Abstract
Psoriasis is an uncontrolled, long-lasting inflammatory dermatosis distinguished by thickened, erythematous, and flaky skin lesions. Massive amounts of inflammatory cytokines are produced when immune system imbalances are driven by genetic and environmental triggers. Vinpocetine (VNP), a man-made analogue of the compound vincamine found in the dwarf periwinkle herb, has robust anti-inflammatory, immunomodulatory, and anti-oxidative effects; alleviates the epidermal penetration of immune cells, such as eosinophils and neutrophils; and abolishes the generation of pro-inflammatory molecules.
Objective
This study was aimed at exploring the effects of long-term topical VNP, both alone and co-administered with clobetasol propionate, in an imiquimod-induced mouse model of psoriasiform dermatitis.
Methods
The study protocol consisted of 48 Swiss albino mice, randomly divided into six groups of eight mice each. In group I, petroleum jelly was administered daily for 8 days. In group II, imiquimod was administered topically at 62.5 mg daily for 8 days. In groups III, VI, V, and VI, 0.05% clobetasol propionate, 1% VNP, 3% VNP, and 3% VNP plus 0.05% clobetasol were administered topically for an additional 8 days after the induction, thus resulting in a total trial length of 16 days.
Results
Topical VNP at various doses alleviated the severity of imiquimod-induced psoriatic lesions—including erythema, silvery-white scaling, and thickening—and reversed the histopathological abnormalities. Moreover, imiquimod-exposed animals treated with VNP showed markedly diminished concentrations of inflammatory biomarkers, including tumour necrosis factor-α, interleukin (IL)-8, IL-17A, IL-23, IL-37, nuclear factor-kappa B (NF-κB), and transforming growth factor-β1.
Conclusion
This research provides new evidence that VNP, alone and in combination with clobetasol, may serve as a potential adjuvant for long-term management of autoimmune and autoinflammatory skin diseases, particularly psoriasis, by attenuating psoriatic lesion severity, suppressing cytokine generation, and limiting NF-κB-mediated inflammation.
Keywords: Antipsoriatic therapy, Inflammatory skin diseases, Imiquimod, Immune-mediated dermatoses, Mouse model of psoriasis, Vinpocetine
الملخص
أهداف البحث
الصدفية هي مرض جلدي التهابي غير منضبط وطويل الأمد يتميز بآفات جلدية سميكة وحمامية ومتقشرة. يتم إنتاج كميات هائلة من السيتوكينات الالتهابية عندما تكون اختلالات الجهاز المناعي مدفوعة بمحفزات وراثية وبيئية. فينبوسيتين، وهو نظير من صنع الإنسان للفينكامين الموجود في عشبة النكة القزمة، هو دواء قوي مضاد للالتهابات، ومعدل للمناعة، ومضاد للأكسدة يمكن أن يخفف من تغلغل الخلايا المناعية، مثل الحمضات والعدلات في البشرة وإلغاء الجيل من العناصر المؤيدة للالتهابات.
طريقة البحث
لاستكشاف التأثير المخفف للفينبوسيتين الموضعي طويل الأمد بمفرده أو مع بروبيونات كلوبيتاسول على نماذج الفئران التي لديها التهاب الجلد الصدفي. يتكون بروتوكول الدراسة من 48 فأرا سويسريا من النوع الأبيض، تم تجميعها عشوائيا في 6 مجموعات تضم كل منها 8 فئران. في المجموعة الأولى، تم إعطاء الفازلين يوميا لمدة ثمانية أيام. في المجموعة الثانية، تم إعطاء إيميكيمود موضعيا بجرعة 62.5 ملغ يوميا لمدة ثمانية أيام. في مجموعات العلاج 3، و 4، و 5، و6 تم إعطاء بروبيونات كلوبيتاسول 0.05٪، فينبوسيتين 1٪، فينبوسيتين 3٪، وفينبوسيتين 3٪ بالإضافة إلى كلوبيتاسول 0.05٪ موضعيا لمدة 8 أيام إضافية بعد التحريض، ليصل إجمالي طول التجربة إلى 16 يوما.
النتائج
خفف الفينبوسيتين الموضعي بجرعات مختلفة من شدة آفات الصدفية الناجمة عن الإيميكيمود، مثل الحمامي، والقشور البيضاء الفضية، والسماكة، وعكس التشوهات المرضية. علاوة على ذلك، فإن الحيوانات المعرضة للإيميكيمود والتي عولجت بالفينبوسيتين قللت بشكل كبير من تركيزات المؤشرات الحيوية الالتهابية، بما في ذلك عامل نخر الورم-ألفا، والإنترلوكين-8، والإنترلوكين-17أ، والإنترلوكين-23، والإنترلوكين-37، والعامل النووي-كابا بي، وتحويل عامل النمو-بيتا1.
الاستنتاجات
يقدم البحث الحالي دليلا جديدا على أن الفينبوسيتين وحده مع كلوبيتاسول هو مادة مساعدة محتملة للإدارة طويلة الأمد لأمراض المناعة الذاتية والالتهابات الجلدية الذاتية، وخاصة الصدفية، عن طريق تخفيف شدة الآفات الصدفية، وقمع توليد السيتوكينات، والحد من العامل النووي-كابا بي.
الكلمات المفتاحية: العلاج المضاد للصدفية, إيميكيمود, نموذج الفأر للصدفية, أمراض الجلد الالتهابية الذاتية, الأمراض الجلدية بوساطة المناعة, أمراض المناعة الذاتية, فينبوسيتين, فينكامين, العناقية الصغير-فينكا الصغرى
Introduction
Psoriasis is a long-lasting, non-contagious, incurable inflammatory disorder characterized by the presence of reddened plaques coated with silvery-white scaling on extensor surfaces, such as the elbows, knees, trunk, scalp, or lumbosacral area. The disease can also manifest as pus-filled lesions on the lips, nails, palms, or soles, or as a widespread outbreak of sterile pustules in some cases. Psoriasis is believed to affect 2–4% of people worldwide; it may appear at any age, and it substantially varies in scope and severity among seasons and individuals.1 Its incidence is associated with several other factors, including geographical region, race, sex, and environment.2 Five types of psoriasis have been recognized: guttate-type; pustular-type; erythrodermic; inverse or intertriginous; and psoriasis vulgaris or plaque-type psoriasis. Psoriasis vulgaris is most prominent and is followed by guttate psoriasis.3 Researchers have demonstrated links between psoriasis and other conditions, including diabetes, cardiac disease, ulcerative colitis, depression, and malignancy.4
The complex interplay among genetic, environmental, and immunological molecules has been implicated in the pathogenesis of psoriasis.5 Psoriasis may have an initial phase—likely to be caused by traumatic accidents (i.e., the Koebner phenomenon), infection attacks, stressful situations, drug use, or smoking—and a maintenance phase marked by continuing clinical progression.6 Psoriatic plaque histology indicates acanthosis, as a result of immune cell infiltration involving dendritic cells (DCs), macrophages, and T lymphocytes. Neovascularization is another clear characteristic.7
The pathogenesis of psoriasis includes autoimmune components, as demonstrated by the detection of autoantigen-specific T cells, which contribute to the onset and chronic persistence of the disease.8,9 LL-37, or cathelicidin, attaches to self-DNA, thereby forming LL-37-DNA complexes. Toll-like receptor (TLR)-7 and TLR-9 recognize these complexes and cause plasmacytoid DCs (pDCs) to release different subtypes of interferon (IFN)-α and IFN-β.10,11 These IFNs, via TLR8, stimulate myeloid DCs (mDCs) to produce TNF-α, IL23, and IL12, which in turn stimulate the maturation of T helper (Th) 1 and Th17 cells and diverse cytokine cascades.12,13 Consequently, extensive keratinocyte multiplication and defective differentiation, the two critical hallmarks of the disorder, result.14, 15, 16
Th17 cells and their cytokine molecules are believed to be integral in the occurrence of psoriasis. IL-17A is an essential cytokine implicated in keratinocyte overgrowth, and neutrophil and macrophage aggregation.15,17,18 Certain TLRs are typically expressed on the outer layer of DCs during infections, thus resulting in cytokine release.19,20 Imiquimod (IMQ), a TLR7 and TLR8 ligand, and a potent immune-stimulating drug, is administered locally to manage cancerous and contagious dermatological conditions, including genital and perianal warts, cutaneous basal cell cancers, and solar keratoses.21 Repeated application of IMQ triggers psoriasiform-like dermatitis in mice.22, 23 Notably, the IMQ-provoked exacerbation of psoriasis occurs not only in the affected region but also in previously unaffected distant skin sites.24 This mouse model bears numerous similarities to human psoriasis, including the proliferation of pDC; generation of type I IFN-γ, NF-κB, TNF-α, and IL-1β; and secretion of IL-17 and IL-23.24,25 Furthermore, IL-17 promotes improper keratinocyte proliferation and stimulates a network of events resulting in the release of Antimicrobial peptides (AMPs), such as cathelicidin and β-defensins, and chemotactic cytokines, such as IL-8 and IL-36, which recruit neutrophils and DCs to lesional skin and perpetuate inflammation.26,27 Increasing evidence implicates TNF-α in the emergence of IMQ-provoked psoriasis inflammation.28 TGF-β1 is a pleiotropic cytokine that is overproduced in the epidermis and serum in people with psoriasis.29,30
Psoriasis manifestations are managed through various treatment approaches, including topical therapy, phototherapy, and systemic therapy.31 Topical treatment is suitable for mild psoriasis, whereas severe or advanced forms of psoriasis may require phototherapy or systemic therapies.32,33 The use of older topical medications, such as dithranol and coal tar, is gradually declining. Salicylic acid is sometimes coupled with topical steroidal preparations. People with facial or intertriginous psoriasis are prescribed low- to moderate-potency topical steroids, such as fluocinol-one acetonide, whereas those with palmoplantar pustular psoriasis are prescribed high-potency steroids, such as clobetasol propionate (CLO). Tazarotene is often used in combination with topical glucocorticoids to alleviate stationary psoriatic plaques. Vitamin D derivatives help treat extremity lesions and palmoplantar pustulosis.34,35
Additional treatment options for psoriatic skin lesions include phototherapy, traditional systemic medications (methotrexate, cyclosporine, and acitretin), oral small-molecule drugs (dimethyl fumarate and apremilast), standard systemic drugs (methotrexate, cyclosporine, and acitretin), and oral small-molecule drugs (dimethyl fumarate, and apremilast), and injectable therapies involving biological agents.36 The widespread use of targeted biological drugs over the past 20 years has substantially enhanced therapeutic approaches to psoriasiform dermatitis and psoriatic arthritis. Before biological therapies, psoriasis treatments were restricted to oral medications, such as methotrexate, which carries more than ten “black box” warnings, several of which are catastrophic. Retinoic derivatives and cyclosporine are additional oral alternatives with black box warnings. Biological drugs surpass traditional oral systemic therapies and do not carry black box warnings.37 The biological treatment choices for severe psoriasis consist of TNF-α antagonists (e.g., infliximab, etanercept, adalimumab, and certolizumab pegol), IL-17 blockers (e.g., secukinumab, ixekizumab, and brodalumab), and the IL-12/IL-23 antagonist ustekinumab.38,39 Furthermore, spesolimab and imsidolimab, which are selective IL-36 inhibitors, have been approved as orphan drugs to treat generalized pustular psoriasis.40, 41, 42 Finally, bimekizumab, a specific blocker of IL-17A and IL-17F, and deucravacitinib, an orally bioavailable blocker of tyrosine kinase 2 (TYK2), are currently approved biologic agents. These advances in therapeutic options have added complexity to the treatment decision-making process.43,44
Vinpocetine (VNP) originates from the compound vincamine, which is present in the herb Vinca minor, often known as lesser periwinkle (Apocynaceae). VNP is also extracted from tabersonine, an alkaloid identified in Voacanga africana seeds.45 Since 1978, the vasodilatory and anti-inflammatory effects of VNP have been known to enhance functional cognition and memory among individuals with dementia and cerebrovascular ischaemia (Khalil et al., 2022). VNP's vast pharmacological uses have been a focus of comprehensive clinical investigations and practical applications. A therapeutic guideline regimen has outlined the optimal management of strokes, diabetes mellitus, epilepsy, digestive problems, glaucoma, cardiopulmonary diseases, neurological disorders, deafness, and autoimmune conditions.46,47 VNP is frequently offered as a prescription medicine under the brand names Cavinton® or Intelectol®, and as a constituent of several products for weight-reduction and muscular-building demands.48 Furthermore, accumulating evidence indicates that VNP has anti-inflammatory properties in numerous types of cells, primarily vascular endothelium cells, macrophages,49 neutrophils,50 cerebral microglia,51 astrocytes,52 and pDCs.53 This medicine has shown promising results in controlling many inflammatory disorders, notably atherosclerosis,54 pulmonary inflammation,49 retinal inflammatory disease,55 and brain ischaemic reperfusion impairment.56 In this regard, VNP decreases the activity of NF-κB and its detrimental byproducts, such as TNF-α, as demonstrated in laboratory animal experiments and/or TNF-α-driven lung inflammation, lipopolysaccharide (LPS), and Streptococcus pneumoniae-triggered otitis media.57 In addition, VNP's anti-inflammatory advantages have been verified in a forthcoming multi-centre clinical research study involving 60 patients with cerebrovascular infarction. VNP restricts the NF-κB transcriptional pathway and inflammatory cytokines, thereby improving cognitive recovery and clinical results.58 VNP also has anti-proliferative activity, by inhibiting cyclooxygenase (COX)-2 activation and decreasing the amounts of IL1β, IL-6, and IL-10 biomarkers in dimethylhydrazine-induced colorectal cancer.59 VNP promotes cell death and has anti-cancer activity via the mitochondrial pathway.60 In addition, it markedly prevents the growth of diverse malignant cells from human breasts via arresting the cell cycle in G0 and G1 phases, and mediating cell apoptosis.61 VNP additionally restores renal function, boosts antioxidant defence, and attenuates inflammation after ischaemic reperfusion injury.62, 63
Various dosage forms of VNP are available, with daily doses ranging from 15 to 40 mg.64 Given its limited water solubility and high first-pass effect, VNP has low oral bioavailability (7%) unless it is taken with meals (60% bioavailability). It has a short half-life of 1–2 h, is well absorbed by the GIT, may readily penetrate the blood–brain barrier, and may be taken three times daily. The drug's pKa of 7.31 indicates that it is weakly basic.65,66 It undergoes rapid de-esterification into apovincaminic acid along with additional byproducts in the liver, followed by renal elimination. Additionally, it exhibits characteristics of first-order pharmacokinetics with a volume of distribution of 3.2–0.9 L/kg.67, 68 VNP is generally tolerated during prolonged use, and is associated with relatively minimal adverse effects, such as nausea, headache, stomach ache, and facial flushing.69 However, topical VNP may be the most effective treatment at optimum concentrations.70
VNP's pharmacodynamic actions may stem from its ability to bind multiple receptors, enzymes, and channels, as well as its diverse range of targets and mechanisms involving blockage of voltage-dependent Na+ channels, antagonism of Ca2+/calmodulin-dependent phosphodiesterase 1 (PDE1), and coupling to peripheral benzodiazepine receptors.71 The drug also blocks voltage-gated calcium channels and glutamate receptors, enhances GABAA transmission, scavenges hydroxyl free radicals, and boosts dopamine metabolism.45 It has an excellent safety profile with low toxicity in humans, and has shown anti-inflammatory action in both laboratory settings and living organisms through several pathways, notably via diminished NF-κB and TNF-α expression.45,72 Furthermore, VNP inhibits TLR-4 and the transcriptional process of NF-κB, thus restricting the production of chemotactic cytokines.73,74 Because of its recognized safety, VNP has recently been explored for its specific medicinal properties and modes of action in many different types of cells and disease prototypes. These findings may support the use of VNP in human disease prevention and therapy.75,68 Therefore, we tested the antipsoriatic potential of VNP in a mouse model of psoriasis induced by IMQ. We examined the effects of VNP on psoriasis symptoms, including redness, flaking/peeling, and thickening/induration of the skin, as well as on histopathological changes and inflammatory biomarkers, such as NF-κB and tissue cytokine levels.
