Abstract
Background & Aims:
Ductular reaction expansion is associated with poor prognosis in patients with advanced liver disease. However, the mechanisms promoting biliary cell proliferation are largely unknown. Here, we identify neutrophils as drivers of biliary cell proliferation and the defective wound-healing response.
Methods:
The intrahepatic localization of neutrophils was evaluated in patients with chronic liver disease. Neutrophil dynamics were analyzed by intravital microscopy and neutrophil-labeling assays in DDC-treated mice. Neutrophil depletion or inhibition of recruitment was achieved using a Ly6g antibody or a CXCR1/2 inhibitor, respectively. Mice deficient in PAD4 (peptidyl arginine deiminase 4) and ELANE/NE (neutrophil elastase) were used to investigate the mechanisms underlying ductular reaction expansion.
Results:
In this study we describe a population of ductular reaction-associated neutrophils (DRANs), which are in direct contact with biliary epithelial cells in chronic liver diseases and whose numbers increased in parallel with disease progression. We show that DRANs are immobilized at the site of ductular reaction for a prolonged period of time. In addition, liver neutrophils display a unique phenotypic and transcriptomic profile, showing a decreased phagocytic capacity and increased oxidative burst. Depletion of neutrophils or inhibition of their recruitment reduces DRANs and the expansion of ductular reaction, while mitigating liver fibrosis and angiogenesis. Mechanistically, neutrophils deficient in PAD4 and ELANE abrogate neutrophil-induced biliary cell proliferation, thus indicating the role of neutrophil extracellular traps and elastase release in ductular reaction expansion.
Conclusions:
Overall, our study reveals the accumulation of DRANs as a hallmark of advanced liver disease and a potential therapeutic target to mitigate ductular reaction and the maladaptive wound-healing response.
Keywords: Ductular reaction, biliary cells, neutrophils, chronic injury, elastase, neutrophil extracellular traps, organoids
Graphical abstract
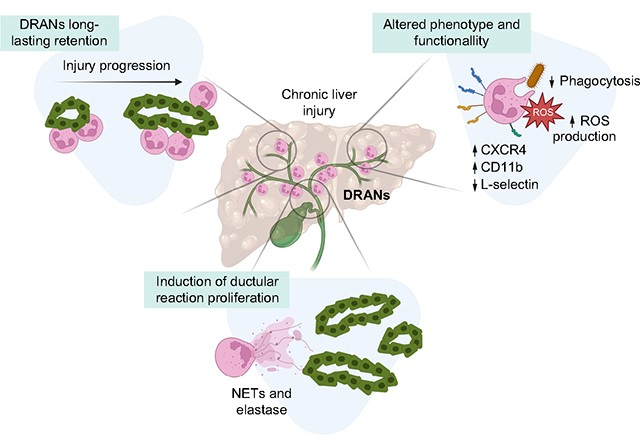
Introduction
Ductular reaction is a maladaptive regenerative response of the liver that occurs during advanced chronic liver diseases. Ductular reaction release pro-inflammatory mediators that lead to neutrophil recruitment,1–3 lymphocyte accumulation,4,5 hepatic stellate cell activation6,7 and new vessel formation,8 thus contributing to an abnormal wound-healing response. In advanced chronic liver diseases, ductular reaction expansion is associated with disease progression and poor patient outcomes.9–11
Neutrophils are the first line of defense against pathogens and infection but they also play important functions in tissue response to injury in both acute and chronic injury conditions.12–15 In acute sterile liver injury, recruited neutrophils perform key restorative functions and return to the circulation, migrating back to the bone marrow.16,17 However, neutrophil recruitment and function in chronic liver injury is still largely unexplored.
Chronic liver diseases have an important effect on circulating neutrophil function, with neutrophils showing reduced phagocytic capacity and enhanced superoxide production. 18–20 Recruited neutrophils are associated with increased liver injury, cholestasis and portal hypertension,3,21,22 and secretion of hydrolytic and oxidative molecules by neutrophils has been shownt to exacerbate tissue injury.23–26
Although infiltrating neutrophils are a common histological feature in advanced liver diseases, little is known about their recruitment dynamics, phenotype and role in liver wound-healing. In this study, we describe ductular reaction-associated neutrophils (DRANs), which accumulate at the biliary epithelium cells and display altered dynamics, phenotype and function. We show that neutrophils mediate biliary epithelium proliferation, contributing to the maladaptive wound-healing response. These findings suggest that targeting DRANs may be an appealing approach to promote an effective wound-healing response in liver disease.
Materials and methods
Patients
Liver paraffin-embedded sections of explants and biopsies from patients with different etiologies of liver disease admitted to the Liver Unit of the Hospital Clinic of Barcelona were used. Signed informed consent was obtained from all the patients, and the study was approved by the Ethics Committee of the Hospital Clinic. The alcohol-related liver disease cohort included patients at different disease stages: pre-cirrhosis (n = 5, F2–F3), compensated cirrhosis (n = 5, F4), decompensated cirrhosis (n = 5, F4) and alcohol-related hepatitis (n = 5, F4).
Animal models
Twelve-week-old C57BL/6J mice (Charles River) were fed with standard diet supplemented with 0.1% 3,5-diethoxycarbonyl-1,4-dihydro-collidin (DDC) (Sigma-Aldrich, St. Louis, MO) for 1 or 3 weeks. The 1-week DDC treatment, carbon tetrachloride (CCl4) injury model and isolation of control mice cells were performed using both male and female mice, whereas long-term (3-week) treatment with DDC and bile duct ligation were performed solely in male mice. Inhibition of CXCR1/2 receptor activity was performed with SCH-527123 (MedChemExpress) and neutrophil depletion was achieved by anti-Ly6G (1A8) antibody (BioXcell) administration. Neutrophil elastase- and protein arginine deiminase 4-deficient mice (Elane−/− and Pad4−/−) were obtained from Jackson Laboratory. All animal experiments were approved by the Ethics Committee of Animal Experimentation of the University of Barcelona. See supplementary data for details.
Results
Neutrophils are immobilized at the biliary epithelium in advanced chronic liver disease
We first determined the localization of infiltrating neutrophils in liver samples from patients with chronic liver disease. In advanced chronic liver disease, neutrophils were predominantly recruited at the periportal area and were closely associated with cytokeratin (KRT)7+ ductular reaction biliary cells in all the examined etiologies, including hepatitis C and B virus, non-alcoholic fatty liver disease, alcohol-related liver disease and primary biliary cholangitis (Fig. 1A and Fig. S1A). This observation suggests that the presence of ductular reaction-associated neutrophils, which we named DRANs, is a common feature in advanced liver diseases, independently of the cause or etiology. Moreover, we found that the extent of DRANs and their proximity to the site of ductular reaction increases with disease progression and correlates with ductular reaction expansion (Fig. 1B and Fig. S1B). Liver tissue clearing from patients with advanced chronic liver disease showed that DRANs are in cell contact with biliary epithelial cells (Fig. 1C and Supplementary Video 1).
Fig. 1. DRANs are recruited to the biliary epithelium.
