Abstract
The rabbit carcasses used in this study were buried at depths of 20 and 40 cm, were examined to construct a fly succession database on buried carrion in Riyadh, Saudi Arabia. Twenty-four rabbits were buried, 12 at 20 cm and 12 at 40 cm. One carcass at each depth was exhumed at 10-day intervals up to 120 days. The degradation rate varied among the carcasses. Differences in species and their colonization were also found in the superficial and exhumed carcasses. Eleven species of flies were recorded on carcasses interred at a depth of 20 cm and seven species at 40 cm, while 13 species were recorded on the carcasses over the top of the soil. Species Rhyncomya sp (Diptera: Calliphoridae), Sarcophaga dux Thomson, and Dolichotachina marginella (Wiedemann) (Diptera: Sarcophagidae) were dominant at both depths, while Chrysomya albiceps (Wiedeman), Chrysomya rufifaces (Macquart) (Diptera: Calliphoridae), Musca domestica Linnaeus, and Musca sorbens Wiedemann (Diptera: Muscidae) were dominant in surface carcasses. Megaselia scalaris (Loew) ((Diptera: Phoridae) is a common and typical forensic indicator that was found in the decay/advanced decay and dry stages at a depth of 20 cm. These findings are possibly useful in forensic investigations involving buried bodies in Riyadh, Saudi Arabia.
Keywords: Buried carrion, Forensic entomology, Dolichotachina marginella Megaselia scalaris, Sarcophaga dux
1. Introduction
Insects invade carcasses soon after death (Catts and Goff, 1992) albeit internal and external variables can influence their presence and behavior on the remains (George et al., 2013). The rate of decay and diversity of species is influenced by temperature, humidity, and the surrounding environment (Gilbert and Bass, 1967, Shean et al., 1993). Bodies are often disposed of by burial. According to previous research, buried carcasses disintegrate more slowly than those exposed to air (Moses, 2012, Payne et al., 1968). Graves are seldom deep as digging takes time and energy. The longer a perpetrator remains in touch with the body, the further probable he is to be detained or leave evidence linking them to the murder. As a result, attackers typically dig shallow graves for their victims.
Previous studies on buried carcasses and pieces of meat found numerous necrophagous species, the majority of which belonged to groups of Diptera, including Muscidae, Phoridae, and Sarcophagidae (Bourel et al., 2004, Huchet, 2014, Huchet and Greenberg, 2010, VanLaerhoven and Anderson, 1999), Leptoceridae, Sphaeroceridae, Psychodidae, Calliphoridae, and Leiodidae (George et al., 2013, Gilbert and Bass, 1967).
In the case of buried carcasses, forensic entomology practitioners may struggle to appropriately quantify PMI due to a lack of reference data and the influence of local variables. These obstacles might be overcome by effectively combining fieldwork and laboratory research, as well as using animal models for experimental studies in circumstances pertinent to forensic entomology. These experiments may offer valuable information for forensic investigations in the future. Except for a study on rat carcasses done by (Al-Mekhlafi, 2021), we are aware of no studies in the Kingdom of Saudi Arabia that attempted to catalog the different kinds of flies found on buried bodies. To identify species and succession of flies and provide information about the decomposition rate on buried remains, we carried out this study in the city of Riyadh, Saudi Arabia. We used rabbit carcasses as an animal model for this investigation.
2. Methods
The examination was conducted from 28 January to 30 May 2021 in an area of ksu campus. The area was roughly 175 m by 250 m. Except for a limited acacia trees and about common wild herbs, the area is virtually barren of vegetation. Experimental animals were purchased from the local market in Riyadh. The Research Ethics Committee approved the use of rabbits for the project at IMSIU, under project No (35–2021). All rabbits were killed on the morning of burial using chloroform. The day before burial, 24 holes (60 × 30 cm) were dug at depths of 20 and 40 cm using a short-handled spade. To avoid interfering with the succession fauna, the pits were separated from one another on all sides by at least 10 m. Carcasses were packed into yard trash bags in order to inhibit colonization and transported by a covered pickup truck. A total of 24 mature rabbits Oryctolagus cuniculus L. (Leporida: Lagomorpha) were buried. Each Carcass was weighed before and after burial to determine the Weight loss. Rabbits were placed on a 30 × 60 piece of chicken wire the holes of which were 25 mm, for make removal simpler and to stop animal scavenging. The interval between the moment of death and the burial of the carcasses was 25 min. Data loggers (EM50G data logger, Ecotone, Gdynia, Poland) capable of measuring soil humidity and temperature were buried alongside the rabbits' bodies. this logger were positioned across the midsection of the rabbits and programmed to record temperature and humidity readings every hour. Rabbits in pits were buried immediately after being placed in the hole (Pastula and Merritt, 2013).
Exposed bodies were positioned inside robust steel 2-cm mesh boxes with a layer of wire screening, each measuring 60 × 50 × 30 cm3 to prevent animal scavenging. using a Lascar EL-USB-2 data recorder. The site's ambient temperature and relative humidity were recorded hourly for the duration of the experiment (Al-Mekhlafi et al., 2020).
