Abstract
Background:
In the Phase III OlympiAD study, olaparib significantly prolonged progression-free survival versus chemotherapy treatment of physician’s choice (TPC) in patients with germline BRCA-mutated (gBRCAm), HER2-negative metastatic breast cancer (mBC). In the final pre-specified analysis (64% maturity), median overall survival (OS) was 19.3 months for olaparib and 17.1 months for TPC (P = 0.513). Post-hoc extended follow-up, 25.7 months longer than previously reported for OS, is reported.
Patients and methods:
Patients with gBRCAm, HER2-negative mBC, who had received ≤2 lines of chemotherapy for metastatic disease were randomised 2:1 to olaparib (300 mg bid) or TPC. During extended follow-up, OS was analysed every 6 months using the stratified log-rank test (overall population) and Cox proportional hazards model (pre-specified subgroups).
Results:
In the overall population (302 patients; 76.8% maturity), median OS was 19.3 months for olaparib and 17.1 months for TPC (hazard ratio [HR] 0.89, 95% confidence interval [CI] 0.67–1.18); median follow-up was 18.9 and 15.5 months, respectively. Three-year survival was 27.9% for olaparib versus 21.2% for TPC. With olaparib, 8.8% of patients received study treatment for ≥3 years versus none with TPC. In first-line (1L) mBC, median OS was longer for olaparib than TPC (22.6 vs 14.7 months; HR 0.55, 95% CI 0.33–0.95) and three-year survival was 40.8% for olaparib versus 12.8% for TPC. No new serious adverse events related to olaparib were observed.
Conclusions:
OS was consistent with previous analyses from OlympiAD. These findings support the possibility of meaningful long-term survival benefit with olaparib, particularly in 1L mBC.
Keywords: Breast cancer, Germline BRCA mutation, Olaparib, Overall survival, PARP inhibitor
1. Introduction
Approximately 5% of patients with breast cancer (BC) carry a germline BRCA1 and/or BRCA2 mutation [1–3], which are associated with a younger age at diagnosis, aggressive disease characteristics, and human epidermal growth factor receptor 2 (HER2)-negative tumours [1, 4–6].
In the primary analysis of the Phase III OlympiAD study (NCT02000622), the oral poly(ADP-ribose) polymerase (PARP) inhibitor olaparib significantly increased median progression-free survival compared with chemotherapy treatment of physician’s choice (TPC) (7.0 vs 4.2 months, respectively) in patients with germline BRCA-mutated, HER2-negative metastatic breast cancer (mBC) [7]. A delay in the time to deterioration of health-related quality of life, functioning, and symptoms was also reported [8]. At the time of the final pre-specified overall survival (OS) analysis (25 September 2017), median follow-up for OS in censored patients was 25.3 months and 26.3 months in the olaparib and TPC groups, respectively (18.9 versus 15.5 months overall, respectively). At this data-cut, 192 of the 302 patients randomised had died (64% data maturity), and there was no statistically significant difference in median OS between olaparib (19.3 months) and TPC (17.1 months) [9]. However, it was notable that some patients, restricted to the olaparib arm, were continuing to receive study treatment, suggesting a long-term survival benefit.
Long-term responders to olaparib who derive prolonged survival benefit have been previously reported in the treatment of ovarian cancer [10]. Given the numbers of patients alive at the time of the final pre-specified OS analysis (on or off treatment), it was of interest to determine survival status at later time points. In March 2018, the OlympiAD study protocol was amended to extend the follow-up of patients by a minimum of 2 years for survival status. Here, we have completed a follow-up that is 25.7 months longer than that previously reported for OS and safety. We report findings for the overall randomised population as well as pre-specified subgroups of interest, including patients receiving olaparib in the first-line setting for metastatic disease. We also present baseline characteristics for patients who had extended time on olaparib treatment owing to positive outcomes.
2. Methods
2.1. Study design and patients
Methodological details of the OlympiAD study have been reported previously [7, 9]. In brief, OlympiAD was a randomised, controlled, open-label, multicentre, international, Phase III trial in adults with germline BRCA-mutated, HER2-negative mBC. Patients had previously received an anthracycline (unless contraindicated) and a taxane in the neoadjuvant, adjuvant, or metastatic setting, and no more than two chemotherapy regimens for mBC. Patients were randomised (2:1) to receive olaparib tablets (300 mg twice daily) or predeclared single-agent TPC (capecitabine, vinorelbine, or eribulin) until objective disease progression (Response Evaluation Criteria in Solid Tumours, version 1.1) or unacceptable toxic effects. The study protocol, and its amendment to allow an extended follow-up period, were approved by ethics review committees at the participating institutions. Patients were re-consented prior to participation in the extended follow-up period.
