Highlights
-
•
We identified a high rate of past arboviral infection among postpartum women.
-
•
172 (57%) of the participants tested positive for anti-Zika virus immunoglobulin G.
-
•
Most of the participants remain susceptible to future chikungunya virus infection.
Keywords: Zika virus, Dengue virus, Chikungunya virus
Abstract
Objectives
Arboviruses represent a major challenge to public health in Brazil. Dengue (DENV) virus has been endemic for decades, and the introduction of Zika (2015) and Chikungunya (2014) viruses (CHIKV) has imposed a significant burden on the country. The present study aimed to investigate the seroprevalence of Zika virus (ZIKV), DENV and CHIKV in women in Salvador, Bahia-Brazil.
Methods
Cross-sectional study involving postpartum women admitted to a maternity hospital in Salvador, Brazil. Anti-ZIKV, anti-DENV and anti-CHIKV immunoglobulin G was measured by enzyme-linked immunosorbent assay.
Results
A total of 302 women were enrolled with a median age: 26 years, interquartile range (21-33). Most self-declared as mixed-race or black skin color (92.4%). The seroprevalence was 57% for ZIKV); 91.4% for DENV, and 7.6% for CHIKV. Most participants denied awareness of previous arboviral infection, although 67 (22.3%) reported a previous history of ZIKV infection, 34 (11.1%) DENV infection and 9 (3%) CHIKV infection.
Conclusion
Our data indicate a high prevalence of past ZIKV and DENV infections in the population studied. Most of the participants remain susceptible to future CHIKV infection, highlighting the need for preventive and educational interventions. Our results suggest the need for continuous epidemiological surveillance of arboviral diseases, particularly among women residing in at-risk regions.
Introduction
Arboviruses represent a major challenge for public health in Brazil. Up to epidemiological week 21 in 2023, the Pan American Health Organization (PAHO) reported that 1,515,460 suspected cases of Dengue (DENV), 124,270 suspected cases of Chikungunya (CHIKV) and close to 7352 suspected Zika virus (ZIKV) cases had occurred in Brazil [1]. Maternal infection with any of these arboviruses during pregnancy has the potential to also infect the fetus and result in congenital disease.
Dengue is endemic in the country, characterized by numerous outbreaks related to different viral serotypes in recent decades [2]. DENV was first described in pregnancy in 1948, and since then reports have linked maternal infection with premature delivery and low birthweight [3].
Following its introduction in Brazil in 2014, the first autochthonous cases of Chikungunya virus were reported in the north and northeastern regions of the country [4]. Pregnant women infected late in pregnancy or close to delivery can transmit CHIKV to the fetus, with outcomes such as myelitis, encephalomyelitis, meningitis or irritability being associated with infection in newborns [5].
In May 2015, the transmission of ZIKV was confirmed in Brazil [6]. Infection had previously been considered benign and self-limiting; however, in late 2015, an unexpected outbreak of newborns with microcephaly in major cities in northeastern Brazil led the Ministry of Health to declare a public health emergency. Later, the association between microcephaly and maternal Zika infection was confirmed [7]. Since 2015, 3563 cases of microcephalic newborns were reported in Brazil, mostly in the state of Bahia (584 cases) [8].
Despite a substantial drop in the number of ZIKV cases since 2017, the case rates for DENV and CHIKV have remained high in the country [1]. Estimating the seroprevalence of arbovirus infection in pregnant and postpartum women can aid our understanding of the dynamics and scale of disease transmission. It can also provide information regarding populational susceptibility for future infection, especially in the context of congenital transmission [9,10]. Accordingly, the present study aimed to investigate the seroprevalence of ZIKV, DENV and CHIKV in puerperal women at a maternity hospital located in Salvador, Bahia-Brazil.
Methods
The present cross-sectional study was conducted between September and November 2017 at the Instituto de Perinatologia da Bahia (IPERBA), a public maternity hospital located in Salvador, Brazil, more than a year after the Zika virus outbreak in northeastern Brazil.