2.1. Sampling of insects and decay of carcasses
A carcass was extracted from each depth (20 and 40) every 10 days for 120 days. Before excavation, the soil covering the buried carcasses underwent inspection for the presence of insects. The soil was regularly hand-sorted for insects while being removed with a shovel. The chicken wire was used to help raise the carcasses directly from the pit and was meticulously examined for about 15 min. Insects were collected from the uncovered soil at the bottommost of the hole and the layers of soil cover in addition to the body of the rabbit. Forceps were used to delicately remove larval specimens. The remaining 50 % of the collected larvae were then allowed to mature into adult flies to aid in their identification, with the remaining 50 % being kept in 70 % ethanol for future reference. The construction of rearing containers followed the guidelines provided by Byrd and Tomberlin, (2010). Before transporting the live fly larvae to the laboratory, they were placed directly on chicken liver. The larvae were then reared in a growth chamber maintained at a constant temperature of 27 °C, with 50 % humidity and constant illumination. The adults of the newly emerging Diptera were kept in 70 % ethanol. Adult and larval species were identified, whenever feasible, using (Smith, 1986), (Marshall et al., 2011), (Courtney, 2000) and (Setyaningrum and Al Dhafer, 2014), or referred to experts in the Insect Museum, Faculty of Food and Agricultural Sciences - ksu. The database recorded only insects that were significant for forensic purposes; hence, soil-associated insects were excluded.
Additionally, insects were gathered daily from the surface of control carcasses. A trap that was filled with a solution of water, soap, and salts was used to catch adult insects according to the method provided by (Mashaly and Al-Mekhlafi, 2016). The larvae were collected using the same methods as before. Each carcass's rate of decomposition as well as the duration of each decomposition stage were observed and recorded.
2.2. Data analysis
To determine the statistical differences in the abundance of Dipteran groups between the different depths in carcasses buried. One-way ANOVA, then Tukey's test, was performed and P < 0.05 was considered statistically significant. by IBM SPSS Statistics 28.0.0.0.
3. Results
3.1. Soil analysis
The soil's grain size, analyzed by the Laboratory of Soil Department, College of Food and Agriculture Sciences, King Saud University, revealed a composition of 76.52 %, 67.02 % sand, 10 %, 18.5 % silt, 13.48 %, and 14.48 % clay before and after burial, respectively (Table 1). Therefore, the soil was described as “sandy loam”, according to the categorization by the US Department of Agriculture.
Table 1.
Results of soil tests as determined by the King Saud University's College of Food and Agriculture Sciences' Laboratory of Soil Department.
| Specimen | Clay % | Silt % | Sand % | Calcium Carbonate CaCO3 % | PH | Electrical Conductivity (EC) ds/m | Organic Matter % |
|---|---|---|---|---|---|---|---|
| Before burial | 13.48 | 10 | 76.52 | 15.455 | 8.24 | 2.06 | 0.91 |
| After burial | 14.48 | 18.5 | 67.02 | 18.69 | 8.29 | 2.70 | 1.21 |
3.2. Temperature and humidity
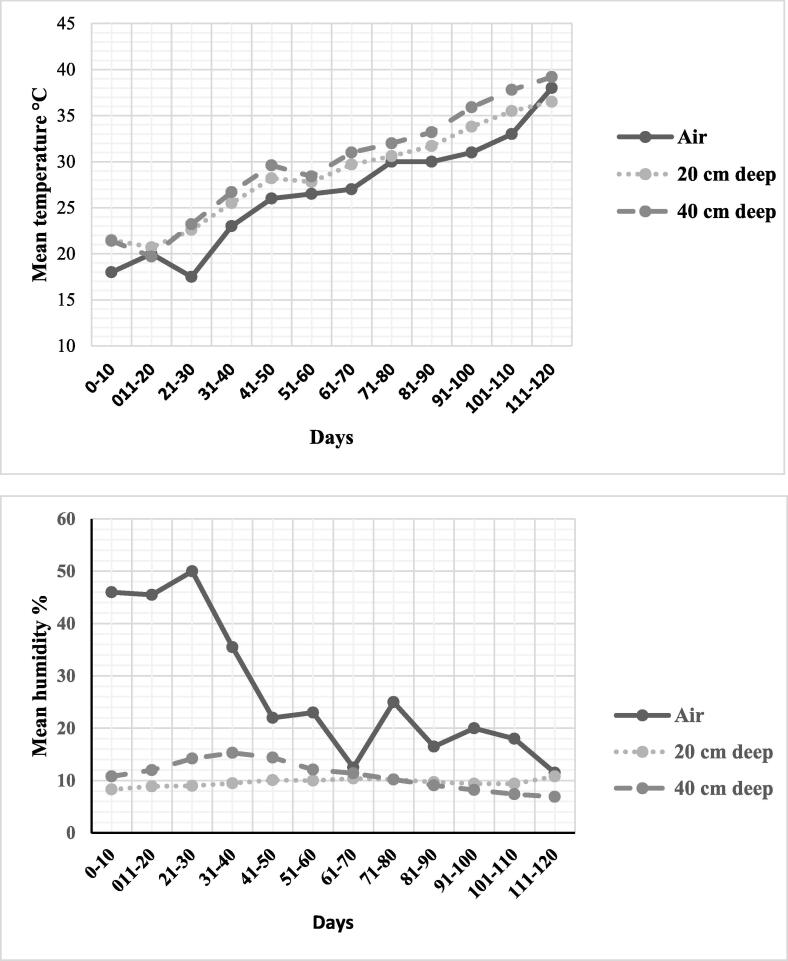
During the course of the experiment, the daily average air temperature displayed fluctuations between 18 and 38 degrees Celsius. Likewise, the daily average soil temperature at a depth of 40 cm ranged from 21.4 to 39.2 °C, while at a depth of 20 cm, it fluctuated between 21.5 and 36.5 °C. Notably, both ambient air humidity and the humidity of the soil surrounding the grave exhibited variations at different depths. However, it was observed that the soil humidity at both depths demonstrated lower variability compared to the surrounding air humidity, as depicted in Fig. 1.