2.2. Endpoints and assessments
OS, treatment duration, time to first subsequent cancer therapy or death (TFST), time to second subsequent cancer therapy or death (TSST), serious adverse event (SAEs), and adverse events of special interest (AESIs; myelodysplastic syndrome, acute myeloid leukaemia, new primary malignancies, and pneumonitis) for olaparib were endpoints in the extended follow-up period. On-treatment study visits during the extended survival follow-up were performed as per local clinical practice. SAEs and AESIs were recorded during the treatment period and for 30 days after discontinuation of study treatment. Investigators were also required to report any SAE (including death) occurring at any time after a patient had completed the study if it was considered causally related to investigational product. Any case of myelodysplastic syndrome, acute myeloid leukaemia, or new primary malignancy occurring after the 30-day follow-up period was to be reported as an SAE for pharmacovigilance purposes.
2.3. Statistical analysis
Exploratory analyses of OS, TFST, and TSST were performed at 6-monthly intervals during the extended follow-up period. For OS analyses, patients not known to have died at the time of the data-cut were censored at the last recorded date on which they were known to be alive. Patients confirmed to be alive were censored at the date of the data-cut. These analyses were not powered to detect differences between the treatment groups; no multiplicity adjustment was applied, and P values are nominal. For the analyses of OS, TFST, and TSST in the overall population, a hazard ratio (HR) and confidence intervals (CIs) were estimated using a stratified log-rank test. The Kaplan–Meier method was used to generate time-to-event curves, from which medians were calculated. Landmark OS analyses (percentage of patients known to be still alive) were performed on the full analysis set and pre-specified subgroups at 12, 24, 36 and 48 months post-randomisation. A single Cox proportional hazards model was used for the OS analyses in pre-specified subgroups: hormone receptor status (hormone receptor-positive vs triple-negative), prior chemotherapy for mBC (yes vs no), and prior platinum-based chemotherapy (yes vs no). Safety data are summarised descriptively.
3. Results
3.1. Patients
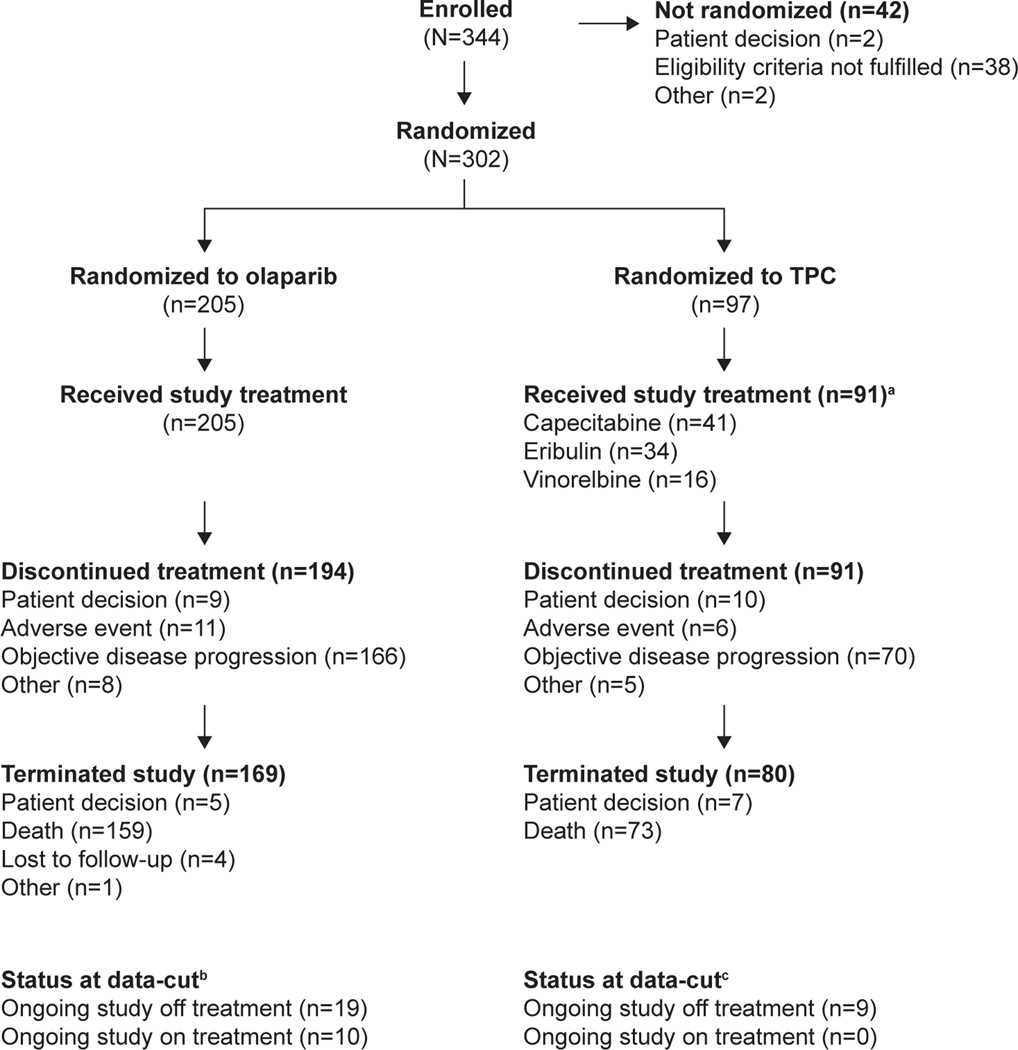
Fifteen patients (5.0%) did not consent to participate in the extended follow-up (olaparib: n = 7, 3.4%; TPC: n = 8, 8.2%). At the time of data-cut off (DCO, 17 November 2019), of the 302 patients randomised (olaparib: n = 205; TPC: n = 97), 38 patients (12.6%) remained in the study. Of these, 10 patients (all in the olaparib arm) were still receiving study treatment. The remaining 28 patients (olaparib: n = 19; TPC: n = 9) were ongoing in the study off treatment because they had discontinued treatment but remained in the study under survival follow-up. A total of 249 patients (82.5%) were off study (olaparib: 82.4%; TPC: 82.5%). The main reason for study termination was death (n = 232; 76.8% OS data maturity) (Fig. 1).
