Abstract
To determine whether the infection-preventing capability of the neutrophil-activating agent poly-(1-6)-β-d-glucopyranosyl-(1-3)-β-d-glucopyranose glucan (PGG-glucan) can be enhanced with antibiotic prophylaxis, we administered PGG-glucan and cefazolin, alone and in combination, to guinea pigs inoculated with isolates of staphylococci. Guinea pigs receiving both PGG-glucan and cefazolin had 50% infective doses that were 8- to 20-fold higher than those obtained with cefazolin alone and 100- to 200-fold higher than those obtained with PGG-glucan alone. PGG-glucan and cefazolin are synergistic in their ability to prevent staphylococcal wound infection.
Antibiotics have proven to be dramatically effective in preventing and treating bacterial infections. Nevertheless, these agents only provide support for the essential immunological functions of phagocytosis and intracellular killing. The enhancements provided by combining phagocyte-stimulating agents with antibiotics have only recently been explored (12, 18, 19). PGG-glucan (poly-[1-6]-β-d-glucopyranosyl-[1-3]-β-d-glucopyranose glucan) (Betafectin; Alpha-Beta Technology, Inc., Worcester, Mass.) is a complex carbohydrate derived from Saccharomyces cerevisiae (6). PGG-glucan primes neutrophils to exhibit greater phagocytosis and a stronger oxidative burst in response to subsequent stimulation (2, 17). While this agent has no innate antibacterial activities, it has been demonstrated to have both prophylactic and therapeutic activity in in vivo models (3, 11, 14, 17), presumably through its stimulation of polymorphonuclear activity. The present study was designed to evaluate whether the prophylactic properties of PGG-glucan could be enhanced with antibiotic prophylaxis in a guinea pig model of staphylococcal wound infection.
Staphylococcus aureus 3094, S. aureus 5030, and Staphylococcus epidermidis 9021 were recovered from wound infections complicating cardiac surgery. Previous in vitro studies have found the MICs of methicillin for S. aureus 3094 and 5030 and S. epidermidis 9021 to be 4, 32, and 16 μg/ml, respectively (11). None of these isolates are inhibited in vitro by PGG-glucan at concentrations as high as 500 μg/ml (11).
Details of the low-inoculum guinea pig model and the methods of administering PGG-glucan (Betafectin) (Alpha-Beta Technology, Inc.) and cefazolin (Eli Lilly & Company, Indianapolis, Ind.) in this model have been described previously (7–11). All in vivo experiments were approved by the institutional committee for animal care at the Nashville Veterans Affairs Medical Center. Five days prior to inoculation, after surgical and anesthetic preparation, the internal jugular veins of albino Hartley guinea pigs, 500 ± 50 g, of either sex (Kingstar, Kingston, N.H.) were cannulated with saline-filled polyethylene catheters (PE-50; Becton Dickinson and Company, Sparks, Md.). The distal end of the jugular cannula was tunneled through the subcutaneous tissue to exit the skin of the dorsal neck and clamped. After placement of the catheter, each guinea pig was housed separately.
On the day of in vivo experimentation, bacteria and sterile dextran microbeads (Cytodex; Sigma Chemical Co., St. Louis, Mo.) were mixed to prepare a range of inocula that produced abscesses from 0 to 100% at the time. When the guinea pigs were prepared for inoculation, the dorsal hair was removed and a grid designating 12 sites was drawn. The experimental design required that on the day of bacterium-microbead inoculation (experimental day 0), prepared guinea pigs first received either PGG-glucan (Betafectin lot no. 2610054), 1 mg/kg of body weight, or placebo intravenously over 2 min, followed by cefazolin, 100 mg/kg, or placebo given subcutaneously. Previous investigations have demonstrated that this dose of cefazolin produced levels in serum comparable to those achieved with a 1-g parenteral dose administered in the clinical setting (7). Immediately thereafter, the potential space between the fascia surrounding skin-related muscle groups and truncal muscle groups underlying each site was inoculated with 0.2 ml of one of the bacterium-microbead suspensions. After inoculation the guinea pigs were returned to their cages. Based on our previous report that PGG-glucan administered 1 day postinoculation is effective in preventing infection (11) and subsequent work showing an enhancement of in vivo prophylaxis related to additional postinoculation administration of PGG-glucan (data not shown), PGG-glucan was administered again at the same dose on postinoculation days 1, 2, and 3.