Fig. 1.
Mean daily temperature and humidity of ambient air and soil at 20, 40 cm for 120 days.
3.3. Decay rate
The above-ground carcasses underwent decomposition over a period of 30 days, whereas the buried carcasses at depths of 20 and 40 cm took 120 days to decompose. Both sets of carcasses exhibited distinct stages of decomposition. The exposed carcasses went through four stages: fresh, bloated, decay, and dry. On the other hand, the buried carcasses showed the following stages: fresh, bloated, decay/advanced decay, and dry. Notably, the dry stage was observed in the buried carcasses after 60 days, while the control carcass reached this stage within 14 days, as illustrated in Fig. 2. The of weight loss of the buried bodies differed in both depths 20 and 40 cm, as it reached 10 and 20 % in the first ten days, while it was 87.55 %, and 76.59 % at 120 day, respectively (Fig. 3).
Fig. 2.
Stages of decomposition of buried rabbits carcass at 20 cm (A), at 40 cm (B) and carcass above the surface of the soil (C).
Fig. 3.
Weight loss of bodies buried at both depths (20,40 cm).
3.4. Fly succession
A total of 1604 flies were collected, 270 at a depth of 20, 202 at a depth of 40, and 1132 on surface carcasses. The dominant species at depths of 20 and 40 cm were D. marginella (26 and 34 %), Rhyncomya sp (27 and 32 %), and S. dux (24 and 22 %), respectively, while they differed in surface carcasses where Ch. albiceps (33 %), M. domestica (30 %), Ch. rufifaces (18 %), and M. sorbens (7 %) were the dominant species (Table 2). The results of the statistical analysis showed significant differences between the numbers of flies on the surface and buried bodies (p ≤ 0.05F = 1.73) while there were no differences between both depths.
Table 2.
Percentage of presence of the different species of flies in buried and superficial carcasses.
| family | Genus and species |
buried |
Soil surface | |
|---|---|---|---|---|
| 20 cm | 40 cm | |||
| Calliphoridae | Chrysomya albiceps | 8 (3 %) | 0 (0 %) | 378 (33 %) |
| Chrysomya rufifaces | 4 (1 %) | 0 (0 %) | 200 (18 %) | |
| Chrysomya megacephala | 4 (1 %) | 0 (0 %) | 0 (0 %) | |
| Hemipyrellia pulchra | 4(1 %) | 2 (1 %) | 6 (1 %) | |
| Rhyncomya sp. | 54 (20 %) | 64 (32 %) | 26 (2 %) | |
| Bengalia sp. | 0 (0 %) | 0 (0 %) | 16 (1 %) | |
| Calliphora vicina | 0 (0 %) | 0 (0 %) | 2 (0 %) | |
| Sarcophagidae | Sarcophaga sp. | 0 (0 %) | 0 (0 %) | 32 (3 %) |
| Sarcophaga dux | 64 (24 %) | 44 (22 %) | 0 (0 %) | |
| Wohlfahrtia nuba | 20 (7 %) | 10 (5 %) | 18 (2 %) | |
| Dolichotachina marginella | 70 (26 %) | 68 (34 %) | 30 (3 %) | |
| Muscidae | Musca domestica | 30 (11 %) | 9 (3 %) | 334 (30 %) |
| Musca sorbens | 0 (0 %) | 0 (0 %) | 76 (7 %) | |
| Phoridae | Megaselia scalaris | 6 (2 %) | 0 (0 %) | 0 (0 %) |
| Ulidiidae | Physiphora alceae | 6(2 %) | 8 (4 %) | 8 (1 %) |
| Ephydridae | Eremotrichoma perspiciendum | 0 (0 %) | 0 (0 %) | 6 (1 %) |
| Total | 270 | 202 | 1132 | |
| Mean | 8.70 ± 1.07b | 6.52 ± 1.30b | 45.28 ± 6.50 a | |
| F-value | 1.73 | |||
Sixteen species of flies belonging to six families were identified, 11 and seven species of it on carcasses buried at depths of 20 and 40 cm, respectively, and 13 species on surface carcasses, where five species of flies Hemipyrellia pulchra, Rhyncomya sp, Wohlfahrtia nuba, Dolichotachina marginella, Musca domestica, and Physiphora alceae were identified on carcasses buried at both depths and surface carcasses. Species Chrysomya megacephala and Megaselia scalaris were recorded at a depth of 20 only, and types Bengalia sp, Calliphora vicina, Sarcophaga sp, Musca sorbens, and Eremotrichoma perspiciendum on surface carcasses only. While species Sarcophaga dux were found at both depths only, species Ch. albiceps and Ch. rufifaces were found at depth 20 and on surface carcasses (Table 3).