Consecutive asymptomatic pregnant women admitted for delivery were recruited during hospitalization on weekdays (Monday through Friday). Informed consent was obtained from all participants, or from the mother's legal guardian if the mother was not of legal age (i.e., a minor).
Sociodemographic data and clinical information were obtained through interviews. Data entry and data management were performed using REDCap (Vanderbilt University, Nashville, TN, USA).
Biological samples were collected by health professionals at the maternity hospital following childbirth. For participants who consented to blood draw, a single serum separator tube was used for collection, then centrifuged and aliquoted, followed by storage at −80°C. Serological testing was performed at the Gonçalo Moniz Institute, Oswaldo Cruz Foundation (IGM-FIOCRUZ) in Salvador-Brazil.
For serological analysis, the detection of specific anti-ZIKV, anti-CHIKV and anti-DENV immunoglobulin G (IgG) antibodies was performed using indirect enzyme-linked immunosorbent assays (ELISA) (Euroimmun; Lüberg, Germany) in accordance with the manufacturer's protocols. Results were obtained by calculating the ratio between the optical density of each patient sample compared to that of the calibrator supplied with the kit, in which samples with a ratio <0.8 were considered negative, samples between ≥0.8 to <1.1 were considered borderline, and samples with a ratio ≥1.1 were considered positive. All samples with initial borderline results were retested twice.
Serological results for HIV, hepatitis B and C and syphilis were obtained from medical records.
Ethical approval
This study was approved by the Institutional Review Board of the Gonçalo Muniz Institute, Oswaldo Cruz Foundation (protocol no. 51889315.7.0000.0040 /2016). All participants or their legal guardians provided written informed consent.
Results
During the study period, 308 postpartum women were enrolled and 302 consented to blood draw. All were asymptomatic at the time of hospital admission/enrollment.
Demographic and clinical characteristics
The median participant age was 26 years, interquartile range (21-33). Most (92.4)% were mixed-race or black. Around 50% were single and had completed high school. With regard to obstetric history, 108 (35.8%) were primigravidae and 76 (25.2%) reported previous miscarriage or abortion. The majority (90.7%) had no previous history of chronic disease or comorbidities. Twenty (6.6%) participants reported having hypertension, and four (1.3%) were diabetic. Twenty-five cases (8.3%) of syphilis infection were detected, as well as one case of hepatitis B, but no cases of HIV infection were identified. The participants’ sociodemographic and clinical data are presented in Table 1.
Table 1.
Sociodemographic and clinical data on 302 postpartum women (Salvador, Brazil-2017).
| Characteristics | n (%) |
|---|---|
| Median age, year (interquartile range) | 26 (21-33) |
| Self-reported skin color | |
| Black | 122 (40.4) |
| Mixed-race | 157 (51.9) |
| White | 23 (7.6) |
| Other | 0 (0) |
| Education level | |
| Grade school | 93 (30.8) |
| High school | 168 (55.6) |
| University | 41 (13.6) |
| Household monthly income (units of Minimum Wage—MW)a | |
| <1 MW | 37 (12.3) |
| 1 to 2 MW | 157 (52.0) |
| 3 to 4 MW | 52 (17.2) |
| 4 to 5 MW | 11 (3.6) |
| >5 MW | 3 (1.0) |
| Unknown | 42 (13.9) |
| Marital status | |
| Single | 148 (49.8) |
| Married | 49 (16.2) |
| Divorced | 1 (0.3) |
| Common-law marriage | 104 (34.4) |
| Obstetric history | |
| Primigravida | 107 (35.5) |
| Secundigravida | 93 (30.9) |
| Multigravida | 101 (33.6) |
| History of miscarriage/abortion | 76/194 (39.2) |
| History of previous or chronic disease | |
| None | 273 (90.7) |
| Diabetes | 4 (1.3) |
| Hypertension | 20 (6.6) |
| Hepatitis B | 1 (0.3) |
| Hepatitis C | 0 (0.0) |
| Syphilis | 25 (8.1) |
| HIV | 0 (0.0) |
| Previous history of arboviral infection | |
| Self-declared Zika infection | 67 (22.3) |
| Self-declared Dengue infection | 34 (11.3) |
| Self-declared Chikungunya infection | 9 (3.0) |
Based on monthly minimum wage in 2017 (R$ 937.00, equivalent to US$ 282.8)
The majority of the participants denied previous arboviral infection. However, 67 (22.3%) reported a previous history of ZIKV infection, 34 (11.1%) DENV and 9 (3%) CHIKV infection.