On day 6 following inoculation of the bacterium-microbead suspensions, the guinea pigs were sacrificed and the lesions were harvested by a sterile technique and cultured as previously described (7). Bacterial growth in samples from each site was recorded. Binary logistic regression was used to calculate inoculum-response (dose-response) curves for each staphylococcal isolate-and-prophylactic regimen combination and statistical differences by using JMP, version 3.1.6. (SAS Institute, Cary, N.C.) (13). The mean infective dose (ID50) was determined as exp(−intercept/slope of log back count). A total of 1,081 lesions among 101 animals, divided almost evenly among the 12 staphylococcal isolate-prophylactic regimen combinations, provided the data for analysis.
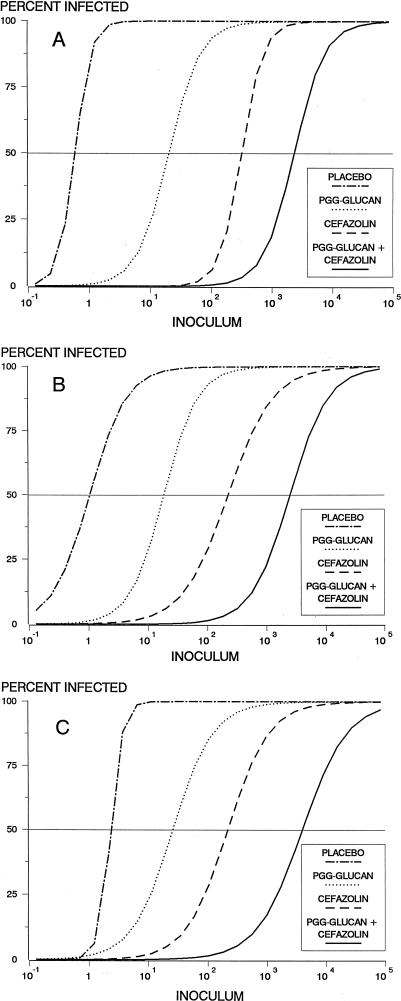
The in vivo prophylaxis studies revealed a significant association between inoculum size and subsequent infection rate after prophylaxis with placebo, PGG-glucan, cefazolin, and PGG-glucan–cefazolin for all three isolates (P < 0.01) (Fig. 1). For a given isolate the number of bacteria required to cause infection differed significantly among the four prophylactic regimens (P < 0.0001). An ID50 for each strain-prophylactic regimen combination was calculated from the logistic regression curves (Table 1). For the three strains, the ID50 was approximately a log higher for PGG-glucan than for placebo, for cefazolin than for PGG-glucan, and for PGG-glucan–cefazolin than for cefazolin. These findings were particularly impressive as all three organisms exhibit borderline or full resistance to methicillin. Importantly, the combination of cefazolin and PGG-glucan yielded an ID50 which far exceeded the ID50 of either agent used alone against the respective strains. Although standardized definitions of antibacterial synergy have yet to be fully established for in vivo studies (16), we believe that the highly significant improvements in efficacy of the PGG-glucan–cefazolin combinations, as demonstrated by the logistic regression analysis as well as by the higher-than-additive MIC results, reflect clear synergy against all three bacterial isolates used in this model (4).
FIG. 1.
Semilog graph of infection rate versus inoculum size (in CFU) for animals inoculated with strains 3094 (A), 5030 (B), and 9021 (C). The curves are idealized constructs derived from the logistic model. The ID50s were determined from the regression equation and correspond to the points at which each curve intersects a horizontal line extending from the 50% infection mark on the y axis. The ID50s are reported in Table 1.