Materials and Methods
Drugs and reagents
IMQ (5% w/w) was manufactured by MEDA Pharmaceutical Co. (Sweden), and VNP was provided by MedChem Express (USA) for animal investigations. GlaxoSmithKline (UK) supplied the 0.05% CLO ointment. Chemical ingredients administered in the current study for the topical VNP formulations were obtained from standard companies. The prepared VNP (1% and 3%) was found not to cause any cutaneous irritation after application to volunteers' skin. Moreover, the formulation had excellent uniformity.
Design and evaluation of VNP emulgel
Different amounts of polymers were used to prepare the gel phase. Carbopol 940 (1%) was mixed and dissipated in distilled water under constant agitation at a reasonable speed. The pH of the solution was modified to match the pH of the skin by gradual addition of triethanolamine to a concentration of 1% in the formulation. The system was exposed to heating until a homogeneous gel-like base was obtained, cooled, and subsequently homogenized for 48 h. The oily component of the emulsion was formulated by dispersion of a specific amount of Span 20 in a certain quantity of carrier oil (coconut oil). The ethanol solution containing VNP was added to the oil phase under magnetic stirring.76 For the aqueous component, Tween 20 was dissolved in propylene glycol, propylparaben, and methylparaben in distilled water. Both component were individually heated at 75 °C. Next, the oily component was steadily blended into the aqueous component and continuously mixed until the entire oily fraction had been incorporated. Finally, an emulsified liquid containing VNP was mixed into a previously prepared Carbopol 940 gel base in a 1:1 ratio under gentle stirring.77
Properties of formulations
Emulgels can be easily prepared, have excellent spreadability, are easily removable, do not leave stains, and are inexpensive, because their production methods are simple and do not require special equipment.78 After storage at room temperature for weeks, the colour, homogeneity, consistency, and phase separation of each emulgel formula were examined to determine its stability. Centrifugation was performed to verify its physical stability, and a pH meter was used to assess the pH.79,80 The formulations with blended VNP appeared off-white, dense, and viscous, and demonstrated favourable physicochemical features, bio-adhesive properties, and rheology behaviour, as well as nearly no changes in colour, pH, and drug delivery characteristics.
Drug content
Drug content was estimated by mixture of a known quantity of gelified emulsion in phosphate-buffered sodium chloride, pH 7.4, in a cylindrical container, agitation for an adequate period to make it entirely soluble, and filtration to obtain a clear solution. A UV–visible spectrophotometer with a wavelength in the 320 nm range was used to determine the VNP content.81
Skin prick tests
The topical formulations were applied in skin prick tests on human volunteers to identify any irritation that might render them inappropriate for topical application. Each topical formula was tested for skin irritation in ten participants. After the application of VNP emulgels to a 2-inch square region encompassing the wrists on the hand, no indications of erythema, blistering, irritation, burning, or itching were observed.82
Design of animal experiments
Al-Nahrain University's College of Medicine's Institutional Review Board authorized the proposed research protocol. The research investigation was conducted from September 2021 to December 2022. The Al-Nahrain University Centre for Biotechnology Research provided model mice 9–11 weeks of age with a weight range of 27–40 g. The mice had been housed in polypropylene boxes at 25 °C, and given access to pelleted feed and water ad libitum. Under the framework of the current study, a total of 48 albino mice were divided into six groups of eight animals each. The hair of the dorsal mouse skinfold was removed with an electric shaver 2 days before the commencement of the investigation. Subsequently, a depilatory lotion was applied to eliminate any remaining strands of hair. The experimental groups were as follows.
Group I (negative control group): An 8-day regimen of medicinal petroleum jelly was applied.
Group II: Induction (or IMQ) group: IMQ cream was topically applied at an average dosage of 62.5 mg each day, which is equivalent to an ordinary 5% strength, for a total of eight successive days.25 Mice were received a topical vehicle solution 1 h before IMQ administration.
Group III (positive control group): CLO ointment at a strength of 0.05% was topically applied twice daily for an additional 8 days after the initial induction.
Group IV (1% VNP group): After the initial induction, 1% VNP emulgel was applied topically twice daily for 8 days.
Group V (3% VNP group): After the initial 8-day induction phase, 3% VNP emulgel was topically applied twice daily for a further 8 days.
Group VI (CLO + VNP group): A combination of 0.05% CLO ointment and 3% VNP emulgel was independently applied twice per day for a total of 8 days beyond the original 8-day induction period.
The entire trial period lasted 16 days.
Evaluating the severity of psoriasis-associated lesions
Clinical parameters were tracked and scored throughout the study. Epidermal thickness, redness, grey peeling of the exposed dorsal skinfolds, and ear thickening were measured daily for the 16 days of the experiment and used to compute the PASI, which estimates the severity of psoriatic inflammation. The following criteria were assessed: scaling, erythema, and epidermal thickening on a rating system ranging from 0 to 4 (0, none; 1, slight; 2, moderate; 3, marked; 4, very marked). A grading system involving red blots was used to establish the level of erythema present (0 = no lesion; 1 = slightly pink; 2 = pink; 3 = red; 4 = dark red). The total score (erythema/redness plus scaling/desquamation plus thickening/induration) was used to assess the intensity of psoriatic plaques (scale of 0–12).25 Epidermal thickening on the right ear was determined on two separate occasions on the indicated dates, with digital micrometre callipers. The increase in ear thickness indicated the extent of inflammation.24 Skin lesions were documented each day with photographs, beginning on day 1 after IMQ cream application, and ear thickening was measured.
Tissue handling and specimen preparation
After receiving the last topical dose on day 16 of the experiment, the mice were euthanized. Tissue specimens were collected and processed into homogenates for use in biomarker assays, and haematoxylin and eosin staining of histopathology slides.25
Preparation of skin tissue homogenates
A quantity of 1 g of newly excised dorsal skin was preserved in a solution of 9 mL of phosphate-buffered saline (pH = 7.2). After homogenization by grinding with a mortar and pestle, the tissues were centrifuged at 5000 rpm for 10 min at a cold setting. For further analysis, the remaining supernatants were flash-frozen at a temperature of −80 °C.25
Histological examination
According to standard methods, tissue samples obtained from multiple groups of mice were preserved in a solution of 10% neutralized buffered formalin before being subjected to paraffin fixation. Sections of tissues were made by cutting the paraffin-embedded specimens and staining them with eosin and haematoxylin. Samples were then examined under a microscope and graded on a scale of 0–10 according to Barker's scoring method to determine the presence of any pathologic alterations.83
Biomarker data analysis
A sandwich enzyme-linked immunosorbent assay (ELISA) was used to measure IL-8, IL-17A, IL-37, IL-23, TGF-β1, and TNF-α biomarker concentrations in tissues, according to the manufacturer's instructions (MyBioSource). An ELISA developed by MyBioSource was also used to measure the presence of NF-κB, with a specialized antibody targeting the specific protein.84 Anti-interleukin antibodies were coated on 96 well plates. Afterward, multi-biotin molecule-labelled antibodies specifically targeting the indicated cytokines were used for detection. Standards and test specimens were placed in the designated wells and subsequently washed with a detergent buffer. After the addition of HRP-streptavidin, any remaining conjugates were eliminated with lysis buffer. HRP enzymatic activity was observed by using TMB substrates. The HRP facilitated TMB activation, thus yielding a soluble blue product. Subsequently, the application of an acidifying stop solution caused a colour change from blue to yellow. The intensity of the yellow colour corresponded to the concentration of a particular cytokine.85
Basic statistics for data analysis
The data sets were entered in the latest version of SPSS 24, a statistical program designed for social scientists. The descriptive statistical analysis included mean ± standard deviation (SD). The data were displayed in charts and tables, and subjected to the relevant statistical testing. One-way analysis of variance (ANOVA) analysis was used for more than two means, and a posthoc test (Tukey HSD test) was used to compare two means. In every statistical analysis, the significance threshold was p ≤ 0.05.86
Results
Effects of investigated drugs on PASI scores
Eight days after IMQ application, all mice, in contrast with the healthy controls, developed classical symptoms of psoriasiform dermatitis, including redness, thickened epidermis (acanthosis), inflammatory infiltration, and scaly skin. Erythema and induration became apparent on the second day, and gradually increased in severity until the eighth day. Scaling (desquamation) started on the fourth day, progressively intensified, and eventually peaked on the eighth day. By the eighth day, the desquamation resembled flagstone mosaics, with dome patterns visible on the layers of the skin. Group II (IMQ) showed significantly greater erythema, epidermal back thickness, grey or silvery scaling, and ear thickness than the healthy controls.
However, the PASI levels for redness, scaling, induration (thickening), and the final cumulative scores of animals with topically applied 1% or 3% VNP alone, 0.05% CLO, or a combination of both (groups III, IV, V, and VI, respectively) gradually decreased over time, particularly with respect to those in group II.