Fig. 1.
Patient disposition at the data-cut for the extended OS analysis (17 November 2019). aSix patients in the TPC arm declined study treatment because of treatment allocation. bSeven patients did participate in the extended follow-up. cEight patients did not participate in the extended follow-up.
Abbreviations: OS, overall survival; TPC, chemotherapy treatment of physician’s choice.
The baseline characteristics of the overall randomised population and the 10 patients who remained on study treatment in the olaparib arm at the time of the data-cut are shown in Table S1. Compared with the overall randomised population, those patients who were still receiving olaparib tended to have better Eastern Cooperative Oncology Group performance status (ECOG PS) at study entry and were less heavily pretreated with chemotherapy for mBC and platinum-based chemotherapy for BC. The response data reported for these patients in Table S1 are limited to the DCO for the primary analysis (09 December 2016).
3.2. Study treatment duration and subsequent treatments
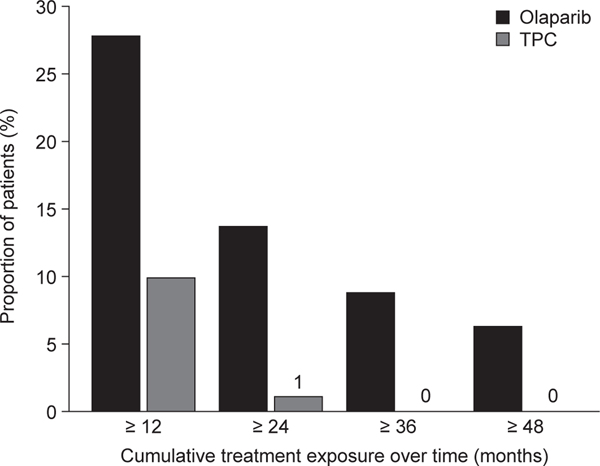
The median total study treatment duration was 8.2 months in the olaparib arm (range, 14 days–4.7 years) versus 3.4 months in the TPC arm (range, 21 days–2.1 years). Of the 205 patients who received at least one dose of olaparib, 27.8% (n = 57) received study treatment for at least 1 year, with 13.7% (n = 28) of these patients continuing for 2 years, 8.8% (n = 18) for 3 years, and 6.3% (n = 13) for 4 years, compared with 9.9% (n = 9) for 1 year, 1.1% (n = 1) for 2 years, and none for 3 or 4 years among the patients who received at least one dose of study treatment in the TPC arm (Fig. 2).
Fig. 2.
Cumulative treatment exposure, including interruptions, in patients who received study treatment in the olaparib arm (n = 205) or TPC arm (n = 91).
Abbreviations: TPC, chemotherapy treatment of physician’s choice.