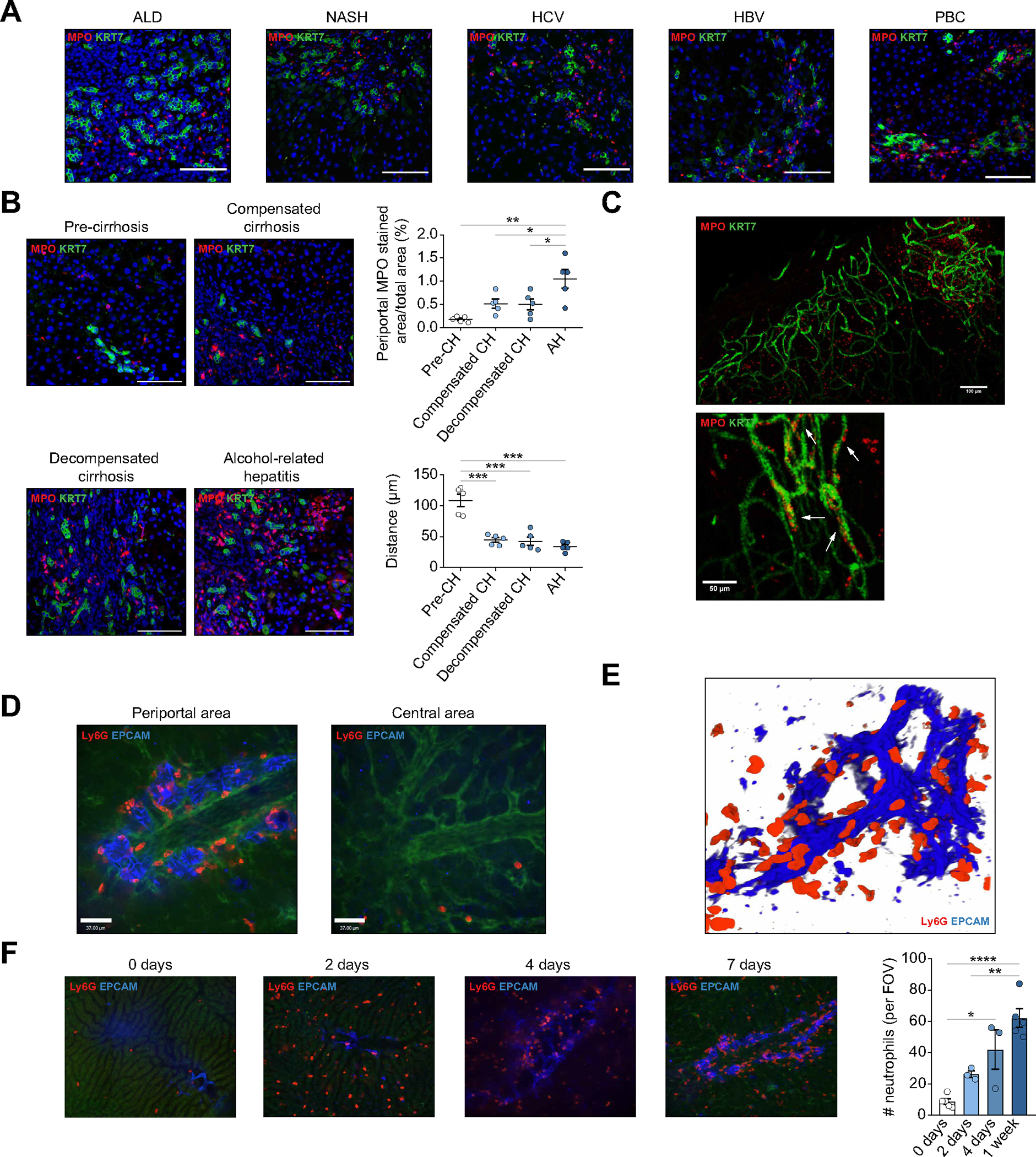
(A) Immunofluorescence of MPO and KRT7 in liver sections of patients with ALD, NASH, HCV, HBV and PBC. Scale bar: 100 μm. (B) Immunofluorescence of DRANs (MPO) at biliary epithelium (KRT7) in hepatic biopsies of patients with ALD. Percentage of MPO+ cells at periportal areas and minimum distance of MPO+ cells to KRT7+ cells. Scale bar: 100 μm. (C) Clearing of 3 mm-liver section of a patient with cirrhosis. Arrows show neutrophils (MPO) attached to biliary cells (KRT7). (D) SD-IVM images of periportal and central areas in DDC-treated mice. (E) 3D-reconstruction of neutrophils (Ly6G) recruited to the biliary epithelium (EpCAM) in mice treated with DDC for 1 week. (F) SD-IVM images of DDC-treated mouse showing the progression of neutrophil (Ly6G) recruitment to ductular reaction (EpCAM). All data is presented as mean ± SEM. *p <0.05, **p <0.01, ***p <0.001 as determined by one-way ANOVA with Tukey’s multiple comparison test (B, F). AH, alcohol-related hepatitis; ALD, alcohol-related liver disease; CH, cirrhosis; DDC, 3,5-diethoxycarbonyl-1,4-dihydrocollidine; DRANs, ductular reaction-associated neutrophils; EpCAM, epithelial cell adhesion molecule; FOV, field of view; KRT, cytokeratin; Ly6G, lymphocyte antigen 6 complex locus G6D; MPO, myeloperoxidase; NASH, non-alcoholic steatohepatitis; PBC, primary biliary cholangitis; SD-IVM, spinning-disk confocal intravital microscopy.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2023.05.045.
To evaluate the dynamic behavior of DRANs, we tracked neutrophils in vivo in mice treated with DDC diet.27 In DDC-treated mice (1 and 3 weeks of diet), neutrophils were located at the site of ductular reaction (73.3%±1.76) rather than within the sinusoids in the central area (Fig. S1A and 1C). Intravital microscopy analysis revealed that DRANs surrounded the network of epithelial cell adhesion molecule (EpCAM)+ biliary epithelial cells (Fig. 1D). Moreover, 3D reconstruction of Z-stack images showed that DRANs are in close contact with EpCAM+ cells (Fig. 1E and Supplementary Video 2). On the contrary, neutrophils were rarely seen at the central area or at sinusoids (Fig. 1D). The accumulation of DRANs at the biliary epithelium structures was confirmed in bile duct-ligated mice as a second cholestatic experimental model (Fig. S1E,F). On the contrary, in a carbon tetrachloride (CCl4) mouse model, wherein mice exhibit a mild ductular reaction, neutrophils were found at the central area (Fig. S1F). Neutrophil recruitment to the liver started as early as 2 days after the initiation of a DDC diet. Of note, although the number of liver neutrophils slightly increased at day 4 of DDC treatment, there was a prominent change in their location when compared to day 2. At day 4, DRANs appeared closely associated with EpCAM+ cells and their accumulation at the site of ductular reaction increased even further at day 7 of DDC diet (Fig. 1F).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2023.05.045.
DRANs are long-lasting and show a static behavior
Next, we aimed to visualize the dynamics of DRANs by using intravital microscopy. A 60-minute-long video of DDC-fed mice shows that Ly6G-positive neutrophils are located at the site of ductular reaction and display a static behavior, with little or no movement (Fig. 2A and Supplementary Video 3). This contrasts with previous work reported in acute liver injury where neutrophils were very dynamic.17 Acute focal thermal injury on DDC-treated animals induced a rapid recruitment of neutrophils to the site of injury, while the number of DRANs at EpCAM+ cells was unaltered (Fig. 2B and Supplementary Video 4). These data indicate that sterile injury could not recruit DRANs away from ductular reaction, suggesting the presence of an overriding stop signal.
Fig. 2. DRANs are immobilized at the biliary epithelium and remain static.
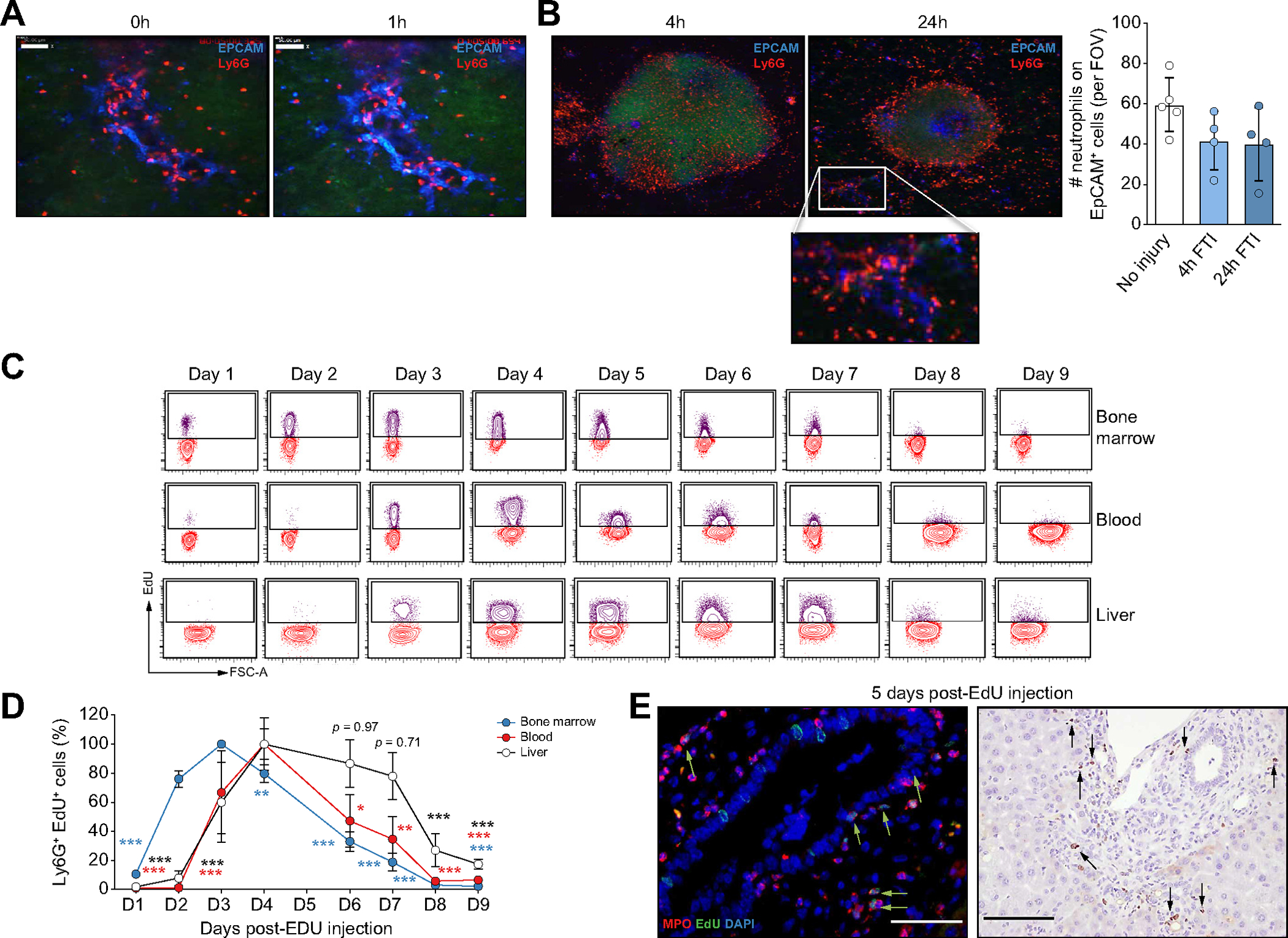
(A) Time-lapse SD-IVM images showing neutrophil (Ly6G) static behaviour at ductular reaction (EpCAM) sites. (B) SD-IVM images of FTI (Sytox Green) in mice treated with DDC for 1 week. Quantification of neutrophils (Ly6G) retained at EpCAM+ cells per FOV. (C) Flow cytometry plots showing Ly6G+ EdU+ neutrophils. (D) Percentage of Ly6G+ EdU+ neutrophils (n = 3–4 mice per time point). Data presented as mean ± SEM. Each time point was compared by two-way ANOVA with Dunnett’s multiple comparison test vs. day 3 (bone marrow samples) or day 4 (liver and blood samples). *p <0.05, **p <0.01 and ***p <0.001. (E) Immunofluorescence (scale bar: 50 μm) and EdU immunohistochemistry (scale bar: 100 μm) of liver sections of mice fed with DDC after 5 days of EdU injection. EdU, 5-ethynyl-2’-deoxyuridine; FOV, field of view; FTI, focal thermal injury; Ly6G, lymphocyte antigen 6 complex locus G6D; SD-IVM, spinning-disk confocal intravital microscopy.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2023.05.045.