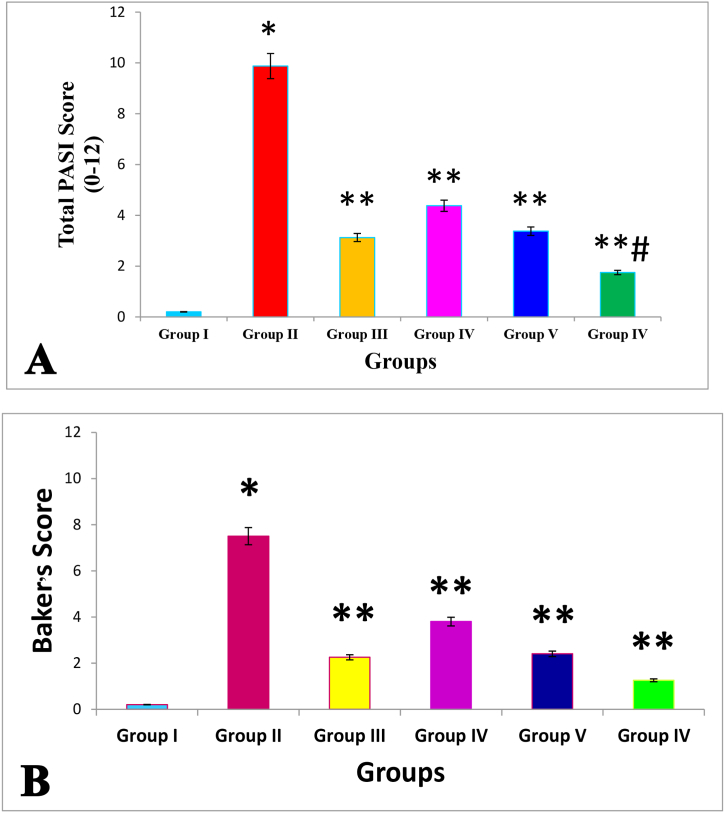
Figure 1, Figure 2 show different types of psoriatic lesions and levels of erythematous papules induced by IMQ. Figure 3 illustrates the intensity scores of cutaneous plaques associated with psoriasis in various groups of mice. Figure 4 depicts the effects of the investigated drugs on redness/erythema, induration/thickening, and scaling/desquamation. Figure 5 demonstrates the effects of the examined pharmaceutical agents on the final PASI rating scores after a continuous 16-day treatment regimen.
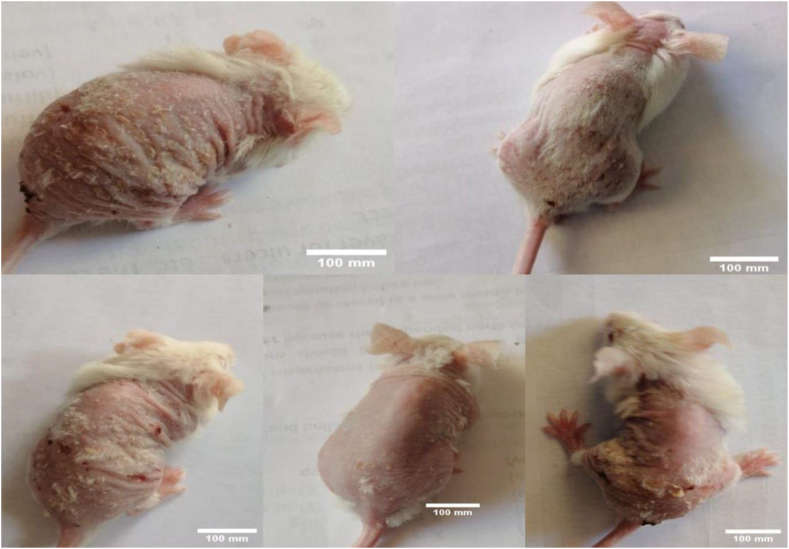
Figure 1.
Severity of imiquimod-triggered psoriasiform dermatitides. Scale bars = 100 mm.
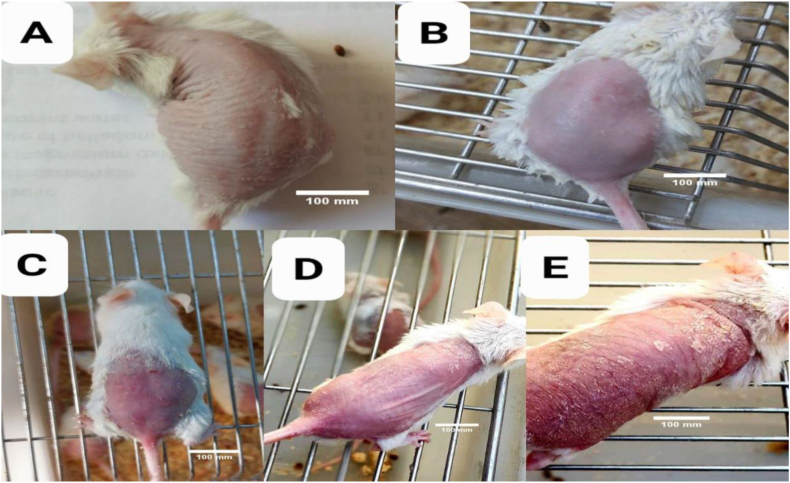
Figure 2.
Degree of imiquimod-induced erythema. A: No lesion (0 days), B: slightly pink (2nd day), C: pink (4th day), D: red (6th day), E: dark red (8th day). Scale bars = 100 mm.
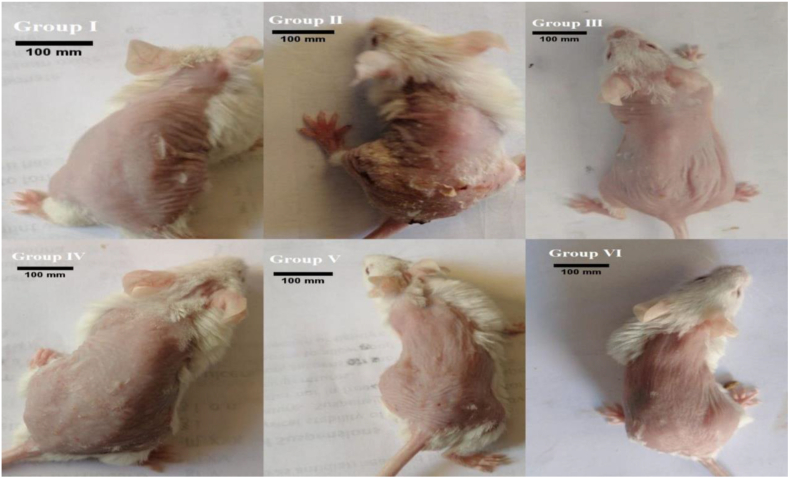
Figure 3.
Severity scores of psoriasiform skin lesions in mouse groups. Scale bars = 100 mm.
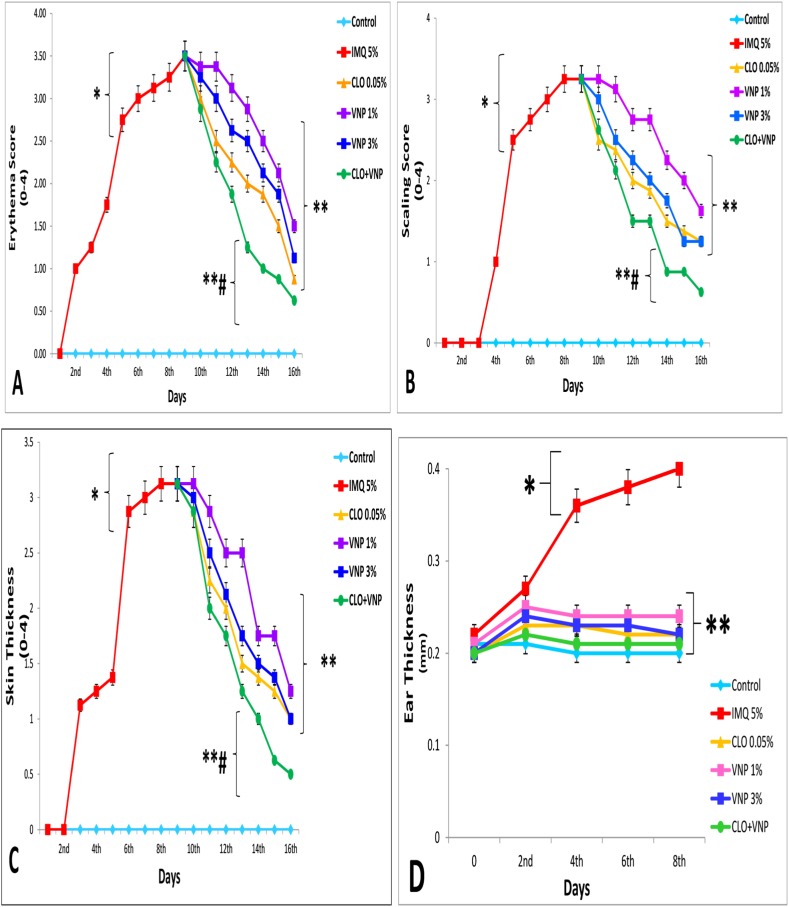
Figure 4.
A: Effects of the investigated drugs on erythema. B: Effects of the investigated drugs on scaling. C: Effects of the investigated drugs on dorsal skin thickness. D: Effects of the investigated drugs on right ear skin thickness. Data are presented as mean ± SD. IMQ = imiquimod, CLO = clobetasol, and CLO + VNP = clobetasol plus vinpocetine. ∗p < 0.001 compared with healthy controls; ∗∗p < 0.01 compared with the IMQ 5% induction group; #p < 0.05 compared with the 0.05% CLO group, n = 8.
Figure 5.
A: Effects of the investigated drugs on total cumulative PASI scores after a continuous 16-day therapeutic regimen. B: Effects of the investigated drugs on Baker's scores after a continuous 16-day therapeutic regimen. Data are presented as mean ± SD. ∗p < 0.001 compared with healthy controls; ∗∗p < 0.01 compared with the IMQ 5% induction group; #p < 0.05 compared with the 0.05% CLO group, n = 8.
Most mice in groups IV and V showed a high degree of tolerance towards the recommended dosages of the topically applied VNP-containing emulgel formulation, thus suggesting that VNP has a favourable safety profile.
Unexpectedly, when VNP, CLO, or combinations thereof, were administered to IMQ-exposed animals, the psoriatic skin changes were markedly ameliorated. Topical VNP at 1% and 3% doses in the VI and V groups, respectively, led to a skin morphology similar to that in the mice administered CLO ointment in group III. However, mice receiving combined topical application of VNP and CLO in group VI, in comparison with the 0.05% CLO group, had smoother skin texture, thinner dorsal and ear skin, less visible erythema, and little or no scaling (Figure 3, Figure 4). Group V, which received a higher dose of VNP than group IV, showed considerably less visible erythema, thinner dorsal and ear skin, less silvery white scaling, and lower cumulative PASI scores than observed in group IV. However, no substantial differences in these psoriatic skin lesions were observed between groups III and V. Meanwhile, group V showed markedly fewer psoriatic lesions than group III. Therefore, as shown in Figure 3, a prototype of IMQ-induced psoriasiform dermatitis in mice responds well to a combination of VNP and CLO, and thus may offer greater anti-inflammatory and immunosuppressive benefits than CLO alone.
Analysis of biomarker concentrations in skin tissues
In this investigation, 48 albino mice were divided into six groups with eight mice each. We calculated the concentrations of numerous inflammatory markers (TNF-α, IL-17A, IL-23, IL-8, TGF-β1, and NF-κB) in tissue biopsy specimens by using ELISA.
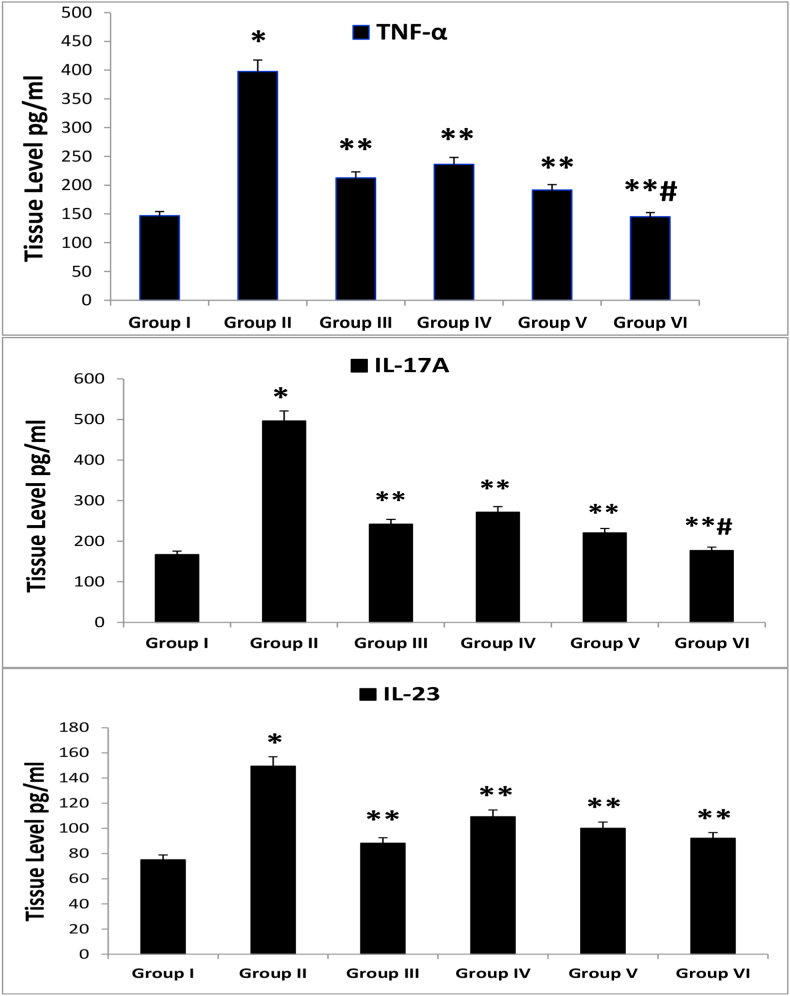
Group II (induction group) had significantly greater tissue concentrations of inflammatory molecules (TNF-α, IL-17A, and IL-23) than group I (p < 0.001). However, groups III (0.05% CLO), IV (1% VNP), V (3% VNP), and VI (CLO + VNP) had significantly lower concentrations of TNF-α, IL-17A, and IL-23 than group II (p < 0.01). Moreover, the tissue concentrations of TNF-α and IL-17A were considerably lower in group VI than group III (p < 0.05), although no substantial difference in IL-23 was found between groups III and VI (p > 0.05) as shown in Figure 6.