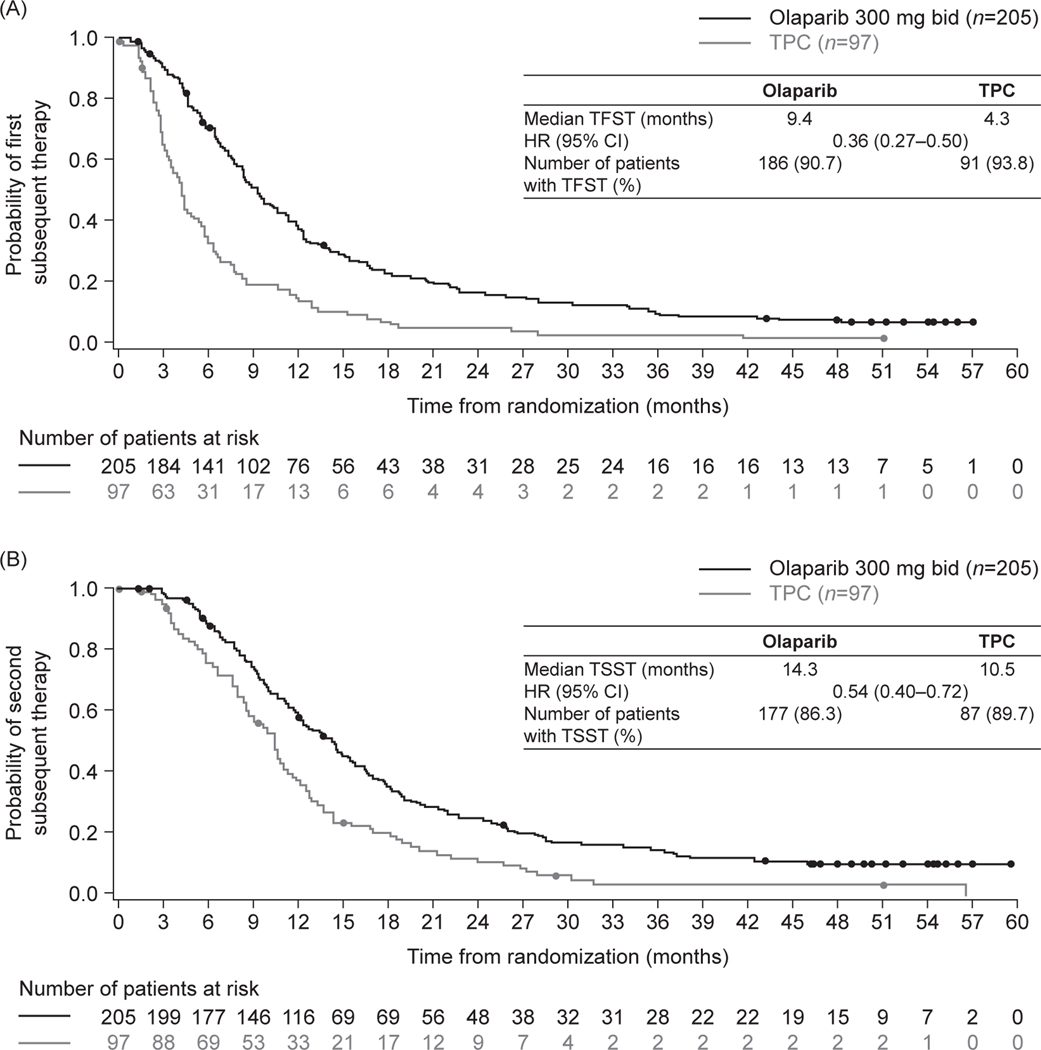
For the patients who discontinued study treatment at any point during the study, subsequent treatments in the olaparib and TPC arms included a PARP inhibitor in 2.0% and 12.4% of patients, and platinum-based chemotherapy in 42.9% and 48.5% of patients, respectively (Table 1). Median TFST was especially favourable for patients randomised to olaparib (9.4 months, 95% CI 8.3–10.6 months) compared with TPC (4.3 months, 95% CI 3.4–5.4 months), HR 0.36 (95% CI 0.27–0.50) (Fig. 3A). Median TSST was 14.3 months (95% CI 12.3–15.7 months) in the olaparib arm and 10.5 months (95% CI 8.5–11.3 months) in the TPC arm, HR 0.54 (95% CI 0.40–0.72) (Fig. 3B).
Table 1.
Subsequent cancer therapies
| Olaparib (n = 205) | TPC (n = 97) | |
|---|---|---|
| PARP inhibitor | 4 (2.0) | 12 (12.4) |
| Platinum-based chemotherapy | 88 (42.9) | 47 (48.5) |
| Other cytotoxic chemotherapy | 136 (66.3) | 71 (73.2) |
| Hormonal therapy | 42 (20.5) | 25 (25.8) |
| Targeted/biologics | 38 (18.5) | 24 (24.7) |
| Other | 8 (3.9) | 4 (4.1) |
| No subsequent cancer therapy | 33 (16.1) | 17 (17.5) |
| Continuing study treatmenta | 10 (4.9) | 0 |
Data are presented as n (%). Patients may have received a subsequent cancer therapy in more than one category.
As of DCO 17 November 2019
DCO, data cut-off; PARP, poly(ADP-ribose) polymerase; TPC, chemotherapy treatment of physician’s choice.
Fig. 3.
Kaplan–Meier estimates for (A) time to first subsequent cancer therapy and (B) second subsequent cancer therapy.
Abbreviations: bid, twice daily; CI, confidence interval; TFST, time to first subsequent cancer therapy; TPC, chemotherapy treatment of physician’s choice; TSST, time to second subsequent cancer therapy.
3.3. OS and landmark analyses
The overall median follow-up for OS was 18.9 months in the olaparib arm and 15.5 months in the TPC arm.