Table 3.
Species of flies collected from buried carcasses at a depth of 20 and 40 cm compared to surface carcasses.
| family | Genus and species | soil surface |
buried |
|
|---|---|---|---|---|
| 20 cm | 40 cm | |||
| Calliphoridae | Chrysomya albiceps | √ | √ | – |
| Chrysomya rufifaces | √ | √ | – | |
| Chrysomya megacephala | – | √ | – | |
| Hemipyrellia pulchra | √ | √ | √ | |
| Rhyncomya sp. | √ | √ | √ | |
| Bengalia sp. | √ | – | – | |
| Calliphora vicina | √ | – | – | |
| Sarcophagidae | Sarcophaga sp. | √ | – | – |
| Sarcophaga dux | – | √ | √ | |
| Wohlfahrtia nuba | √ | √ | √ | |
| Dolichotachina marginella | √ | √ | √ | |
| Muscidae | Musca domestica | √ | √ | √ |
| Musca sorbens | √ | – | – | |
| Phoridae | Megaselia scalaris | – | √ | – |
| Ulidiidae | Physiphora alceae | √ | √ | √ |
| Ephydridae | Eremotrichoma perspiciendum | √ | – | – |
Flies species presence (√) or absence (--).
The fly larvae began to appear in the buried carcasses after 30 days, and no type of fly was recorded in the buried rabbit carcasses on days 10 and 20, and the carcasses were in the stage of decay/advanced decay. The carcasses continued at this stage until day 50. All classified species in our study were recorded at this stage. Some of continued throughout the dry stage. The first appearance of species Rhyncomya sp (L20) and types W. nuba (L6) and D. marginella (L3) were recorded on day 30 at a depth of 20 only. In addition to the previous species, the emergence of eight species was recorded on day 40. Species H. pulchra, M. domestica, and S. dux were recorded at the two depths, while the five species were recorded at depth 20 only (Table 4).
Table 4.
Timing and succession of fly species collected from rabbit carcasses buried at depths of 20 and 40 cm.
| Days | family | Genus and species | D. stage | 20 cm | 40 cm |
|---|---|---|---|---|---|
| 10 | – | – | Bloated | – | – |
| 20 | – | – | Decay and advanced decay | – | – |
| 30 | Calliphoridae | Rhyncomya sp. | L(40) | – | |
| Sarcophagidae | Wohlfahrtia nuba | L(12) | – | ||
| Dolichotachina marginella | L(6) | – | |||
| 40 | Calliphoridae | Chrysomya albiceps | L(8) | – | |
| Chrysomya megacephala | L(4) | – | |||
| Chrysomya rufifaces | L(4) | – | |||
| Hemipyrellia pulchra | L(4) | L(2) | |||
| Rhyncomya sp. | L(12) | – | |||
| Sarcophagidae | Sarcophaga dux | L(8) | – | ||
| Wohlfahrtia nuba | L(8) | – | |||
| Dolichotachina marginella | L(30) | – | |||
| Muscidae | Musca domestica | L(28) | L(6) | ||
| Phoridae | Megaselia scalaris | L(2) | – | ||
| Ulidiidae | Physiphora alceae | L(6) | – | ||
| 50 | Sarcophagidae | Sarcophaga dux | L(34) | L(6) | |
| Dolichotachina marginella | L(32) | – | |||
| Ulidiidae | Physiphora alceae | – | L(8) | ||
| Total decay | 238 | 22 | |||
| Mean | 14.00 ± 1.59 a | 1.30 ± 0.32c | |||
| 60 | Calliphoridae | Rhyncomya sp. | – | L(52) | |
| Sarcophagidae | Sarcophaga dux | Dry | L(22) | L(38) | |
| Wohlfahrtia nuba | – | L(8) | |||
| Dolichotachina marginella | L(2) | L(56) | |||
| Muscidae | Musca domestica | L(2) | – | ||
| 70 | Calliphoridae | Rhyncomya sp. | L(2) | – | |
| 80 | – | – | – | – | |
| 90 | Calliphoridae | Rhyncomya sp. | – | L(4) | |
| Sarcophagidae | Dolichotachina marginella | – | L(6) | ||
| Wohlfahrtia nuba | – | L(2) | |||
| 100 | Calliphoridae | Rhyncomya sp. | – | L(8) | |
| Sarcophagidae | Dolichotachina marginella | – | L(6) | ||
| 110 | – | – | – | – | |
| 120 | Phoridae | Megaselia scalaris | L(4) | – | |
| Total dry | 32 | 180 | |||
| Mean | 2.28 ± 0.78 bc | 12.86 ± 2.67 ab | |||
| F-value | 4.91 | ||||
L = larvae, A = adult. Number of insects collected in parenthesis.