Figure 6.
Mean tissue concentrations of TNF-α and IL-17A. IMQ = imiquimod, CLO = clobetasol, VNP = vinpocetine, CLO + VNP = clobetasol plus vinpocetine. Data are shown as mean ± SD. ∗p < 0.001 compared with the control group; ∗∗p < 0.01 compared with the IMQ induction group; #p < 0.05 compared with the 0.05% CLO group, n = 8.
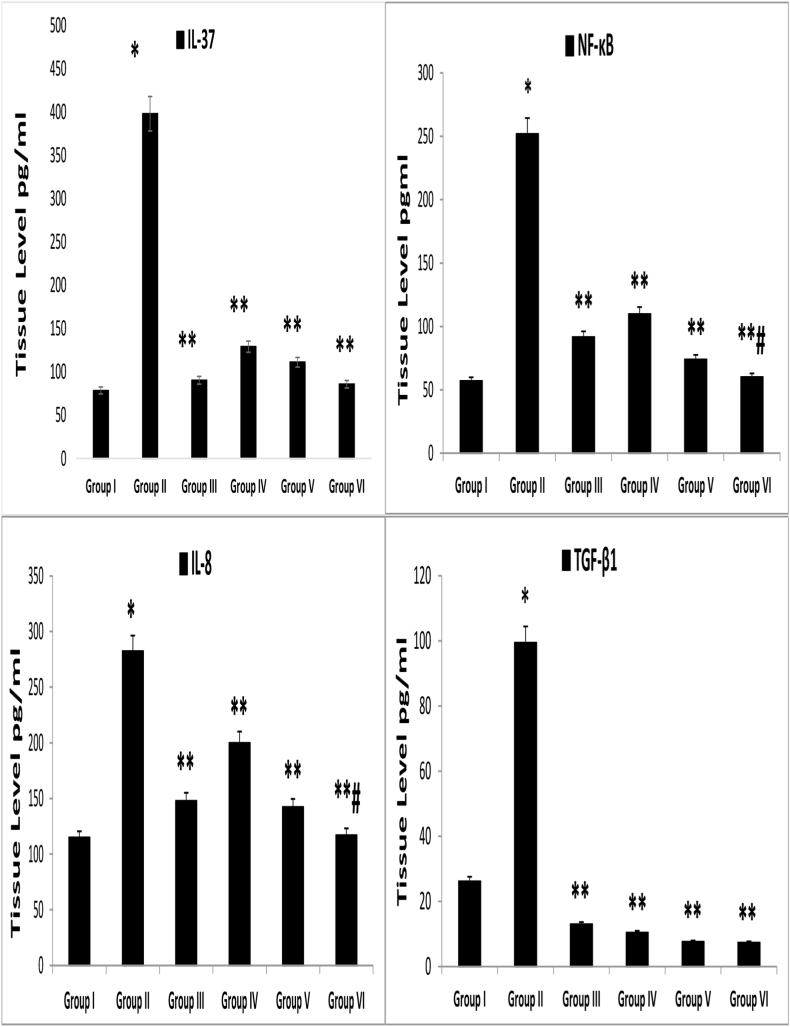
Likewise, the current results indicated that the mice in group II, compared with the healthy mice in group I, had significantly greater tissue scores of the chemokine IL-8, immunoregulatory molecules IL-37 and TGF-β1, and the inducible transcription factor NF-κB (p < 0.001). Meanwhile, in comparison with model group II, groups IIII (0.05% CLO), IV (1% VNP), V (3% VNP), and VI (CLO + VNP) had significantly lower concentrations of IL-8, IL-37, TGF-β1, and NF-κB (p < 0.01). Moreover, the IL-8 and NF-κB tissue concentrations were considerably lower in group VI than group III (p < 0.05). Nonetheless, as shown in Figure 7, no significant correlations in IL-37 or TGF-β1 were observed between groups III and VI (p > 0.05).
Figure 7.
Mean tissue concentrations of IL-8, NF-κB, and TGF-β1. IMQ = imiquimod, CLO = clobetasol, VNP = vinpocetine, CLO + VNP = clobetasol plus vinpocetine. Data are shown as mean ± SD. ∗p < 0.001 compared with the control group; ∗∗p < 0.01 compared with the IMQ induction group; #p < 0.05 compared with the 0.05% CLO group, n = 8.
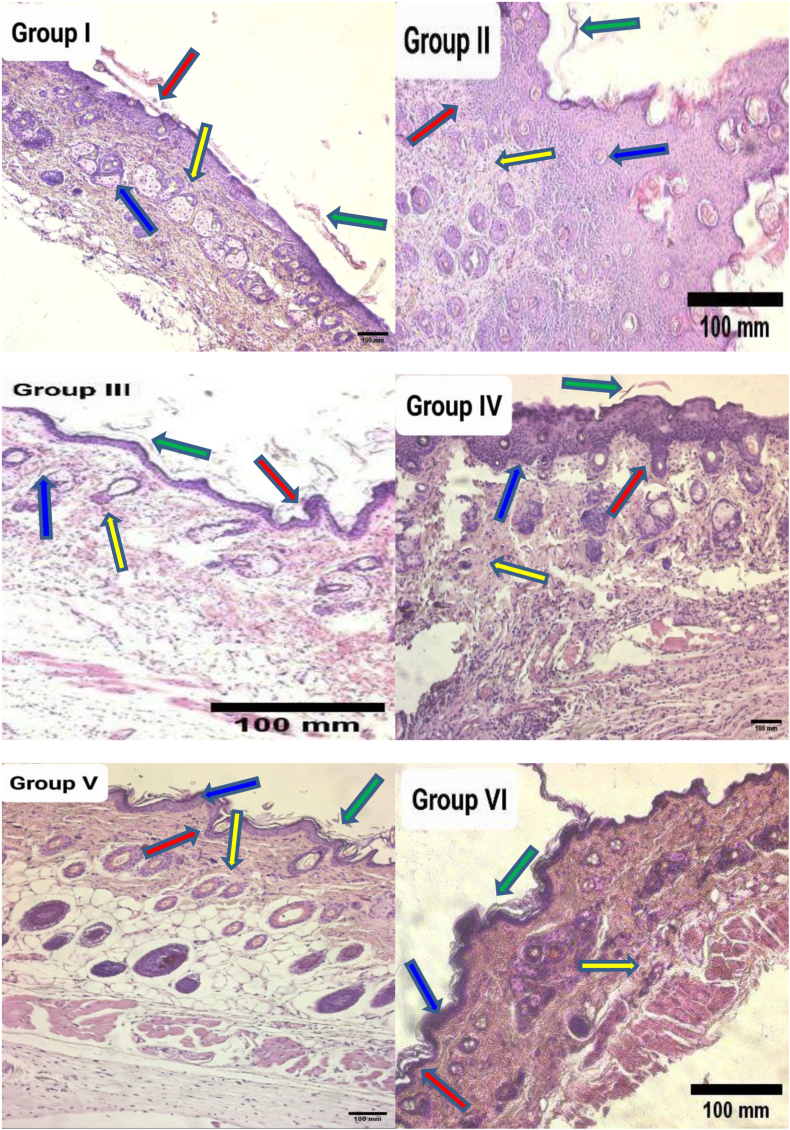
Effects of the investigated drugs on histopathological alterations
The dorsal skin biopsies from multiple mouse groups underwent haematoxylin and eosin staining. The scoring system developed by Barker was used to examine the relevance of the histological differences among experimental animal groups. Group I mice (healthy controls) had normal skin architecture: the keratin skin layers, the outermost layer of the dermis, sebaceous glands, and hair-growing follicles of the healthy controls all appeared normal under a microscope (Figure 8). In contrast to the controls (group I), group II (IMQ group) showed a thicker cutaneous layer and downward stretching of the skin. In addition, IMQ caused notable histopathological changes (p < 0.05) indicated by skin barrier breakdown, a Munro abscess, marked hyperkeratosis, parakeratosis, acanthosis, aberrant epidermal thickness, epidermal atrophic changes, upstream papillary dermis, the appearance of rete processes, and prominent lymphocyte aggregation and papillary congestion, as shown in Figure 8. Simultaneously, VNP therapy significantly ameliorated psoriatic condition measures, such as skin thickness. However, the histological features of groups IV, V, and VI were notably devoid of Munro abscesses, dermal congestion, hyperkeratotic lesions, parakeratotic pustulosa, acanthotic changes, rete pegs, and lymphocytic infiltration, similarly to observations in the negative control group, as shown in Figure 8. CLO plus 3% VNP in group VI resulted in significantly greater protection and restoration than observed in group II (p < 0.05). Importantly, VNP decreased CLO-induced atrophy and other skin-associated adverse reactions.
Figure 8.
Histological skin sections of mouse groups stained with haematoxylin and eosin at 10× magnification. Group 1 (control) showed normal skin structures, including the keratin skin layers (yellow line), epidermis (red line), dermis (green line), and sebaceous glands (blue line). Group II (IMQ group) tissue demonstrated hallmarks of psoriasis, including marked epidermal inflammatory processes (yellow line), prominent rete processes thinning (red line), intense hyperkeratotic lesions (green line), and marked acanthotic alterations (blue line). Group III (CLO group) showed mild psoriasis inflammation (yellow line), moderate rete peg lengthening (red line), mild hyperkeratotic lesions (green line), and mild acanthotic changes (blue line). Mice subjected to 1% VNP (group IV) exhibited modest inflammation (yellow line), mild extension of rete pegs (red line), minor hyperkeratotic lesions (green line), and modest acanthotic modifications (blue line). Group V (3% VNP) mice had mild skin inflammation (yellow line), absence of rete ridge processes (red line), absence of hyperkeratotic signs (green line), and absence of acanthotic alterations (blue line). Mice in group V (CLO + VNP) displayed modest epidermal inflammation (yellow line), absence of rete ridge processes (red line), absence of hyperkeratotic signs (green line), and a lack of acanthotic changes (blue line).
Discussion
The principal goal of this investigation was to determine the medicinal prospects of VNP in treating psoriasis in a mouse model induced by IMQ. This goal was achieved by analysis of VNP's effects on psoriasiform-like skin lesions, characterized by erythema/redness, silvery-white scaling, and skin thickening/induration; NF-κB and Th1 and Th17 cytokine concentrations in skin tissue; and histopathological changes. IMQ-induced psoriasis is the mouse model most frequently used in research on molecular mechanisms, antipsoriatic drug discovery, and transdermal drug uptake.87,88 In agreement with previous research findings, daily IMQ application to dorsal skin in mice for 8 days elicited psoriasiform lesions with erythema, scaling/desquamation, and epidermal thickening/induration, as well as increased production of pro-inflammatory molecules.25,89, 90 According to prior research, erythema alludes to the extent of epidermal vasodilation exacerbated by DCs, mast cells, and keratinocytes, which release inflammatory chemicals including TNF-α, NO, and histamine. Skin becomes inflamed and thickened as a result of the multiplication of keratinocytes initiated by pro-inflammatory cytokines. A failure of keratinocytes to correctly differentiate results in the accumulation of grey or silvery skin flakes.90, 91
Topically applied corticosteroids are currently the treatment of choice for psoriasis. CLO, a prednisone predecessor, is the most frequently recommended steroidal antipsoriatic medication.92,93 A recent study has indicated notable improvements in the unique characteristics of psoriasis-associated lesions with CLO treatment. These positive outcomes may potentially be attributed to the medication's anti-inflammatory, immunosuppressive, apoptotic, vasoconstricting, and anti-proliferative activities.88,94,95 Topically applied CLO appears to mitigate psoriasiform dermatitis via promoting vasoconstriction, decreasing the synthesis of phospholipase A2, hindering the stimulation of immune system cells and consequent cytokine discharge, and obstructing DNA and signalling components such as NF-κB.88,96,97
Although topical steroids are the preferred treatment for psoriasis, their excessive use is associated with many deleterious skin effects, including telangiectasis, hypertrichosis, striae, rosacea, impaired wound healing, and acne exacerbation.98,99 Additionally, the application of topical corticosteroids, particularly CLO, may lead to dermal atrophy, owing to a deficit in TGF-β-induced collagen formation.100 Another drawback of topical steroids is the potential for an intense rebound reaction in the event of sudden withdrawal of therapy.101 Therefore, the exploration of unique psoriasis treatments capable of counteracting steroid-induced adverse effects is urgently needed102, 103. This research examining the efficacy of VNP as a topical agent was undertaken because of the need to investigate novel topically applied medicines with minimal adverse effects and promising outcomes for the treatment of psoriasis.