Although neutrophils have a short lifespan in homeostasis, several reports suggest that this can be significantly increased in injury or infection.28 Thus, to evaluate the retention of DRANs and to track their recruitment and persistence within the liver tissue, we labeled bone marrow-proliferating neutrophils with 5-ethynyl-2’-deoxyuridine (EdU) in DDC-treated mice.29 At day 2 after EdU administration, 80% of neutrophils at the bone marrow were positive for EdU, while EdU+ neutrophils where still not present in the circulation or liver. The number of labeled neutrophils at the bone marrow peaked at day 3 and declined until day 8. In the blood or liver, EdU+ neutrophils started to be detected at day 3 and reached peak levels at day 4. Interestingly, while circulating EdU+ neutrophils then declined completely, mirroring the decline in bone marrow, the amount of liver EdU+ neutrophils remained at peak levels and unchanged for a period of 3 days, suggesting that these cells were not being continuously replaced by new neutrophils, but rather remained immobilized to the EpCAM+ cells. At day 8, EdU+ liver neutrophils did decline (Fig. 2C,D and Fig. S2A). Fig. 2E shows a representative image of EdU+ DRANs at day 5 after EdU injection. The strikingly different kinetics of neutrophils between the circulation and the liver tissue reveals that DRANs are retained at the liver and have a prolonged lifespan.
Liver neutrophils in DDC mice adopt a unique phenotypic and functional profile
Liver neutrophils from DDC mice showed increased CXCR4 and reduced CD62L (L-Selectin) expression when compared to bone marrow or circulating neutrophils (Fig. 3A–C), and CXCR4high liver neutrophils were increased compared to control mice (Fig. 3D). Based on previous studies, these results indicate that liver neutrophils show an aged-related phenotype.30–32
Fig. 3. Liver neutrophils in chronic liver damage acquire an aged and pro-inflammatory phenotype.
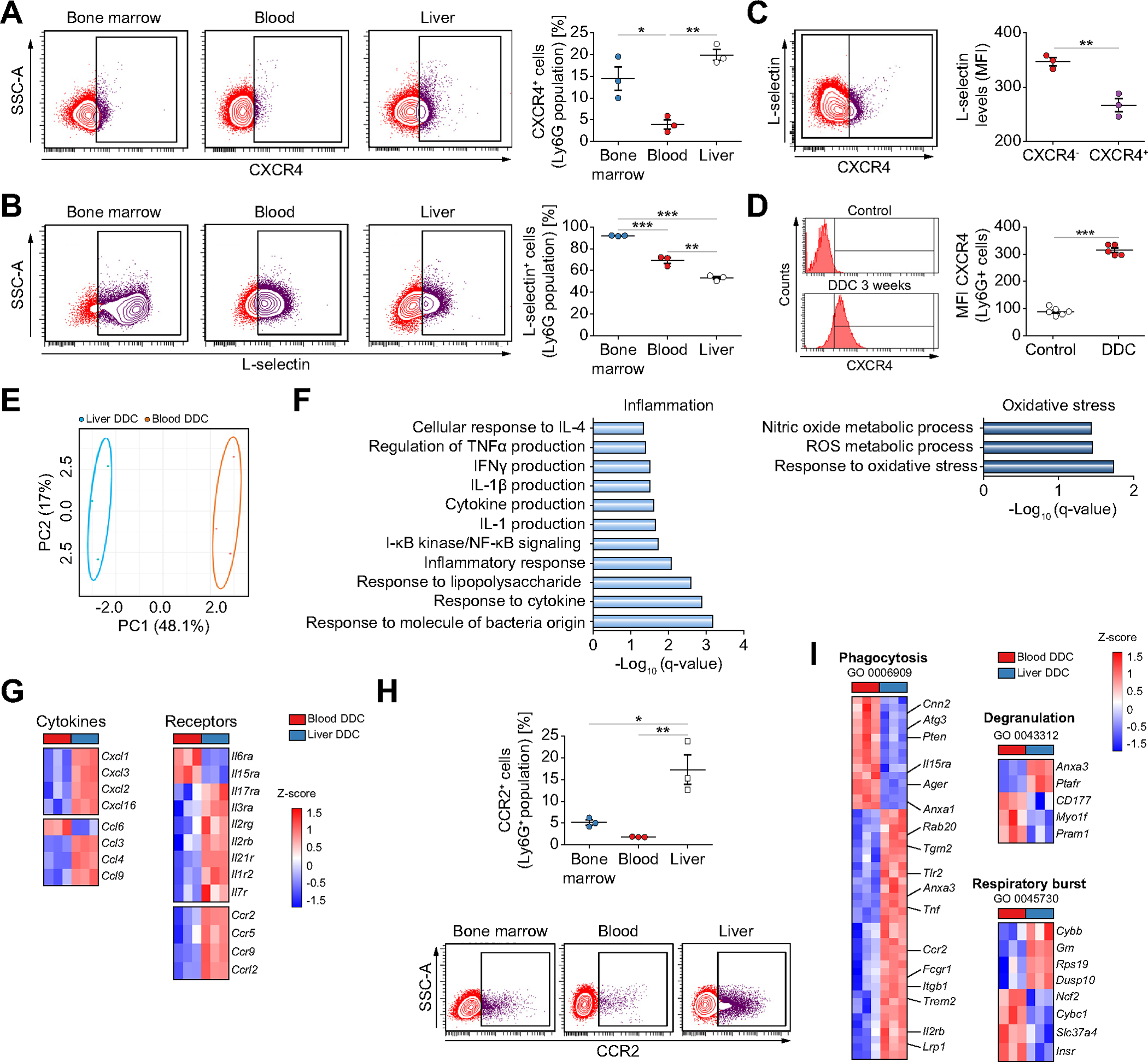
Flow cytometry analysis of the expression of CXCR4 (A) and L-selectin (B) in Ly6G+ cells (n = 3 mice per group). (C) Flow cytometry analysis of DDC-treated liver neutrophils (n = 3 mice per group). (D) Flow cytometry analysis of liver neutrophils (n = 5 mice per group). (E) Principal component analysis of transcriptomic data of neutrophils from DDC-treated mice (n = 3 mice per group). (F) Enriched gene ontology biological processes in liver neutrophils compared to blood neutrophils. (G) Heat map of differentially expressed inflammatory cytokines and receptors (n = 3 mice per group). (H) Flow cytometry analysis of CCR2 expression in Ly6G+ neutrophils in DDC-fed mice (n = 3 mice per group). (I) Heat map of differentially expressed genes associated with significantly enriched gene ontology annotations (n = 3 mice per group). All data is presented as mean ± SEM. *p <0.05, **p <0.01 and ***p <0.001 as determined by one-way ANOVA with Tukey’s multiple comparison test (A, B, H) and Student’s t test (C, D). CCR2, C-C Motif Chemokine Receptor 2; CXCR4, C-X-C motif chemokine receptor 4; DDC, 3,5-diethoxycarbonyl-1,4-dihydrocollidine; Ly6G, lymphocyte antigen 6 complex locus G6D; MFI, median fluorescence intensity.
RNA-sequencing of Ly6G+ neutrophils isolated from the liver (highly enriched in DRANs, 73.3±1.76%) and blood from DDC-treated mice showed clear differences in their transcriptomic profile (Fig. 3E and Fig. S3A). Liver neutrophils were enriched in inflammation- and oxidative stress-related pathways and chemokines and cytokine receptors (Fig. 3F and 3G). Flow cytometry analysis confirmed the expression of CCR2, a marker of tissue-infiltrating neutrophils,33,34 in liver neutrophils (Fig. 3H). Furthermore, a protein-protein interaction analysis with upregulated genes in liver neutrophils identified Tnfa as a central player in neutrophil-related inflammatory networks (Fig. S3B). Genes involved in phagocytosis, degranulation and respiratory burst were altered between liver and blood neutrophils (Fig. 3I). Interestingly, liver neutrophils from DDC-treated mice showed an enrichment in inflammatory and wound-healing biological processes compared to control liver neutrophils. These results suggest that neutrophil gene expression profiles are altered as a result of injury and infiltration (Fig. S3C and 3D).