Arboviral serology
Antibodies against one or more of the three viruses were present in 283 (93.7%) out of 302 puerperal women; 172 (57%) tested positive for anti-ZIKV IgG, 276 (91.4%) for anti-DENV IgG and 23 (7.6%) for anti-CHIKV IgG (Table 2).
Table 2.
Arboviral serology of 302 postpartum women (Salvador, Brazil-2017).
| Serology positivity | N (%) | Confidence interval |
|---|---|---|
| Anti-ZIKV IgG | 172 (57) | (51.33-62.44) |
| Anti-DENV IgG | 275 (91.36) | (87.44-93.89) |
| Anti-CHIKV IgG | 23 (7.6) | (4.90-11.20) |
CHIKV, Chikungunya virus; DENV, Dengue virus; Ig, immunoglobulin; ZIKV, Zika virus.
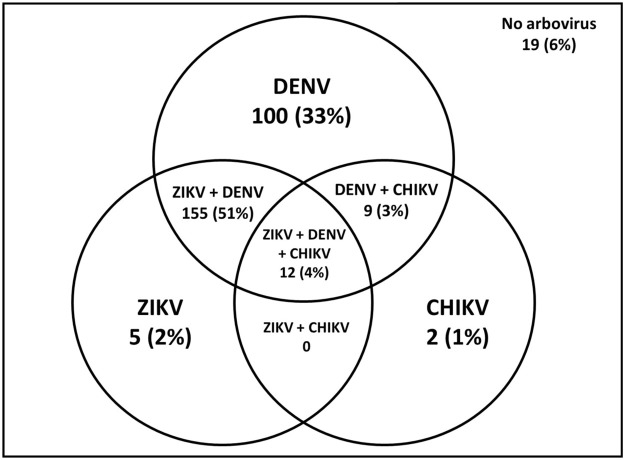
Most samples were positive for at least two arboviruses, with 155 (51.3%) were positive for both anti-Zika IgG and anti-Dengue-IgG, 9 (3.0%) were positive for DENV and CHIKV and 12 (4.0%) were positive for all three arboviruses. (Figure 1).
Figure 1.
Serological detection of specific ZIKV, DENV and CHIKV immunoglobulin G antibodies in 302 postpartum women (Salvador-Brazil, 2017).
CHIKV, Chikungunya vírus; DENV, Dengue vírus; ZIKV, Zika virus.
Discussion
After the introduction of CHIKV and ZIKV viruses in Brazil, in 2014 [4] and 2015 [6] respectively, few studies have focused on the seroprevalence of these arboviruses in the country. The present investigation sought to provide information on previous exposure to arboviruses in a region of the country that was greatly impacted by previous epidemics. Moreover, the present sample consisted of women of childbearing age, which serves to enhance our understanding of prior arbovirus transmission in a population vulnerable to severe outcomes associated with arbovirus infection, i.e., congenital infection due to ZIKV and CHIKV infection during pregnancy.
