Abstract
Background
Mycoplasma pneumoniae (M. pneumoniae) is widely recognised as an important cause of community‐acquired lower respiratory tract infection (LRTI) in children. Pulmonary manifestations are typically tracheobronchitis or pneumonia but M. pneumoniae is also implicated in wheezing episodes in both asthmatic and non‐asthmatic individuals. Although antibiotics are used to treat LRTIs, a review of several major textbooks offers conflicting advice for using antibiotics in the management of M. pneumoniae LRTI in children.
Objectives
To determine whether antibiotics are effective in the treatment of childhood LRTI secondary to M. pneumoniae infections acquired in the community.
Search methods
We searched CENTRAL (2014, Issue 3), MEDLINE (1966 to July week 4, 2014), EMBASE (1980 to July, 2014), and both WHO ICTRP and ClinicalTrials.gov (13 August 2014).
Selection criteria
Randomised controlled trials (RCTs) comparing antibiotics commonly used for treating M. pneumoniae (i.e. macrolide, tetracycline or quinolone classes) versus placebo, or antibiotics from any other class in the treatment of children under 18 years of age with community‐acquired LRTI secondary to M. pneumoniae.
Data collection and analysis
The review authors independently selected trials for inclusion and assessed methodological quality. We extracted and analysed relevant data separately and resolved disagreements by consensus.
Main results
A total of 1912 children were enrolled from seven studies. Data interpretation was limited by the inability to extract data that referred to children with M. pneumoniae. In most studies, clinical response did not differ between children randomised to a macrolide antibiotic and children randomised to a non‐macrolide antibiotic. In one controlled study (of children with recurrent respiratory infections, whose acute LRTI was associated with Mycoplasma, Chlamydia or both, by polymerase chain reaction and/or paired sera) 100% of children treated with azithromycin had clinical resolution of their illness compared to 77% not treated with azithromycin at one month.
Authors' conclusions
There is insufficient evidence to draw any specific conclusions about the efficacy of antibiotics for this condition in children (although one trial suggests macrolides may be efficacious in some children with LRTI secondary to Mycoplasma). The use of antibiotics has to be balanced with possible adverse events. There is still a need for high quality, double‐blinded RCTs to assess the efficacy and safety of antibiotics for LRTI secondary to M. pneumoniae in children.
Plain language summary
Antibiotics to treat respiratory infections caused by the bacteria Mycoplasma pneumoniae in children
Review question This review sought to answer the question of whether antibiotics are effective in the treatment of LRTIs caused by the bacteria Mycoplasma pneumoniae (M. pneumoniae) in children.
Background M. pneumoniae is a bacterial infection, often responsible for lower respiratory tract infections (LRTIs) in children. The infection can present in a number of different ways and the most common respiratory manifestations are acute bronchitis, pneumonia or wheezing. The illness is generally self limiting, with symptoms that can last several weeks but may (occasionally) also cause severe pneumonia. Antibiotics are often given to children with M. pneumoniae LRTI.
Search date We searched for trials published and pending as at July 2014.
Study characteristics Randomised controlled trials (RCTs) comparing either two types of antibiotic therapy or an antibiotic versus a placebo in children with pneumonia.
Key results We identified seven studies (1912 children). Within each study, there were some children who had M. pneumoniae but we could not extract relevant data relating to efficacy or adverse events relating only to children with M. pneumoniae.
Quality of evidence Overall the quality of the evidence for each of the main outcomes is very low as there are insufficient data for any outcome. Hence, currently, there is insufficient evidence to show conclusively that antibiotics are effective in children with LRTI caused by M. pneumoniae.
Background
Description of the condition
Mycoplasma pneumoniae (M. pneumoniae) is widely recognised as an important cause of community‐acquired lower respiratory tract infection (LRTI) in children, accounting for 14% to 34% of cases (Kogan 2003; Michelow 2004; Nelson 2002; Principi 2002). The highest attack rates are reported to occur in five to 20‐year olds and the infection is usually self limiting, with symptoms lasting several weeks (Nelson 2002; Rudolph 2003). More recently, M. pneumoniae has been identified as an important cause of LRTI in children under five years of age (Principi 2001). Pulmonary manifestations are typically tracheobronchitis or pneumonia but can be complicated by pleural effusion, lung abscess, pneumothorax, bronchiectasis and respiratory distress syndrome (Principi 2002). M. pneumoniae is also implicated in wheezing episodes in both asthmatic and non‐asthmatic individuals (Phelan 1994; Principi 2001). Uncommon extrapulmonary manifestations may include erythema multiforme, myocarditis, encephalitis, Guillain‐Barré syndrome, transverse myelitis and haemolytic anaemia (Nelson 2002; Waites 2003). Radiographic findings are quite variable and non‐diagnostic (Principi 2001). In some cases there can be significant radiological changes in the absence of clinical signs on auscultation of the chest (so‐called 'walking pneumonia') (Rudolph 2003).
Description of the intervention
Antibiotics are frequently used to treat LRTIs and empiric antibiotic therapy is often chosen to cover both bacteria and atypical organisms (Kogan 2003). A review of several major textbooks offers conflicting advice for management of M. pneumoniae LRTI. The chapter on M. pneumoniae in a paediatric respiratory textbook mentions that there is little evidence of beneficial effect from antibiotic therapy (Phelan 1994). This is in contrast to the recommendations in a major general paediatric textbook (Rudolph 2003), and paediatric infectious disease textbook (Katz 1998), which state that erythromycin is the treatment of choice.
How the intervention might work
The use of antibiotics in treating LRTI in children would be expected to reduce the severity or duration (or both) of the infection and its associated symptoms.
Why it is important to do this review
The conclusion that antibiotics are effective in M. pneumoniae chest infections seems to have been drawn from trials of antibiotic therapy for community‐acquired or atypical pneumonia, where M. pneumoniae was identified as a causative organism in a subgroup of cases. In these studies, macrolide antibiotics, to which M. pneumoniae is susceptible, have been compared to non‐macrolide antibiotics. However, it is not always possible to draw meaningful conclusions from the results, as the numbers of individuals with M. pneumoniae are small in most trials (Block 1995; Kogan 2003; Wubbel 1999).
Identification of M. pneumoniae infection as the causative infectious agent may, however, pose difficulties. Serological tests are the most common method used to diagnose M. pneumoniae infections, but can lead to difficulties with interpretation (Principi 2001). Measurement of immunoglobulin M (IgM) is used to diagnose acute infection, but the accuracy of the test depends on the method used. Not all methods are specific for IgM and an elevated IgM may persist for months after the acute infection (Murray 2003). Immuno‐fluorescent antibody (IFA) assay is more sensitive and specific than the complement fixation (CF) test (Murray 2003; Principi 2001). Identification of M. pneumoniae in nasopharyngeal secretions by culture or polymerase chain reaction (PCR) may also cause difficulties with interpretation as this organism can persist for variable periods following the acute infection (Murray 2003). The 'gold standard' for diagnosis of M. pneumoniae infection is a four‐fold increase in total antibody titre as measured in paired sera (Katz 1998; Murray 2003).
Objectives
To determine whether antibiotics are effective in the treatment of childhood LRTI secondary to M. pneumoniae infections acquired in the community.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing antibiotics from the macrolide, tetracycline or quinolone class (i.e. antibiotics that are efficacious for Mycoplasma) versus placebo, or antibiotics from any other class (i.e. medications that are not efficacious for mycoplasma).
Types of participants
Trials that included children under 18 years of age with community‐acquired LRTI secondary to M. pneumoniae. Diagnosis of M. pneumoniae infection was via either a four‐fold rise in total antibody titre from paired sera or total antibody titre ≥ 1:512 on a single specimen. We included other methods of diagnosis, such as culture or PCR of M. pneumoniae in nasopharyngeal secretions or demonstration of elevated IgM on a single specimen (IgM titre ≥ 1:10), and analysed these separately as a subgroup.
Exclusion criteria
Children with underlying chronic cardiorespiratory illnesses, such as cystic fibrosis, bronchiectasis, immunodeficiency, chronic neonatal lung disease and symptomatic congenital heart disease.
Children with non‐community‐acquired LRTI.
Types of interventions
We evaluated two separate treatment regimes.
Any antibiotic versus placebo.
Antibiotics from the macrolide, tetracycline or quinolone class versus placebo or antibiotics from any other class.
We included trials that allowed the use of other medications or interventions in addition to antibiotic therapy if all participants had equal access to such medications or interventions.
Types of outcome measures
We made attempts to obtain data on at least the following outcome measures.
Primary outcomes
Proportions of participants who were not improved at follow‐up. We measured failure to improve according to the hierarchy listed below.
Secondary outcomes
Mean difference in symptoms and signs (mean improvement in clinical state).
Proportions requiring hospitalisation.
Proportions experiencing pulmonary complications (empyema, pleural effusion, air leak).
Proportions experiencing non‐pulmonary complications.
Proportions experiencing adverse effects (for example, nausea, diarrhoea, abdominal pain, rash).
