specific response to LPS endotoxemia,17 sCD14 is also involved in more general immune responses involving monocyte activation and cellular metabolism.36 The mechanistic significance of their divergence with respect to L-arginine bioavailability will require further investigation.
This study has several strengths. We analyzed a large, prospective cohort with a high prevalence of malaria and preterm birth, providing adequate power to interrogate the associations reported. Furthermore, as a substudy of a clinical trial, there was a high degree of rigor in enrollment, data collection, and outcome ascertainment. The results of this study are generalizable to HIV-negative pregnant women at high risk of P. falciparum infection living in sub-Saharan Africa. However, the study is not without limitations. First, pregnant women living with HIV were not included in the parent intervention trial, thus further studies in these populations are required. Second, we were limited by the volume of participant plasma and only had sufficient quantities to optimize two markers of microbial translocation for this study. We note that there are several other plasma markers of gut leak reported in the literature which may provide further insights into the mechanisms of gut leak with malaria in pregnancy. Third, the study design does not permit determination of causation with respect to gut leak and preterm birth, nor the mechanistic role of L-arginine in intestinal barrier integrity. Moreover, the L-arginine studies were conducted on samples from a subset of primigravid participants, thus the effect of gravidity on the associations between L-arginine bioavailability and gut leak could not be assessed. We did observe increased concentrations of sCD14 and LBP among primigravid with malaria compared with multigravidae (Supplementary Figure S2). Finally, clinical data on infections in pregnancy were limited to P. falciparum and HIV, and dietary assessments were not performed. This precludes analysis of other potential contributors to gut leak and preterm birth. The important mechanisms linking nutrition, immunity, malaria, and pregnancy outcomes need to be further explored in clinical trials to augment L-arginine bioavailability in pregnant women at risk for malaria.
In conclusion, this study provides evidence linking malaria-associated intestinal barrier dysfunction in pregnancy and systemic inflammation, disrupted angiogenic mediators important for placental development, and an increased risk of preterm birth. Further studies will be required to determine if interventions to enhance L-arginine bioavailability during pregnancy can mitigate P. falciparum-induced gut leak and improve birth outcomes in populations living in malaria-endemic regions.
Contributors
JW, AW, MN and KK defined the research question and designed the study.
JW, AW, MN, VS, KZ conducted the sCD14 and LBP assays.
FtK and MM were the lead investigators of the parent-RCT. ST conducted the malaria PCR testing for parent trial.
RE, CM, AW, KZ conducted the inflammatory and angiogenic biomarker assays from the parent RCT (published in PLoS Med 2019 Oct; 16 (10): e1002914.) CM led the quantification of L-arginine pathway components from the parent RCT (published in Sci Transl Med, 2018 Oct; 10 (431):eaan6007).
JW led the data analysis; AW, MN, CM, RE, AC, BC, FtK, KK contributed to the data analysis. In addition to JW, AW and KK accessed and verified the underlying data.
JW drafted the initial version of the manuscript and figures, which was then edited and approved by all of the co-authors.
All of the authors read and approved the final version of the manuscript.
Data sharing statement
Deidentified participant data and data dictionary will be made available upon publication by contacting the corresponding author.
Declaration of interests
None of the authors have conflicts of interest to declare.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (CIHR) Foundation grant (FDN-148439) [KCK], Canada Research Chair [KCK], Open Philanthropy [KCK], Vanier Canada Graduate Scholarships [JW, REE] and the Eliot Phillipson Clinician Scientist Training Program, University of Toronto [JW]. The parent trial was supported by the European and Developing Countries Clinical Trials Partnership (Award Number IP.2007.31080.003), and the Malaria in Pregnancy Consortium which was funded by the Bill & Melinda Gates Foundation grant to the Liverpool School of Tropical Medicine (Award Number 46099).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104808.
Contributor Information
Julie K. Wright, Email: julie.wright@mail.utoronto.ca.
Andrea M. Weckman, Email: andrea.weckman@mail.utoronto.ca.
Michelle Ngai, Email: michelle.c.ngai@gmail.com.
Veselina Stefanova, Email: veselina.stefanova@hotmail.com.
Kathleen Zhong, Email: kathleen.zhong@uhnresearch.ca.
Chloe R. McDonald, Email: chloe.mcdonald@mail.utoronto.ca.
Robyn E. Elphinstone, Email: robyn.elphinstone@mail.utoronto.ca.
Andrea L. Conroy, Email: conroya@iu.edu.
Bryan A. Coburn, Email: bryan.coburn@utoronto.ca.
Mwayi Madanitsa, Email: mmadanitsa@must.ac.mw.
Steve M. Taylor, Email: steve.taylor@duke.edu.
Feiko O. ter Kuile, Email: Feiko.terKuile@lstmed.ac.uk.
Kevin C. Kain, Email: kevin.kain@uhn.ca.