In regions with a previous history of documented ZIKV outbreaks, few serosurveys have been conducted. However, the available data is suggestive of high viral circulation in affected communities. In French Polynesia, a populational study found a seroprevalence of ZIKV IgG antibodies in 49% of participants [11], while a study of blood donors in Martinique revealed a seroprevalence of 42% [12]. Our results indicate a higher level of seroprevalence (57%) in the population studied, which is similar to another study carried out in Salvador, Brazil reporting 63% seropositivity in a community cohort [13]. Information on the prevalence of ZIKV IgG antibodies in pregnant women is even more scarce [14]. In Colombia, 89% of screened pregnant women tested positive for ZIKV anti-IgG [15] compared to just 31% in Thailand [16].
The seroprevalence of dengue has been observed to be heterogenous between continents, as well as among different regions inside Brazil [17]. Regions in the Caribbean and Latin America have shown some of the highest prevalence of dengue IgG, including 100% positivity among a sample of pregnant women [18]. In Brazil, dengue seroprevalence studies have reported widely discrepant results among different regions and over different periods, ranging from 3% to 97% [17,19]. The present rate of positivity for dengue (91.4%) in pregnant women is similar to that reported by other Brazilian studies carried out in the country's northeastern region [20].
With regard to CHIKV seroprevalence, highly variable results have been observed worldwide, ranging from 0.4% to 82% [15,17]. Studies in Brazil carried out in the northeast have identified frequencies between 20% to 57% [21,22]. A cohort study conducted in the city of Fortaleza (Ceará-Brazil) identified that 37.2% of women of childbearing age tested positive for CHIKV IgG antibodies [23]. Herein, we observed a much lower frequency of CHIKV IgG antibodies (7.6%), suggesting relatively low CHIKV circulation in the city of Salvador, which corroborates findings from a local populational study that reported a seroprevalence of 11.8% for CHIKV [24]. This finding is notable in that a high frequency of women remain susceptible to future infection in the region presently studied.
We observed a high discrepancy between the degree of awareness of previous arboviral infection reported by our participants and the rates of IgG positivity for each respective arbovirus. For example, just 11.1% of the women believed that they had prior exposure to dengue, while we observed a seropositivity rate of 91.4%. It is known that many arboviral infections can present asymptomatically. Serosurveys investigating the rates of asymptomatic vs symptomatic patient presentations have indicated that the majority of infections went unnoticed [25], [26], [27]. In French Guiana, the proportion of symptomatic Zika infection reportedly varied from 17% to 35% in pregnant women [28], with a much higher seroprevalence (77%). These data stand in agreement with the discrepant results found herein: just 22.3% of the women reported a previous history of ZIKV infection, compared to 57% identified by serology for anti-ZIKV IgG.
For serological evaluations, we employed commercial ELISA kits with satisfactory specificities [29]. Even though, cross-reactivity among flaviviruses (ZIKV and DENV) may have occurred since DENVs and ZIKV share a high degree of similarity on the E and NS1 structural proteins, which are used as antigens in indirect serological ELISA tests [30]. One limitation of our study is the fact that neutralization testing, such as the Plaque Reduction Neutralization Test (PRNT), was not performed. Nonetheless, we believed that the prevalence rates for anti-ZIKV and anti-DENV IgG we observed are pretty accurate since it's similar to another study carried out in Salvador, which performed both ELISA for anti-ZIKV IgG and ZIKV PRNT. These authors identified a 63.3% prevalence of anti-ZIKV IgG and also found a 62.5% rate of neutralizing antibodies against ZIKV, with high agreement (85.3%) between both methodologies [31].
Our results indicate a very high prevalence of past ZIKV and DENV infections in postpartum women, reinforcing the notion that the ZIKV outbreak in 2015-2016 affected a large swath of the region's population, in addition to the known endemic circulation of DENV. The high frequency of antibodies against these flaviviruses in the studied population may be linked to the dramatic decline in ZIKV case numbers since 2017, despite the continued presence of the vector responsible for transmission and the continued sporadic transmission of ZIKV reported in the region.