Next, we functionally evaluated neutrophils from DDC-treated mice. As shown in Fig. 4A and 4B, liver neutrophils showed an increased baseline expression of Tnfa, Cxcl2, Il6 and Il10 and secretion of TNFa and IL-6. In addition, cytokine production upon lipopolysaccharide stimulation was higher in liver neutrophils than in blood neutrophils (Fig. 4B). Phagocytic capacity and burst index of circulating neutrophils was reduced in DDC-treated mice compared to healthy mice (Fig. S4, Fig. 4C,D), and phagocytosis was further reduced in liver neutrophils from DDC-treated mice (Fig. 4C). By contrast, burst capacity was increased in liver neutrophils from DDC-treated mice at a basal level and after stimulation with phorbol 12-myristate 13-acetate when compared with circulating neutrophils (Fig. 4D).
Fig. 4. Liver neutrophils in chronic injury present an altered functionality.
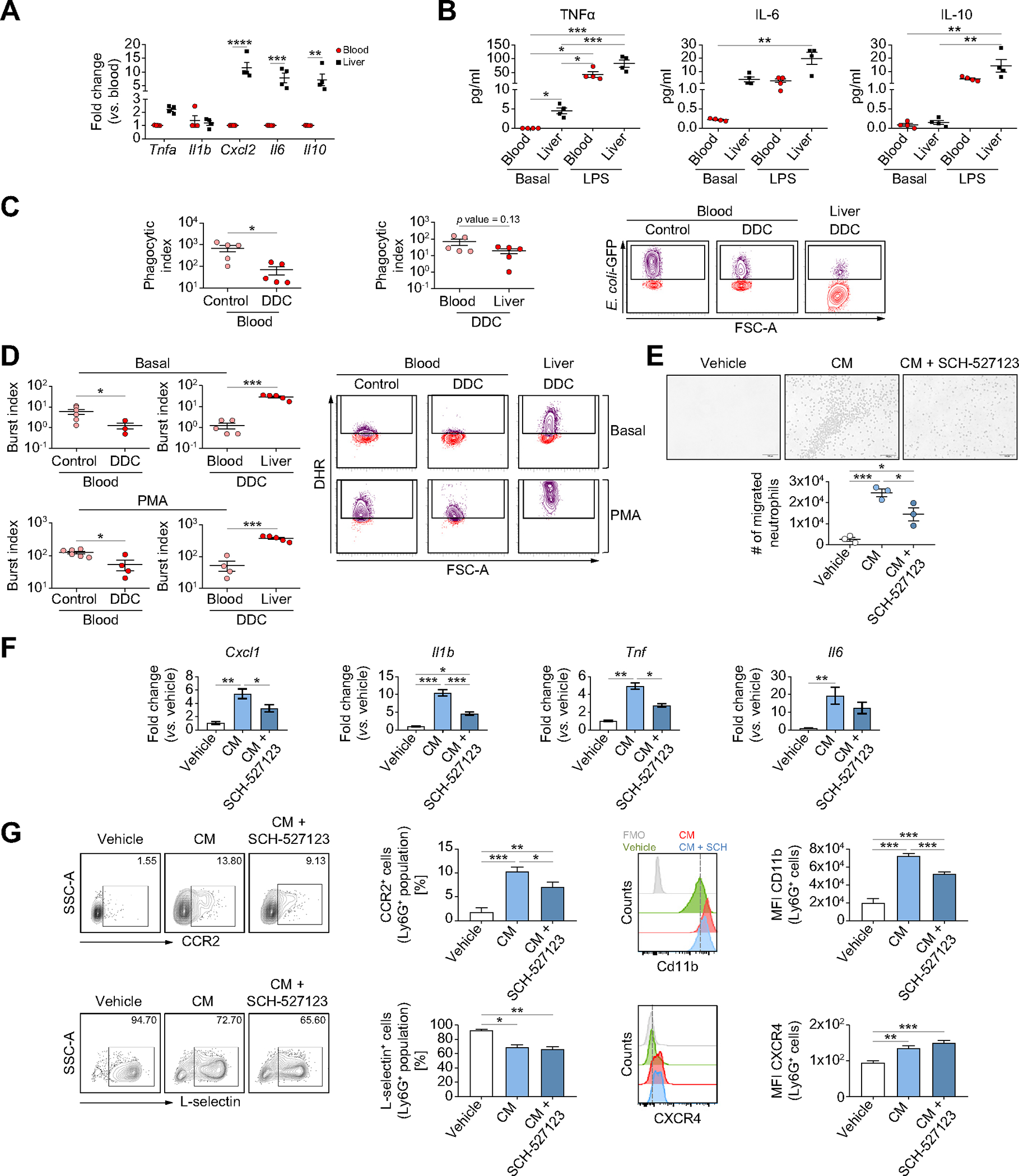
(A) Gene expression analysis of liver and circulating neutrophils from DDC-treated mice (n = 4 mice per group). (B) Supernatant levels of cytokines in neutrophils stimulated with LPS (100 ng/ml) or vehicle for 6 h (n = 4 mice per group). (C) Phagocytic activity of neutrophils isolated from DDC and control mice (n = 5 mice per group). Phagocytic index: % of Ly6G+E. coli-FITC+ cells × MIF/100. (D) Neutrophil oxidative burst at basal or PMA-stimulated contexts in neutrophils from DDC and control mice (n = 5–6 mice per group). Burst index: % of Ly6G+ Rhodamine+ cells × MIF/100. (E) Images and number of migrated neutrophils (n = 3) when exposed to biliary organoid-conditioned media (n = 3) and SCH-527123 (50 μm) for 2 h. Scale bar: 100 μm (F) Gene expression analysis of neutrophils (n = 3) treated with organoid-conditioned media (n = 3) and SCH-527123 (50 μm) for 6 h. (G) Flow cytometry analysis of CCR2, L-selectin, CD11b and CXCR4 expression in neutrophils (n = 4–5) treated for 18 h with organoid-conditioned media (n = 3) and SCH-527123 (50 μm). All data is presented as mean ± SEM. *p <0.05, **p <0.01 and ***p <0.001 as determined by two-way ANOVA with Sidak’s multiple comparison (A), one-way ANOVA with Tukey’s multiple comparison (Cxcl1 and Il1b in E, F, CCR2, CD11b and CXCR4 in G), Kruskal-Wallis test with Dunn’s multiple comparison (B, Tnf and Il6 in E; L-selectin in G) or Student’s t test (C, D). CCR2, C-C Motif Chemokine Receptor 2; CM, conditioned media; CXCR4, C-X-C motif chemokine receptor 4; DDC, 3,5-diethoxycarbonyl-1,4-dihydrocollidine; GFP, green fluorescent protein; LPS, lipopolysaccharide, Ly6G, lymphocyte antigen 6 complex locus G6D; MFI, median fluorescence intensity; PMA, phorbol 12-myristate 13-acetate.
We previously demonstrated the existence of a crosstalk between neutrophils and ductular reaction and described ductular reaction as an important source of CXCL ligands in chronic liver disease,1 thus suggesting that ductular reaction recruits neutrophils and alters their phenotype through CXCR1/2-dependent signaling. To address this hypothesis we used conditioned medium from DDC mice-biliary organoids as an in vitro model of ductular reaction.1,8 First, we observed that organoid-conditioned medium induced neutrophil migration, which was abrogated by SCH-527123, a CXCR1/2 inhibitor (Fig. 4E). Neutrophils stimulated with organoid-conditioned medium showed lower levels of L-selectin, increased expression of CD11b, CXCR4 and CCR2 and increased expression of Il6, Tnfa, Il1b and Cxcl1, mimicking the phenotype observed in DDC mice. This phenotype was partially prevented by SCH-527123 (Fig. 4F,G), but changes in CXCR4 and L-selectin expression were not avoided (Fig. 4G).
Overall, these results indicate that ductular reaction recruits neutrophils and promotes changes in their phenotype and function through the CXCL-CXCR1/2 axis.
Liver neutrophils promote biliary epithelium cell proliferation and expansion
To determine the role of neutrophils we: i) depleted neutrophils, and ii) inhibited neutrophil recruitment in the DDC mouse model. Depletion of neutrophils with anti-Ly6G antibody, as assessed by myeloperoxidase (MPO) and Ly6G staining, resulted in a reduction of ductular reaction expansion, fibrosis deposition and angiogenesis (Fig. 5A and Fig. S5A). Neutrophil depletion reduced the number of SOX9- and EpCAM-proliferating cells(Fig. 5B,C), which agrees with changes in proliferating KRT7 cells in human samples (Fig. S1D). The reduction of ductular reaction cells was not associated with increased apoptosis or senescence (Fig. S5B). No proliferating endothelial cells or differences in hepatocyte proliferation were observed, suggesting that changes in angiogenesis may be mediated by sprouting and cell migration processes rather than cell expansion35 (Fig. S5C,D). Neutrophil depletion reduced the expression of Epcam, but not of inflammatory mediators (Fig. S5E). This effect was not due to reduced liver injury, as serum transaminase levels increased after neutrophil depletion(Fig.S5F).