VNP is an innovative anti-inflammatory agent enabling novel strategies for the amelioration of NF-κB-triggered immune-related and inflammatory events. VNP suppresses monocytic cell adherence and chemotaxis during inflammation.49,104 The combination of VNP and its derivatives with other immunosuppressive medicines, particularly corticos-teroids, has been documented in pharmaceutical formulations.105 In light of these investigations, the co-administration of VNP and steroidal dexamethasone has been observed to ameliorate the synthesis of TNF-α, IL-8, IL-20, and IFN-γ, and to modulate the activity of TLR2 and TLR4, thus offering greater immunosuppressive, anti-inflammatory, and anti-oxidative benefits than corticoste-roids alone, and ameliorating mental deterioration in individuals with radiation-damaged nasopharyngeal cancer.106
Herein, VNP at two strengths (1% and 3%) in combination with CLO attenuated the severity of lesional psoriasis of erythema, scaling, and skin thickening; diminished NF-κB and Th1 and Th17 cytokine concentrations in dermal tissue; and restored normal histopathology of the epidermal layers. These improvements may be attributable to a vast array of anti-inflammatory, antioxidant, antifibrotic, immunomodulating, anti-proliferating, and PDE-inhibiting character-stics.107
VNP has recently been demonstrated to achieve excellent neuro-protective, cardio-protective, hepato-protective, antithrombotic, anti-ageing, and anti-fibrotic outcomes.107,108 VNP can repair and sustain the skin's defensive barriers in addition to treating immune-mediated diseases. According to Xiao-Xiao et al. (2013), VNP enhances the survival of random skin flaps, diminishes the necrotic area, and encourages neovascularization.109 Another study has reported that VNP restores natural skin morphology in carrageenan-induced paw skin, possibly by inhibiting tissue oedema, congestion, immune cell invasion, NF-κB expression, and the generation of detrimental inflammatory molecules comprising TNF-α, myeloperoxidase, COX-2, and prostaglandin (PGE)-2.110 This agent also relieves pain associated with inflammatory conditions by eliminating NF-κB activation, paw skin cytokine generation, and cellular oxidative damage.111 Additionally, the medication accelerates wound healing in diabetic rat skin and regenerates the skin's structural features, by decreasing epidermal thickness, renewing dermal granulation, and increasing collagen fibre formation.112 Interestingly, IMQ in psoriatic lesions activates pDCs, which in turn respond to TLR7 or TLR9 by releasing large quantities of IFN-α, thereby inducing psoriasis.113 Given that VNP markedly blocked TLR9 signalling, attenuated pDC activation, and downregulated the expression of CD40, CD80, and CD86 on pDCs, thus decreasing generation of IL-12p40, TNF-α, and IL-6, VNP might potentially be used to treat immune-mediated diseases involving pDCs, such as psoriasis.53 Moreover, immune cell-targeted therapies have yielded encouraging results in the treatment of psoriatic inflammation, notably decreasing Th17/Th1 lymphocytes and increasing Treg cells, and thus significantly ameliorating psoriasis.114,115 In addition, neutrophils, another immune cell type, contribute to psoriatic skin lesions by favouring inflammation and its harmful consequences.116,117 VNP has also been found to alleviate LPS-challenged neutrophil trafficking by impeding the activity of NF-κB target genes, specifically the chemotactic cytokine peptides TNF-α and IL-33.118 According to a more recent study, the medication downregulates the influx of neutrophils, macrophages, mononuclear cells, and total leukocytes into the skin.111 Nevertheless, Kang et al. (2022) have reported that topical VNP substantially mitigates itching, induration, peeling, and reddened skin in mice with atopic dermatitis, by ameliorating inflammatory cell influx into the skin, and decreasing plasma levels of immunoglobulins (IgE and IgG) and inflammatory cytokines (IL-13 and IL-4); moreover, VNP's ability to alleviate tissue eosinophilia was considered responsible for the clear decrease in the epidermal thickening of dorsal skin tissues.70 In another study, inhibition of tissue eosinophil infiltration as well as IL-13 and IgE levels was ameliorated by VNP in a mouse model of allergic inflammation.119 Similarly, promising evidence has indicated that VNP might alleviate autoimmune ulcerative colitis in rats by hindering the extent of mucosal damage and the secretion of pro-inflammatory molecules.48 Furthermore, VNP attenuates myelin expression and oligodendroglial cell maturation in individuals with autoimmune-related multiple sclerosis.120 When used as a PDE inhibitor, VNP has fewer adverse effects than PDE4 inhibitors.121,122
More importantly, potent antioxidants that eliminate multiple types of free radicals may be an effective treatment for psoriatic inflammation.123 VNP has remarkable antioxidant capacity, thereby preventing the formation of free radicals in macrophages and neutrophils.50 It decreases concentrations of malondialdehyde and inducible NO synthase in the CNS while increasing superoxide dismutase activity, neurotransmitter levels, glucose utilization, and oxygen uptake.124 The drug also mitigates oxidative stress by decreasing lipid peroxidation; restoring the endogenous antioxidant defence; and scavenging singlet oxygen, superoxide, and hydroxyl radicals.111,125 Anti-inflammatory agents and oxidative stress biomarkers offer exciting therapeutic alternatives for psoriasis.126 Similarly, VNP markedly decreases TNF-α in neural tissue in mice with paraquat-induced parkinsonism.127 The drug also has been found to decrease inflammation in a murine prototype of recurring otitis media. VNP's ability to increase cylindromatosis, a key inflammatory suppressor, and block MAPK and ERK activity, thereby decreasing S. pneumoniae-induced inflammation, clarifies these findings.128 Likewise, Zhou and colleagues have found that VNP decreases levels of TNF-α and IL-6 cytokine peptides in murine microgliocytes challenged by LPS.129 Notably, the medication suppresses the mRNA levels of TLR4 and TLR2 and their key signalling molecules, including the mitogen-activated protein kinase (MAPK) cascade and MYD88.130 Moreover, VNP has been found to prevent SARS infection and COVID-19 via modulating IL1β, IL-8, IL-12, and TNF-α/MAPK/NF-κB transcription pathways.131 Remarkably, VNP has been reported to alleviate the biological activities of TGF-β, phospho-SMAD2/SMAD3, and PDE-1 in an animal model of diabetes-related cardiomyopathy.132 Adalimumab and cyclosporine, drugs used to treat psoriasis, are likely to exert their medicinal benefits by inhibiting the signaling pathway of TGF-β.133 Corroborating the above findings, VNP has been found to inhibit TGF-β-mediated fibroblast activation and matrix gene expression,134, 135 and to block TNF-α, IL-1β, and IL-33 secretion in LPS-challenged murine models.50 Important implications in controlling NF-κB signalling may be drawn on the basis of VNP's ability to mitigate brain ischaemic damage via substantially hindering NF-κB/MyD88/TLR4 activation.136
In contrast, calcium/calmodulin-dependent protein kinase has been reported to drive IMQ-induced psoriatic disease via dermal macrophages and epidermal keratinocytes in mice.137 Recently, signal transducer and activator of transcription 3 (STAT3) has been recognized as an essential factor triggering the onset of psoriasis and various inflammatory dermatoses.138 Thus, VNP may ameliorate psoriasis-like dermatitis via decreasing the activity of protein kinase and STAT3.61 Given that IL-23 activity largely depends on the Janus kinase (JAK) class of tyrosine kinase proteins (TYK), JAK inhibition may be an attractive therapeutic option for psoriasis.139 JAK1, JAK2, and TYK2 phosphorylate STAT proteins, thus resulting in improper gene transcription that is visible in psoriatic lesions.140 VNP has been found to hinder JAK2 and STAT3 phosphorylation, and therefore serves as a potent STAT3 antagonist, which is believed to affect the generation of inflammation-associated cytokines such as IFN-α, IL-6, and IL-10.141 Finally, VNP acts as a PDE-1 blocker, thus resulting in destruction of cGMP and cAMP131 The PDE enzyme is abundantly distributed in keratinocytes, T lymphocytes, and DCs, and is overexpressed in lesional psoriasis. PDE inhibition increases cGMP and cAMP levels, and attenuates psoriasiform-like lesions by decreasing key immunostimulatory molecules, notably IL-17, IL-23, and TNF-α.142, 143, 144
VNP also hinders the phosphorylation of JAK2 and STAT3 and the secretion of IL-6, IL-10, and IFN-α in adipose tissue.141 The medication also exhibits anti-proliferative activity, as indicated by its blockade of COX-2 activation, and diminished amounts of IL1β, IL-2, IL-6, and IL-10; therefore, it may serve as a useful agent in preventing the growth of colorectal cancer, potentially because of its antioxidant and anti-inflammatory abilities.59
Our results, together with those from other studies in clinical settings, suggest that VNP has favourable tolerance, remarkable safety, and no adverse effects during prolonged treatment.64,67,70
As far as we know, this present study represents the first investigation into the potential anti-inflammatory effects of VNP in experimental animal models of psoriasis. Nevertheless, this investigation had several limitations. First, although diminished TGF-β1, TNF-α, IL-23, IL-17A, and IL-8 cytokine levels, together with NF-κB signaling pathway, were observed in back skinfold specimens of mice receiving VNP (1% and 3%) alone and in combination with CLO, immune system cell phenotypes, such as splenocytes, and drainage of lymphatic nodes were not examined. Prospective studies are necessary to further understand the effects of VNP in controlling immune system responses in vivo. Second, in the current analysis, the effects of VNP on psoriasis were examined, but its exact mode of action was not determined. The recognition of multiple targets for VNP necessitates further investigation to clarify the mechanisms underpinning its anti-psoriatic effects. Third, activated STAT3 signalling may participate in Th17 survival and differentiation; however, we were unable to confirm additional potential pharmacological actions of VNP, such as anti-oxidant effects, suppression of STAT3 stimulation, or anti-inflammatory effects, which may either entirely or partially govern Th17 development. Finally, whereas VNP ameliorated aspects of psoriasis skin lesions, such as redness/erythema, flaking/desquamation, and epidermal induration or thickening in mice, our study did not involve clinical examination; therefore, further evidence from biological patient collections or clinical trials is required for assessing and establishing the medical benefits of VNP.
Conclusions
The current study demonstrated that VNP, alone or in combination with CLO, successfully attenuated psoriasis-linked lesions, decreased tissue concentrations of the TNF-α, IL-8, IL-17A, IL-23, IL-37, and TGF-β1 cytokines; and limited NF-κB-triggered inflammatory cascades. Therefore, VNP may be an attractive candidate for future research on the prolonged management of immune-related inflammatory skin conditions involving psoriasis.
Data availability statement
The dataset corroborating the outcomes of the current investigation is available from corresponding author on reasonable request.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
We have no conflicts of interest to declare.
Ethical approval
The institution's approving council at the College of Medicine at Al-Nahrain University evaluated the study and approved the latest instalment. The research was performed according to the Declaration of Helsinki guidelines for ethics. A regional ethical commission assessed the study protocol, participant data, and consent documentation according to document 1025 on 11 April, 2021, and subsequently provided study authorization.