No approved vaccines for CHIKV and ZIKV are available. However, several preclinical candidates have been evaluated and some clinical trials are in progress [32], [33], [34]. A licensed CHIKV vaccine is expected to be released soon [35]. Currently, two licensed vaccines for DENV are available, and others have been tested in clinical trials [36]. It's noteworthy to mention that the determination of DENV pre-exposure (by IgG antibodies) has been considered relevant in the context of dengue vaccine Dengvaxia® since there is evidence of an excess risk of severe dengue in IgG seronegative vaccine recipients [37,38]. With these new perspectives on vaccination against DENV, CHIKV, and ZIKV, seroprevalence studies are necessary and can contribute to guiding the prioritization of target audiences for vaccine campaigns.
Our data shed light on the high rate of past arboviral infection among postpartum women living in Salvador, Brazil. Considering that most arboviral infections occur asymptomatically, together with the evidence on potential risks to pregnancy and infant outcomes, the present results highlight the need for continuous epidemiological surveillance of arboviral disease, particularly among women residing in at-risk regions. In addition, the findings further serve to indicate that many postpartum women likely remain susceptible to future CHIKV infection, thus necessitating the implementation of preventive and educational interventions.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This work has been supported by the National Council for Scientific and Technological Development [CNPq 443875/2018 and 316456/2021-7], theWHO HRP/TDR/PAHO Joint Small Grants Program for research on the Zika virus outbreak in the Americas [770084-0/2017], State of Bahia Research Support Foundation [Fapesb 19.573.201.5418] and by NIH NIAID U01AI151698, United World Antiviral Research Network, part of the NIAID CREID network.
Acknowledgments
The authors are grateful to the physicians and nurses involved in the patients’ clinical care and also thank Andris K. Walter who provided critical analysis, English language revision and manuscript copyediting assistance.
Author contributions
ICS and JR contributed to the study design. ICS, PPBF, MVF, BG, BLA contributed to data analysis and writing of the manuscript. PPBF, DVBR and IMBA tributed to participant enrollment, medical records review, and collection of samples and data. MVF, CSS and MSJS contributed to laboratory analysis.
References
- 1.Pan American Health Organization /World Health Organization . PAHO/WHO; Washington: 10 June 2023. Epidemiological update. Dengue, chikungunya and Zika; p. 2023. [Google Scholar]
- 2.Teixeira MG, Costa MDCN, Barreto F, Barreto ML. Dengue: twenty-five years since reemergence in Brazil. Cad Saude Publica. Fundacao Oswaldo Cruz. 2009;25:S7–18. doi: 10.1590/s0102-311x2009001300002. [DOI] [PubMed] [Google Scholar]
- 3.Basurko C, Carles G, Youssef M, Guindi WE. Maternal and fetal consequences of dengue fever during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009;147:29–32. doi: 10.1016/j.ejogrb.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Nunes MRT, Faria NR, de Vasconcelos JM, Golding N, Kraemer MU, de Oliveira LF, et al. Emergence and potential for spread of Chikungunya virus in Brazil. BMC Med. 2015;13:102. doi: 10.1186/s12916-015-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritel X, Rollot O, Gerardin P, Gauzere BA, Bideault J, Lagarde L, et al. Chikungunya virus infection during pregnancy, Reunion, France, 2006. Emerg Infect Dis. 2010;16:418–425. doi: 10.3201/eid1603.091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanluca C, De Melo VCA, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Araújo TVB, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo APL, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016;16:1356–1363. doi: 10.1016/S1473-3099(16)30318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Health, Brazil. Epidemiological bulletim: epidemiological Situation of Congenital Syndrome Associated to Zika Vírus Infection in 2020, until week 45, https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/boletins-epidemiologicos/edicoes/2020/boletim_epidemiologico_svs_47.pdf; 2020 [accessed 19 December 2022] [in Portuguese].