Fig. 5. Long-term depletion of neutrophils in chronic liver injury leads to a decrease in proliferating biliary epithelial cells.
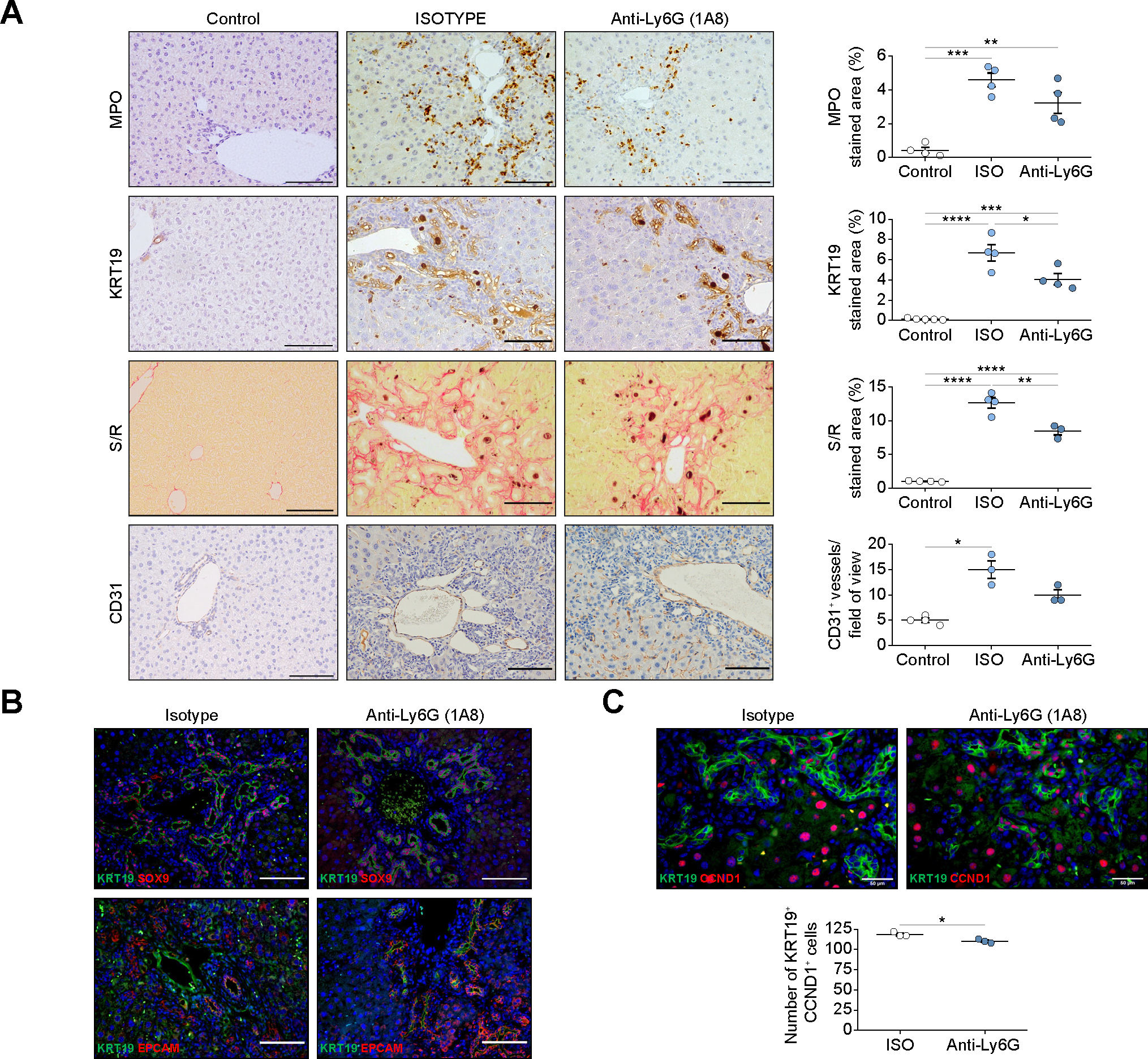
(A) Immunohstochemistry analysis of livers of control- and DDC-treated mice with anti-Ly6G antibody (1A8) or isotype (n = 4 mice per group). Scale bars: 100 μm. (B) Staining of progenitor markers in mice treated with anti-Ly6G antibody (1A8) and isotype. Scale bars: 100 μm. (C) Immunofluorescence of KRT19 and CCND1 of liver sections of mice treated with anti-Ly6G antibody (1A8) and isotype (n = 3 mice per group). Scale bars: 50 μm. All data is presented as mean ± SEM. *p <0.05, **p <0.01 and ***p <0.001 as determined by one-way ANOVA with Tukey’s multiple comparison test (MPO, KRT19, S/R in A), one-way ANOVA Kruskal-Wallis with Dunn’s multiple comparison test (CD31 in A) and Mann-Whitney test (C). CCND1, cyclin D1; CD31, cluster of differentiation 31; DDC, 3,5-diethoxycarbonyl-1,4-dihydrocollidine; EpCAM, epithelial cell adhesion molecule; ISO, isotype; KRT, cytokeratin; MPO, myeloperoxidase; Ly6G, lymphocyte antigen 6 complex locus G6D; SOX9, SRY-Box transcription factor 9; S/R, sirius red.
We have previously shown that ductular reaction cells recruit neutrophils and alter their phenotype through CXCR1/2. Thus, we inhibited in vivo neutrophil recruitment with the CXCR1/2 inhibitor SCH-527123. Intravital imaging showed that SCH-527123 reduced the accumulation of DRANs at EpCAM+ cells (Fig. S6A). Inhibition of CXCR1/2 for 3 weeks in DDC-treated mice (Fig. 6A) resulted in a reduction in the expression of pro-inflammatory and pro-fibrogenic mediators, as well as biliary markers such as Epcam and Hnf1b (Fig. S6B).CXCR1/2 inhibition reduced DRANs, proliferating ductular reaction cells, the deposition of fibrosis and the degree of intrahepatic angiogenesis (Fig. 6B–D). Apoptosis or senescence was not detected in ductular reaction (Fig. S6C). We did not observe Ki67+ endothelial cells (Fig. S6D). Hepatocyte proliferation did not change after SCH-527123 treatment (Fig. S6E). No changes in liver injury levels were observed (Fig. S6F). These results indicate that CXCR1/2-dependent recruitment of neutrophils leads to ductular reaction expansion.
Fig. 6. Blocking neutrophil recruitment by CXCR1/2 inhibitor reduces biliary epithelium expansion in chronic injury.
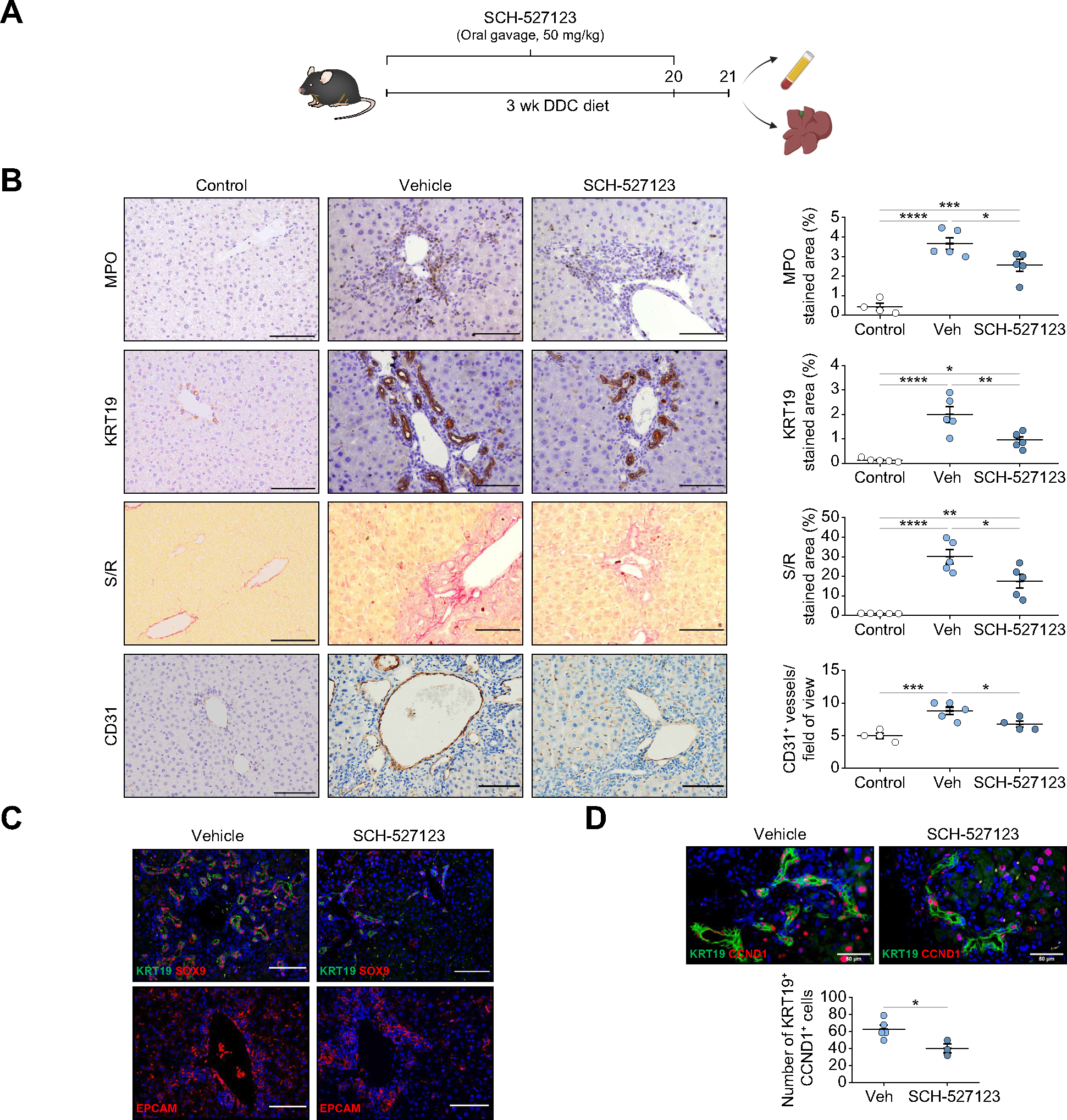
(A) Mice were fed with DDC and treated daily with CXCR1/2 inhibitor (SCH-527123) for 3 weeks at a dose of 50 mg/kg. (B) Immunohistochemistry analysis of liver sections of control and 3-weeks DDC-fed mice treated with SCH-527123 or vehicle (n = 5–6 mice per group). Scale bars: 100 μm. (C) Immunofluorescence of progenitor markers in liver sections of mice fed with DDC for 3 weeks and treated with SCH-527123 inhibitor or vehicle. Scale bars: 100 μm. (D) Immunofluorescence of KRT19 and CCND1 in mice treated with SCH-527123 or vehicle (n = 3–5 mice per group). Scale bars: 50 μm. Data presented as mean ± SEM. *p <0.05, **p <0.01 and ***p <0.001 as determined by one-way ANOVA with Tukey’s multiple comparison test (B) and Student’s t test (D). CCND1, cyclin D1; CD31, cluster of differentiation 31; DDC, 3,5-diethoxycarbonyl-1,4-dihydrocollidine; EpCAM, epithelial cell adhesion molecule; KRT, cytokeratin; MPO, myeloperoxidase; SOX9, SRY-Box transcription factor 9; S/R, sirius red; Veh, vehicle; wk, weeks.