The dry stage began on day 60 and continued until day 120. Six species continued to appear, namely Rhyncomya sp, S. dux, W. nuba, D. marginella, M. domestica, and Me. scalaris. While five species were not recorded in this stage of decomposition, which is Ch. albiceps, Ch. megacephala, Ch. rufifaces, H. pulchra, and P. alceae. Where its presence was limited to the decay/advanced decay stage only. The results of the statistical decomposition showed significant differences (p ≤ 0.05, F = 4.91) between the decay and dry stages of decomposition and, also, between both depths in terms of the abundance of fly species (Table 4). In terms of decomposition time, fly populations, and the species observed, there is a definite difference between buried and surface carcasses (Table 5).
Table 5.
Timing and succession of fly species collected from control rabbit carcasses placed on the soil surface.
| Days | family | Genus and species | D. stage | Numbers |
|---|---|---|---|---|
| 1–2 | – | – | Fresh | – |
| 3–7 | Calliphoridae | Chrysomya albiceps | Bloated | A(1 5 6) |
| Hemipyrellia pulchra | A(4) | |||
| Sarcophagidae | Sarcophaga sp. | A(20) | ||
| Muscidae | Musca domestica | A(1 8 8) | ||
| Musca sorbens | A(32) | |||
| Ephydridae | Eremotrichoma perspiciendum | A(4) | ||
| 8–12 | Calliphoridae | Chrysomya albiceps | Decay | A(1 0 4)+L(50) |
| Chrysomya rufifaces | L(2 0 0) | |||
| Hemipyrellia pulchra | A(2) | |||
| Sarcophagidae | Sarcophaga sp. | A(6) | ||
| Wohlfahrtia nuba | L(16) | |||
| Muscidae | Musca domestica | A(14) | ||
| Musca sorbens | A(36) | |||
| Ephydridae | Eremotrichoma perspiciendum | A(2) | ||
| Ulidiidae | Physiphora alceae | A(6) | ||
| 13–30 | Calliphoridae | Chrysomya albiceps | Dry | A(68) |
| Bengalia sp. | A(16) | |||
| Calliphora vicina | A(2) | |||
| Rhyncomya sp. | A(26) | |||
| Sarcophagidae | Wohlfahrtia nuba | A(2) | ||
| Sarcophaga sp. | A(6) | |||
| Dolichotachina marginella | A(30) | |||
| Muscidae | Musca domestica | A(6) | ||
| Musca sorbens | A(8) | |||
| Ulidiidae | Physiphora alceae | A(2) |
L = larvae, A = adult. Number of insects collected in parenthesis.
4. Discussion
Criminals frequently bury bodies to conceal them, but soil hardness and burial depth act as physical barriers that have a substantial impact on temperature and insect succession (Payne et al., 1968). In our investigation, rabbit carcasses were buried at a depth of 20 and 40 cm, and these depths were adequate to reduce the pace of decay. These findings agree with those made on pig carcasses by Bonacci et al. in Italy in 2021 (Bonacci et al., 2021). During the study period, there was a difference in the rate of decomposition between buried and surface corpses. In comparison to the buried corpses, the control carcass decomposed more quickly. Carcasses placed on the surface of the ground are in direct touch with some abiotic elements, such as temperatures, wind, and rainfall, which makes succession and arthropod colonization by specific arthropod species easier to happen. The rate of a cadaver's decomposition is known to be significantly influenced by abiotic variables (Byrd and Tomberlin, 2019, George et al., 2013). Buried cadavers are exposed to different sets of abiotic factors and biotic elements driving decomposition processes, such as increased bacterial activity, fungal growth, and various sets of arthropod colonization, in contrast to above-ground scenarios, where they are exposed to ambient temperatures, wind, and rain (Haglund and Sorg, 1997, Tibbett and Carter, 2008). In excessively dry soils, microbial and soil organism activity is reduced, according to (Janaway, 2013). The cold weather and dry soils prevented enhanced activity of soil organisms and microbes, which resulted in comparatively slow decomposition and, consequently, buried carcasses losing biomass at a slow rate.
The first signs of below-ground colonization were observed 30 days after burial, indicating that dipteran succession occurred on the buried carcasses at a significantly slower rate than on the control carcasses. Although they were placed in their placements simultaneously, the uncovered carcasses had a distinct insect successional pattern compared to buried ones. Species such as Rhyncomya sp, W. nuba, and D. marginella first arrived on buried carcasses on deep 20 cm. In contrast to what happened in the surface carcasses, the first appearance of the following species Ch. albiceps, H. pulchra, Sarcophaga sp, M. domestica, and M. sorbens was recorded. Species Rhyncomya sp was not recorded in any study in Saudi Arabia on surface carcasses, while it was recorded in South Africa (Tembe and Mukaratirwa, 2020) and Nigeria (Izuma Joshua 2019). It was also found to be attracted to termite nests (Zumpt, 1958, Zumpt and Tsacas, 1978). This was also observed in our study. Also, type W. nuba was not recorded except on surface carcasses only (Al-Qahtni et al., 2020, Haddadi et al., 2019). As for type D. marginella, it was not recorded in the Saudi fauna but was documented in the Middle East in the United Arab Emirates and Egypt (Verves and Khrokalo, 2018). It was recorded on buried carcasses in Sudan (Yassin and Mohamed, 2015). The appearance of these types on buried corpses is very curious and requires further verification in Saudi Arabia.