- 9.Alves LV, Leal CA, Alves JGB. Zika virus seroprevalence in women who gave birth during Zika virus outbreak in Brazil - a prospective observational study. Heliyon. 2020;6:e04817. doi: 10.1016/j.heliyon.2020.e04817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duarte AO, Oliveira JV, Carvalho TCX, Pessoa LB, Filho CM, Lima JGS, et al. Maternal and congenital infections arising from Zika, dengue and chikungunya arboviruses in Salvador, Brazil. Trans R Soc Trop Med Hyg. 2020;114:222–225. doi: 10.1093/trstmh/trz098. [DOI] [PubMed] [Google Scholar]
- 11.Aubry M, Teissier A, Huart M, Merceron S, Vanhomwegen J, Roche C, et al. Zika virus seroprevalence, French Polynesia, 2014–2015. Emerg Infect Dis. 2017;23:669–672. doi: 10.3201/eid2304.161549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallian P, Cabié A, Richard P, Paturel L, Charrel RN, Pastorino B, et al. Zika virus in asymptomatic blood donors in Martinique. Blood. 2017;129:263–266. doi: 10.1182/blood-2016-09-737981. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Barraquer I, Costa F, Nascimento EJM, Júnior Nery N, Castanha PMS, Sacramento GA, et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science. 2019;363:607–610. doi: 10.1126/science.aav6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell PK, Mier-Y-Teran-Romero L, Biggerstaff BJ, Delorey MJ, Aubry M, Cao-Lormeau VM, et al. Reassessing serosurvey-based estimates of the symptomatic proportion of Zika virus infections. Am J Epidemiol. 2019;188:206–213. doi: 10.1093/aje/kwy189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marbán-Castro E, Arrieta GJ, Martínez MJ, González R, Bardají A, Menéndez C, et al. High seroprevalence of antibodies against arboviruses among pregnant women in rural Caribbean Colombia in the context of the Zika virus epidemic. Antibodies (Basel) 2020;9:56. doi: 10.3390/antib9040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phatihattakorn C, Wongsa A, Pongpan K, Anuwuthinawin S, Mungmanthong S, Wongprasert M, et al. Seroprevalence of Zika virus in pregnant women from central Thailand. PLoS One. 2021;16 doi: 10.1371/journal.pone.0257205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritzell C, Rousset D, Adde A, Kazanji M, Van Kerkhove MD, Flamand C. Current challenges and implications for dengue, chikungunya and Zika seroprevalence studies worldwide: a scoping review. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood H, Drebot MA, Dewailly E, Dillon L, Dimitrova K, Forde M, et al. Seroprevalence of seven zoonotic pathogens in pregnant women from the Caribbean. Am J Trop Med Hyg. 2014;91:642–644. doi: 10.4269/ajtmh.14-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teixeira MG, Siqueira JB, Jr, Ferreira GLC, Bricks L, Joint G. Epidemiological trends of dengue disease in Brazil (2000–2010): a systematic literature search and analysis. PLoS Negl Trop Dis. 2013;7:e2520. doi: 10.1371/journal.pntd.0002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leite RC, Souza AI, Castanha PM, Cordeiro MT, Martelli CT, Ferreira AL, et al. Dengue infection in pregnancy and transplacental transfer of anti-dengue antibodies in Northeast, Brazil. J Clin Virol. 2014;60:16–21. doi: 10.1016/j.jcv.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Dias JP, Costa MDCN, Campos GS, Paixão ES, Natividade MS, Barreto FR, et al. Seroprevalence of Chikungunya virus after its emergence in Brazil. Emerg Infect Dis. 2018;24:617–624. doi: 10.3201/eid2404.171370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barreto FKA, Alencar CH, Araújo FMC, Oliveira RMAB, Cavalcante JW, Lemos DRQ, et al. Seroprevalence, spatial dispersion and factors associated with Flavivirus and chikungunha infection in a risk area: a population-based seroprevalence study in Brazil. BMC Infect Dis. 2020;20:881. doi: 10.1186/s12879-020-05611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correia F, Kerr L, Frota C, Barreto I, Almeida R, Pamplona L, et al. Factors associated with chikungunya infection in a cohort of women aged 15–39 y in Fortaleza. Brazil. Trans R Soc Trop Med Hyg. 2021;115:1070–1079. doi: 10.1093/trstmh/traa182. [DOI] [PubMed] [Google Scholar]
