This cohort study uses Medicare data to assess the number of patients with cardiac implantable electronic device infection who underwent device extraction from 2006 to 2019 and all-cause mortality associated with lead extraction.
Key Points
Question
What is the frequency of guideline-recommended complete hardware removal for cardiac implantable electronic device (CIED) infections?
Findings
In this cohort study of 1 065 549 Medicare patients from 2006 to 2019, rates of guideline-recommended device extraction among patients older than 65 years with CIED infections were low, such that only 18.6% underwent extraction within 30 days of diagnosis. Earlier CIED extraction was associated with lower mortality.
Meaning
The findings suggest that a minority of patients with CIED infection undergo extraction and that there is a need to improve guideline-directed care of patients with CIED infection.
Abstract
Importance
Complete hardware removal is a class I recommendation for cardiovascular implantable electronic device (CIED) infection, but practice patterns and outcomes remain unknown.
Objective
To quantify the number of Medicare patients with CIED infections who underwent implantation from 2006 to 2019 and lead extraction from 2007 to 2019 to analyze the outcomes in these patients in a nationwide clinical practice cohort.
Design, Setting, and Participants
This cohort study included fee-for-service Medicare Part D beneficiaries from January 1, 2006, to December 31, 2019, who had a de novo CIED implantation and a CIED infection more than 1 year after implantation. Data were analyzed from January 1, 2005, to December 31, 2019.
Exposure
A CIED infection, defined as (1) endocarditis or infection of a device implant and (2) documented antibiotic therapy.
Main Outcomes and Measures
The primary outcomes of interest were device infection, device extraction, and all-cause mortality. Time-varying multivariable Cox proportional hazards regression models were used to evaluate the association between extraction and survival.
Results
Among 1 065 549 patients (median age, 78.0 years [IQR, 72.0-84.0 years]; 50.9% male), mean (SD) follow-up was 4.6 (2.9) years after implantation. There were 11 304 patients (1.1%) with CIED infection (median age, 75.0 years [IQR, 67.0-82.0 years]); 60.1% were male, and 7724 (68.3%) had diabetes. A total of 2102 patients with CIED infection (18.6%) underwent extraction within 30 days of diagnosis. Infection occurred a mean (SD) of 3.7 (2.4) years after implantation, and 1-year survival was 68.3%. There was evidence of highly selective treatment, as most patients did not have extraction within 30 days of diagnosed infection (9202 [81.4%]), while 1511 (13.4%) had extraction within 6 days of diagnosis and 591 (5.2%) had extraction between days 7 and 30. Any extraction was associated with lower mortality compared with no extraction (adjusted hazard ratio [AHR], 0.82; 95% CI, 0.74-0.90; P < .001). Extraction within 6 days was associated with even lower risk of mortality (AHR, 0.69; 95% CI, 0.61-0.78; P < .001).
Conclusions and Relevance
In this study, a minority of patients with CIED infection underwent extraction. Extraction was associated with a lower risk of death compared with no extraction. The findings suggest a need to improve adherence to guideline-directed care among patients with CIED infection.
Introduction
Indications for cardiovascular implantable electronic devices (CIEDs) and their rates of implantation have increased over time, with more than 300 000 patients receiving a new CIED implantation every year in the US alone.1,2 However, as the rates of CIED implantation have increased, so have device infections.3 These infections can result in substantial morbidity, and patients with CIED infections have over 3-fold higher mortality compared with patients without infections.4 Additionally, CIED infections are associated with significant financial burden to the health care system.4,5
Antibiotics alone are not effective for treatment of CIED infection and can have significant adverse consequences, including higher rates of infection relapse and mortality.6,7,8 Complete device and lead removal in patients with definite CIED infection (systemic or local), bacteremia, or infective endocarditis is standard of care and carries a class I recommendation in clinical practice guidelines.8,9,10 Studies have shown that extractions performed earlier after diagnosis are associated with significantly lower 1-year mortality rates as well as shorter length of hospital stay and fewer physician or hospital visits.7,11,12 There have been concerns about lack of guideline adherence with respect to hardware removal among patients with CIED infections.13,14 However, there are limited data on the rates of complete device and lead removal in patients with CIED infections.