Neutrophil extracellular traps and neutrophil elastase mediates biliary epithelium expansion
In chronic liver damage, infiltrating neutrophils release neutrophil extracellular traps (NETs) contributing to liver injury.36–38 Moreover, NETs have been linked to cell proliferation in tumor development.39 Mice deficient for PAD4, a molecule required for histone citrullination and NET formation, were fed with DDC diet. Deficient NETosis in Pad4−/− mice was validated by citrullinated histone H3 and MPO co-staining (Fig. 7A). DDC-treated Pad4−/− mice showed a slight increase in DRANs, but an important reduction in ductular reaction expansion and proliferating KRT19+ cells (Fig. 7B–D). However, no expression of cleaved caspase 3 or p21 in ductular reaction cells was observed (Fig. S7A). Hepatocyte proliferation increased in Pad4−/− mice, but no Ki67+ endothelial cells were observed (Fig. S7B and 7C). To explore whether neutrophil elastase, a NET-associated protease, mediates biliary epithelium expansion, we examined elastase-deficient mice (Elane−/−) fed with DDC for 3 weeks. No changes in NETosis were observed as assessed by citrullinated histone H3 and MPO co-staining (Fig. 7E). Although liver injury in Elane−/− DDC-treated mice was not affected (Fig. S7D), we observed a reduction in the expression of fibrosis and progenitor cell genes (Fig. S7E). Histologically, Elane−/− mice presented no major differences in the number of DRANs but a reduction in proliferation and the expansion of ductular reaction cells (Fig. 7F–H). No expression of cleaved caspase 3 and p21 in ductular reaction cells was observed (Fig. S7F). While hepatocyte proliferation decreased in Elane−/− mice, no proliferation was observed in endothelial cells (Fig. S7G,H). To provide evidence for a direct effect of neutrophil elastase on ductular reaction proliferation, mouse biliary organoids were co-cultured for 1 week in low-attachment plates with phorbol 12-myristate 13-acetate-stimulated neutrophils from control and Elane−/− mice. As observed in vivo and in patients with liver disease, neutrophils increased the proliferation of organoids. Although not totally prevented, the induction of organoid proliferation was lower when elastase-deficient neutrophils were used (Fig. 7I).
Fig. 7. Depletion of NETosis and elastase release reduces liver progenitor cell expansion.
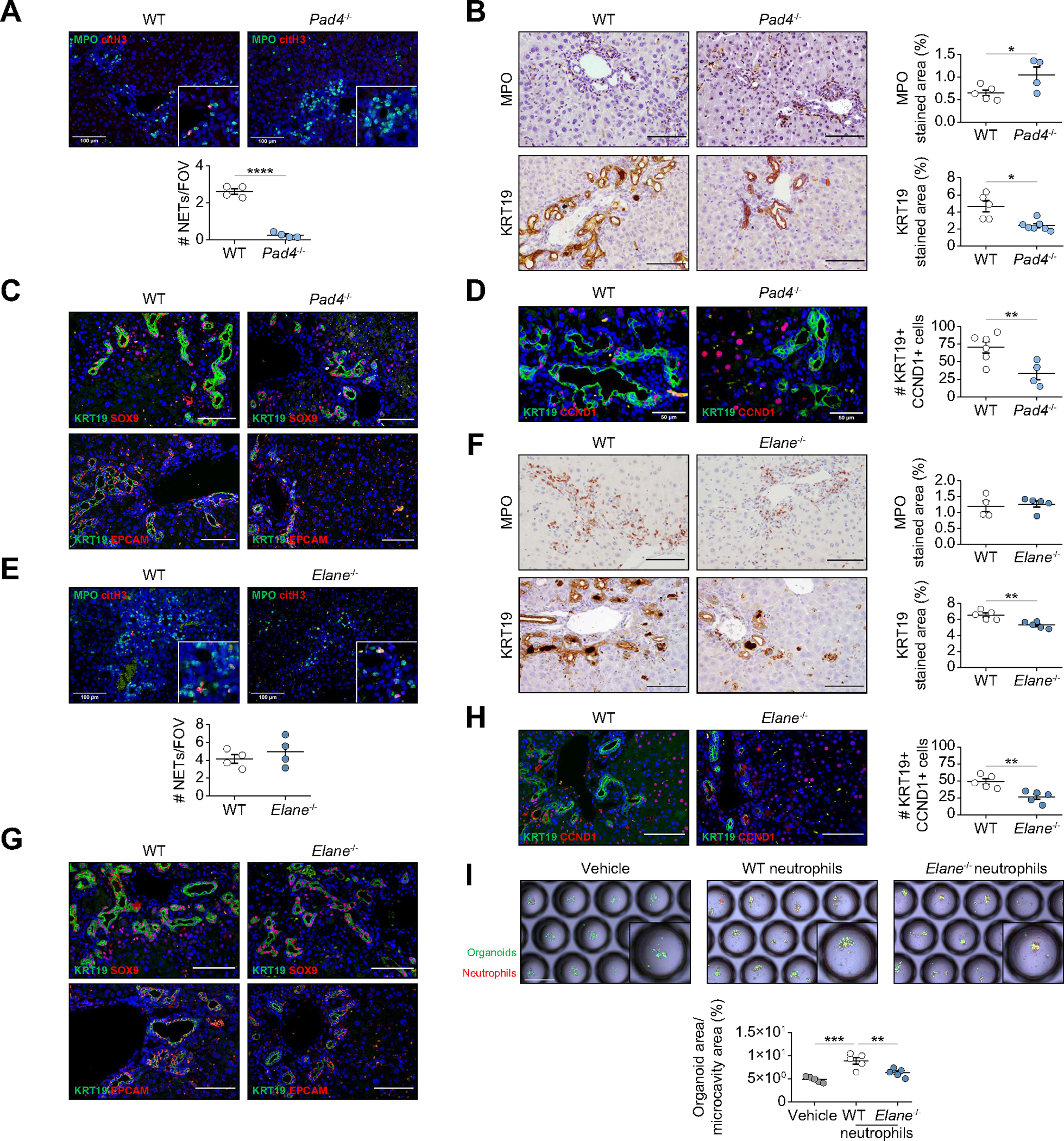
(A) Immunofluorescence of MPO and citH3 in WT and Pad4−/− liver sections (n = 4 mice per group). Scale bars: 100 μm. (B) Immunohistochemistry analysis of WT and Pad4−/− mice liver sections (n = 4–7 mice per group). Scale bars: 100 μm. (C) Immunofluorescence of KRT19-EpCAM and KRT19-SOX9 in WT and Pad4−/− mouse liver sections. Scale bars: 100 μm. (D) Immunofluorescence of KRT19 and CCND1 in WT and Pad4−/− mice (n = 4–6 mice per group). Scale bars: 50 μm. (E) Immunofluorescence of NETs in WT and Elane−/− liver sections (n = 4 mice per group). Scale bars: 100 μm. (F) Immunohistochemistry analysis of MPO and KRT19 in WT and Elane−/− mouse liver sections (n = 4–5 mice per group). Scale bars: 100 μm. (G) Immunofluorescence of progenitor markers in WT and Elane−/− mouse liver sections. Scale bars: 100 μm. (H) Immunofluorescence of KRT19 and CCND1 in WT and Elane−/− mice. Scale bars: 50 μm. (I) Images of biliary organoids and WT or Elane−/− neutrophil co-culture. Organoid area is normalized per microcavity area. Each measurement represents a technical replicate consisting of the average area of at least 40 microcavities (n = 3 mice-derived organoids). Scale bar: 500 μm. Data presented as mean ± SEM. *p <0.05, **p <0.01 and ***p <0.001 as determined by Student’s t test (A, B, D, E, F, H) and one-way ANOVA with Tukey’s multiple comparison test (I). CCND1, cyclin D1; citH3, citrullinated histone H3; DDC, 3,5-diethoxycarbonyl-1,4-dihydrocollidine; Elane, neutrophil elastase; EpCAM, epithelial cell adhesion molecule; FOV, field of view; KRT, cytokeratin; MPO, myeloperoxidase; NETs, neutrophil extracellular traps; Pad4, peptidyl arginine deiminase 4; SOX9, SRY-Box transcription factor 9; WT, wild-type.