Flesh fly S. dux were present in abundance, at both 20 and 40 cm depths, and this might be a result of the sandy soil, which facilitates the larvae's ability to dig and access the carcasses for feeding. (Rodriguez and Bass, 1985) saw a similar event when they observed different species of flesh flies larvipositing close to soil fissures and the immatures burrowed down to the carcass. Therefore, it appears that the sandy soil plays a role in easing the tunneling process and enabling access to carcasses. At a depth of 60 cm, other species of the genus Sarcophaga that are unknown were found (Botham 2016). The presence of S. africa and S. bullata were previously reported in buried remains at 20–40 cm depth (Albushabaa, 2016) (Pastula and Merritt, 2013). Ch. albiceps, Ch. Rufifaces, and Ch. megacephala were collected from carcasses buried at a depth of 20 cm and have been reported in different previous studies (Albushabaa, 2016, Leşinin and Bölgesinden, 2018, Sharif and Qamar, 2021). Ch. rufifacies larvae can burrow several inches into the soil to colonize buried remains (Byrd and Tomberlin, 2019). In addition, P. alceae was also found at both depths in our study and was not previously recorded on buried carcasses to our knowledge, but was only reported in surface carcasses (Al-Mekhlafi et al., 2020, Al-Qahtni et al., 2020, Haddadi et al., 2019). The presence of P. alceae at both depths suggests that this species may exhibit a more diverse foraging behavior than previously thought, and it highlights the importance of considering various ecological factors and adaptations that can influence the behavior and distribution of carrion-feeding insects. Further research may be needed to understand the specific factors driving this behavior in P. alceae.
Two fly species, H. pulchra and M. domestica, were collected 40 days after burial at a depth of 40 cm, indicating that insects would take longer to colonize carcasses buried at 40 cm compared to 20 cm. H. pulchra was not recorded on carcasses, but (Nandi, 2002) stated that it was attracted to dead animals. It was attracted to the bait consisting of 250 g of beef offal (Klong-Klaew et al., 2018), while M. domestica was recorded on buried carcasses (Bala and Kaur, 2015, Kekillioğlu and Başar, 2021).
Species M. scalaris is a common and typical forensic indicator reported in buried remains (Botham, 2016, Bourel et al., 2004, Byrd and Tomberlin, 2019, Pastula and Merritt, 2013, Sharif and Qamar, 2021, Turner and Wiltshire, 1999). It was found in the decay/advanced decay and dry stages at a depth of 20 cm in very low numbers. This may be attributed to soil texture or other factors. This is also consistent with the study of (Bonacci et al., 2021). According to reports, the only species inhabiting a human corpse buried in a wooden coffin at a depth of 30 to 40 cm in Italy are coffin flies, M. scalaris. Campobasso et al 2004 also found these species in pig carcasses that were buried 60 cm deep (Pastula and Merritt, 2013). M. scalaris has been found colonizing remnants at depths of up to 1.8 m (Merritt et al., 2007), so it is not unexpected that it can colonize a cadaver at a depth of 20 cm. (Al-Qahtni et al., 2019) recorded M. scalaris in Riyadh, Saudi Arabia, on human corpses inside the building and did not record it on corpses outside the building. It was also found on human corpses inside a car (Al-Khalifa et al., 2020). The Phoridae family prefers enclosed environments as rooms with closed doors and windows or sealed plastic bags (Reibe and Madea, 2010). Overall, these findings may be useful in forensic investigations concerning buried bodies in Riyadh, Saudi Arabia.
5. Conclusion
Our findings constitute an experimental investigation of the impacts of burial on diptera colonization of carcasses, which should be expanded to various habitats, including suburban, urban, and forest ones, as well as various seasons, to support future forensic investigations. Other factors besides depth and time may also play a role in insect community separation, such as the kind of soil and the presence of microbes. It is necessary to conduct more research on the influences of soil type and microbial activity on insect arrival and succession. In addition to studying the succession of various necrophagous insects, such as Coleoptera, Lepidoptera, and Hymenoptera, it would be valuable to conduct a comprehensive investigation encompassing these insect groups. This expanded research approach will provide a more holistic understanding of the ecological dynamics surrounding carcass decomposition and its relevance to forensic applications.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors express their sincere appreciation to the Researchers Supporting Project number (RSP2023R112), King Saud University, Riyadh, Saudi Arabia.
Funding
This project was funded by Researchers Supporting Project number (RSP2023R112), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Osama Al-Zahrani, Email: 441105606@student.ksu.edu.sa.
Mohammed S. Al-Khalifa, Email: mkhalifa@ksu.edu.sa.
Abdulmani H. Al-Qahtni, Email: abdumani@ncw.gov.sa.
Fahd A. AL-Mekhlafi, Email: falmekhlafi@ksu.edu.sa.