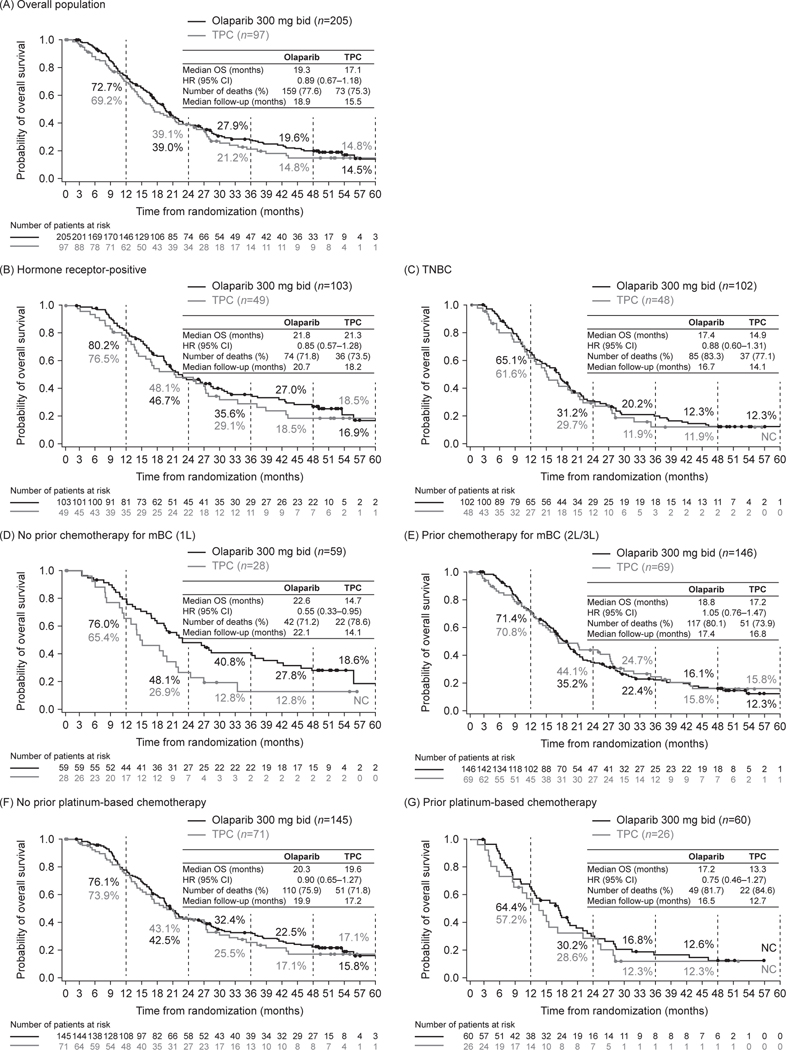
Fig. 4 shows Kaplan–Meier estimates of OS for the overall population and for pre-specified subgroups (hormone receptor status, prior chemotherapy for mBC, and prior platinum-based chemotherapy). In the overall population, median OS was 19.3 months for olaparib and 17.1 months for TPC (HR 0.89, 95% CI 0.67–1.18) (Fig. 4A). Landmark analyses showed that 27.9% of patients in the olaparib arm were alive at 3 years compared with 21.2% of patients in the TPC arm (Fig. 4A).
Fig. 4.
Kaplan–Meier estimates for overall survival (A) in the overall population and (B–G) in pre-specified subgroups: hormone receptor-positive (B), TNBC (C), no prior chemotherapy for mBC, (D) prior chemotherapy for mBC (E), no prior platinum-based chemotherapy for BC (F), and prior platinum-based chemotherapy for BC (G). Vertical grey dashed lines indicate the landmark analyses timepoints; percentages represent the proportions of patients still alive at each of these timepoints.
Abbreviations: BC, breast cancer; bid, twice daily; CI, confidence interval; HR, hazard ratio; L, line; mBC, metastatic breast cancer; mo, month; NC, non-calculable; no., number; TNBC, triple-negative breast cancer; TPC, chemotherapy treatment of physician’s choice.
Median OS was consistently numerically longer for olaparib than for TPC across the pre-specified subgroups (Fig. 4B–G). OS benefit was numerically greater among patients receiving olaparib versus TPC in the first-line setting (i.e., no prior chemotherapy for mBC subgroup), median 22.6 vs 14.7 months, respectively; HR 0.55, 95% CI 0.33–0.95 (Fig. 4D). Of the patients receiving study treatment in the first-line setting for mBC, 40.8% of patients in the olaparib arm were alive at 3 years compared with 12.8% of patients in the TPC arm (Fig. 4D).