Proportions experiencing complications (for example, requirement for medication change).
We determined the proportions of participants who failed to improve on treatment and the mean clinical improvement using the following hierarchy of assessment measures. (We reported all outcomes, but where two or more assessment measures were reported in the same study and we obtained conflicting results, we used the outcome measure that was listed first in the hierarchy).
Objective measurements of cough indices (cough frequency).
Symptomatic (cough, wheeze, dyspnoea, malaise, general well‐being, headache): assessed by the child (Likert scale, visual analogue scale, level of interference of symptoms, diary, quality of life).
Symptomatic (cough, wheeze, dyspnoea, malaise, general well‐being, headache): assessed by the parents/carers (Likert scale, visual analogue scale, level of interference of symptoms, diary, quality of life).
Symptomatic (cough, wheeze, dyspnoea, malaise, general well‐being, headache): assessed by the clinician (Likert scale, visual analogue scale, level of interference of symptoms, diary, quality of life).
Fever.
Non‐clinical outcomes (chest radiology, white cell count, C‐reactive protein, erythrocyte sedimentation rate, lung function).
Eradication of M. pneumoniae by PCR evaluation.
Search methods for identification of studies
Electronic searches
For this 2014 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 3) (accessed 8 July 2014) limited to year published 2011 to 2014, which contains the Acute Respiratory Infection Group's Specialised Register, MEDLINE (1 January 2012 to June week 4, 2014) and EMBASE (1 January 2012 to July 2014).
Previously we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2012, Issue 2) (accessed 13 March 2012), which contains the Acute Respiratory Infection Group's Specialised Register, MEDLINE (1966 to February Week 5, 2012) and EMBASE (1980 to March 2012). Details of earlier searches are described in Appendix 1.
We used the search terms in Appendix 2 to search MEDLINE and CENTRAL. We combined the MEDLINE search with a sensitive search strategy for identifying child studies (Boluyt 2008) and the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search terms for EMBASE (Appendix 3).
We imposed no language or publication restrictions.
Searching other resources
We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and the US National Institute of Health ClinicalTrials.gov platform (Appendix 4) for all registered trials, both published and pending, as at 13 August 2014. We further manually checked all references for reports of trials.
Data collection and analysis
Selection of studies
In the first publication of this review, three review authors (JG, AC, SM) independently reviewed the literature searches from the title, abstract or descriptions, to identify potentially relevant trials for full review. We conducted searches of bibliographies and texts to identify additional studies. Three review authors (JG, AC, SM) independently selected trials for inclusion from the full text using specific criteria. In the 2012 update two review authors (MG, AC) reviewed the literature searches. For this 2014 update two review authors (SG, AC) independently assessed the literature searches for inclusion and exclusion.
Data extraction and management
In the first publication of this review, three review authors (JG, AC, SM) independently extracted data and resolved disagreement by consensus. We reviewed trials that satisfied the inclusion criteria and recorded the following information: study setting; year of study; source of funding; patient recruitment details (including number of eligible children); inclusion and exclusion criteria; randomisation and allocation concealment method; numbers of participants randomised; blinding (masking) of participants, care providers and outcome assessors; intervention (type of antimicrobials, dose, duration); control (type, dose, duration); co‐interventions; numbers of patients not followed up; reasons for withdrawals from study protocol (clinical, side effects, refusal and other); details on side effects of therapy; and whether intention‐to‐treat (ITT) analyses were possible. We extracted data on the outcomes described previously. The review authors requested further information from the study authors where required.
Assessment of risk of bias in included studies
In the original review, two review authors (JG, AC) utilised the Jadad quality assessment scores (Gavranich 2005). In the 2012 update three review authors (JG, AC, SM) independently assessed the quality of studies included in the review using the 'Risk of bias' table available in Review Manager 5 (RevMan 2014), in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed five components of quality.
Adequate sequence generation. This assesses the quality of the method of randomisation.
Allocation concealment. This assesses whether or not enrolling staff were aware of the group to which participants would be allocated.
Blinding. This assesses the extent of blinding, with participant/caregiver and outcome assessor blinding taken into account.
Follow‐up. This assesses whether the proportion of participants lost to follow‐up is admissible, and whether adequate reasons for the losses were made available.
Reporting of participants by allocation group. This assesses whether the results were reported relative to the treatment groups.
Measures of treatment effect
In the protocol we planned to calculate relative and absolute risk reductions using an ITT analysis for the dichotomous outcome variables of each individual study. However, data were unavailable.
Dealing with missing data
The review authors wrote to the trial authors to enquire about availability of data but we did not receive any replies.
Assessment of heterogeneity
In the protocol we planned to describe any heterogeneity between the study results and, depending upon the number of trials included in the review, we had planned to use a funnel plot to look for publication bias. However, data were unavailable and we were unable to include any studies in a meta‐analysis.
Data synthesis
In the protocol we planned to include the results from studies that met the inclusion criteria and report any of the outcomes of interest in the subsequent meta‐analysis. We planned to calculate the summary weighted risk ratio (RR) and 95% confidence interval (CI) (fixed‐effect model) using the inverse of the variance of each study result for weighting. We planned to calculate the number needed to treat to benefit using the summary odds ratio (OR) and the average control event rate described in the relevant studies. We stated in the protocol that the cough indices were assumed to be normally distributed continuous variables so the mean difference (MD) in outcomes could be estimated. In studies that reported outcomes using different measurement scales, we would have estimated the standardised MD. However, data were unavailable.
Subgroup analysis and investigation of heterogeneity
In the protocol we intended to perform an a priori subgroup analysis for the following.
Children aged seven years and older.
Intervention type (class of antibiotics).
Diagnostic criteria used for identification of M. pneumoniae.
However, data were unavailable.
Sensitivity analysis
In the protocol we planned a sensitivity analysis to assess the impact of the potentially important factors on overall outcomes.
Study quality.
Study size.
Variation in the inclusion criteria.
Differences in the medications used and duration of treatment in the intervention and comparison groups.
Differences in outcome measures.
Analysis by 'treatment received' rather than ITT.
However, data were unavailable.
Results
Description of studies
Results of the search
We identified 91 potentially relevant titles in the initial search. After reviewing the abstracts, we obtained 17 papers in full text for consideration for inclusion in the review. We included seven studies and details are provided in the Characteristics of included studies table. Three of the included studies were non‐English: German (Ruhrmann 1982) and Spanish (Gomez Campdera 1996; Saez‐Llorens 1998).
In the updated search in 2009 we identified 20 new records, of which we considered 11 for inclusion, but only included one (Esposito 2005). We excluded two as they did not meet the inclusion criteria (Bradley 2007; Lee 2008), two had no focus on the aetiology of the LRTI (Bradley 2007; Fonseca‐Aten 2006), and three were review papers including the most recent review (Atkinson 2007). One only focused on upper respiratory tract infections (URTIs) (Esposito 2006), one result was the previous version of this review (Gavranich 2005), and one paper was unavailable for evaluation (Simon 2006). In the 2012 search we identified 77 studies, though none fulfilled the inclusion criteria.
In this 2014 search we identified a total of 22 studies and none fulfilled the specified inclusion criteria.
Included studies
Participants
The studies involved children diagnosed with LRTI ranging in age from one month to 16 years. In all except three studies (Esposito 2005; Gomez Campdera 1996; Soderstrom 1991) children had pneumonia supported with abnormal chest X‐ray, and apart from two studies (Esposito 2005; Ruhrmann 1982) the children were described as having community‐acquired pneumonia. The study by Gomez Campdera 1996 did not define pneumonia and the study by Soderstrom 1991 included participants with acute bronchitis. The number of children with M. pneumoniae causing LRTI was not stated in four studies (Esposito 2005; Gomez Campdera 1996; Ruhrmann 1982; Saez‐Llorens 1998). In one study there were 12 children with M. pneumoniae infections and six were in the subgroup randomised to either azithromycin or amoxycillin‐clavulanate, but the number assigned to each therapy was not available (Wubbel 1999). In two other studies the number of children with M. pneumoniae infections in each intervention group was provided. In the study by Harris 1998 there were 30 children who had M. pneumoniae infections randomised to either azithromycin or amoxycillin‐clavulanate (21 in the azithromycin group and nine in the amoxycillin‐clavulanate group) and there were eight children in the study by Kogan 2003 (five in the azithromycin group and three in the amoxycillin‐clavulanate group). In the study by Soderstrom 1991 there were only seven patients with LRTI (bronchitis) and one case of M. pneumoniae, but the age of the participants with M. pneumoniae was not provided. The study by Esposito 2005 did not distinguish between upper and lower respiratory tract infections in their analysis of results, although the number of M. pneumoniae infections (which included both URTIs and LRTIs) was made available.