This analysis was designed to quantify the number of Medicare patients with CIED infections who underwent lead extraction from 2007 to 2019 and analyze the outcomes in these patients in a nationwide clinical practice cohort. We hypothesized that lead extraction would be associated with lower mortality and that earlier lead extraction would be associated with lower mortality than delayed lead extraction.
Methods
Data Sources
In this cohort study, we obtained 100% of Medicare fee-for-service (FFS) data from January 1, 2004, through December 31, 2019, for beneficiaries with a pacemaker or implantable cardioverter defibrillator (ICD) implantation procedure. These data contain all of the administrative claims for patients who received a pacemaker or ICD in the US and were enrolled in FFS Medicare between January 1, 2004, and December 31, 2019. Data were accessed through the Centers for Medicare & Medicaid Services (CMS) Chronic Conditions Warehouse Virtual Research Data Center.15 The study was approved by the Duke University institutional review board, which waived informed consent because data sets were deidentified prior to analysis as defined by the Health Insurance Portability and Accountability Act per 45 CFR §164.514(b). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Medicare claims included the inpatient, outpatient, carrier, Master Beneficiary Summary File (MBSF), home health (HH), durable medical equipment (DME), and skilled nursing facility (SNF) files. We also used Part D files, which include prescription drug fill records from January 1, 2006, through December 31, 2019; the Part D plan election type beneficiary summary; and the Part D drug characteristics files. The Medicare inpatient file contains institutional claims for hospital inpatient services. The Medicare outpatient file contains institutional claims for outpatient services. The carrier file contains noninstitutional claims submitted by health care clinicians for services in any setting. Each of these files includes diagnosis and procedure codes, dates of service, and institution- or clinician-specific information. The HH, DME, and SNF files include claims for procedures and services and dates of service in each respective setting. The Medicare prescription drug event data and the Part D drug characteristics file contain information on prescriptions covered by the Medicare prescription drug plan. The MBSF contains information on demographics, birth and death dates, and program eligibility and enrollment.
Study Population
Medicare FFS patients who underwent a de novo pacemaker or ICD implantation procedure (eTable 1 in Supplement 1) from January 1, 2005, through December 31, 2019, were identified using the carrier file. Patients were considered to not have a de novo implantation and were excluded based on the following criteria: (1) a Current Procedural Terminology (CPT) code of 33212, 33213, 33214, 33215, 33218, 33220, 33221, 33224, 33227, 33228, 33229, 33226, 33222, 33223, 33230, or 33231 in the year prior to implantation; (2) a CPT code of 33206, 33207, 33208, or 33249 in the year prior to implantation; or (3) a CPT code of 33225 without 33206, 33207, 33208, or 33249. The study population was required to have continuous Medicare FFS for the 12 months before de novo implantation to ensure that they had no prior device and to have Medicare FFS and Part D coverage in the 12 months after implantation to assess infection. Those with an infection within 12 months after device implantation were excluded as these acute infections may represent stitch abscesses or other minor wound healing abnormalities. Infections within 12 months of implantation were also excluded because these cases are considered device removals and do not require extraction and the use of specialized services.9 Patients with an infection were required to have (1) a primary diagnosis of infection of a device implantation and (2) documented antibiotic therapy within 30 days after device infection. Device infection was identified by searching the outpatient, inpatient, and carrier files for the diagnosis of endocarditis or infection of a device implantation (codes are listed in eTable 2 in Supplement 1). The outpatient, inpatient, carrier, HH, DME, and SNF files were used to identify patients with a Healthcare Common Procedure Coding System code for intravenous (IV) antibiotics. Additionally, patients using IV antibiotics or oral linezolid were identified by searching for a generic drug name and dosage form in the Part D event file (eTable 3 in Supplement 1). Patients were required to have at least 30 days of continuous FFS and Part D coverage after the date of device infection or until the date of death.
Cohort With CIED Infection
The study population was further restricted to those who developed a CIED infection at least 1 year after device implantation. Patients identified as having a CIED infection were required to have continuous FFS and Part D coverage from the time of device implantation to device infection and 30 days of continuous FFS and Part D coverage after the date of device infection or until the date of death. To account for a possible delay in CIED infection diagnosis, patients with a CIED extraction in the 30 days prior to the infection diagnosis date were additionally excluded. Device extraction was identified by searching the carrier and outpatient files for the codes listed in eTable 4 in Supplement 1. The study design is shown in the eFigure in Supplement 1.