3.4. Safety
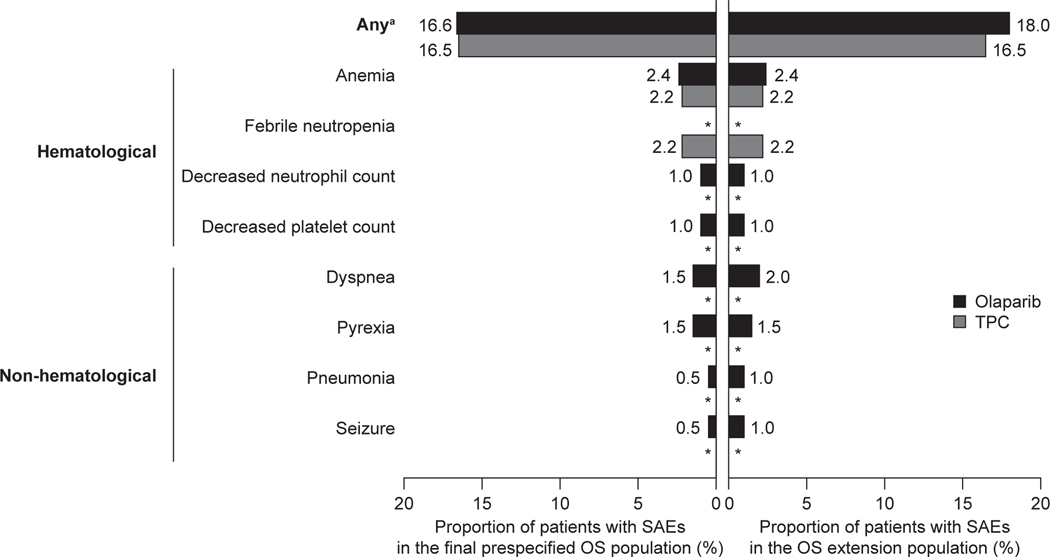
No new SAEs causally related to olaparib treatment or AESIs were reported during the extended follow-up period, as compared to previous analyses. No cases of myelodysplastic syndrome, acute myeloid leukaemia, or pneumonitis were reported throughout the study. SAEs were reported at similar frequencies per treatment arm in the final pre-specified OS population (16.6% of patients treated with olaparib, 16.5% treated with TPC) and OS extension population (18.0% of patients treated with olaparib, 16.5% treated with TPC). The most common SAE, anaemia, was reported by the same proportion of patients in the final pre-specified OS and extended populations per treatment arm: olaparib (2.4% of patients) and TPC (2.2% of patients) (Fig. 5). Throughout the study, 11 patients (5.4%) in the olaparib arm and six patients (6.6%) in the TPC arm discontinued treatment because of an adverse event (Fig. 1).
Fig. 5.
Incidence of SAEs occurring in two or more patients in either treatment arm in the final pre-specified OS population (DCO 25 September 2017) or in the OS extension population (DCO 17 November 2019). Data are shown as the proportion of patients with SAEs (categorised as haematological or non-haematological) in each treatment arm. aIncluding SAEs that occurred one or more times. *Indicates no recorded SAE.
Abbreviations: DCO, data cut-off; OS, overall survival; SAEs, serious adverse events; TPC, chemotherapy treatment of physician’s choice.
4. Discussion
This extended follow-up of patients enrolled in the OlympiAD study provides clinically important long-term observations of a PARP inhibitor, olaparib, in the treatment of germline BRCA-mutated, HER2-negative mBC. The numeric OS benefit between olaparib and TPC was consistent across the overall population and pre-specified subgroups, with the possibility of greatest long-term benefit among patients who had not received prior chemotherapy for metastatic disease: 40.8% of patients receiving first-line treatment for mBC in the olaparib arm were alive at 3 years compared with 12.8% of patients in the TPC arm. In the olaparib arm, a numerically larger subset of patients received study treatment for at least 1 year (27.8%, n = 57), 2 years (13.7%, n = 28), 3 years (8.8%, n = 18), and 4 years (6.3% n = 13), compared with 9.9% (n = 9) for 1 year, 1.1% (n = 1) for 2 years, and none at 3 or 4 years in the TPC arm. This sustainability of the treatment effect of olaparib in the overall population was supported by longer median times to subsequent treatments in the olaparib arm than in the TPC arm (TFST, 9.4 months vs 4.3 months; TSST, 14.3 months vs 10.5 months).
Prolongation of OS is a desirable goal when cancer has progressed beyond curative treatment. In the overall population and across the pre-specified subgroups (prior chemotherapy for mBC, hormone receptor status, and prior platinum-based chemotherapy), landmark survival analyses numerically favoured the olaparib arm, where 27.9% of patients were alive at 3 years compared with 21.2% of patients in the TPC arm. Furthermore, eighteen patients (8.8%) in the olaparib arm received study treatment for at least 3 years in the absence of clinical progression, compared with no patients in the TPC arm. The long-term OS findings of this exploratory analysis are concordant with the final pre-specified OS analysis in OlympiAD [9]. The OlympiAD study was not powered to identify differences in OS (a secondary endpoint), but rather to detect improvement in progression-free survival with olaparib (the primary endpoint), and the extended OS analysis was exploratory. This survival analysis may also have been confounded by subsequent treatments following discontinuation of one of the study treatments [7], for example PARP inhibitor use (12.4% [TPC arm] vs 2.0% [Olaparib arm]), although the sustainable effect of olaparib therapy was supported by median TFST and TSST being consistently longer in the olaparib arm.