References
- Albushabaa S.H.H. Insect succession and decomposition of buried rabbits during two seasons in Al Kufa City-Iraq. Res. J. Pharma. Biol. Chem. Sci. 2016;7(5):2976–2985. [Google Scholar]
- Al-Khalifa M.S., Mashaly A.M., Al-Qahtni A.H. Insect species colonized indoor and outdoor human corpses in Riyadh, Saudi Arabia. J. King Saud Univ.-Sci. 2020;32(3):1812–1817. [Google Scholar]
- Al-Mekhlafi F.A. Decomposition process for buried rat (Rattus norvegicus, Berkenhout 1769) carcasses in Riyadh city, Saudi Arabia: A preliminary study. Saudi J. Biol. Sci. 2021;28(7):3745–3750. doi: 10.1016/j.sjbs.2021.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mekhlafi, F.A., Alajmi, R.A., Almusawi, Z., Abd Al GAlil, F.M., Kaur, P., Al-Wadaan, M., Al-Khalifa, M.S., 2020. A study of insect succession of forensic importance: Dipteran flies (diptera) in two different habitats of small rodents in Riyadh City, Saudi Arabia. Journal of King Saud University-Science 32(7), 3111-3118.
- Al-Qahtni A.H., Mashaly A.M., Alajmi R.A., Alshehri A.A., Al-Musawi Z.M., Al-Khalifa M.S. Forensic insects attracted to human cadavers in a vehicular environment in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2019;26(7):1499–1502. doi: 10.1016/j.sjbs.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qahtni A.H., Al-Khalifa M.S., Mashaly A.M. Two human cases associated with forensic insects in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2020;27(3):881–886. doi: 10.1016/j.sjbs.2019.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala M., Kaur P. Entomofauna on decomposed piece of pork: Study on delayed burial. J. Entomol. Res. 2015;39(1):77–84. [Google Scholar]
- Bonacci T., Mendicino F., Bonelli D., Carlomagno F., Curia G., Scapoli C., Pezzi M. Investigations on arthropods associated with decay stages of buried animals in Italy. Insects. 2021;12(4):311. doi: 10.3390/insects12040311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botham J.L. University of the Free State; 2016. Decomposition and arthropod succession on buried remains during winter and summer in Central South Africa: Forensic implications and predictive analyses. [Google Scholar]
- Bourel B., Tournel G., Hédouin V., Gosset D. Entomofauna of buried bodies in northern France. Int. J. Leg. Med. 2004;118:215–220. doi: 10.1007/s00414-004-0449-0. [DOI] [PubMed] [Google Scholar]
- Byrd J.H., Tomberlin J.K. CRC Press; 2019. Forensic entomology: the utility of arthropods in legal investigations. [Google Scholar]
- Catts E.P., Goff M.L. Forensic entomology in criminal investigations. Annu. Rev. Entomol. 1992;37(1):253–272. doi: 10.1146/annurev.en.37.010192.001345. [DOI] [PubMed] [Google Scholar]
- Courtney G. Morphology and terminolgoy of Diptera larvae. Contrib. Manual Palaearctic Diptera. 2000;1:85–161. [Google Scholar]
- George K.A., Archer M.S., Toop T. Abiotic environmental factors influencing blowfly colonisation patterns in the field. Forensic Sci. Int. 2013;229(1–3):100–107. doi: 10.1016/j.forsciint.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Gilbert B.M., Bass W.M. Seasonal dating of burials from the presence of fly pupae. Am. Antiq. 1967;32(4):534–535. [Google Scholar]
- Haddadi R., Alajmi R., Abdel-Gaber R. A comparative study of insect succession on rabbit carrion in three different microhabitats. J. Med. Entomol. 2019;56(3):671–680. doi: 10.1093/jme/tjy235. [DOI] [PubMed] [Google Scholar]
- Haglund W.D., Sorg M.H. CRC Press; 1997. Forensic taphonomy: the postmortem fate of human remains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchet J.-B., Greenberg B. Flies, Mochicas and burial practices: a case study from Huaca de la Luna, Peru. J. Archaeol. Sci. 2010;37(11):2846–2856. [Google Scholar]
- Huchet, J.-B., 2014. Insect remains and their traces: relevant fossil witnesses in the reconstruction of past funerary practices. Anthropologie (1962-) 52(3), 329-346.
- Izuma Joshua , N., M. Aline E, 2019. Succession Patterns and Diversity of Arthropods Associated with Decomposing Domestic Rabbit (Oryctolagus cuniculus L, 1758) in Different Habitats. Environment and Ecology Research 7(6), 303 - 312.