Interventions
Studies included in this review involved patients with LRTI randomised to either a macrolide antibiotic or another antibiotic, usually a different macrolide or non‐macrolide antibiotic. In two studies the entire study population was randomised to either a macrolide or non‐macrolide antibiotic (Ruhrmann 1982; Soderstrom 1991). Ruhrmann 1982 included children with pneumonia who received either erythromycin 70 to 80 mg/kg/day or amoxycillin 60 to 70 mg/kg/day. The duration of therapy was not stated. The study by Soderstrom 1991 had a subgroup of participants (number of children not stated) with acute bronchitis who received either erythromycin 500 mg twice daily for seven days or phenoxymethylpenicillin 800 mg twice daily for seven days. Four studies randomised a subgroup of children under five years of age to azithromycin or amoxycillin‐clavulanate (Gomez Campdera 1996; Harris 1998; Saez‐Llorens 1998; Wubbel 1999). The dose of amoxycillin‐clavulanate was 40 mg/kg/day in three divided doses for 10 days in all studies. The dose of azithromycin was 10 mg/kg once daily for three days in one study (Gomez Campdera 1996) and 10 mg/kg on day one followed by 5 mg/kg once daily for day two to five in three studies (Harris 1998; Saez‐Llorens 1998; Wubbel 1999). In the study by Kogan 2003 the intervention for the subgroup with classic pneumonia was either azithromycin 10 mg/kg once daily for three days or amoxycillin 75 mg/kg/day in three divided doses for seven days. The Esposito 2005 study compared azithromycin with symptom‐specific agents to symptom‐specific agents alone; the azithromycin that was given was 10 mg/kg/day, three days per week for three weeks and acetaminophen (at 10 mg/kg/dose) was the symptom‐specific agent.
Outcome measures
Clinical
Clinical response was the main outcome but was not defined in three studies (Gomez Campdera 1996; Ruhrmann 1982; Soderstrom 1991). In three studies clinical cure was defined as complete resolution of symptoms and signs by day 15 to 19 (Harris 1998), day 10 to 25 (Saez‐Llorens 1998) and day 10 to 37 (Wubbel 1999). In the study by Kogan 2003 the clinical response was defined as the proportion of children without fever on day three. The Esposito 2005 study evaluated clinical responses at both one month (defined as the complete resolution of the acute symptoms, with no relapse) and six months (defined as the presence of no more than two respiratory relapses).
Radiological
Radiological outcome was recorded in three studies (Gomez Campdera 1996; Harris 1998; Kogan 2003), but was not defined in the study by Gomez Campdera 1996. Bacteriological outcome was recorded in three studies (Esposito 2005; Harris 1998; Saez‐Llorens 1998), but was not defined in the study by Saez‐Llorens 1998. Adverse events were recorded in four studies (Gomez Campdera 1996; Harris 1998; Saez‐Llorens 1998; Wubbel 1999), but were only defined in the study by Harris 1998.
We made attempts to obtain individual patient data from four studies (Esposito 2005; Harris 1998; Kogan 2003; Wubbel 1999), where the number of children with LRTI due to M. pneumoniae was not identified, but we did not receive a reply at the time this review was completed.
Excluded studies
We have excluded 19 papers and details are provided in the Characteristics of excluded studies table. The main reasons for exclusion were the non‐randomised nature of the study (Jensen 1967; Sakata 2001; Vasilos 1995), or use of inadequate placebo or comparator (Block 1995; Bradley 2007; Chien 1993; Jensen 1967; Lee 2008; Lee 2012; Manfredi 1992; Nogeova 1997; Ronchetti 1994; Schonwald 1990; Sempertegui 2014; Wu 2014; Yin 2002). Three of the excluded studies were non‐English: Japanese (Sakata 2001), Russian (Vasilos 1995) and Chinese (Yin 2002).
Risk of bias in included studies
We assessed risk of bias using the 'Risk of bias' tables for included studies (see Characteristics of included studies) (Higgins 2011). We generated a graph and summary for the information, and the combined results for the different categories of risk are highlighted. Approximately 50% of included studies were not blinded, but good results were seen for both follow‐up and reporting of participants by allocation group overall (i.e. in more than half the included studies these were not found to be a source of bias).
Allocation
All studies were described as randomised and the method of randomisation was clearly described and appropriate in three studies where a random number list was used (Esposito 2005; Ruhrmann 1982; Saez‐Llorens 1998). The method of randomisation was unclear in one study where the method used was described as a list of randomised therapy assignments (Wubbel 1999). In the trial Soderstrom 1991, the method used was sequential patient numbers and we thought this to be inadequate. Three studies did not describe the method of randomisation (Gomez Campdera 1996; Harris 1998; Kogan 2003). Concealment of allocation was unclear in all except three studies; two assigned therapy by pharmacy (Saez‐Llorens 1998; Wubbel 1999), and one allocated the duties of enrolment and randomisation to separate investigators (Esposito 2005).
Blinding
There was no blinding in four studies (Gomez Campdera 1996; Ruhrmann 1982; Saez‐Llorens 1998; Wubbel 1999). In three studies the blinding involved only the participant (Harris 1998), clinician (Kogan 2003) or radiologist (Soderstrom 1991). The Esposito 2005 study blinded the participant, caregiver, clinical outcome assessors and data/statistical analysts.
Incomplete outcome data
Five of the included studies adequately followed up their participants. Three of the eight included studies had unclear levels of follow‐up. Gomez Campdera 1996 and Ruhrmann 1982 made no mention of losses to follow‐up. While Saez‐Llorens 1998 mentioned that 30 were lost to follow‐up, there was no mention of why or from which groups these losses occurred.
Selective reporting
Although selective reporting was not readily identified, possible issues are highlighted in Other potential sources of bias.
Other potential sources of bias
Three of the eight included studies were funded by Pfizer Incorporated, a large pharmaceutical company responsible for producing Zithromax, a popular azithromycin (Esposito 2005; Harris 1998; Wubbel 1999). This association may have influenced the subjective outcome measures of these studies (i.e. 'clinical success'). All three studies were concerned with the efficacy of azithromycin in treating LRTIs, and none found it to be a less effective drug than alternative antimicrobial therapy. Wubbel 1999 found no difference and Esposito 2005 and Harris 1998 found it to be a superior treatment.
Effects of interventions
Ideally the primary and secondary outcomes should be reported here but the lack of data relevant for M. pneumoniae within each study precludes this and hence the data described below relate to the studies themselves where the subgroup was reported.
We identified seven trials with 1912 children. The number of children from one study was unavailable (Soderstrom 1991).
Data interpretation was significantly limited by the inability to extract data that specifically referred to children with lower respiratory tract infection (LRTI) caused by M. pneumoniae. There was only one study of children randomised to any antibiotic versus placebo (Esposito 2005). Most of the included studies comprised a subgroup of children who were randomised to a macrolide versus non‐macrolide antibiotic. The total number of children in this subgroup was not known as the numbers were only available in four studies (Harris 1998; Kogan 2003; Ruhrmann 1982; Wubbel 1999). The number of children with LRTI secondary to M. pneumoniae in this subgroup was only available in two studies (Harris 1998; Kogan 2003), and the lack of individual patient data did not allow for inclusion of results in a meta‐analysis. There was a total of 26 in the azithromycin group and 12 in the amoxycillin‐clavulanate group.
In the study by Gomez Campdera 1996 the rate of clinical cure was 95.12% in the azithromycin group and 90.41% in the amoxycillin‐clavulanate group. Radiological improvement was noted in 90.6% of the azithromycin group. Adverse events were recorded in 11.25% of the azithromycin group and 17.14% in the amoxycillin‐clavulanate group. Harris 1998 reported no difference in the rate of clinical cure at day 15 to 19 (67.2% versus 66.7%) and four to six weeks (85.1% versus 85.4%) in children randomised to azithromycin or amoxycillin‐clavulanate. M. pneumoniae was identified in 16% (30 of 188 children under five years of age). Eradication of M. pneumoniae occurred in 3/3 in the azithromycin group and in 0/1 in the amoxycillin‐clavulanate group. Adverse events in those children under five years of age were 12.1% in the azithromycin group and 42.3% in the amoxycillin‐clavulanate group.
One participant in each group discontinued treatment because of adverse events. In the study by Kogan 2003, which compared azithromycin to amoxicillin in children with classical pneumonia (eight children of 47 had M. pneumoniae), X‐ray resolution was significantly better in those treated with azithromycin (81% versus 60.9% at day seven), but there was no difference in clinical symptoms or signs between groups. In those with atypical pneumonia (23 children of 59 had M. pneumoniae) there was no significant difference between children treated with azithromycin or erythromycin (Kogan 2003). Ruhrmann 1982 reported clinical cure after 3.79 days in the erythromycin group and 3.96 days in the amoxycillin group. Saez‐Llorens 1998 reported a similar clinical response (99% versus 98%) in children under five years who were randomised to azithromycin or amoxycillin‐clavulanate. Eradication of M. pneumoniae occurred in 23 out of 24 in the azithromycin group. Adverse events were reported in 11% on azithromycin, 30% on amoxycillin‐clavulanate and 27% on erythromycin. Soderstrom 1991 did not report the clinical response in the subgroup of patients with bronchitis. In the study by Wubbel 1999, where 7% (12 of 168 children) had M. pneumoniae, no difference was found in children randomised to azithromycin or amoxicillin‐clavulanate. Adverse events were reported in 14% on azithromycin, 67% on amoxycillin‐clavulanate and 25% on erythromycin. Eleven patients did not complete the prescribed therapy. Esposito 2005, which grouped Chlamydia pneumoniae (C. pneumoniae) and M. pneumoniae together (and did not distinguish between upper and lower respiratory tract infections) when reporting clinical success rates (with a total of 200/560 infected children), found a 100% success rate in the short term with azithromycin and symptomatic therapy, and a 73.2% success rate at the six‐month follow‐up. Symptomatic treatment alone showed a success rate of 77.2% at one month and 56.0% at six months. Adverse events were not reported in this study.