- 24.Anjos RO, Mugabe VA, Moreira PSS, Carvalho CX, Portilho MM, Khouri R, et al. Transmission of Chikungunya virus in an urban slum. Brazil. Emerg Infect Dis. 2020;26:1364–1373. doi: 10.3201/eid2607.190846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 26.Yoon IK, Alera MT, Lago CB, Tac-An IA, Villa D, Fernandez S, et al. High rate of subclinical Chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon A, Kuan G, Mercado JC, Gresh L, Avilés W, Balmaseda A, et al. The Nicaraguan pediatric dengue cohort study: incidence of inapparent and symptomatic dengue virus infections, 2004–2010. PLoS Negl Trop Dis. 2013;7:e2462. doi: 10.1371/journal.pntd.0002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flamand C, Fritzell C, Matheus S, Dueymes M, Carles G, Favre A, et al. The proportion of asymptomatic infections and spectrum of disease among pregnant women infected by Zika virus: systematic monitoring in French Guiana, 2016. Euro Surveill. 2017;22:17–00102. doi: 10.2807/1560-7917.ES.2017.22.44.17-00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low SL, Leo YS, Lai YL, Lam S, Tan HH, Wong JCC, et al. Evaluation of eight commercial Zika virus IgM and IgG serology assays for diagnostics and research. PLoS One. 2021;16 doi: 10.1371/journal.pone.0244601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaspar-Castillo C, Rodríguez MH, Ortiz-Navarrete V, Alpuche-Aranda CM, Martinez-Barnetche J. Structural and immunological basis of cross-reactivity between dengue and Zika infections: implications in serosurveillance in endemic regions. Front Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1107496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Netto EM, Moreira-Soto A, Pedroso C, Höser C, Funk S, Kucharski AJ, et al. High Zika virus seroprevalence in Salvador, Northeastern Brazil limits the potential for further outbreaks. mBio. 2017;8:e01390. doi: 10.1128/mBio.01390-17. e01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tebas P, Roberts CC, Muthumani K, Reuschel EL, Kudchodkar SB, Zaidi FI, et al. Safety and immunogenicity of an anti-Zika virus DNA vaccine. N Engl J Med. 2021;385:e35. doi: 10.1056/NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lima Cavalcanti TYV, Pereira MR, de Paula SO, RFdO Franca. A review on Chikungunya virus epidemiology, pathogenesis and current vaccine development. Viruses. 2022;14:969. doi: 10.3390/v14050969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folegatti PM, Harrison K, Preciado-Llanes L, Lopez FR, Bittaye M, Kim YC, et al. A single dose of ChAdOx1 Chik vaccine induces neutralizing antibodies against four Chikungunya virus lineages in a phase 1 clinical trial. Nat Commun. 2021;12:4636. doi: 10.1038/s41467-021-24906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider M, Narciso-Abraham M, Hadl S, McMahon R, Toepfer S, Fuchs U, et al. Safety and immunogenicity of a single-shot live-attenuated chikungunya vaccine: a double-blind, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2023;401:2138–2147. doi: 10.1016/S0140-6736(23)00641-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilder-Smith A. Dengue vaccine development: challenges and prospects. Curr Opin Infect Dis. 2022;35:390–396. doi: 10.1097/QCO.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 37.Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379:327–340. doi: 10.1056/NEJMoa1800820. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization Dengue vaccine: WHO position paper – September 2018. Wkly Epidemiol Rec. 2018;93:457–476. [Google Scholar]