Authors contributions
HRS planned and devised the study's framework, performed the investigation, supplied study resources, and assembled and arranged the findings. AAA analysed and evaluated data. AHA wrote the original manuscript and offered logistical assistance. HAM was accountable for the official evaluation, methods, resources, and development of software. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgement
The research results were gathered as part of a doctoral dissertation submitted to the Department of Pharmacology at Al-Nahrain University's College of Medicine.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Papp KA, Gniadecki R, Beecker J, Dutz J, Gooderham MJ, Hong C-H, et al. Psoriasis prevalence and severity by expert elicitation. Dermatol Ther. 2021;11(3):1053–1064. doi: 10.1007/s13555-021-00518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. doi: 10.1136/bmj.m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medovic MV, Jakovljevic VL, Zivkovic VI, Jeremic NS, Jeremic JN, Bolevich SB, et al. Psoriasis between autoimmunity and oxidative stress: changes induced by different therapeutic approaches. Oxid Med Cell Longev. 2022;2022:2249834. doi: 10.1155/2022/2249834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, van Voorhees AS, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–390. doi: 10.1016/j.jaad.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou A., Kong Q., Sang H. Identification of key apoptosis-related genes and immune infiltration in the pathogenesis of psoriasis. Hereditas. 2022;159(1):26. doi: 10.1186/s41065-022-00233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari D, Casciano F, Secchiero P, Reali E. Purinergic signaling and inflammasome activation in psoriasis pathogenesis. Int J Mol Sci. 2021;22(17):9449. doi: 10.3390/ijms22179449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz-Lopez L.I., Choudhary V., Bollag W.B. Updated perspectives on keratinocytes and psoriasis: keratinocytes are more than innocent bystanders. Psoriasis (Auckl) 2022;12:73–87. doi: 10.2147/ptt.S327310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schön M.P. Adaptive and innate immunity in psoriasis and other inflammatory disorders. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.01764. 1764–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chekol Abebe E, Tilahun Muche Z, Behaile TMA, Mengie Ayele T, Mekonnen Agidew M, Teshome Azezew M, et al. Role of Fetuin-A in the pathogenesis of psoriasis and its potential clinical applications. Clin Cosmet Invest Dermatol. 2022;15:595–607. doi: 10.2147/CCID.S356801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T., Yamasaki K. Psoriasis and antimicrobial peptides. Int J Mol Sci. 2020;21(18):6791. doi: 10.3390/ijms21186791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian D., Lai Y. The relapse of psoriasis: mechanisms and mysteries. JID Innov. 2022;2(3) doi: 10.1016/j.xjidi.2022.100116. 100116–100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prinz I., Sandrock I., Mrowietz U. Interleukin-17 cytokines: effectors and targets in psoriasis-A breakthrough in understanding and treatment. J Exp Med. 2020;217(1) doi: 10.1084/jem.20191397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duvetorp A. Linköping University Electronic Press; 2021. Different aspects of psoriasis: comorbidity, comedication and disease biomarkers. [Google Scholar]
- 14.Kamiya K, Kishimoto M, Sugai J, Komine M, Ohtsuki M., Kamiya K, et al. Risk factors for the development of psoriasis. Int J Mol Si. 2019;20(18):4347. doi: 10.3390/ijms20184347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadeem A, Al-Harbi NO, Al-Harbi MM, El-Sherbeeny AM, Ahmad SF, Siddiqui N, et al. Imiquimod-induced psoriasis-like skin inflammation is suppressed by BET bromodomain inhibitor in mice through RORC/IL-17A pathway modulation. Pharmacol Res. 2015;99:248–257. doi: 10.1016/j.phrs.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Albanesi C, Madonna S, Gisondi P, Girolomoni G. The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front Immunol. 2018;9:1549. doi: 10.3389/fimmu.2018.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadeem A, Ahmad SF, Al-Harbi NO, El-Sherbeeny AM, Al-Harbi MM, Almukhlafi TS. GPR43 activation enhances psoriasis-like inflammation through epidermal upregulation of IL-6 and dual oxidase 2 signaling in a murine model. Cell Signal. 2017;33:59–68. doi: 10.1016/j.cellsig.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Alzahrani KS, Nadeem A, Ahmad SF, Al-Harbi NO, Ibrahim KE, El-Sherbeeny AM, et al. Inhibition of spleen tyrosine kinase attenuates psoriasis-like inflammation in mice through blockade of dendritic cell-Th17 inflammation axis. Biomed Pharmacother. 2019;111:347–358. doi: 10.1016/j.biopha.2018.12.060. [DOI] [PubMed] [Google Scholar]
- 19.Nadeem A, Ahmad SF, El-Sherbeeny AM, Al-Harbi NO, Bakheet SA, Attia SM. Systemic inflammation in asocial BTBR T(+) tf/J mice predisposes them to increased psoriatic inflammation. Prog Neuropsychopharmacol Biol Psychiatry. 2018;83:8–17. doi: 10.1016/j.pnpbp.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Al-Harbi NO, Nadeem A, Ahmad SF, Bakheet SA, El-Sherbeeny AM, Ibrahim KE, et al. Therapeutic treatment with Ibrutinib attenuates imiquimod-induced psoriasis-like inflammation in mice through downregulation of oxidative and inflammatory mediators in neutrophils and dendritic cells. Eur J Pharmacol. 2020;877:173088. doi: 10.1016/j.ejphar.2020.173088. [DOI] [PubMed] [Google Scholar]
- 21.Jamshaid H, Din Fu, Malik M, Mukhtiar M, Choi HG, Ur-Rehman T, et al. A cutback in Imiquimod cutaneous toxicity; comparative cutaneous toxicity analysis of Imiquimod nanotransethosomal gel with 5% marketed cream on the BALB/c mice. Sci Rep. 2022;12(1):14244. doi: 10.1038/s41598-022-18671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang S-Y, Lin C-H, Sung CT, Fang J-Y. Murine models of psoriasis and their usefulness for drug discovery. Expert Opin Drug Discov. 2018;13(6):551–562. doi: 10.1080/17460441.2018.1463214. [DOI] [PubMed] [Google Scholar]
- 23.Al-juhaishi AMR, Al-Zubaidy AAK, Al-Mousawy JMM. Effects of montelukast on imiquimod-induced model of psoriasis In mice. NVEO-Nat Volatiles Essent Oils J|NVEO. 2021;8(6):3160–3171. [Google Scholar]
- 24.Van Der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 25.Jabeen M, Boisgard A-S, Danoy A, El Kholti N, Salvi J-P, Boulieu R, et al. Advanced characterization of imiquimod-induced psoriasis-like mouse model. Pharmaceutics. 2020;12(9):789. doi: 10.3390/pharmaceutics12090789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christmann C, Zenker S, Martens L, Hübner J, Loser K, Vogl T, et al. Interleukin 17 promotes expression of alarmins S100A8 and S100A9 during the inflammatory response of keratinocytes. Front Immunol. 2020;11:599947. doi: 10.3389/fimmu.2020.599947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Chen Y, Cui L, Shi Y, Guo C. Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death Dis. 2022;13(1):81. doi: 10.1038/s41419-022-04523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sajjad MT, Mohammed QY. The effect of lenalidomide ointment on TNF-α tissue levels in mice with imiquimod-induced psoriasis. J Fac Med Baghdad. 2023;64(4):252–260. doi: 10.32007/jfacmedbagdad.6441959. [DOI] [Google Scholar]
- 29.Han G, Williams CA, Salter K, Garl PJ, Li AG, Wang X-J. A role for TGFβ signaling in the pathogenesis of psoriasis. J Invest Dermatol. 2010;130(2):371–377. doi: 10.1038/jid.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karas A, Holmannova D, Borsky P, Fiala Z, Andrys C, Hamakova K, et al. Significantly altered serum levels of NAD, AGE, RAGE, CRP, and elastin as potential biomarkers of psoriasis and aging-a case-control study. Biomedicines. 2022;10(5):1133. doi: 10.3390/biomedicines10051133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajitha P, Shammika P, Aiswarya S, Gopikrishnan A, Jayakumar R, Sabitha M. Chaulmoogra oil based methotrexate loaded topical nanoemulsion for the treatment of psoriasis. J Drug Deliv Sci Technol. 2019;49:463–476. [Google Scholar]
- 32.Aschoff R, Bewley A, Dattola A, De Simone C, Lahfa M, Llamas-Velasco M, et al. Beyond-mild psoriasis: a consensus statement on calcipotriol and betamethasone dipropionate foam for the topical treatment of adult patients. Dermatol Ther. 2021;11(5):1791–1804. doi: 10.1007/s13555-021-00600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrone V, Losi S, Maiorino A, Antonelli S, Giovannitti M, Giacomini E, et al. Treatment patterns and pharmacoutilization in patients affected by psoriasis: an observational study in an Italian real-world setting. Drugs Real World Outcomes. 2022;9(2):243–251. doi: 10.1007/s40801-021-00290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torsekar R., Gautam M.M. Topical therapies in psoriasis. Indian Dermatol Online J. 2017;8(4):235–245. doi: 10.4103/2229-5178.209622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maul J-T, Anzengruber F, Conrad C, Cozzio A, Häusermann P, Jalili A, et al. Topical treatment of psoriasis vulgaris: the Swiss treatment pathway. Dermatology. 2021;237(2):166–178. doi: 10.1159/000512930. [DOI] [PubMed] [Google Scholar]
- 36.Raharja A., Mahil S.K., Barker J.N. Psoriasis: a brief overview. Clin Med (Lond) 2021;21(3):170–173. doi: 10.7861/clinmed.2021-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownstone ND, Hong J, Mosca M, Hadeler E, Liao W, Bhutani T, et al. Biologic treatments of psoriasis: an update for the clinician. Biologics. 2021;15:39–51. doi: 10.2147/btt.S252578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruggiero A, Picone V, Martora F, Fabbrocini G, Megna M. Guselkumab, risankizumab, and tildrakizumab in the management of psoriasis: a review of the real-world evidence. Clin Cosmet Investig Dermatol. 2022;15:1649–1658. doi: 10.2147/ccid.S364640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metko D., Torres T., Vender R. Viewpoint about biologic agents for psoriasis: are they immunosuppressants or immunomodulators? J Int Med Res. 2023;51(6) doi: 10.1177/03000605231175547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campione E, Cosio T, Di Prete M, Lanna C, Dattola A, Bianchi L. Experimental pharmacological management of psoriasis. J Exp Pharmacol. 2021;13:725. doi: 10.2147/JEP.S265632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplon H, Chenoweth A, Crescioli S, Reichert JM. Antibodies to watch in 2022. mAbs. 2022;14(1):2014296. doi: 10.1080/19420862.2021.2014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navarini AA, Prinz JC, Morita A, Tsai TF, Viguier MA, Li L, et al. Spesolimab improves patient-reported outcomes in patients with generalized pustular psoriasis: results from the Effisayil 1 study. J Eur Acad Dermatol Venereol. 2023;37(4):730–736. doi: 10.1111/jdv.18820. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Y, Chen Y, Yu Q, Shi Y. Biologic and small-molecule therapies for moderate-to-severe psoriasis: focus on psoriasis comorbidities. BioDrugs. 2023;37(1):35–55. doi: 10.1007/s40259-022-00569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iorizzo M., Tosti A. Updates in treatment and impact of nail psoriasis. Expert Rev Clin Immunol. 2023:1–10. doi: 10.1080/1744666X.2023.2215987. [DOI] [PubMed] [Google Scholar]
- 45.Billakota S, Andresen JM, Gay BC, Stewart GR, Fedorov NB, Gerlach AC, et al. Personalized medicine: vinpocetine to reverse effects of GABRB3 mutation. Epilepsia. 2019;60(12):2459–2465. doi: 10.1111/epi.16394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vora S.C., Gujar K.N. Vinpocetine: hype, hope and hurdles towards neuroprotection. Asian J Pharm Res Dev. 2013:17–23. [Google Scholar]
- 47.Witika BA, Poka MS, Demana PH, Matafwali SK, Melamane S, Malungelo Khamanga SM, et al. Lipid-based nanocarriers for neurological disorders: a review of the state-of-the-art and therapeutic success to date. Pharmaceutics. 2022;14(4):836. doi: 10.3390/pharmaceutics14040836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Datt N., Patyar R.R., Patyar S. Comparative evaluation of different doses of vinpocetine alone and in combination with sulfasalazine in experimentally induced inflammatory bowel disease in rats. Asian J Pharmaceut Clin Res. 2017:88–93. [Google Scholar]