Appendix A. Supplementary data
References
- 1.Rogerson S.J., Desai M., Mayor A., Sicuri E., Taylor S.M., van Eijk A.M. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis. 2018;18(4) doi: 10.1016/S1473-3099(18)30066-5. [DOI] [PubMed] [Google Scholar]
- 2.De Beaudrap P., Turyakira E., Nabasumba C., et al. Timing of malaria in pregnancy and impact on infant growth and morbidity: a cohort study in Uganda. Malar J. 2016;15:92. doi: 10.1186/s12936-016-1135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai M., ter Kuile F.O., Nosten F., et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7(2):93. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 4.World malaria report 2022. 2022. Geneva. [Google Scholar]
- 5.Elphinstone R.E., Weckman A.M., McDonald C.R., et al. Early malaria infection, dysregulation of angiogenesis, metabolism and inflammation across pregnancy, and risk of preterm birth in Malawi: a cohort study. PLoS Med. 2019;16(10) doi: 10.1371/journal.pmed.1002914. http://dx.plos.org/10.1371/journal.pmed.1002914 Seidlein L von. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald C.R., Cahill L.S., Gamble J.L., et al. Malaria in pregnancy alters l-arginine bioavailability and placental vascular development. Sci Transl Med. 2018;10(431) doi: 10.1126/scitranslmed.aan6007. http://stm.sciencemag.org/lookup/doi/10.1126/scitranslmed.aan6007 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran V., Weckman A.M., Crowley V.M., et al. The Angiopoietin-Tie2 axis contributes to placental vascular disruption and adverse birth outcomes in malaria in pregnancy. eBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103683. [cited 2022 Feb 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chau J.Y., Tiffany C.M., Nimishakavi S., et al. Malaria-associated L-arginine deficiency induces mast cell-associated disruption to intestinal barrier defenses against nontyphoidal Salmonella bacteremia. Infect Immun. 2013;81(10):3515. doi: 10.1128/IAI.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubach M.P., Zhang H., Florence S.M., et al. Kinetic and cross-sectional studies on the genesis of hypoargininemia in severe pediatric Plasmodium falciparum malaria. Infect Immun. 2019;87(4) doi: 10.1128/IAI.00655-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngai M., Weckman A.M., Erice C., et al. Malaria in pregnancy and adverse birth outcomes: new mechanisms and therapeutic opportunities. Trends Parasitol. 2020;36(2):127–137. doi: 10.1016/j.pt.2019.12.005. [cited 2022 Feb 19] [DOI] [PubMed] [Google Scholar]
- 11.Schleiffer R., Raul F. Prophylactic administration of L-arginine improves the intestinal barrier function after mesenteric ischaemia. Gut. 1996;39(2):194. doi: 10.1136/gut.39.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ersin S., Tuncyurek P., Esassolak M., et al. The prophylactic and therapeutic effects of glutamine- and arginine-enriched diets on radiation-induced enteritis in rats. J Surg Res. 2000;89(2) doi: 10.1006/jsre.1999.5808. [DOI] [PubMed] [Google Scholar]
- 13.Sukhotnik I., Mogilner J., Krausz M.M., et al. Oral arginine reduces gut mucosal injury caused by lipopolysaccharide endotoxemia in rat. J Surg Res. 2004;122(2):256. doi: 10.1016/j.jss.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Viana M.L., Santos R.G.C., Generoso S.V., Arantes R.M.E., Correia M.I.T.D., Cardoso V.N. Pretreatment with arginine preserves intestinal barrier integrity and reduces bacterial translocation in mice. Nutrition. 2010;26(2):218. doi: 10.1016/j.nut.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Wang W.W., Qiao S.Y., Li D.F. Amino acids and gut function. Amino Acids. 2009;37(1):105. doi: 10.1007/s00726-008-0152-4. [DOI] [PubMed] [Google Scholar]
- 16.Duggan C., Gannon J., Walker W.A. Protective nutrients and functional foods for the gastrointestinal tract. Am J Clin Nutr. 2002;75(5):789. doi: 10.1093/ajcn/75.5.789. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S.S., Wang J., Yannie P.J., Ghosh S. Intestinal barrier dysfunction, LPS translocation, and disease development. J Endocr Soc. 2020;4(2) doi: 10.1210/jendso/bvz039. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=32099951&retmode=ref&cmd=prlinks Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haussner F., Chakraborty S., Halbgebauer R., Huber-Lang M. Challenge to the intestinal mucosa during sepsis. Front Immunol. 2019;10(APR):891. doi: 10.3389/fimmu.2019.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenchley J.M., Price D.A., Schacker T.W., et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. https://www.nature.com/articles/nm1511 [cited 2022 Apr 21]. Available from: [DOI] [PubMed] [Google Scholar]