TABLE 1.
ID50s for staphylococcal strains in guinea pigs receiving different prophylactic regimens
| Regimen | ID50 (CFU) of straina
|
||
|---|---|---|---|
| 3094 (BSSA) | 5030 (MRSA) | 9021 (MRSE) | |
| Placebo | 0.5 | 0.8 | 1.9 |
| PGG-glucan | 18 | 17 | 23 |
| Cefazolin | 315 | 220 | 212 |
| PGG-glucan–cefazolin | 2,468 | 2,642 | 4,256 |
ID50s are calculated from the results of the logistic regression equation. Abbreviations: BSSA, borderline susceptible S. aureus; MRSA, methicillin-resistant S. aureus; MRSE, methicillin-resistant S. epidermidis. For each staphylococcal strain, significant differences among the four prophylactic regimens were obtained by logistic regression analysis (P < 0.0001).
The importance of synergism between phagocyte activity and antimicrobial agents was noted early in the antimicrobial era. Alexander and Good demonstrated that when a variety of antibiotics were joined with leukocytes, a marked enhancement of bactericidal activity occurred (1). While the mechanism of such synergy has yet to be fully defined, it is known that after exposure to antibiotics, bacteria are more susceptible to phagocytosis and intracellular killing (1, 5, 15). More recently, the enhancements provided by combining granulocyte colony-stimulating factors with antibiotics have been explored. In the presence of polymorphonuclear leukocytes, these agents have been shown to act synergistically with antibiotics in both in vivo and in vitro studies (12, 19).
Prior studies in this laboratory with S. aureus 3094 have demonstrated that the addition of a second dose of cefazolin (50 mg/kg administered 2 h after the 100-mg/kg dose) or combining the cefazolin with a β-lactamase inhibitor, sulbactam, substantially raises the ID50 to 1,690 or 1,519 CFU, respectively (8). This contrasts with an ID50 of 2,468 CFU when PGG-glucan and a single dose of cefazolin are employed as the prophylactic regimen. Thus, for this strain, which exhibits borderline susceptibility to methicillin, the addition of PGG-glucan to cefazolin provides prophylactic activity which is at least equal to the activity seen when additional cefazolin or a second antibiotic is used. More importantly, its presumed mechanism of action (i.e., enhancement of phagocytic activity) suggests that the use of PGG-glucan in conjunction with antibiotics can achieve clinical outcomes which are unattainable with antibiotics alone. A better understanding of the importance of PGG-glucan in prophylaxis will await the results of ongoing clinical trials.
Acknowledgments
This work was supported by NIH AI32126 and a grant from Alpha-Beta Technology, Inc.
We thank Hiriam Gates for technical assistance.
REFERENCES
- 1.Alexander J W, Good R A. Effect of antibiotics on the bactericidal activity of human leukocytes. J Lab Clin Med. 1968;71:971–983. [PubMed] [Google Scholar]
- 2.Brunke-Reese D, Gu Y, Crotty K, Fisette L, Mackin W M. Enhanced microbicidal activities of human peripheral blood monocytes and neutrophils (PMN) after pre-treatment with PGG-glucan (Betafectin™) FASEB J. 1994;8:488. [Google Scholar]
- 3.Cisneros R L, Gibson F C, Tzianabos A O. Passive transfer of poly-(1-6)-β-glucotriosyl-(1-3)-β-glucopyranose glucan protection against lethal infection in an animal model of intra-abdominal sepsis. Infect Immun. 1996;64:2201–2205. doi: 10.1128/iai.64.6.2201-2205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fantin B, Carbon C. In vivo antibiotic synergism: contribution of animal models. Antimicrob Agents Chemother. 1992;36:907–912. doi: 10.1128/aac.36.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman H, Warren G H. Enhanced susceptibility of penicillin-resistant staphylococci to phagocytosis after in vitro incubation with low doses of nafcillin. Proc Soc Exp Biol Med. 1974;146:707–711. doi: 10.3181/00379727-146-38177. [DOI] [PubMed] [Google Scholar]
- 6.Jamas, S., D. Easson, and G. Ostroff. June 1994. U.S. patent 5,322,841.