Discussion
Summary of main results
This review failed to find any randomised controlled trials (RCTs) that specifically looked at the effectiveness of antibiotics for lower respiratory tract infection (LRTI) secondary to M. pneumoniae. There was only one study of antibiotics versus placebo (Esposito 2005), but this study defined success rates relative to LRTI secondary to M. pneumoniae and Chlamydia defined by polymerase chain reaction (PCR) or paired sera. In this study significantly more children in the azithromycin group had 'clinical success' on follow‐up than the placebo group. From the other studies, in the subgroup of children with LRTI secondary to M. pneumoniae the intervention was a macrolide antibiotic versus a non‐macrolide antibiotic, usually amoxycillin‐clavulanate. This subgroup identified only 38 children with M. pneumoniae infection and there were insufficient data to analyse the efficacy of macrolide antibiotics in this group. Adverse events were common: reported in 11% to 67% of children. The majority of adverse events related to the gastrointestinal tract (diarrhoea, vomiting, abdominal pain, nausea, anorexia) and where reported were more common in younger children (under five years of age).
Overall completeness and applicability of evidence
There were significant difficulties in interpreting the data from the included studies. Firstly, although all studies (except Soderstrom 1991) enrolled children with LRTI, only a proportion had M. pneumoniae infection. It was not possible to obtain information on the subgroup with M. pneumoniae. Secondly, the dose and type of antibiotics differed among studies. Thirdly, application of diagnostic criteria (serology versus PCR) varied and these are not necessarily interchangeable. Fourthly, the inclusion criteria differed (various types of LRTI manifestation) between studies. Furthermore, the outcomes measured were variable and in some papers clinical cure was undefined.
Quality of the evidence
In addition to the above, the quality of the studies varied (Figure 1; Figure 2), with non‐blinded outcomes in the majority of the included studies.
1.
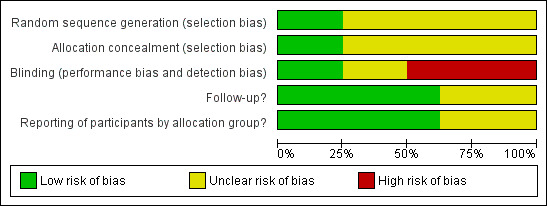
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
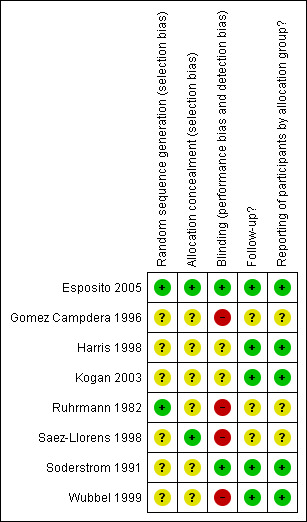
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Potential biases in the review process
We did not identify any potential biases in the review process.
Agreements and disagreements with other studies or reviews
Despite the commonality of M. pneumoniae LRTI in children (attributed in up to 40% of community‐acquired pneumonia as reported by Waites 2003), there is surprisingly no RCT that has specifically evaluated the efficacy of antibiotics for the treatment of childhood LRTI secondary to M. pneumoniae infections acquired in the community. Such disparity in knowledge is exemplified by the conflicting advice given in paediatric textbooks (Phelan 1994; Rudolph 2003). This systematic review, as well as that conducted by Biondi 2014 showing concordant findings, reaffirms the need for such trials to be carried out.
Authors' conclusions
Implications for practice.
Based on a single randomised controlled trial (RCT), it is likely that macrolides are efficacious in (at least) a small group of children with lower respiratory tract infection (LRTI) secondary to M. pneumoniae. However, there is insufficient evidence to draw any specific conclusions about the efficacy of antibiotics for this condition in children. The use of antibiotics for M. pneumoniae LRTI has to be individualised and balanced with possible adverse events associated with antibiotic use.
Implications for research.
M. pneumoniae infection is relatively common and its clinical manifestations range from being asymptomatic to death from its complications. As respiratory symptoms are the most common symptoms, there is a need for high quality, double‐blinded RCTs to assess the efficacy and safety of antibiotics for LRTI secondary to M. pneumoniae in children. Studies should consider the various clinical and microbiological diagnostic criteria of M. pneumoniae infection and utilise clear outcome criteria. Community studies using polymerase chain reaction (PCR) for rapid early diagnosis would be useful in evaluating the efficacy of antibiotics forM. pneumoniae for respiratory and non‐respiratory manifestations, as well as for prevention of complications and microbiological clearance of M. pneumoniae.
What's new
| Date | Event | Description |
|---|---|---|
| 8 July 2014 | New search has been performed | A new author joined the review team. |
| 8 July 2014 | New citation required but conclusions have not changed | Searches updated. We did not include any new trials and we excluded three new trials (Lee 2012; Sempertegui 2014; Wu 2014). |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 15 March 2012 | New citation required but conclusions have not changed | A new author joined the review team. |
| 13 March 2012 | New search has been performed | Searches conducted. |
| 22 February 2010 | New search has been performed | Searches conducted. One new included trial (Esposito 2005) and six new excluded trials (Atkinson 2007; Bradley 2007; Esposito 2006; Fonseca‐Aten 2006; Lee 2008; Simon 2006) have been added to the update. |
| 22 February 2010 | New citation required and conclusions have changed | A new author joined the review team. The conclusion has changed to reflect the new included trial. |
| 22 July 2008 | Amended | Converted to new review format. |
| 23 May 2005 | Amended | Review first published Issue 3, 2005 |
Acknowledgements
We thank Igor Bezuglov, Tan Yook Hua and Hiroshi Ito for reviewing the Russian, Chinese and Japanese articles, and special thanks to Julio Clavijo and Andreas Schibler for extracting data from the Spanish and German articles. We thank Michael Nissen and Jennifer Robson for their advice on microbiological testing for M. pneumoniae. We thank Liz Dooley, Chris Del Mar and Sarah Thorning from the Acute Respiratory Infections Group for their assistance with the preparation of this systematic review. We wish to acknowledge the peer referees who commented on the draft protocol: Amy Zelmer, Imtiaz Jehan, Nicola Principi, Mark Jones and Richmal Oates‐Whitehead. We wish to thank the referees who commented on the 2012 updated review: Amy Zelmer, Imtiaz Jehan, Mark Griffin and Taixiang Wu. Finally we wish to thank the previous co‐authors, Selamawit Mulholland and Malcolm Gillies, who contributed to the progressive works of this review.