This long-term exploratory assessment of OS has confirmed the findings of the final pre-specified analysis in OlympiAD [9] that suggested a possible benefit in the first-line setting for mBC. Among the 10 patients (n = 4 hormone receptor-positive, n = 6 triple-negative breast cancer) who remained on study treatment in the olaparib arm at the time of the data-cut, only one patient had received platinum-based chemotherapy for BC and only half had previously received chemotherapy for mBC. There are, however, some important caveats to be taken into consideration. The progression-free survival benefit for olaparib relative to TPC was similar across subgroups, including those defined by extent of prior therapy. Also, the median OS was unexpectedly lower for patients without prior chemotherapy for mBC compared with the overall population in the TPC arm (14.7 months vs 17.1 months), although it was higher in the olaparib arm (22.6 months vs 19.3 months). Furthermore, the final OS analysis of the phase 3 EMBRACA trial did not demonstrate any first-line OS benefit for the PARP inhibitor talazoparib in a similar patient population [11].
Long-term exposure to olaparib was generally well tolerated, with no evidence of cumulative toxicity and no new safety signals compared with earlier data-cuts [7, 9]. Specifically, there were no new SAEs considered by the investigators to be causally related to olaparib treatment and no AESIs. In the previously reported primary analysis, one patient in the olaparib arm had a nonserious event of melanoma in situ [7, 9]. No cases of myelodysplastic syndrome, acute myeloid leukaemia, or pneumonitis were reported throughout the study. The most common SAE, anaemia, was reported by the same proportion of patients in the extended and final pre-specified OS populations. The low rates of discontinuation from olaparib treatment due to adverse events (<6%), such as anaemia, reported in the current and previous analyses of the OlympiAD study [7, 9] show that supportive treatment, dose interruptions, and reductions can be used effectively to manage tolerability, ensuring that patients remain on treatment for as long as they are receiving benefit.
In summary, this extended follow-up of patients enrolled in the OlympiAD study provides clinically important observations of a PARP inhibitor in the treatment of mBC, with a subset (8.8%) of patients receiving treatment with olaparib for at least 3 years. These findings are consistent with evidence of prolonged responses to olaparib in the treatment of ovarian cancer [10]. In this extended follow-up, although OS did not differ significantly between the two treatment arms, the HR favoured olaparib over TPC. The finding that 40.8% of olaparib-treated patients who had not previously received chemotherapy for mBC were alive at 3 years, compared with 12.8% in the TPC arm, raises the possibility of a meaningful OS benefit for some patients receiving olaparib in the first-line setting for mBC. These exploratory findings should be investigated in further studies, including those employing real-world data.
Supplementary Material
Highlights.
Median OS was 19.3 months for olaparib and 17.1 months for chemotherapy treatment of physician’s choice (TPC).
A subset of patients (8.8%) received at least 3 years of study treatment in the olaparib arm versus no patients in the TPC arm.
In the mBC first-line setting, median OS was numerically longer for olaparib (22.6 months) than for TPC (14.7 months).
In the mBC first-line setting, 3-year survival rate was 40.8% in the olaparib arm and 12.8% in the TPC arm.
These data confirm the findings of the final pre-specified OlympiAD analysis, suggesting a possible survival benefit in the first-line setting.
Highlights.
Median OS was 19.3 and 17.1 months for olaparib versus chemotherapy respectively
8.8% of patients received at least 3 years of olaparib versus none in the TPC arm
In first-line, median OS was numerically longer for olaparib (22.6 vs 14.7 months)
In first-line, 3-year OS rate was 40.8% for olaparib vs 12.8% in the TPC arm
These data confirm previous findings and suggest a possible OS benefit in first-line
Acknowledgements
We thank Wendy Bannister and Carsten Goessl for providing statistical and medical input while at Phastar, London, UK (contracted to AstraZeneca) and AstraZeneca, Gaithersburg, MD, USA, respectively. We also thank Dr Wei Li for their help in the development of this manuscript.
Funding
This study was supported by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD). Medical writing assistance was provided by Elizabeth Gandhi, PhD and Martin Dalziel, PhD, from Oxford PharmaGenesis Ltd, UK, funded by AstraZeneca and MSD.