Study Variables
Device extraction was categorized into (1) no extraction within 30 days of the date of device infection, (2) extraction at less than 7 days after the date of device infection, and (3) extraction at 7 to 30 days after the date of device infection. Information on age, sex, race, and region were derived from the MBSF. Race (ascertained in the Medicare files from the Social Security Administration, which transfers demographic information to Medicare’s enrollment database at the time of Medicare eligibility) was included in the analysis because it was hypothesized to be a potential confounder in the treatment-outcome association; categories were Black, White, and other (included Asian, Hispanic, North American Native, unknown, and other—not broken down further due to small cell sizes and complying with the CMS cell suppression policy). We searched inpatient, outpatient, and carrier claims until 1 year after the implantation date or date of device infection and from 2 years prior for comorbid conditions using validated algorithms.16,17 A claims-based frailty indicator was derived by searching the inpatient, outpatient, and carrier claims.18
Outcomes
The primary outcomes of interest were device infection, device extraction, and all-cause mortality. All-cause mortality was defined using death dates in the MBSF. Follow-up time for device infection started 1 year after the date of device implantation and was censored at (1) 1, 2, or 5 years after the index date; (2) the end of claims availability (December 31, 2019); (3) the date when enrollment in FFS Medicare ended; or (4) the date when Part D enrollment ended. Follow-up time for device extraction started at the date of device infection and was censored at (1) 1, 2, or 5 years after the infection date; (2) the end of claims availability (December 31, 2019); or (3) the date when enrollment in FFS Medicare ended. Death from any cause was treated as a competing risk for the outcomes of infection and extraction. To compare all-cause mortality between extraction groups, 2 approaches were used with different index dates. Follow-up time until all-cause mortality was calculated from the date of device infection for the time-varying analysis or 30 days after device infection for the landmark analysis. Follow-up time was censored at (1) 1 year after each respective index date, (2) the end of claims availability (December 31, 2019), or (3) the date when enrollment in FFS Medicare ended.
Statistical Analysis
Summary statistics are presented as percentages for categorical variables and medians with IQRs for continuous variables. Group differences were assessed using χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables. The Kaplan-Meier method was used to calculate the rate of all-cause mortality, and group differences were tested using log-rank tests in a landmark analysis excluding patients with less than 30 days of follow-up. For the outcomes of infection and extraction, the cumulative incidence function was used to account for the competing risk of mortality and group differences were tested using the Gray test. Cox proportional hazards regression models were then used to estimate the association between extraction groups and all-cause mortality in the full cohort with infection. First, extraction was included in the model as a time-dependent indicator defined as extracted (vs not extracted). In a separate Cox proportional hazards regression model, the time-dependent indicators of extraction at less than 7 days after infection (vs no extraction within the first 6 days) and extraction at 7 to 30 days after infection (vs no extraction within 7 to 30 days) were included. The proportional hazards assumption was tested. All models adjusted for demographics, comorbid conditions, geographic region, device type, time since implantation, and year of device infection. A robust sandwich estimator was used to account for the clustering of patients within hospitals. Separate sensitivity analyses excluding patients with end-stage kidney disease and age younger than 65 years and restricting to patients with endocarditis were performed. Since patient frailty might be expected to influence decisions regarding infection management and extraction, an additional sensitivity analysis was conducted adjusting for baseline frailty with the Segal frailty index in the Cox proportional hazards regression models.18 All statistical analyses were performed using SAS Enterprise Guide, version 7.15 (SAS Institute Inc). Two-sided P < .05 was considered significant. Data were analyzed from January 1, 2005, to December 31, 2019.
Results
Baseline Characteristics
Among 1 065 549 patients with a CIED, the median age was 78.0 years (IQR, 72.0-84.0 years) and the mean (SD) follow-up was 4.6 (2.9) years after implantation; 49.1% were female; 50.9%, male; 7.6%, Black; 87.2%, White; and 5.2%, other race. A total of 7724 (68.3%) had diabetes. As shown in Table 1, there were 11 304 patients (1.1%) with CIED infection with a median age of 75.0 years (IQR, 67.0-82.0 years); 39.9% were female; 60.1%, male; 15.7%, Black; 77.2%, White; and 7.2%, other race. The patients with CIED infection had higher rates of comorbidities including diabetes, ischemic heart disease, peripheral vascular disease, heart failure, chronic obstructive pulmonary disease, and kidney disease compared with the overall population with a CIED. The CIED infections were more commonly seen in patients with a cardiac resynchronization therapy defibrillator (CRTD) (13.2% vs 8.2%) or ICD (29.5% vs 17.4%).