Appendices
Appendix 1. Previous searches
2012 search details
MEDLINE (Ovid)
1 Pneumonia, Mycoplasma/ 2 (mycoplasma adj3 pneumonia*).tw. 3 primary atypical pneumonia.tw. 4 or/1‐3 5 Mycoplasma pneumoniae/ 6 (mycoplasma pneumoniae or "M. pneumoniae").tw. 7 Mycoplasma Infections/ 8 mycoplasma.tw. 9 or/5‐8 10 exp Pneumonia/ 11 (pneumon* or bronchopneumon* or pleuropneumon*).tw. 12 exp Bronchitis/ 13 (bronchit* or tracheobronchit*).tw. 14 Respiratory Sounds/ 15 wheez*.tw. 16 exp Respiratory Tract Infections/ 17 (respiratory tract infection* or acute respiratory infection* or lower respiratory infection* or lower respiratory tract infection* or lrti).tw. 18 or/10‐17 19 9 and 18 20 4 or 19 21 exp Anti‐Bacterial Agents/ 22 exp Macrolides/ 23 exp Quinolones/ 24 exp Tetracyclines/ 25 antibiotic*.tw,nm. 26 (macrolide* or erythromycin* or roxithromycin* or clarithromycin* or azithromycin*).tw,nm. 27 or/21‐26 28 20 and 27
EMBASE
#36 #27 AND #35 #35 #30 NOT #34 #34 #31 NOT #33 #33 #31 AND #32 #32 'human'/de AND [embase]/lim #31 'animal'/de OR 'nonhuman'/exp OR 'animal experiment'/de AND [embase]/lim #30 #28 OR #29 #29 random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR trial:ti OR crossover*:ab,ti OR 'cross over':ab,ti OR (doubl* NEXT/1 blind*):ab,ti AND [embase]/lim #28 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim #27 #21 AND #26 #26 #22 OR #23 OR #24 OR #25 #25 erythromycin*:ab,ti OR roxithromycin*:ab,ti OR clarithromycin*:ab,ti OR azithromycin*:ab,ti OR macrolide*:ab,ti AND [embase]/lim #24 antibiotic*:ab,ti AND [embase]/lim #23 'macrolide'/exp OR 'quinolone derivative'/exp OR 'tetracycline derivative'/exp AND [embase]/lim #22 'antibiotic agent'/exp AND [embase]/lim #21 #4 OR #20 #20 #9 AND #19 #19 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 #18 lrti:ab,ti AND [embase]/lim #17 (infection* NEAR/1 ('respiratory tract' OR 'acute respiratory' OR 'lower respiratory' OR 'lower respiratory tract')):ab,ti AND [embase]/lim #16 'respiratory tract infection'/de OR 'lower respiratory tract infection'/de AND [embase]/lim #15 wheez*:ab,ti AND [embase]/lim #14 'wheezing'/de AND [embase]/lim #13 bronchit*:ab,ti OR tracheobronchit*:ab,ti AND [embase]/lim #12 'bronchitis'/exp AND [embase]/lim #11 pneumon*:ab,ti OR bronchopneumon*:ab,ti OR pleuropneumon*:ab,ti AND [embase]/lim #10 'pneumonia'/exp AND [embase]/lim #9 #5 OR #6 OR #7 OR #8 #8 mycoplasma:ab,ti AND [embase]/lim #7 'mycoplasmosis'/de AND [embase]/lim #6 'mycoplasma pneumoniae':ab,ti OR 'm. pneumoniae':ab,ti AND [embase]/lim #5 'mycoplasma pneumoniae'/de AND [embase]/lim #4 #1 OR #2 OR #3 #3 'primary atypical pneumonia':ab,ti AND [embase]/lim #2 (mycoplasma NEAR/3 pneumonia):ab,ti AND [embase]/lim #1 'mycoplasma pneumonia'/de AND [embase]/lim
2010 search details
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 1), which contains the Acute Respiratory Infection Group's Specialised Register, MEDLINE (1966 to February Week 2, 2010) and EMBASE (1980 to February 2010).
We used the following search terms for MEDLINE and CENTRAL and adapted them for EMBASE. We combined the search terms used in MEDLINE with a sensitive search strategy for identifying child studies (Boluyt 2008) and the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2008).
MEDLINE (Ovid)
1 exp MYCOPLASMA/ 2 exp Mycoplasma pneumoniae/ 3 mycoplasma.tw. 4 "m. pneumoniae".tw. 5 or/1‐4 6 exp BRONCHITIS/ 7 exp PNEUMONIA/ 8 exp Respiratory Tract Infections/ 9 bronchit*.tw. 10 pneumon*.tw. 11 wheez*.tw. 12 tracheobronchit*.tw. 13 respiratory tract infection*.tw. 14 acute respiratory infection*.tw. 15 or/6‐14 16 exp Anti‐Bacterial Agents/ 17 exp MACROLIDES/ 18 exp QUINOLONES/ 19 exp TETRACYCLINES/ 20 antibiotic*.tw,nm. 21 (macrolide* or erythromycin or roxithromycin or clarithromycin or azithromycin).tw,nm. 22 or/16‐21 23 5 and 15 and 22 24 exp Infant/ 25 (infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur*).tw. 26 exp Child/ 27 (child* or schoolchild* or school age* or preschool* or kid or kids or toddler*).tw. 28 Adolescent/ 29 (adoles* or teen* or boy* or girl*).tw. 30 Minors/ 31 Puberty/ 32 (minor* or pubert* or pubescen*).tw. 33 exp Pediatrics/ 34 (pediatric* or paediatric*).tw. 35 exp Schools/ 36 (nursery school* or kindergar* or primary school* or secondary school* or elementary school* or high school* or highschool*).tw. 37 or/24‐36 38 37 and 23
EMBASE
1. 'mycoplasma'/de OR 'mycoplasma pneumoniae'/de 2. 'm. pneumoniae':ab,ti OR mycoplasma:ab,ti 3. #1 OR #2 4. 'bronchitis'/exp OR 'pneumonia'/exp 5. bronchit*:ab,ti OR pneumon*:ab,ti OR wheez*:ab,ti OR tracheobronchit*:ab,ti 6. 'respiratory tract infection'/de OR 'lower respiratory tract infection'/de 7. 'respiratory tract infection':ab,ti OR 'respiratory tract infections':ab,ti OR 'acute respiratory infection':ab,ti OR 'acute respiratory infections':ab,ti 8. #4 OR #5 OR #6 OR #7 9. 'antibiotic agent'/exp 10. antibiotic*:ab,ti 11. 'macrolide'/exp OR 'quinolone derivative'/exp OR 'tetracycline derivative'/exp 12. macrolide*:ab,ti OR quinolone*:ab,ti OR tetracycline*:ab,ti OR erythromycin*:ab,ti OR roxithromycin*:ab,ti OR clarithromycin*:ab,ti OR azithromycin*:ab,ti 13. #9 OR #10 OR #11 OR #12 14. #3 AND #8 AND #13 15. 'child'/exp 16. child*:ab,ti OR schoolchild*:ab,ti OR 'school age':ab,ti OR 'school aged':ab,ti OR 'school ages':ab,ti OR preschool*:ab,ti OR kid:ab,ti OR kids:ab,ti OR toddler*:ab,ti 17. 'adolescent'/exp 18. adoles*:ab,ti OR teen*:ab,ti OR boy*:ab,ti OR girl*:ab,ti 19. 'puberty'/exp 20. minor*:ab,ti OR juvenile*:ab,ti OR pubert*:ab,ti OR pubescen*:ab,ti 21. 'pediatrics'/exp 22. pediatric*:ab,ti OR paediatric*:ab,ti 23. 'school'/exp 24. (school* NEAR/2 (nursery OR primary OR secondary OR high OR elementary)):ab,ti OR kindergar*:ab,ti OR highschool*:ab,ti 25. #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 26. #14 AND #25 27. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp 28. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR ((singl* OR doubl*) NEAR/2 (blind* OR mask*)):ab,ti 29. #27 OR #28 30. #26 AND #29
We imposed no language or publication restrictions.
2005 search details
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2005, Issue 1), which contains the Acute Respiratory Infections Group's Specialised Register; MEDLINE (1966 to February 2005) and EMBASE (1980 to December 2004).
We used the following search terms for MEDLINE and CENTRAL and adapted them for EMBASE. We combined the search terms used in MEDLINE with the highly sensitive strategy devised by Dickersin 1994.
MEDLINE
1 exp MYCOPLASMA/ 2 exp Mycoplasma pneumoniae/ 3 mycoplasma 4 or/1‐3 5 exp BRONCHITIS/ 6 exp PNEUMONIA/ 7 exp Respiratory Tract Infections/ 8 bronchitis 9 pneumonia 10 atypical pneumonia 11 respiratory tract infection$ 12 acute respiratory infection$ 13 or/5‐12 14 exp Anti‐Bacterial Agents/ 15 exp MACROLIDES/ 16 exp QUINOLONES/ 17 exp TETRACYCLINES/ 18 antibiotic$ 19 (macrolide$ or erythromycin or roxithromycin or clarithromycin or azithromycin) 20 or/14‐19 21 exp CHILD/ 22 (child or children) 23 (paediatric or pediatric) 24 or/21‐23 25 4 and 13 and 20 and 24
We imposed no language or publication restrictions.