- 20.Seethaler B., Basrai M., Neyrinck A.M., et al. Biomarkers for assessment of intestinal permeability in clinical practice. Am J Physiol Gastrointest Liver Physiol. 2021;321(1):G11–G17. doi: 10.6084/m9.figshare. [cited 2023 Jan 30]. Available from: [DOI] [PubMed] [Google Scholar]
- 21.Vanuytsel T., Tack J., Farre R. The Role of Intestinal Permeability in Gastrointestinal Disorders and Current Methods of Evaluation. Front Nutr. 2021;8:717925. doi: 10.3389/fnut.2021.717925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madanitsa M., Kalilani L., Mwapasa V., et al. Scheduled intermittent screening with rapid diagnostic tests and treatment with dihydroartemisinin-piperaquine versus intermittent preventive therapy with sulfadoxine-pyrimethamine for malaria in pregnancy in Malawi: an open-label randomized controlled trial. PLoS Med. 2016;13(9) doi: 10.1371/journal.pmed.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bode-Böger S.M., Scalera F., Ignarro L.J. The L-arginine paradox: importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther. 2007;114(3):295–306. doi: 10.1016/j.pharmthera.2007.03.002. https://pubmed.ncbi.nlm.nih.gov/17482266/ [cited 2023 May 28]. Available from: [DOI] [PubMed] [Google Scholar]
- 24.Shivakoti R., Gupte N., Kumar N.P., et al. Intestinal barrier dysfunction and microbial translocation in human immunodeficiency virus–infected pregnant women are associated with preterm birth. Clin Infect Dis. 2018;67(7):1103–1109. doi: 10.1093/cid/ciy253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weckman A.M., McDonald C.R., Baxter J.A.B., Fawzi W.W., Conroy A.L., Kain K.C. Perspective: L-arginine and L-citrulline supplementation in pregnancy: a potential strategy to improve birth outcomes in low-resource settings. Adv Nutr. 2019;10(5):765–777. doi: 10.1093/advances/nmz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milner D.A., Lee J.J., Frantzreb C., et al. Quantitative assessment of multiorgan sequestration of parasites in fatal pediatric cerebral malaria. JID (J Infect Dis) 2015;212(8):1317–1321. doi: 10.1093/infdis/jiv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molyneux M.E., Looareesuwan S., Menzies I.S., et al. Reduced hepatic blood flow and intestinal malabsorption in severe falciparum malaria. Am J Trop Med Hyg. 1989;40(5):470–476. doi: 10.4269/ajtmh.1989.40.470. [cited 2022 Apr 13] [DOI] [PubMed] [Google Scholar]
- 28.Wilairatana P., Meddings J.B., Ho M., Vannaphan S., Looareesuwan S. Increased gastrointestinal permeability in patients with Plasmodium falciparum malaria. Clin Infect Dis. 1997;24(3):430–435. doi: 10.1093/clinids/24.3.430. [DOI] [PubMed] [Google Scholar]
- 29.Leopold S.J., Ghose A., Allman E.L., et al. Identifying the components of acidosis in patients with severe P. falciparum malaria using metabolomics. J Infect Dis. 2019;219:1766–1776. doi: 10.1093/infdis/jiy727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uche I.V., MacLennan C.A., Saul A. A systematic review of the incidence, risk factors and case fatality rates of invasive nontyphoidal Salmonella (iNTS) disease in Africa (1966 to 2014) PLoS Negl Trop Dis. 2017;11(1) doi: 10.1371/journal.pntd.0005118. https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0005118 [cited 2022 Apr 9]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weckman A.M., Ngai M., Wright J., McDonald C.R., Kain K.C. The impact of infection in pregnancy on placental vascular development and adverse birth outcomes. Front Microbiol. 2019;10:1924. doi: 10.3389/fmicb.2019.01924. https://www.frontiersin.org/article/10.3389/fmicb.2019.01924/full Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang Y.S., Chen C.N., Jeng S.F., et al. The sFlt-1/PlGF ratio as a predictor for poor pregnancy and neonatal outcomes. Pediatr Neonatol. 2017;58(6):529–533. doi: 10.1016/j.pedneo.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 33.López M., Figueras F., Coll O., et al. Inflammatory markers related to microbial translocation among HIV-infected pregnant women: a risk factor of preterm delivery. J Infect Dis. 2016;213(3):343–350. doi: 10.1093/infdis/jiv416. [cited 2022 Apr 7] [DOI] [PubMed] [Google Scholar]
- 34.Weinberg J.B., Lopansri B.K., Mwaikambo E., Granger D.L. Arginine, nitric oxide, carbon monoxide, and endothelial function in severe malaria. Curr Opin Infect Dis. 2008;21(5):468. doi: 10.1097/QCO.0b013e32830ef5cf. pmc/articles/PMC2732119/ [cited 2023 Feb 8]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darling A., McDonald C.R., Urassa W.S., Kain K.C., Mwiru R.S., Fawzi W.W. Maternal dietary L-arginine and adverse birth outcomes in Dar es Salaam, Tanzania. Am J Epidemiol. 2017;186(5):603–611. doi: 10.1093/aje/kwx080. [cited 2021 Nov 25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanoni I., Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol. 2013;3:32. doi: 10.3389/fcimb.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.