- 7.Kaiser A B, Kernodle D S, Parker R A. Low-inoculum model of surgical wound infection. J Infect Dis. 1992;166:393–399. doi: 10.1093/infdis/166.2.393. [DOI] [PubMed] [Google Scholar]
- 8.Kernodle D S, Kaiser A B. Comparative prophylactic efficacy of cefazolin and vancomycin in a guinea pig model of Staphylococcus aureus wound infection. J Infect Dis. 1993;168:152–157. doi: 10.1093/infdis/168.1.152. [DOI] [PubMed] [Google Scholar]
- 9.Kernodle D S, Kaiser A B. Efficacy of prophylaxis with β-lactams and β-lactam–β-lactamase inhibitor combinations against wound infection by methicillin-resistant and borderline-susceptible Staphylococcus aureus in a guinea pig model. Antimicrob Agents Chemother. 1993;37:702–707. doi: 10.1128/aac.37.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kernodle D S, Kaiser A B. Comparative prophylactic efficacies of ciprofloxacin, ofloxacin, cefazolin, and vancomycin in experimental model of staphylococcal wound infection. Antimicrob Agents Chemother. 1994;38:1325–1330. doi: 10.1128/aac.38.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kernodle D S, Gates H, Kaiser A B. Prophylactic anti-infective activity of poly-[1-6]-β-d-glucopyranosyl-[1-3]-β-d-glucopyranose glucan in a guinea pig model of staphylococcal wound infection. Antimicrob Agents Chemother. 1998;42:545–549. doi: 10.1128/aac.42.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kropec A, Lemmen S W, Grundmann H J, Engels I, Daschner F D. Synergy of simultaneous administration of ofloxacin and granulocyte colony-stimulating factor in killing of Escherichia coli by human neutrophils. Infection. 1995;23:298–300. doi: 10.1007/BF01716290. [DOI] [PubMed] [Google Scholar]
- 13.McCullagh P, Nelder J A. Generalized linear models. 2nd ed. London, England: Chapman and Hall; 1989. [Google Scholar]
- 14.Onderdonk A B, Cisneros R L, Hinkson P, Ostroff G. Anti-infective effect of poly-β1-6-glucotriosyl-β1-3-glucopyranose glucan in vivo. Infect Immun. 1992;60:1642–1647. doi: 10.1128/iai.60.4.1642-1647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pruul H, McDonald P J. Damage to bacteria by antibiotics in vitro and its relevance to antimicrobial chemotherapy: a historical perspective. J Antimicrob Chemother. 1988;21:695–698. doi: 10.1093/jac/21.6.695. [DOI] [PubMed] [Google Scholar]
- 16.Renneberg, J. 1993. Definitions of antibacterial interactions in animal infection models. J. Antimicrob. Chemother. 31(Suppl. D):167–175. [DOI] [PubMed]
- 17.Stashenko P, Wang C Y, Riley E, Wu Y, Ostroff G, Niederman R. Reduction of infection-stimulated periapical bone resorption by the biological response modifier PGG glucan. J Dent Res. 1995;74:323–330. doi: 10.1177/00220345950740010701. [DOI] [PubMed] [Google Scholar]
- 18.Tzianabos A O, Cisneros R L. Prophylaxis with the immunomodulator PGG glucan enhances antibiotic efficacy in rats infected with antibiotic-resistant bacteria. Ann N Y Acad Sci. 1996;797:285–287. doi: 10.1111/j.1749-6632.1996.tb52980.x. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda H, Ajiki Y, Shimozato T, Kasahara M, Kawada H, Iwata M, Shimizu K. Therapeutic efficacy of granulocyte colony-stimulating factor alone and in combination with antibiotics against Pseudomonas aeruginosa infections in mice. Infect Immun. 1990;58:2502–2509. doi: 10.1128/iai.58.8.2502-2509.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]