Appendix 2. MEDLINE, CENTRAL, WHO ICTRP and ClinicalTrials.gov search strategy
MEDLINE (Ovid)
1 Pneumonia, Mycoplasma/ 2 (mycoplasma adj3 pneumon*).tw. 3 primary atypical pneumonia.tw. 4 or/1‐3 5 Mycoplasma pneumoniae/ 6 (mycoplasma pneumoniae or "M. pneumoniae").tw. 7 Mycoplasma Infections/ 8 mycoplasma.tw. 9 or/5‐8 10 exp Pneumonia/ 11 (pneumon* or bronchopneumon* or pleuropneumon*).tw. 12 exp Bronchitis/ 13 (bronchit* or tracheobronchit*).tw. 14 Respiratory Sounds/ 15 wheez*.tw. 16 exp Respiratory Tract Infections/ 17 (respiratory tract infection* or acute respiratory infection* or lower respiratory infection* or lower respiratory tract infection* or lrti).tw. 18 or/10‐17 19 9 and 18 20 4 or 19 21 exp Anti‐Bacterial Agents/ 22 exp Macrolides/ 23 exp Quinolones/ 24 exp Tetracyclines/ 25 antibiotic*.tw,nm. 26 (macrolide* or erythromycin* or roxithromycin* or clarithromycin* or azithromycin*).tw,nm. 27 or/21‐26 28 20 and 27
Appendix 3. EMBASE search strategy
#36 #27 AND #35 #35 #30 NOT #34 #34 #31 NOT #33 #33 #31 AND #32 #32 'human'/de AND [embase]/lim #31 'animal'/de OR 'nonhuman'/exp OR 'animal experiment'/de AND [embase]/lim #30 #28 OR #29 #29 random*:ab,ti OR placebo*:ab,ti OR allocat*:ab,ti OR trial:ti OR crossover*:ab,ti OR 'cross over':ab,ti OR (doubl* NEXT/1 blind*):ab,ti AND [embase]/lim #28 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim #27 #21 AND #26 #26 #22 OR #23 OR #24 OR #25 #25 erythromycin*:ab,ti OR roxithromycin*:ab,ti OR clarithromycin*:ab,ti OR azithromycin*:ab,ti OR macrolide*:ab,ti AND [embase]/lim #24 antibiotic*:ab,ti AND [embase]/lim #23 'macrolide'/exp OR 'quinolone derivative'/exp OR 'tetracycline derivative'/exp AND [embase]/lim #22 'antibiotic agent'/exp AND [embase]/lim #21 #4 OR #20 #20 #9 AND #19 #19 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 #18 lrti:ab,ti AND [embase]/lim #17 (infection* NEAR/1 ('respiratory tract' OR 'acute respiratory' OR 'lower respiratory' OR 'lower respiratory tract')):ab,ti AND [embase]/lim #16 'respiratory tract infection'/de OR 'lower respiratory tract infection'/de AND [embase]/lim #15 wheez*:ab,ti AND [embase]/lim #14 'wheezing'/de AND [embase]/lim #13 bronchit*:ab,ti OR tracheobronchit*:ab,ti AND [embase]/lim #12 'bronchitis'/exp AND [embase]/lim #11 pneumon*:ab,ti OR bronchopneumon*:ab,ti OR pleuropneumon*:ab,ti AND [embase]/lim #10 'pneumonia'/exp AND [embase]/lim #9 #5 OR #6 OR #7 OR #8 #8 mycoplasma:ab,ti AND [embase]/lim #7 'mycoplasmosis'/de AND [embase]/lim #6 'mycoplasma pneumoniae':ab,ti OR 'm. pneumoniae':ab,ti AND [embase]/lim #5 'mycoplasma pneumoniae'/de AND [embase]/lim #4 #1 OR #2 OR #3 #3 'primary atypical pneumonia':ab,ti AND [embase]/lim #2 (mycoplasma NEAR/3 pneumonia):ab,ti AND [embase]/lim #1 'mycoplasma pneumonia'/de AND [embase]/lim
Appendix 4. WHO ICTRP and ClinicalTrials.gov searches
1 mycoplasma AND pneumonia AND antibiotics 2 (condition ‐ mycoplasma pneumonia) + (intervention ‐ antibiotics) + (type ‐ interventional study)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Esposito 2005.
| Methods |
|
|
| Participants | 560 children, aged 1 to 14 years. 352 had acute respiratory infections and a history of recurrent respiratory tract infections (mean age = 3.6, 57.1% male, 136 with acute M. pneumoniae infection), and 208 were in the control group (mean age = 3.9, 57.2% male, 5 with acute M. pneumoniae infection) | |
| Interventions | Patients were randomised to receive azithromycin (n = 177, 10 mg/kg/day, 3 days/week for 3 weeks) with symptom‐specific agents (acetaminophen, 10 mg/kg per dose) or symptom‐specific agents alone (n = 175) | |
| Outcomes |
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All the patients were randomised in a blinded manner with a computerized list, by the only investigator responsible for randomisation" Comment: randomisation was appropriate |
| Allocation concealment (selection bias) | Low risk | The enrolment officer was different to the investigator assigned to randomisation. Consequently, the enroller was unaware of which treatment group the participants would be allocated to |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Clinical outcome assessor blinded. Although patients and caregivers were not blinded, caregivers were "instructed not to inform the evaluator, who was blinded with respect to randomisation, whether the child had received azithromycin" Quote: "Data entry and statistical analyses were carried out in a blinded manner, with SAS software" Comment: raw data analyses were also blinded |
| Follow‐up? All outcomes | Low risk | Quote: "All of the enrolled patients completed the 1‐month follow‐up evaluation" Quote: "A total of 339 patients (96.3%) completed the 6‐month follow‐up evaluation" Comment: a high proportion of participants were followed up |
| Reporting of participants by allocation group? All outcomes | Low risk | The progress of all the children in both groups was described, although at 6 months 13 children were noted to be lost to follow‐up. The tables of results (both 1‐month and 6‐month follow‐ups) account for all available children |
Gomez Campdera 1996.
| Methods |
|
|
| Participants | 155 children aged 6 months to 16 years with pneumonia. Males = 84. Number of children with M. pneumoniae infection in each group not stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not provided |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of outcome assessor |
| Follow‐up? All outcomes | Unclear risk | There was no description of withdrawals or drop‐outs |
| Reporting of participants by allocation group? All outcomes | Unclear risk | No mention of withdrawals or drop‐outs |
Harris 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment were not identified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Although the study design noted it was a "double blinded trial", most methods of blinding used were not specified. Participants and their caregivers were probably blinded because "the placebo and study drug formulations were similar in texture, color and taste" |
| Follow‐up? All outcomes | Low risk | Clinical and laboratory outcomes were measured in 92.1% Quote: "A total of 36 patients [of 456] ... were excluded from efficacy analysis for methodologic reasons such as no follow‐up evaluation or concomitant antibiotic use" |
| Reporting of participants by allocation group? All outcomes | Low risk | The progress of all randomised children in each group was described, with numbers lost to exclusion and follow‐up noted |
Kogan 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Patients with classic pneumonia:
|
|
| Outcomes |
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment were not identified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Almost no methods of blinding were specified. Participants and caregivers may have been aware of their treatment group, as the frequency and duration of drug administrations were different between the groups Quote: "All chest X‐rays done ... were seen by the same radiologist, who was not familiar with the patients' clinical history and treatment group" Comment: radiology assessment was blinded |
| Follow‐up? All outcomes | Low risk | Quote: "Of the 110 enrolled patients, 4 children developed severe pneumonia in the first 12 hr of enrolment and were excluded from the study... The remaining 106 children completed the study" Comment: no participants were lost to follow‐up |
| Reporting of participants by allocation group? All outcomes | Low risk | The progress of all randomised children in each group was described. Results tables compared outcomes between groups |
Ruhrmann 1982.
| Methods | Participants were selected at the children's hospital in Hamburg, Germany. Patients were diagnosed with pneumonia based on chest X‐ray. The study compared erythromycin therapy with amoxycillin therapy. The duration of the study was not specified, nor were the inclusion and exclusion criteria. Although the treatment allocation was randomised, there was no blinding of the outcome assessor or the participant. Baseline measurements were recorded using temperature, full blood examination, chest X‐ray and cough presence. Outcome measures were noted over 10 days and were not well described, with 'clinical improvement' being documented without any clear definition | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Clinical presentations | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A list of randomised numbers was used to allocate participants into treatment groups |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants or outcome assessors |
| Follow‐up? All outcomes | Unclear risk | No description of losses to follow‐up was included in the paper |
| Reporting of participants by allocation group? All outcomes | Unclear risk | Unclear mention of withdrawals or drop‐outs |
Saez‐Llorens 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | List of randomised numbers assigned to therapy. Unclear how randomised numbers were generated but medication given by pharmacy |
| Allocation concealment (selection bias) | Low risk | Medications provided by pharmacy |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of outcome assessor |
| Follow‐up? All outcomes | Unclear risk | 30 drop‐outs but no description of withdrawals or drop‐outs were provided in accordance to groups |
| Reporting of participants by allocation group? All outcomes | Unclear risk | No mention of withdrawals or drop‐outs relative to allocated groups |
Soderstrom 1991.