- Janaway R. The decay of buried human remains and their associated materials, Studies in crime: an introduction to forensic archaeology. Routledge. 2013:58–85. [Google Scholar]
- Kekillioğlu A., Başar M. Research on the ecological success role of the muscidae (Insecta: diptera) species. Eurasian J. Sci. Eng. Technol. 2021;2(1):36–42. [Google Scholar]
- Klong-Klaew T., Ngoen-Klan R., Moophayak K., Sukontason K., Irvine K.N., Tomberlin J.K., Somboon P., Chareonviriyaphap T., Kurahashi H., Sukontason K.L. Predicting geographic distribution of forensically significant blow flies of subfamily Chrysomyinae (Diptera: Calliphoridae) in Northern Thailand. Insects. 2018;9(3):106. doi: 10.3390/insects9030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leşinin O.Ü.Y.S.D., Bölgesinden D. Assessment of Entomological Remains from Soil Samples Collected from a Pig (Sus scrofa domestica) Carcass Decomposition Site after 13 Years. Turkiye Parazitol. Derg. 2018;42(4):281–285. doi: 10.5152/tpd.2018.5917. [DOI] [PubMed] [Google Scholar]
- Marshall S., Whitworth T., Roscoe L. Blow flies (Diptera: Calliphoridae) of eastern Canada with a key to Calliphoridae subfamilies and genera of eastern North America, and a key to the eastern Canadian species of Calliphorinae, Luciliinae and Chrysomyiinae. Canad. J. Arthropod. Identification. 2011;11(11):1–93. [Google Scholar]
- Mashaly A.M.A., Al-Mekhlafi F.A. Differential Diptera succession patterns on decomposed rabbit carcasses in three different habitats. J. Med. Entomol. 2016;53(5):1192–1197. doi: 10.1093/jme/tjw079. [DOI] [PubMed] [Google Scholar]
- Merritt R.W., Snider R., De Jong J.L., Benbow M.E., Kimbirauskas R.K., Kolar R.E. Collembola of the grave: a cold case history involving arthropods 28 years after death. J. Forensic Sci. 2007;52(6):1359–1361. doi: 10.1111/j.1556-4029.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- Moses R.J. Experimental adipocere formation: implications for adipocere formation on buried bone. J. Forensic Sci. 2012;57(3):589–595. doi: 10.1111/j.1556-4029.2011.02032.x. [DOI] [PubMed] [Google Scholar]
- Nandi B. Blow flies (Diptera: Calliphoridae) of West Bengal, India with a note on their biodiversity. Rec. Zool. Surv. India. 2002;100(1–2):117–129. [Google Scholar]
- Pastula E., Merritt R. Insect arrival pattern and succession on buried carrion in Michigan. J. Med. Entomol. 2013;50(2):432–439. doi: 10.1603/me12138. [DOI] [PubMed] [Google Scholar]
- Payne J.A., King E.W., Beinhart G. Arthropod succession and decomposition of buried pigs. Nature. 1968;219(5159):1180–1181. doi: 10.1038/2191180a0. [DOI] [PubMed] [Google Scholar]
- Reibe S., Madea B. Use of Megaselia scalaris (Diptera: Phoridae) for post-mortem interval estimation indoors. Parasitol. Res. 2010;106:637–640. doi: 10.1007/s00436-009-1713-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez W.C., Bass W.M. Decomposition of buried bodies and methods that may aid in their location. J. Forensic Sci. 1985;30(3):836–852. [PubMed] [Google Scholar]
- Setyaningrum H., Al Dhafer H.M. The calliphoridae the blow flies (Diptera: Oestroidea) of Kingdom of Saudi Arabia. Egypt Acad. J. Biol. Sci. A, Entomol. 2014;7(1):49–139. [Google Scholar]
- Sharif S., Qamar A. Insect faunal succession on buried goat carcass in Aligarh Region of Uttar Pradesh, India, with implications in forensic entomology. Egypt. J. Forensic Sci. 2021;11:1–8. [Google Scholar]
- Shean B.S., Messinger L., Papworth M. Observations of differential decomposition on sun exposed v. shaded pig carrion in coastal Washington State. J. Forensic Sci. 1993;38(4):938–949. [PubMed] [Google Scholar]
- Smith K.G. A manual of forensic entomology. Parasitol. Today. 1986;3:163–164. [Google Scholar]
- Tembe D., Mukaratirwa S. Forensic entomology research and application in southern Africa: A scoping review. S. Afr. J. Sci. 2020;116(5–6):1–8. [Google Scholar]
- Tibbett M., Carter D.O. CRC Press; 2008. Soil analysis in forensic taphonomy: chemical and biological effects of buried human remains. [Google Scholar]
- Turner B., Wiltshire P. Experimental validation of forensic evidence: a study of the decomposition of buried pigs in a heavy clay soil. Forensic Sci. Int. 1999;101(2):113–122. doi: 10.1016/s0379-0738(99)00018-3. [DOI] [PubMed] [Google Scholar]
- VanLaerhoven S., Anderson G. Insect succession on buried carrion in two biogeoclimatic zones of British Columbia. J. Forensic Sci. 1999;44(1):32–43. [PubMed] [Google Scholar]
- Verves Y., Khrokalo L. The Sarcophagidae (Diptera) of the Middle East. Zool. Middle East. 2018;64(3):273–282. [Google Scholar]
- Yassin, A.E.T., Mohamed, E.A.E.R., 2015. A review of the family Sarcophagidae (Diptera) in the Suda.
- Zumpt F. Calliphoridae (Diptera Cyclorrhapha). Part II.: Rhiniini. Explor. Parc Natn. Albert Miss. GF De Witte. 1958;92:1–207. [Google Scholar]
- Zumpt, F., Tsacas, L., 1978. Taxonomic notes on Higher Díptera placed by E. Séguy in the genus Rhyncomya Robineau-Desvoidy [Dipt. Calliphoridae Rhiniinae]. Bulletin de la Société entomologique de France 83(3), 85-93.