- 49.Jeon KI, Xu X, Aizawa T, Lim JH, Jono H, Kwon DS, et al. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc Natl Acad Sci U S A. 2010;107(21):9795–9800. doi: 10.1073/pnas.0914414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruiz-Miyazawa KW, Pinho-Ribeiro FA, Zarpelon AC, Staurengo-Ferrari L, Silva RL, Alves-Filho JC, et al. Vinpocetine reduces lipopolysaccharide-induced inflammatory pain and neutrophil recruitment in mice by targeting oxidative stress, cytokines and NF-κB. Chem Biol Interact. 2015;237:9–17. doi: 10.1016/j.cbi.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y-Y, Yu J-Z, Li Q-Y, Ma C-G, Lu C-Z, Xiao B-G. TSPO-specific ligand vinpocetine exerts a neuroprotective effect by suppressing microglial inflammation. Neuron Glia Biol. 2011;7(2–4):187–197. doi: 10.1017/S1740925X12000129. [DOI] [PubMed] [Google Scholar]
- 52.Zhao M, Hou S, Feng L, Shen P, Nan D, Zhang Y, et al. Vinpocetine protects against cerebral ischemia-reperfusion injury by targeting astrocytic connexin43 via the PI3K/AKT signaling pathway. Front Neurosci. 2020;14:223. doi: 10.3389/fnins.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng X, Wang Y, Hao Y, Ma Q, Dai J, Liang Z, et al. Vinpocetine inhibited the CpG oligodeoxynucleotide-induced immune response in plasmacytoid dendritic cells. Immunol Invest. 2017;46(3):263–273. doi: 10.1080/08820139.2016.1248561. [DOI] [PubMed] [Google Scholar]
- 54.Cai Y., Li J.-D., Yan C. Vinpocetine attenuates lipid accumulation and atherosclerosis formation. Biochem Biophys Res Commun. 2013;434(3):439–443. doi: 10.1016/j.bbrc.2013.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu RT, Wang A, To E, Gao J, Cao S, Cui JZ, et al. Vinpocetine inhibits amyloid-beta induced activation of NF-κB, NLRP3 inflammasome and cytokine production in retinal pigment epithelial cells. Exp Eye Res. 2014;127:49–58. doi: 10.1016/j.exer.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Zhang K, Zhao L, Tang J, Gao L, Wei Z. Anti-inflammatory effects of vinpocetine on the functional expression of nuclear factor-kappa B and tumor necrosis factor-alpha in a rat model of cerebral ischemia–reperfusion injury. Neurosci Lett. 2014;566:247–251. doi: 10.1016/j.neulet.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 57.Lee J-Y, Komatsu K, Lee B-C, Miyata M, O’Neill Bohn A, Xu H, et al. Vinpocetine inhibits Streptococcus pneumoniae–induced upregulation of mucin MUC5AC expression via induction of MKP-1 phosphatase in the pathogenesis of otitis media. J Immunol. 2015;194(12):5990–5998. doi: 10.4049/jimmunol.1401489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang F, Yan C, Wei C, Yao Y, Ma X, Gong Z, et al. Vinpocetine inhibits NF-κB-dependent inflammation in acute ischemic stroke patients. Transl Stroke Res. 2018;9:174–184. doi: 10.1007/s12975-017-0549-z. [DOI] [PubMed] [Google Scholar]
- 59.Bharti Sonkar A, Kumar P, Kumar A, Kumar Gautam A, Verma A, Singh A, et al. Vinpocetine mitigates DMH-induce pre-neoplastic colon damage in rats through inhibition of pro-inflammatory cytokines. Int Immunopharmacol. 2023;119:110236. doi: 10.1016/j.intimp.2023.110236. [DOI] [PubMed] [Google Scholar]
- 60.Lala R.R., Shinde A.S. Development, optimization, and in vitro evaluation of atorvastatin calcium and vinpocetine codelivery by solid lipid nanoparticles for cancer therapy. Future J Pharm Sci. 2021;7(1):1–14. [Google Scholar]
- 61.Huang EW, Xue SJ, Zhang Z, Zhou JG, Guan YY, Tang YB. Vinpocetine inhibits breast cancer cells growth in vitro and in vivo. Apoptosis. 2012;17(10):1120–1130. doi: 10.1007/s10495-012-0743-0. [DOI] [PubMed] [Google Scholar]
- 62.Azouz AA, Hersi F, Ali FEM, Hussein Elkelawy AMM, Omar HA. Renoprotective effect of vinpocetine against ischemia/reperfusion injury: modulation of NADPH oxidase/Nrf2, IKKβ/NF-κB p65, and cleaved caspase-3 expressions. J Biochem Mol Toxicol. 2022;36(7) doi: 10.1002/jbt.23046. [DOI] [PubMed] [Google Scholar]
- 63.Abbas WJ, Altemimi ML, Al-Mudhafar RH, Zigam QA, Hadi NR. Effects of Vinpocetine on Renal Ischemia Reperfusion Injury in a Male Rat Model. Sys Rev Pharm. 2020;11(12) [Google Scholar]
- 64.Dubey A, Kumar N, Mishra A, Singh Y, Tiwari M. Review on vinpocetine. Int J Pharm Life Sci. 2020;11(5) [Google Scholar]
- 65.Mao YT, Hua HY, Zhang XG, Zhu DX, Li F, Gui ZH, et al. Ethosomes as delivery system for transdermal administration of vinpocetine. Pharmazie. 2013;68(5):381–382. [PubMed] [Google Scholar]
- 66.Ahmed T.A. Formulation and clinical investigation of optimized vinpocetine lyoplant-tabs: new strategy in development of buccal solid dosage form. Drug Des Devel Ther. 2019;13:205–220. doi: 10.2147/dddt.S189105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C., Yan C. Updates of recent vinpocetine research in treating cardiovascular diseases. J Cell Immunol. 2020;2(5):211–219. doi: 10.33696/immunology.2.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khalil NY, Bakheit AH, Alkahtani HM, al-Muhanna T. In: Profiles of drug substances, excipients and related methodology. Al-Majed A.A., editor. Academic Press; 2022. Chapter One - Vinpocetine (A comprehensive profile) pp. 1–54. [DOI] [PubMed] [Google Scholar]
- 69.Gan J, Guo L, Zhang X, Yu Q, Yang Q, Zhang Y, et al. Anti-inflammatory therapy of atherosclerosis: focusing on IKKβ. J Inflamm. 2023;20(1):8. doi: 10.1186/s12950-023-00330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang HS, Song JY, Kim JH, Il Park T, Choi WS, Lee J. Effects of vinpocetine on atopic dermatitis after administration via three different routes in HR-1 hairless mice. Pharmazie. 2022;77(1):9–13. doi: 10.1691/ph.2022.1941. [DOI] [PubMed] [Google Scholar]
- 71.Svab G, Doczi J, Gerencser AA, Ambrus A, Gallyas F, Sümegi B, et al. The mitochondrial targets of neuroprotective drug vinpocetine on primary neuron cultures, brain capillary endothelial cells, synaptosomes, and brain mitochondria. Neurochem Res. 2019;44(10):2435–2447. doi: 10.1007/s11064-019-02871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gutiérrez-Farfán I, Reyes-Legorreta C, Solís-Olguín M, Alatorre-Miguel E, Verduzco-Mendoza A, Durand-Rivera A. Evaluation of vinpocetine as a therapy in patients with sensorineural hearing loss: a phase II, open-label, single-center study. J Pharmacol Sci. 2021;145(4):313–318. doi: 10.1016/j.jphs.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 73.Wu L-R, Liu L, Xiong X-Y, Zhang Q, Wang F-X, Gong C-X, et al. Vinpocetine alleviate cerebral ischemia/reperfusion injury by down-regulating TLR4/MyD88/NF-κB signaling. Oncotarget. 2017;8(46):80315. doi: 10.18632/oncotarget.20699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu H, Wang E, Chen F, Xiao J, Wang M. Neuroprotective phytochemicals in experimental ischemic stroke: mechanisms and potential clinical applications. Oxid Med Cell Longev. 2021;2021:6687386. doi: 10.1155/2021/6687386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y.S., Li J.D., Yan C. An update on vinpocetine: new discoveries and clinical implications. Eur J Pharmacol. 2018;819:30–34. doi: 10.1016/j.ejphar.2017.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sabalingam S., Siriwardhene M.A. A review on emerging applications of emulgel as topical drug delivery system. World J Adv Res Rev. 2022;13(1):452–463. [Google Scholar]
- 77.Khullar R, Kumar D, Seth N, Saini S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm J. 2012;20(1):63–67. doi: 10.1016/j.jsps.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Talat M, Zaman M, Khan R, Jamshaid M, Akhtar M, Mirza AZ. Emulgel: an effective drug delivery system. Drug Dev Ind Pharm. 2021;47(8):1193–1199. doi: 10.1080/03639045.2021.1993889. [DOI] [PubMed] [Google Scholar]
- 79.Jahandideh M, Kharazi P, Jafariazar Z, Fahimi S. Preparation of a topical product from Allium sativum retrieved from Iranian traditional medicine. Res J Pharmacogn. 2019;6(4):3–6. doi: 10.22127/rjp.2019.93491. [DOI] [Google Scholar]
- 80.Sulthana S, Chary PS, Bhavana V, Pardhi E, Singh SB, Mehra NK. Development and evaluation emulgel for effective management of the imiquimod-induced psoriasis. Inflammopharmacology. 2023;31(1):301–320. doi: 10.1007/s10787-022-01131-7. [DOI] [PubMed] [Google Scholar]
- 81.Khan BA, Ullah S, Khan MK, Alshahrani SM, Braga VA. Formulation and evaluation of Ocimum basilicum-based emulgel for wound healing using animal model. Saudi Pharm J. 2020;28(12):1842–1850. doi: 10.1016/j.jsps.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shukr M., Metwally G.F. Evaluation of topical gel bases formulated with various essential oils for antibacterial activity against methicillin-resistant Staphylococcus aureus. Trop J Pharm Res. 2013;12(6):877–884. doi: 10.4314/tjpr.v12i6.3. [DOI] [Google Scholar]
- 83.Cardiff R.D., Miller C.H., Munn R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014;2014(6):655–658. doi: 10.1101/pdb.prot073411. [DOI] [PubMed] [Google Scholar]
- 84.Zhou W, Hu M, Zang X, Liu Q, Du J, Hu J, et al. Luteolin attenuates imiquimod–induced psoriasis-like skin lesions in BALB/c mice via suppression of inflammation response. Biomed Pharmacother. 2020;131:110696. doi: 10.1016/j.biopha.2020.110696. [DOI] [PubMed] [Google Scholar]
- 85.Lin ZC, Hsieh PW, Hwang TL, Chen CY, Sung CT, Fang JY. Topical application of anthranilate derivatives ameliorates psoriatic inflammation in a mouse model by inhibiting keratinocyte-derived chemokine expression and neutrophil infiltration. FASEB J. 2018;32(12):6783–6795. doi: 10.1096/fj.201800354. [DOI] [PubMed] [Google Scholar]
- 86.Nundy S, Kakar A, Bhutta ZA, Nundy S, Kakar A, Bhutta ZA. Understanding medical biostatistics, How to practice academic medicine and publish from developing countries? A practical guide; 2022. pp. 95–116. [Google Scholar]
- 87.Alsaedi HF, Al-zubaidy AA, Ramadhan MA. Effect of metformin gel against imiquimod–induced psoriasis in mice. Int J Res Pharm Sci. 2019 doi: 10.26452/IJRPS.V10I2.255. [DOI] [Google Scholar]
- 88.Salman HR, Al-Zubaidy AA, Abbas AH, Zigam QA. The ameliorative effects of topical gemifloxacin alone or in combination with clobetasol propionate on imiquimod-induced model of psoriasis in mice. Naunyn Schmiedebergs Arch Pharmacol. 2023:1–18. doi: 10.1007/s00210-023-02629-9. [DOI] [PubMed] [Google Scholar]
- 89.Moos S, Mohebiany AN, Waisman A, Kurschus FC. Imiquimod-induced psoriasis in mice depends on the IL-17 signaling of keratinocytes. J Invest Dermatol. 2019;139(5):1110–1117. doi: 10.1016/j.jid.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Abbas A, Abd A, Salman H, Abbas Z. The attenuated effects of cimifugin on imiquimod-induced model of psoriasis in mice. Lat Am J Pharm. 2023;(42(special issue):):362–369. [Google Scholar]
- 91.Khan R, Mirza MA, Aqil M, Alex TS, Raj N, Manzoor N, et al. In vitro and in vivo investigation of a dual-targeted nanoemulsion gel for the amelioration of psoriasis. Gels. 2023;9(2) doi: 10.3390/gels9020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoegsberg T, Iversen L, Lange MM, Bissonette R, Carvalho AVE, van de Kerkhof PC, et al. Topical treatment of psoriasis: questionnaire results on topical therapy as long-term continuous treatment and use on specific body sites. J Dermatol Treat. 2021;32(8):916–921. doi: 10.1080/09546634.2020.1724250. [DOI] [PubMed] [Google Scholar]
- 93.Nair AB, Kumar S, Dalal P, Nagpal C, Dalal S, Rao R, et al. Novel dermal delivery cargos of clobetasol propionate: an update. Pharmaceutics. 2022;14(2) doi: 10.3390/pharmaceutics14020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wolverton S.E., Wu J.J. Elsevier Health Sciences; 2019. Comprehensive dermatologic drug therapy. [Google Scholar]
- 95.Del Rosso J.Q. Topical corticosteroid therapy for psoriasis-a review of clobetasol propionate 0.025% cream and the clinical relevance of penetration modification. J Clin Aesthet Dermatol. 2020;13(2):22–29. DOI: 32308782. [PMC free article] [PubMed] [Google Scholar]
- 96.Tan S.Y., Chandran N.S., Choi E.C.-E. Steroid phobia: is there a basis? A review of topical steroid safety, addiction and withdrawal. Clin Drug Invest. 2021;41(10):835–842. doi: 10.1007/s40261-021-01072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Segaert S, Calzavara-Pinton P, de la Cueva P, Jalili A, Lons Danic D, Pink AE, et al. Long-term topical management of psoriasis: the road ahead. J Dermatol Treat. 2022;33(1):111–120. doi: 10.1080/09546634.2020.1729335. [DOI] [PubMed] [Google Scholar]
- 98.Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457–465. doi: 10.1517/14740338.2016.1140743. [DOI] [PubMed] [Google Scholar]