| Methods |
|
|
| Participants | 138 patients were recruited with age range 10 to 70 years (median 32.5). Males = 56. 2 patients dropped out. There were only 7 with bronchitis (lower respiratory tract infection) and M. pneumoniae was identified in 1 case | |
| Interventions |
|
|
| Outcomes | Clinical presentations | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper states that patients were "randomly assigned", but simply states that "patients were given sequential patient numbers, which indicated which of the two treatments should be given to each patient." It is unclear how treatment groups were indicated by patient number, and so the randomisation cannot be assessed |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment were not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The physician at the first visit and the nurse who met the patient at the follow‐up visit did not know which prescription the patient had had" Comment: the outcome assessor was blinded. The participants and caregivers were presumably not blinded, as they were given prescriptions for their antibiotics |
| Follow‐up? All outcomes | Low risk | Quote: "136/138 patients returned for follow‐up within 10‐12 days ... The 2 remaining patients interrupted the treatment within 2 days" Comment: 98.6% of participants were clinically assessed at the follow‐up visit |
| Reporting of participants by allocation group? All outcomes | Low risk | The results table clearly compared the erythromycin and phenoxymethylpenicillin groups |
Wubbel 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Children under 5 years only Group A (n = 39): azithromycin 10 mg/kg OD day 1, followed by 5 mg/kg OD day 2 to 5 Group B (n = 49): amoxycillin‐clavulanate 40 mg/kg TID day 1 to 10 | |
| Outcomes |
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Using a randomised list of therapy assignments..." Comment: the method of randomisation was not adequately specified |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment were not identified |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "This study was a prospective, randomised, unblinded trial..." Comment: while mostly unblinded, one outcome was partially blinded. Radiographs were secondarily assessed by "radiologists who were not familiar with the patients' clinical history or results of special studies" |
| Follow‐up? All outcomes | Low risk | Quote: "Of the 168 patients who were assessed for etiology of pneumonia, 21 were excluded from clinical evaluation; 10 failed to return for follow‐up examination and 11 did not complete treatment" Comment: 147/168 (87.5%) were continuously followed throughout the study |
| Reporting of participants by allocation group? All outcomes | Low risk | The progress of all randomised children in each group was described |
CAP: community‐acquired pneumonia IgG: immunoglobulin G IgM: immunoglobulin M n: number OD: once daily PCR: polymerase chain reaction TID: three times a day
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atkinson 2007 | Review of studies: cited most recent evidence for treating M. pneumoniae as "inconclusive" |
| Block 1995 | Does not fulfil the inclusion criteria. Inappropriate comparator intervention as comparison between 2 drugs from the macrolide group ‐ clarithromycin versus erythromycin ethylsuccinate |
| Bradley 2007 | Does not fulfil the inclusion criteria and no specified aetiology. Inappropriate comparator intervention as comparison between fluoroquinolone and macrolides ‐ levofloxacin versus clarithromycin/ceftriaxone with clarithromycin/erythromycin lactobionate. M. pneumoniae affecting the LRT and its treatments were not specifically identified |
| Chien 1993 | Does not fulfil the inclusion criteria. Inappropriate comparator intervention as comparison between 2 drugs from the macrolide group ‐ clarithromycin versus erythromycin |
| Esposito 2006 | No focus on LRTIs. URTIs were the focus of this study |
| Fonseca‐Aten 2006 | No specified aetiology. M. pneumoniae affecting the LRT and its treatments were not specifically identified |
| Jensen 1967 | Does not fulfil the inclusion criteria and study not randomised. Study looked at treatment of all affected individuals with oxytetracycline and there was no placebo group. Household contacts were treated with either oxytetracycline or placebo to determine effectiveness of oxytetracycline in secondary prevention of mycoplasma infections. Allocation of treatment of household contacts was not randomised |
| Lee 2008 | Does not meet the inclusion criteria and too few participants. Inappropriate comparator intervention as comparison between 2 drugs from macrolide groups ‐ clarithromycin versus erythromycin. Only 26 participants |
| Lee 2012 | Does not fulfil the inclusion criteria. A small‐scale (36 participants completed), adult‐focused RCT with inappropriate comparator intervention as comparison between fluoroquinolone and combination macrolide and cephalosporin |
| Manfredi 1992 | Does not fulfil the inclusion criteria. Inappropriate comparator intervention as comparison between 2 drugs from the macrolide group ‐ azithromycin versus erythromycin |
| Nogeova 1997 | Does not fulfil the inclusion criteria. Inappropriate comparator intervention as comparison between 2 drugs from the cephalosporin group ‐ ceftibuten versus cefuroxime‐axetil |
| Ronchetti 1994 | Does not fulfil the inclusion criteria. Inappropriate comparator intervention as comparison between 2 drugs from the macrolide group ‐ azithromycin versus josamycin |
| Sakata 2001 | Study participants were not randomised |
| Schonwald 1990 | Does not fulfil the inclusion criteria. Inappropriate comparator intervention as comparison between 2 drugs from the macrolide group ‐ azithromycin versus erythromycin |
| Sempertegui 2014 | Does not fulfil the inclusion criteria. Inappropriate comparator intervention as comparison between standard antimicrobial +/‐ zinc as an adjunct therapy |
| Simon 2006 | Article unavailable for evaluation |
| Vasilos 1995 | Study participants were not randomised |
| Wu 2014 | Does not fulfil the inclusion criteria. Inappropriate comparator intervention as comparison between azithromycin +/‐ glucocorticoids as adjunct therapy |
| Yin 2002 | Does not fulfil the inclusion criteria. Inappropriate comparator intervention as comparison between 2 drugs from the macrolide group ‐ oral azithromycin versus intravenous erythromycin |
LRT: lower respiratory tract LRTI: lower respiratory tract infection RCT: randomised controlled trial URTI: upper respiratory tract infection
Contributions of authors
In the first version, John Gavranich (JG) wrote the protocol, independently selected papers for inclusion, assessed quality, extracted data and wrote the review. Anne Chang (AC) edited and co‐wrote the protocol, independently selected papers for inclusion, assessed quality, extracted data, and edited and co‐wrote the review.
For the 2010 updated version, Selamawit Mulholland (SM) and AC selected papers for inclusion. SM included the 'Risk of bias' tables and figures and updated the included/excluded studies and their characteristics and the text accordingly. These were adapted and checked by AC. The revised version was reviewed by all review authors.
For the 2012 update, Malcolm Gillies (MG) and AC reviewed the literature searches.
In this 2014 review update Samantha Gardiner (SG) and AC independently assessed the literature searches. SG updated the text body and table of excluded studies, which was checked by AC and the revised version reviewed by all authors.
Sources of support
Internal sources
West Moreton Health Service District, Ipswich, Australia.
Royal Children's Hospital, Brisbane, Australia.
External sources
-
NHMRC, Australia.
Practitioner Fellowship salary support for AC (grant 545216)
Declarations of interest
Samantha J Gardiner: none known. John B Gavranich: none known. Anne B Chang is a recipient of a GlaxoSmithKline grant towards the study of microbia in bronchoalveolar lavage (BAL), a topic unrelated to this review. AC has received funding from Johnson and Johnson (J&J) to present at the World Paediatric series, though the talk does not endorse nor make reference to J&J. AC is also the principal investigator on a study examining azithromycin for bronchiolitis in Indigenous children.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Esposito 2005 {published data only}
- Esposito S, Bosis S, Faelli N, Begliatti E, Droghetti R, Tremolati E, et al. Role of atypical bacteria and azithromycin therapy for children with recurrent respiratory tract infections. Pediatric Infectious Disease Journal 2005;24(5):438‐44. [DOI] [PubMed] [Google Scholar]
Gomez Campdera 1996 {published data only}
- Gomez Campdera JA, Navarro Gomez ML, Hernandez‐Sampelayo T, Merello Godino C, Sanchez Sanchez C. Azithromycin in the treatment of ambulatory pneumonia in children. Acta Pediatrica Espanola 1996;54(8):554‐62. [Google Scholar]
Harris 1998 {published data only}
- Harris J‐AS, Kolokathis A, Campbell M, Cassell GH, Hammerschlag MR. Safety and efficacy of azithromycin in the treatment of community‐acquired pneumonia in children. Pediatric Infectious Disease Journal 1998;17(10):865‐71. [DOI] [PubMed] [Google Scholar]
Kogan 2003 {published data only}
- Kogan R, Martinez MA, Rubilar L, Paya E, Quevedo I, Puppo H, et al. Comparative randomized trial of azithromycin versus erythromycin and amoxycillin for treatment of community‐acquired pneumonia in children. Pediatric Pulmonology 2003;35(2):91‐8. [DOI] [PubMed] [Google Scholar]