Combined, these results indicate that neutrophil elastase promotes biliary epithelium expansion, and therefore may be involved in the maladaptive wound-healing and regenerative response of the liver in the context of chronic liver disease.
Discussion
In this study we describe the complex recruitment dynamics of neutrophils in chronic liver injury and we define DRANs as a distinct population of neutrophils immobilized at the site of ductular reaction with an extended half-life. Most of the information on the dynamic recruitment of neutrophils to tissues derives from studies on infection or acute injury. In the healthy liver, neutrophils can be found in the sinusoidal vasculature but very rarely infiltrate the parenchyma. When there is a sterile acute liver insult such as local thermal injury or ischemia-reperfusion, neutrophils are rapidly recruited to the site of damage, where they remain for a short time before returning to the vasculature.17 In this study we show that the recruitment dynamics of neutrophils in chronic liver injury are different and that a population of neutrophils located at ductular reaction, named DRANs, emerges and persists for several days. This observation suggests that DRANs may have an extended lifespan, with the potential to become a stable inflammatory cell pool.
We show that neutrophil recruitment to the liver entails important changes in their phenotype and function. DRANs adopt an aged phenotype but also start to express receptors that may be important for their intrahepatic recruitment. This is in agreement with studies showing that expression of CCR-family members increase in neutrophils recruited to chronic inflammatory sites.34 Moreover, we demonstrate that CXCL-chemokines among other soluble factors secreted by ductular reaction promote neutrophils’ acquisition of a pro-inflammatory and altered phenotype. As previously reported in the literature, our results demonstrate the effect of tissue factors on neutrophils, driving their adaptation and changes in their phenotype, which fine tune tissue recruitment and specialized functions.33,34 Moreover, the aged neutrophil phenotype is associated with the acquisition of a more reactive and inflammatory function.36–32 These results highlight the plasticity of liver-recruited neutrophils and show how neutrophils adapt to a chronic injury environment. A growing number of studies report the existence of heterogeneity within neutrophil populations in different contexts.40,41 Whether liver neutrophils in chronic injury comprise a heterogeneous population and which phenotypic and functional characteristics define each of these populations requires further investigation.
The recruitment of neutrophils in response to infection or acute injury stimulates the secretion of hydrolytic and oxidative molecules at the site of injury, which affect neighboring cells, exacerbating tissue injury.23,24,42 Moreover, the release of NETs also has an impact on the surrounding tissue, promoting vascular thrombosis and enhancing inflammatory responses.21,38,43 However, besides the detrimental role of neutrophils to tissue injury, neutrophils have been shown to participate in tissue healing.23,44,45 Our study expands on the understanding of the role of neutrophils in chronic liver injury and provides evidence that neutrophils are involved in ductular reaction expansion, affecting fibrosis and angiogenesis.
We show important crosstalk between DRANs and ductular reaction cells. The finding that both Pad4−/− and Elane−/− mice showed reduced biliary epithelium cell proliferation but not apoptosis or senescence, indicates that NETosis and specifically, elastase, may be involved in the proliferation of biliary epithelium cells. These results are in agreement with reports describing the role of NETs in triggering cell proliferation in the cancer field where NET-associated proteases (neutrophil elastase and MMP9) sequentially cleave the extracellular matrix protein laminin, promoting proliferation of cancer cells.46 However, due to the opposite effects observed on biliary cells and hepatocytes, further analyses are required to clarify the cell-specific effect of neutrophils in liver regeneration. Besides the role in biliary regeneration, these results expand the concept that neutrophils may functionally interact with epithelial cells to regulate their proliferation.
Herein, we have described the dynamics of DRANs in chronic liver injury and their role in the expansion of ductular reaction. We show that neutrophils are plastic and adapt to tissue injury, acquiring a new phenotype, function and lifespan. Moreover, we show that DRANs promote ductular reaction expansion, thus uncovering their role as players in liver regeneration and wound-healing. All these data suggest that neutrophils could be a good therapeutic target to mitigate ductular reaction expansion and to promote liver regeneration and tissue repair in chronic liver diseases.
Supplementary Material
Impact and implications.
Our results indicate that neutrophils are highly plastic and can have an extended lifespan. Moreover, we identify a new role of neutrophils as triggers of expansion of the biliary epithelium. Overall, the results of this study indicate that ductular reaction-associated neutrophils (or DRANs) are new players in the maladaptive tissue-healing response in chronic liver injury and may be a potential target for therapeutic interventions to reduce ductular reaction expansion and promote tissue repair in advanced liver disease.
Highlights.
Advanced chronic liver diseases are characterized by the presence of ductular reaction-associated neutrophils.
Ductular reaction-associated neutrophils are long-lasting and immobilized to biliary epithelial cells.
Neutrophils recruited to the liver adopt an altered phenotype and function.
Neutrophils mediate biliary cell proliferation contributing to the maladaptive wound-healing response.
Acknowledgments
This work was performed in part at the Centre Esther Koplowitz (CEK) (Barcelona, Spain) and at the Snyder Institute for Chronic Diseases (Calgary, Canada). The authors wish to thank Lori Zbytnuik for her advice and support and Pepa Ros for her excellent laboratory management support. We are indebted to the Biobank Unit and the cytometry and cell sorting facility of Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) for their technical help.
Financial support
This work has been supported by grants from Fondo de Investigación Sanitaria Carlos III (FIS), co-financed by Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, “Una manera de hacer Europa” (FIS PI20/00765 to P.S.-B and FIS 18-PI18/00862 to M.C), from the NIH National Institute on Alcohol Abuse and Alcoholism grant 1U01AA021908–01-33490 to P.S.-B. P.G. is funded by Agencia de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) 2014 SGR 708, Centro de Investigación en Red Enfermedades Hepáticas y Digestivas (CIBERehd) and Institució Catalana de Recerca i Estudis Avançats (ICREA). MC is funded by Ramon y Cajal programme from the Ministerio de Ciencia e Innovación RYC2019–026662-I and by Plan estatal de investigación científica y técnica de innovación PID2021–125195OB-I00. S.A received a grant from the Ministerio de Educación, Cultura y Deporte, FPU programme (FPU17/04992). B.A.-B. is funded by Instituto de Salud Carlos III, PFIS (FI16/00203). SA funded by la Caixa Foundation (ID 100010434) and the European Union’s Horizon 2020 under the Marie Skłodowska-Curie (847648) and by the MCIN/AEI/10.13039/501100011033 by “FEDER Una manera de hacer Europa” (PID2021–124694OA-I00).
Abbreviations
- CCl4
carbon tetrachloride
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- DRANs
ductular reaction-associated neutrophils
- EdU
5-ethynyl-2’-deoxyuridine
- Elane
neutrophil elastase
- EpCAM
epithelial cell adhesion molecule
- KRT19
cytokeratin 19
- KRT7
cytokeratin 7
- MPO
myeloperoxidase
- NETs
neutrophil extracellular traps
- PAD4
peptidyl arginine deiminase 4
Footnotes
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2023.05.045.
Data availability statement
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary information files or from the corresponding author upon request. Raw RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) database under accession code GSE203336. All the other materials and methods are described in the supplementary data.