- 99.Pofi R, Caratti G, Ray DW, Tomlinson JW. Treating the side effects of exogenous glucocorticoids; can we separate the good from the bad? Endocr Rev. 2023 doi: 10.1210/endrev/bnad016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Le QV, Wen SY, Chen CJ, Huang CY, Kuo WW. Reversion of glucocorticoid-induced senescence and collagen synthesis decrease by LY294002 is mediated through p38 in skin. Int J Biol Sci. 2022;18(16):6102–6113. doi: 10.7150/ijbs.73915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fernandes AR, Martins-Gomes C, Santini A, Silva AM, Souto EB. In: Design of nanostructures for versatile therapeutic applications. Grumezescu A.M., editor. William Andrew Publishing; 2018. Chapter 9 - Psoriasis vulgaris—pathophysiology of the disease and its classical treatment versus new drug delivery systems; pp. 379–406. [Google Scholar]
- 102.Mohammed SS, Kadhim HM, Al-Sudani IM, Musatafa WW. Study the topical effect of six days use of different lycopene doses on imiquimod-induce psoriasis-like skin inflammation in mice. Int J Health Sci. 2022;6(S3):171–185. [Google Scholar]
- 103.Ahmed NH, Al-Zubaidy AAK, Qasim BJ. Effect of Topical Dipyridamole Gel in Comparison with Clobetasol on induced Psoriasis in Mice. Inte J Drug Deliv Technol. 2021;2(11):524–529. [Google Scholar]
- 104.Zhang L., Yang L. Anti-inflammatory effects of vinpocetine in atherosclerosis and ischemic stroke: a review of the literature. Molecules. 2014;20(1):335–347. doi: 10.3390/molecules20010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan C, Li J-D, Berk B, Xu X. Google Patents; 2016. Method and compositions for treatment or prevention of inflammatory conditions. [Google Scholar]
- 106.Zhang P, Cao Y, Chen S, Shao L. Combination of vinpocetine and dexamethasone alleviates cognitive impairment in nasopharyngeal carcinoma patients following radiation injury. Pharmacology. 2021;106(1–2):37–44. doi: 10.1159/000506777. [DOI] [PubMed] [Google Scholar]
- 107.Balaha M, Alahmari A, Kandeel S, Balaha M. Vinpocetine’s immunomodulating, anti-oxidant, anti-inflammatory, ant-ifibrotic, and PDE inhibiting potencies ameliorate bleomycin-induced pulmonary fibrosis. Iran J Basic Med Sci. 2023;26(1):13–22. doi: 10.22038/ijbms.2022.64175.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elnfarawy AA, Nashy AE, Abozaid AM, Komber IF, Elweshahy RH, Abdelrahman RS. Vinpocetine attenuates thioacetamide-induced liver fibrosis in rats. Hum Exp Toxicol. 2021;40(2):355–368. doi: 10.1177/0960327120947453. [DOI] [PubMed] [Google Scholar]
- 109.Xiao-Xiao T., Sen-Min W., Ding-Sheng L. Effects of vinpocetine on random skin flap survival in rats. J Reconstr Microsurg. 2013;29(6):393–398. doi: 10.1055/s-0033-1343834. [DOI] [PubMed] [Google Scholar]
- 110.Twafik w, Morad O, Hassan R, nematallah K, El-hamed D, Morad S, et al. Vinpocetine suppresses inflammatory and oxidative machineries in acute model of inflammation—pivotal role of COX-2 signaling. SVU-Int J Vet Sci. 2021;4(3):51–69. doi: 10.21608/svu.2021.70729.1119. [DOI] [Google Scholar]
- 111.Lourenco-Gonzalez Y, Fattori V, Domiciano TP, Rossaneis AC, Borghi SM, Zaninelli TH, et al. Repurposing of the nootropic drug vinpocetine as an analgesic and anti-inflammatory agent: evidence in a mouse model of superoxide anion-triggered inflammation. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/6481812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barakat W., Anwar H., Mahmoud M. Sildenafil and vinpocetine promote wound healing in diabetic rats. J Adv Pharm Res. 2021;5(1):211–221. [Google Scholar]
- 113.Kim T.-G., Kim S.H., Lee M.-G. The origin of skin dendritic cell network and its role in psoriasis. Int J Mol Sci. 2018;19(1):42. doi: 10.3390/ijms19010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nadeem A, Ahmad SF, Al-Harbi NO, Ibrahim KE, Alqahtani F, As Sobeai HM, et al. Inhibition of interleukin-2-inducible T-cell kinase causes reduction in imiquimod-induced psoriasiform inflammation through reduction of Th17 cells and enhancement of Treg cells in mice. Biochimie. 2020;179:146–156. doi: 10.1016/j.biochi.2020.09.023. [DOI] [PubMed] [Google Scholar]
- 115.Young K.Z., Plazyo O., Gudjonsson J.E. Targeting immune cell trafficking and vascular endothelial cells in psoriasis. J Clin Invest. 2023;133(9) doi: 10.1172/jci169450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chiang C-C, Cheng W-J, Korinek M, Lin C-Y, Hwang T-L. Neutrophils in psoriasis. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang W.M., Jin H.Z. Role of neutrophils in psoriasis. J Immunol Res. 2020;2020 doi: 10.1155/2020/3709749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ruiz-Miyazawa KW, Zarpelon AC, Pinho-Ribeiro FA, Pavão-de-Souza GF, Casagrande R, Verri WA., Jr. Vinpocetine reduces carrageenan-induced inflammatory hyperalgesia in mice by inhibiting oxidative stress, cytokine production and NF-κB activation in the paw and spinal cord. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0118942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Choi WS, Kang HS, Kim HJ, Lee WT, Sohn UD, Lee JY. Vinpocetine alleviates lung inflammation via macrophage inflammatory protein-1β inhibition in an ovalbumin-induced allergic asthma model. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0251012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Torres KJ, Göttle P, Kremer D, Rivera JF, Aguirre-Cruz L, Corona T, et al. Vinpocetine inhibits oligodendroglial precursor cell differentiation. Cell Physiol Biochem. 2012;30(3):711–722. doi: 10.1159/000341451. [DOI] [PubMed] [Google Scholar]
- 121.Medina A.E. Vinpocetine as a potent antiinflammatory agent. Proc Natl Acad Sci USA. 2010;107(22):9921–9922. doi: 10.1073/pnas.1005138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giachini FR, Lima VV, Carneiro FS, Tostes RC, Webb RC. Decreased cGMP level contributes to increased contraction in arteries from hypertensive rats: role of phosphodiesterase 1. Hypertension. 2011;57(3):655–663. doi: 10.1161/hypertensionaha.110.164327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Al-Harbi NO, Nadeem A, Ansari MA, Al-Harbi MM, Alotaibi MR, AlSaad AMS, et al. Psoriasis-like inflammation leads to renal dysfunction via upregulation of NADPH oxidases and inducible nitric oxide synthase. Int Immunopharmacol. 2017;46:1–8. doi: 10.1016/j.intimp.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 124.Abu-Elfotuh K, Hamdan AME, Abbas AN, Alahmre ATS, Elewa MAF, Masoud RAE, et al. Evaluating the neuroprotective activities of vinpocetine, punicalagin, niacin and vitamin E against behavioural and motor disabilities of manganese-induced Parkinson’s disease in Sprague Dawley rats. Biomed Pharmacother. 2022;153:113330. doi: 10.1016/j.biopha.2022.113330. [DOI] [PubMed] [Google Scholar]
- 125.Han D, Wang J, Wen L, Sun M, Liu H, Gao Y. Vinpocetine attenuates ischemic stroke through inhibiting NLRP3 inflammasome expression in mice. J Cardiovasc Pharmacol. 2021;77(2):208. doi: 10.1097/FJC.0000000000000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Al-Harbi NO, Nadeem A, Al-Harbi MM, Zoheir KMA, Ansari MA, El-Sherbeeny AM, et al. Psoriatic inflammation causes hepatic inflammation with concomitant dysregulation in hepatic metabolism via IL-17A/IL-17 receptor signaling in a murine model. Immunobiology. 2017;222(2):128–136. doi: 10.1016/j.imbio.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 127.Ishola IO, Akinyede AA, Adeluwa TP, Micah C. Novel action of vinpocetine in the prevention of paraquat-induced parkinsonism in mice: involvement of oxidative stress and neuroinflammation. Metab Brain Dis. 2018;33(5):1493–1500. doi: 10.1007/s11011-018-0256-9. [DOI] [PubMed] [Google Scholar]
- 128.Komatsu K, Nam D-H, Lee J-Y, Yoneda G, Yan C, Li J-D. Vinpocetine suppresses Streptococcus pneumoniae–induced inflammation via inhibition of ERK1 by CYLD. J Immunol. 2020;204(4):933. doi: 10.4049/jimmunol.1901299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou Q, Guo D, Li X, Wang Y, Ye X, Xue S, et al. Anti-inflammatory effects of vinpocetine in LPS-stimulated microglia via activation of AMPK. An Acad Bras Cienc. 2020;92(4) doi: 10.1590/0001-3765202020200241. [DOI] [PubMed] [Google Scholar]
- 130.Heidari A., Yazdanpanah N., Rezaei N. The role of Toll-like receptors and neuroinflammation in Parkinson's disease. J Neuroinflammation. 2022;19(1):135. doi: 10.1186/s12974-022-02496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Al-kuraishy HM, Al-Gareeb AI, Fageyinbo MS, Batiha GE-S. Vinpocetine is the forthcoming adjuvant agent in the management of COVID-19. Future Sci OA. 2022;8(5):FSO797. doi: 10.2144/fsoa-2021-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vishwakarma VK, Shah S, Kaur T, Singh AP, Arava SK, Kumar N, et al. Effect of vinpocetine alone and in combination with enalapril in experimental model of diabetic cardiomyopathy in rats: possible involvement of PDE-1/TGF-β/Smad 2/3 signalling pathways. J Pharm Pharmacol. 2023 doi: 10.1093/jpp/rgad043. [DOI] [PubMed] [Google Scholar]
- 133.Adwent I, Grabarek BO, Kojs-Mrożkiewicz M, Brus R, Staszkiewicz R, Plewka A, et al. The influence of adalimumab and cyclosporine A on the expression profile of the genes related to TGFβ signaling pathways in keratinocyte cells treated with lipopolysaccharide. A, Mediators Inflamm. 2020;2020:3821279. doi: 10.1155/2020/3821279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qin R, Zhao Q, Han B, Zhu H-P, Peng C, Zhan G, et al. Indole-Based small molecules as potential therapeutic agents for the treatment of fibrosis. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.845892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu MP, Zhang YS, Xu X, Zhou Q, Li JD, Yan C. Vinpocetine attenuates pathological cardiac remodeling by inhibiting cardiac hypertrophy and fibrosis. Cardiovasc Drugs Ther. 2017;31(2):157–166. doi: 10.1007/s10557-017-6719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu LR, Liu L, Xiong XY, Zhang Q, Wang FX, Gong CX, et al. Vinpocetine alleviate cerebral ischemia/reperfusion injury by down-regulating TLR4/MyD88/NF-κB signaling. Oncotarget. 2017;8(46):80315–80324. doi: 10.18632/oncotarget.20699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yong L, Yu Y, Li B, Ge H, Zhen Q, Mao Y, et al. Calcium/calmodulin-dependent protein kinase IV promotes imiquimod-induced psoriatic inflammation via macrophages and keratinocytes in mice. Nat Commun. 2022;13(1):4255. doi: 10.1038/s41467-022-31935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Calautti E., Avalle L., Poli V. Psoriasis: a STAT3-centric view. Int J Moci. 2018;19(1) doi: 10.3390/ijms19010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Works MG, Yin F, Yin CC, Yiu Y, Shew K, Tran TT, et al. Inhibition of TYK2 and JAK1 ameliorates imiquimod-induced psoriasis-like dermatitis by inhibiting IL-22 and the IL-23/IL-17 axis. J Immunol. 2014;193(7):3278–3287. doi: 10.4049/jimmunol.1400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rusiñol L., Puig L. Tyk2 targeting in immune-mediated inflammatory diseases. Int J Mol Sci. 2023;24(4) doi: 10.3390/ijms24043391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim NJ, Baek JH, Lee J, Kim H, Song JK, Chun KH. A PDE1 inhibitor reduces adipogenesis in mice via regulation of lipolysis and adipogenic cell signaling. Exp Mol Med. 2019;51(1):1–15. doi: 10.1038/s12276-018-0198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ridha Z, Ouchene L, Netchiporouk E, Gooderham MJ. Topical PDE-4 inhibitors are emerging for psoriasis treatment. J Cutan Med Surg. 2021;25(1):109–110. doi: 10.1177/1203475420960429. [DOI] [PubMed] [Google Scholar]
- 143.Gyldenløve M, Meteran H, Sørensen JA, Fage S, Yao Y, Lindhardsen J, et al. Efficacy and safety of oral roflumilast for moderate-to-severe psoriasis—a randomized controlled trial (PSORRO) Lancet Reg Health Europe. 2023;30:100639. doi: 10.1016/j.lanepe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ahmed N.H., Al-Zubaidy A.A.K., Qasim B.J. Effect of topical tadalafil gel in imiquimod-induced psoriasiform skin inflammation in mice. Indian J Forensic Med Toxicol. 2021;15(3):1572–1577. doi: 10.37506/ijfmt.v15i3.15529. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset corroborating the outcomes of the current investigation is available from corresponding author on reasonable request.