Abbreviations:
- AESI
adverse event of special interest
- BC
breast cancer
- bid
twice daily
- CI
confidence interval
- DCO
data cut-off
- ECOG PS
Eastern Cooperative Oncology Group performance status
- gBRCAm
germline BRCA-mutated
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
- L
line
- mBC
metastatic breast cancer
- mo
month
- NC
non-calculable
- no
number
- OS
overall survival
- PARP
poly(ADP-ribose) polymerase
- SAEs
serious adverse event
- TFST
time to first subsequent cancer therapy
- TNBC
triple-negative breast cancer
- TPC
chemotherapy treatment of physician’s choice
- TSST
time to second subsequent cancer therapy
Footnotes
Conflict of interest statement
Mark. E. Robson reports honoraria from Intellisphere, MJH, Physicians’ Education Resources, and Research to Practice; research grants (to institution) from AstraZeneca, Merck, and Pfizer; personal fees from Change Health Care; consulting (uncompensated) for Epic Sciences, Merck, Pfizer, Zenith Epigenetics; and non-financial support from Pfizer (editorial services). Dr Robson is funded in part through an NIH/NCI Cancer Center Support Grant (P30 CA008748) and the Breast Cancer Research Foundation.
Seock-Ah Im reports consultancy for AstraZeneca, Amgen, Eisai, GSK, Hanmi, Lilly, MSD, Novartis, Pfizer, and Roche; and research funding from AstraZeneca, Roche, Boryung Pharm, and Eisai.
Elzbieta Senkus reports honoraria from Amgen, AstraZeneca, Cancérodigest, Clinigen, Curio Science, Egis, Eli Lilly, Genomic Health, Gilead, high5md, Novartis, Oncompass Medicine, Pfizer, Pierre Fabre, Roche, Sandoz, TLC Biopharmaceuticals; travel support from Amgen, AstraZeneca, Egis, Gilead, Novartis, Pfizer, and Roche; research funding from AstraZeneca, Eli Lilly, Novartis, Pfizer, Roche, and Samsung; and holding stocks of AstraZeneca, Eli Lilly, and Pfizer.
Binghe. Xu reports no conflicts of interest.
Susan M. Domchek reports honoraria from AstraZeneca, Clovis Oncology, and Bristol-Myers Squibb, and research funding to the University of Pennsylvania from AstraZeneca and Clovis Oncology.
Norikazu Masuda reports honoraria from AstraZeneca, Chugai Pharma, Eisai, Eli Lilly, Pfizer, and Takeda; and institutional research funding from AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Eli Lilly, Kyowa Hakka Kirin, MSD, Nippon-Kayaku, Novartis, Pfizer, and Sanofi.
Suzette Delaloge reports honoraria from AstraZeneca, GE Healthcare, Novartis, Pfizer, Puma Biotechnology, and Roche (all institutional); consulting for AstraZeneca, Novartis, Pfizer, Puma Biotechnology, Roche (all institutional); research funding from AstraZeneca, Novartis, Pfizer, Puma Biotechnology, and Roche; and travel expenses, and accommodation from AstraZeneca, Novartis, and Pfizer.
Nadine Tung reports consulting for AstraZeneca; and receiving grant support from Ambry Genetics and Myriad Genetics.
Anne Armstrong reports receiving fees for serving on an advisory board from Roche and Syndax Pharmaceuticals; and her spouse holding stock options in AstraZeneca.
Mike Dymond, Anitra Fielding, and Allison Allen report employment from AstraZeneca and stock and other ownership interests with AstraZeneca.
Pierfranco Conte reports speakers’ bureaus with AstraZeneca, GlaxoSmithKline, Novartis, and Roche; travel, accommodation, and expenses from Celgene, GlaxoSmithKline, and Novartis; and research funding from Merck Serono, Novartis, and Roche.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Manuscript preparation: Mark E. Robson, Elzbieta Senkus and Suzette Delaloge
Manuscript review and approval: All authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Malone KE, Daling JR, Doody DR, Hsu L, Bernstein L, Coates RJ, et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006;66:8297–308. [DOI] [PubMed] [Google Scholar]
- [2].Kurian AW, Gong GD, John EM, Miron A, Felberg A, Phipps AI, et al. Performance of prediction models for BRCA mutation carriage in three racial/ethnic groups: findings from the Northern California Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2009;18:1084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Winter C, Nilsson MP, Olsson E, George AM, Chen Y, Kvist A, et al. Targeted sequencing of BRCA1 and BRCA2 across a large unselected breast cancer cohort suggests that one-third of mutations are somatic. Ann Oncol. 2016;27:1532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee HB, Han W. Unique features of young age breast cancer and its management. J Breast Cancer. 2014;17:301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Evans DG, Lalloo F, Howell S, Verhoef S, Woodward ER, Howell A. Low prevalence of HER2 positivity amongst BRCA1 and BRCA2 mutation carriers and in primary BRCA screens. Breast Cancer Res Treat. 2016;155:597–601. [DOI] [PubMed] [Google Scholar]
- [7].Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33. [DOI] [PubMed] [Google Scholar]
- [8].Robson M, Ruddy KJ, Im SA, Senkus E, Xu B, Domchek SM, et al. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur J Cancer. 2019;120:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Robson ME, Tung N, Conte P, Im SA, Senkus E, Xu B, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Friedlander M, Matulonis U, Gourley C, du Bois A, Vergote I, Rustin G, et al. Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. Br J Cancer. 2018;119:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee KH, Gonçalves A, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020;31:1526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.