References
- [1].Aguilar-Bravo B, Rodrigo-Torres D, Ariño S, Coll M, Pose E, Blaya D, et al. Ductular reaction cells display an inflammatory profile and recruit neutrophils in alcoholic hepatitis. Hepatology 2019;69:2180–2195. 10.1002/hep.30472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Govaere O, Cockell S, Van Haele M, Wouters J, Van Delm W, Van den Eynde K, et al. High-throughput sequencing identifies aetiology-dependent differences in ductular reaction in human chronic liver disease. J Pathol 2019;248:66–76. 10.1002/path.5228. [DOI] [PubMed] [Google Scholar]
- [3].Takeuchi M, Vidigal PT, Guerra MT, Hundt MA, Robert ME, Olave-Martinez M, et al. Neutrophils interact with cholangiocytes to cause cholestatic changes in alcoholic hepatitis. Gut 2021;70:342–356. 10.1136/gutjnl-2020-322540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wiggins BG, Pallett LJ, Li X, Davies SP, Amin OE, Gill US, et al. The human liver microenvironment shapes the homing and function of CD4+ T-cell populations. Gut 2022;71:1399–1411. 10.1136/GUTJNL-2020-323771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zimmer CL, von Seth E, Buggert M, Strauss O, Hertwig L, Nguyen S, et al. A biliary immune landscape map of primary sclerosing cholangitis reveals a dominant network of neutrophils and tissue-resident T cells. Sci Transl Med 2021;13:3107. 10.1126/SCITRANSLMED.ABB3107/SUPPL_FILE/SCITRANSLMED.ABB3107_SM.PDF. [DOI] [PubMed] [Google Scholar]
- [6].Zhang Z, Zhong X, Shen H, Sheng L, Liangpunsakul S, Lok AS, et al. Biliary NIK promotes ductular reaction and liver injury and fibrosis in mice. Nat Commun 2022;13:1–15. 10.1038/s41467-022-32575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology 2014;146:349–356. 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- [8].Coll M, Ariño S, Martínez-Sánchez C, Garcia-Pras E, Gallego J, Moles A, et al. Ductular reaction promotes intrahepatic angiogenesis through Slit2–Roundabout 1 signaling. Hepatology 2022;75:353–368. 10.1002/HEP.32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sancho-Bru P, Altamirano J, Rodrigo-Torres D, Coll M, Millán C, José Lozano J, et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology 2012;55:1931–1941. 10.1002/hep.25614. [DOI] [PubMed] [Google Scholar]
- [10].Roskams T Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene 2006;25:3818–3822. 10.1038/SJ.ONC.1209558. [DOI] [PubMed] [Google Scholar]
- [11].Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol 2019;16:269–281. 10.1038/S41575-019-0125-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019;133:2178–2185. 10.1182/BLOOD-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- [13].Wang J Neutrophils in tissue injury and repair. Cell Tissue Res 2018;371:531–539. 10.1007/S00441-017-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Casanova-Acebes M, Nicolás-Ávila JA, Li JL, García-Silva S, Balachander A, Rubio-Ponce A, et al. Neutrophils instruct homeostatic and pathological states in naive tissues. J Exp Med 2018;215:2778–2795. 10.1084/JEM.20181468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol 2014;9:181–218. 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McDonald B, Kubes P. Neutrophils and intravascular immunity in the liver during infection and sterile inflammation. Toxicol Pathol 2011;40:157–165. 10.1177/0192623311427570. [DOI] [PubMed] [Google Scholar]
- [17].Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017;358:111–116. 10.1126/science.aam9690. [DOI] [PubMed] [Google Scholar]
- [18].Das S, Maras JS, Hussain MS, Sharma S, David P, Sukriti S, et al. Hyperoxidized albumin modulates neutrophils to induce oxidative stress and inflammation in severe alcoholic hepatitis. Hepatology 2017;65:631–646. 10.1002/HEP.28897. [DOI] [PubMed] [Google Scholar]
- [19].Boussif A, Rolas L, Weiss E, Bouriche H, Moreau R, Périanin A. Impaired intracellular signaling, myeloperoxidase release and bactericidal activity of neutrophils from patients with alcoholic cirrhosis. J Hepatol 2016;64:1041–1048. 10.1016/J.JHEP.2015.12.005. [DOI] [PubMed] [Google Scholar]
- [20].Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–1437. 10.1053/J.GASTRO.2013.02.042.1437.e1-e1437. [DOI] [PubMed] [Google Scholar]
- [21].Hilscher MB, Sehrawat T, Arab JP, Zeng Z, Gao J, Liu M, et al. Mechanical stretch increases expression of CXCL1 in liver sinusoidal endothelial cells to recruit neutrophils, generate sinusoidal microthombi, and promote portal hypertension. Gastroenterology 2019;157:193–209.e9. 10.1053/J.GASTRO.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang R, Su L, Fu M, Wang Z, Tan L, Chen H, et al. CD177+ cells produce neutrophil extracellular traps that promote biliary atresia. J Hepatol 2022;77:1299–1310. 10.1016/j.jhep.2022.06.015. [DOI] [PubMed] [Google Scholar]
- [23].Yang W, Tao Y, Wu Y, Zhao X, Ye W, Zhao D, et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat Commun 2019;10:1–14. 10.1038/s41467-019-09046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marwick JA, Mills R, Kay O, Michail K, Stephen J, Rossi AG, et al. Neutrophils induce macrophage anti-inflammatory reprogramming by suppressing NF-κB activation. Cell Death Dis 2018;9:665. 10.1038/s41419-018-0710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, et al. A histologic scoring system for prognosis of patients with AlcoholicHepatitis. Gastroenterology 2014;146:1231. 10.1053/J.GASTRO.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cho Y, Bukong TN, Tornai D, Babuta M, Vlachos IS, Kanata E, et al. Neutrophil extracellular traps contribute to liver damage and increase defective low-density neutrophils in alcoholic hepatitis. J Hepatol 2022;78:28–44. 10.1016/J.JHEP.2022.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pose E, Sancho-Bru P, Coll M. 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine diet: a rodent model in cholestasis Research. Methods Mol Biol 2019;1981:249–257. 10.1007/978-1-4939-9420-5_16. [DOI] [PubMed] [Google Scholar]
- [28].Simon H-U. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev 2003;193:101–110. 10.1034/J.1600-065X.2003.00038.X. [DOI] [PubMed] [Google Scholar]
- [29].Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci 2008;105:2415–2420. 10.1073/PNAS.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim JH, Podstawka J, Lou Y, Li L, Lee EKS, Divangahi M, et al. Aged polymorphonuclear leukocytes cause fibrotic interstitial lung disease in the absence of regulation by B cells. Nat Immunol 2018;19:192–201. 10.1038/s41590-017-0030-x. [DOI] [PubMed] [Google Scholar]
- [31].Peng Z, Liu C, Victor AR, Cao D-Y, Veiras LC, Bernstein EA, et al. Tumors exploit CXCR4hiCD62Llo aged neutrophils to facilitate metastatic spread. Oncoimmunology 2021;10:1870811. 10.1080/2162402X.2020.1870811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chèvre R, A-González N, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013;153:1025. 10.1016/J.CELL.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Metzemaekers M, Gouwy M, Proost P. Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol Immunol 2020;17:433–450. 10.1038/s41423-020-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, Hoffmann F, et al. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol 2008;181:8053–8067. 10.4049/JIMMUNOL.181.11.8053. [DOI] [PubMed] [Google Scholar]
- [35].Mühleder S, Fernández-Chacón Macarena, Garcia-Gonzalez I, Rui Benedito. Endothelial sprouting, proliferation, or senescence: tipping the balance from physiology to pathology. Cell Mol Life Sci 2021;78:1329–1354. 10.1007/s00018-020-03664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tang Y, Li H, Li J, Liu Y, Li Y, Zhou J, et al. Macrophage scavenger receptor 1 contributes to pathogenesis of fulminant hepatitis via neutrophil-mediated complement activation. J Hepatol 2018;68:733–743. 10.1016/J.JHEP.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Windt van der DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018;68:1347–1360. 10.1002/HEP.29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bukong TN, Cho Y, Iracheta-Vellve A, Saha B, Lowe P, Adejumo A, et al. Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J Hepatol 2018;69:1145–1154. 10.1016/J.JHEP.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020;583:133–138. 10.1038/s41586-020-2394-6. [DOI] [PubMed] [Google Scholar]
- [40].Ballesteros I, Rubio-Ponce A, Genua M, Lusito E, Kwok I, Fernández-Calvo G, et al. Co-Option of neutrophil fates by tissue environments. Cell 2020;183:1282–1297.e18. 10.1016/J.CELL.2020.10.003. [DOI] [PubMed] [Google Scholar]
- [41].Xue R, Zhang Q, Cao Q, Kong R, Xiang X, Liu H, et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature 2022;612:141–147. 10.1038/S41586-022-05400-X. [DOI] [PubMed] [Google Scholar]
- [42].Makkar K, Tomer S, Rathi S, Arora SK, Taneja S, Duseja A, et al. Neutrophil dysfunction in acute on chronic liver failure. J Clin Exp Hepatol 2017;7:S12–S13. 10.1016/J.JCEH.2017.05.032. [DOI] [Google Scholar]
- [43].Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern–activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 2015;62:600–614. 10.1002/HEP.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mattos MS, Lopes ME, Araujo AM de, Alvarenga DM, Nakagaki BN, Mafra K, et al. Prolonged neutrophil survival at necrotic sites is a fundamental feature for tissue recovery and resolution of hepatic inflammation. J Leukoc Biol 2020;108:1199–1213. 10.1002/JLB.1MA0420-634R. [DOI] [PubMed] [Google Scholar]
- [45].Calvente CJ, Tameda M, Johnson CD, Pilar H del, Lin YC, Adronikou N, et al. Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA-223. J Clin Invest 2019;129:4091–4109. 10.1172/JCI122258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018;361:eaao4227. 10.1126/SCIENCE.AAO4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article and its Supplementary information files or from the corresponding author upon request. Raw RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) database under accession code GSE203336. All the other materials and methods are described in the supplementary data.