Ruhrmann 1982 {published data only}
- Ruhrmann H, Blenk H. Erythromycin versus amoxicillin for the treatment of pneumonia in children. Infection 1982;10(Suppl):86‐91. [DOI] [PubMed] [Google Scholar]
Saez‐Llorens 1998 {published data only}
- Saez‐Llorens X, Castano E, Wubbel L, Castrejon MM, Morales I, Vallarino D, et al. Importance of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community‐acquired pneumonia. Revista Medica de Panama 1998;23(2):27‐33. [PubMed] [Google Scholar]
Soderstrom 1991 {published data only}
- Soderstrom M, Blomberg J, Christensen P, Hovelius B. Erythromycin and phenoxymethylpenicillin (Penicillin V) in the treatment of respiratory tract infections as related to microbiological findings and serum C‐reactive protein. Scandinavian Journal of Infectious Disease 1991;23:347‐54. [DOI] [PubMed] [Google Scholar]
Wubbel 1999 {published data only}
- Wubbel L, Muniz L, Ahmed A, Trujillo M, Carubelli C, McCoig C, et al. Etiology and treatment of community‐acquired pneumonia in ambulatory children. Pediatric Infectious Disease Journal 1999;18(2):98‐104. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Atkinson 2007 {published data only}
- Atkinson M, Yanney M, Stephenson T, Smyth A. Effective treatment strategies for paediatric community‐acquired pneumonia. Expert Opinion Pharmacotherapy 2007;8(8):1091‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Block 1995 {published data only}
- Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community‐acquired pneumonia: comparative efficacy and safety of clarithromycin vs erythromycin ethylsuccinate. Pediatric Infectious Disease Journal 1995;14(6):471‐7. [DOI] [PubMed] [Google Scholar]
Bradley 2007 {published data only}
- Bradley JS, Arguedas A, Blumer JL, Saez‐Llorens X, Melkote R, Noel GJ. Comparative study of levofloxacin in the treatment of children with community‐acquired pneumonia. Paediatric Infectious Disease Journal 2007;26(10):868‐78. [DOI] [PubMed] [Google Scholar]
Chien 1993 {published data only}
- Chien SM, Pichotta P, Siepman N, Chan CK. Treatment of community‐acquired pneumonia. A multicenter, double‐blind, randomized study comparing clarithromycin with erythromycin. Chest 1993;103(3):697‐701. [DOI] [PubMed] [Google Scholar]
Esposito 2006 {published data only}
- Esposito S, Bosis S, Begliatti E, Droghetti R, Tremolati E, Tagliabue C, et al. Acute tonsillopharyngitis associated with atypical bacterial infection in children: natural history and impact of macrolide therapy. Clinical Infectious Diseases 2006;43:206‐9. [DOI] [PubMed] [Google Scholar]
Fonseca‐Aten 2006 {published data only}
- Fonseca‐Aten M, Okada PJ, Bowlware KL, Chavez‐Bueno S, Meijias A, Rios AM, et al. Effect of clarithromycin on cytokines and chemokines in children with an acute exacerbation of recurrent wheezing: a double‐blind, randomized, placebo‐controlled trial. Annals of Allergy, Asthma and Immunology 2006;97:457‐63. [DOI] [PubMed] [Google Scholar]
Jensen 1967 {published data only}
- Jensen KJ, Senterfit LB, Scully WE, Conway TJ, West RF, Drummy WM. Mycoplasma pneumoniae infections in children. An epidemiological appraisal in families treated with oxytetracycline. American Journal of Epidemiology 1967;86(2):419‐32. [DOI] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee P‐I, Wu M‐H, Huang L‐M, Chen J‐M, Lee C‐Y. An open, randomized, comparative study of clarithromycin and erythromycin in the treatment of children with community‐acquired pneumonia. Journal of Microbiology, Immunology and Infection 2008;41:54‐61. [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee JH, Kim SW, Kim JH, Ryu YJ, Chang JH. High does levofloxacin in community‐acquired pneumonia: a randomized, open‐label study. Clinical Drug Investigation 2012;32(9):569‐76. [DOI] [PubMed] [Google Scholar]
Manfredi 1992 {published data only}
- Manfredi R, Jannuzzi C, Mantero E, Longo L, Schiavone R, Tempesta A, et al. Clinical comparative study of azithromycin versus erythromycin in the treatment of acute respiratory tract infections in children. Journal of Chemotherapy 1992;4(6):364‐70. [DOI] [PubMed] [Google Scholar]
Nogeova 1997 {published data only}
- Nogeova A, Galova K, Krizan L, Sufliarska S, Cizmarova E, Raskova J, et al. Ceftibuten vs cefuroxime‐axetil in initial therapy for community‐acquired bronchopneumonia: randomized multicentric study in 140 children. Infectious Diseases in Clinical Practice 1997;6(2):133‐4. [Google Scholar]
Ronchetti 1994 {published data only}
- Ronchetti R, Blasi F, Grossi E, Pecori A, Bergonzi F, Ugazio A, et al. The role of azithromycin in treating children with community‐acquired pneumonia. Current Therapeutic Research and Clinical Experimentation 1994;55(4):965‐70. [Google Scholar]
Sakata 2001 {published data only}
- Sakata H. Clinical study on azithromycin in lower respiratory infections in children. Japanese Journal of Chemotherapy 2001;49(6):363‐8. [Google Scholar]
Schonwald 1990 {published data only}
- Schonwald S, Gunjaca M, Kolacny‐Babic L, Car V, Gosev M. Comparison of azithromycin and erythromycin in the treatment of atypical pneumonia. Journal of Antimicrobial Chemotherapy 1990;25:123‐6. [DOI] [PubMed] [Google Scholar]
Sempertegui 2014 {published data only}
- Sempertegui F, Estrella B, Rodriguez O, Gomez D, Salgado G, Sabin LL, et al. Zinc as an adjunct to the treatment of severe pneumonia in Ecuadorian children: a randomized controlled trial. American Journal of Clinical Nutrition 2014;99(3):497‐505. [DOI] [PubMed] [Google Scholar]
Simon 2006 {published data only}
- Simon A, Schildgen O. Antimicrobial therapy in childhood asthma and wheezing. Treatments in Respiratory Medicine 2006;5(4):255‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vasilos 1995 {published data only}
- Vasilos LV, Rumel NB, Shchuka SS. Chemotherapeutic effectiveness of erythromycin, rifampicin and tetracyclines in chlamydiosis and mycoplasmosis in children. Antibiotiki i Khimiterapiia 1995;40(6):40‐2. [PubMed] [Google Scholar]
Wu 2014 {published data only}
- Wu YJ, Sun J, Zhang JH, Feng LL. Clinical efficacy of adjuvant therapy with glucocorticoids in children with lobar pneumonia caused by mycoplasma pneumonia. Chinese Journal of Contemporary Paediatrics 2014;16(4):401‐5. [PubMed] [Google Scholar]
Yin 2002 {published data only}
- Yin T, Jiang Y‐F. Comparison of azithromycin and erythromycin in the treatment of pediatric mycoplasmal pneumonia. Chinese Journal of Antibiotics 2002;27(4):240‐2. [Google Scholar]
Additional references
Biondi 2014
- Biondi E, McCulloh R, Alverson B, Klein A, Dixon A, Ralston S. Treatment of mycoplasma pneumonia: a systematic review. Paediatrics 2014;133(6):1081‐91. [DOI] [PubMed] [Google Scholar]
Boluyt 2008
- Boluyt N, Tjosvold L, Lefebvre C, Klassen T. Usefulness of systematic review search strategies in finding child health systematic reviews in MEDLINE. Archives of Pediatrics and Adolescent Medicine 2008;162(2):111‐6. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Katz 1998
- Katz SL, Gershon AA, Hoetz PJ. Krugman's Infectious Disease of Children. 10th Edition. St. Louis: Mosby, 1998. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Michelow 2004
- Michelow IC, Olsen K, Lozano J, Rollins NK, Duffy LB, Ziegler T, et al. Epidemiology and clinical characteristics of community‐acquired pneumonia in hospitalized children. Pediatrics 2004;113(4):701‐7. [DOI] [PubMed] [Google Scholar]
Murray 2003
- Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH. Manual of Clinical Microbiology. 8th Edition. Vol. 1, Washington DC: ASM Press, 2003. [Google Scholar]
Nelson 2002
- Nelson CT. Mycoplasma and Chlamydia pneumonia in pediatrics. Seminars in Respiratory Infections 2002;17(1):10‐4. [DOI] [PubMed] [Google Scholar]
Phelan 1994
- Phelan PD, Olinsky A, Robertson CF. Respiratory Illness in Children. 4th Edition. Oxford: Blackwell Scientific Publications, 1994. [Google Scholar]
Principi 2001
- Principi N, Esposito S. Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory‐tract infections. Lancet Infectious Diseases 2001;1:334‐44. [DOI] [PubMed] [Google Scholar]
Principi 2002
- Principi N, Esposito S. Mycoplasma pneumoniae and Chlamydia pneumoniae cause lower respiratory tract disease in paediatric patients. Current Opinion in Infectious Diseases 2002;15(3):295‐300. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rudolph 2003
- Rudolph CD, Rudolph AM, Hostetter MK, Lister G, Siegel NJ. Rudolph's Pediatrics. 21st Edition. USA: McGraw‐Hill Medical Publishing Division, 2003. [Google Scholar]
Waites 2003
- Waites KB. New concepts of Mycoplasma pneumoniae infections in children. Pediatric Pulmonology 2003;36:267‐78. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gavranich 2004
- Gavranich JB, Chang AB. Antibiotics for community acquired lower respiratory tract infections (LRTI) secondary to Mycoplasma pneumoniae in children. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004875] [DOI] [PubMed] [Google Scholar]
Gavranich 2005
- Gavranich JB, Chang AB. Antibiotics for community acquired lower respiratory tract infections (LRTI) secondary to Mycoplasma pneumoniae in children. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004875.pub2] [DOI] [PubMed] [Google Scholar]
Mulholland 2010
- Mulholland S, Gavranich JB, Chang AB. Antibiotics for community‐acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD004875.pub2] [DOI] [PubMed] [Google Scholar]
Mulholland 2012
- Mulholland S, Gavranich JB, Gillies MB, Chang AB. Antibiotics for community‐acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD004875.pub4] [DOI] [PubMed] [Google Scholar]