Table 1. Baseline Patient Characteristics.
| Characteristic | Patientsa | |||
|---|---|---|---|---|
| Overall population (N = 1 065 549) | Cohort with CIED infection (n = 11 304) | Standardized difference, % | P value | |
| Age, median (IQR), y | 78.0 (72.0-84.0) | 75.0 (67.0-82.0) | 41.3 | <.001 |
| Age group, y | ||||
| <65 | 79 225 (7.4) | 2380 (21.1) | 42.5 | <.001 |
| 65 to <75 | 290 003 (27.2) | 3112 (27.5) | ||
| 75 to <85 | 439 681 (41.3) | 4021 (35.6) | ||
| ≥85 | 256 640 (24.1) | 1791 (15.8) | ||
| Sex | ||||
| Female | 522 877 (49.1) | 4505 (39.9) | 18.6 | <.001 |
| Male | 542 672 (50.9) | 6799 (60.1) | ||
| Race | ||||
| Black | 80 827 (7.6) | 1773 (15.7) | 27.6 | <.001 |
| White | 929 276 (87.2) | 8722 (77.2) | ||
| Otherb | 55 446 (5.2) | 809 (7.2) | ||
| Comorbidities | ||||
| Dementia | 120 890 (11.3) | 1372 (12.1) | 2.5 | .008 |
| Diabetes | 525 584 (49.3) | 7724 (68.3) | 39.4 | <.001 |
| Ischemic heart disease | 846 343 (79.4) | 10 280 (90.9) | 32.8 | <.001 |
| Peripheral vascular disease | 508 214 (47.7) | 7359 (65.1) | 35.7 | <.001 |
| Congestive heart failure | 691 251 (64.9) | 9826 (86.9) | 53.4 | <.001 |
| Cerebrovascular disease | 489 286 (45.9) | 5913 (52.3) | 12.8 | <.001 |
| Hypertension | 1 030 436 (96.7) | 11 115 (98.3) | 10.4 | <.001 |
| COPD | 585 915 (55.0) | 7989 (70.7) | 32.9 | <.001 |
| Kidney disease | 403 603 (37.9) | 8000 (70.8) | 70.0 | <.001 |
| Stroke or TIA | 315 595 (29.6) | 4053 (35.9) | 13.3 | <.001 |
| Myocardial infarction | 356 961 (33.5) | 5302 (46.9) | 27.6 | <.001 |
| Cancer | 225 905 (21.2) | 2822 (25.0) | 8.9 | <.001 |
| Valvular heart disease | 679 327 (63.8) | 9153 (81.0) | 39.2 | <.001 |
| Device type | ||||
| CRTD | 87 552 (8.2) | 1488 (13.2) | 36.6 | <.001 |
| CRTP | 27 143 (2.5) | 243 (2.1) | ||
| Pacemaker | 765 432 (71.8) | 6241 (55.2) | ||
| ICD | 185 422 (17.4) | 3332 (29.5) | ||
| Geographic region | ||||
| Midwest | 264 663 (24.8) | 2695 (23.8) | 3.8 | .006 |
| Northeast | 196 430 (18.4) | 2046 (18.1) | ||
| South | 437 659 (41.1) | 4776 (42.3) | ||
| West or other | 166 797 (15.7) | 1787 (15.8) | ||
| Dual Medicaid eligibility | 237 070 (22.2) | 4147 (36.7) | 32.1 | <.001 |
| Claims-based frailty indicator | 308 558 (29.0) | 4176 (36.9) | 17.0 | <.001 |
| Frailty index score, median (IQR) | 0.2 (0.1-0.3) | 0.2 (0.1-0.3) | 18.1 | <.001 |
Abbreviations: CIED, cardiac implantable electronic device; COPD, chronic obstructive pulmonary disease; CRTD, cardiac resynchronization therapy defibrillator; CRTP, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter defibrillator; TIA, transient ischemic attack.
Data are presented as the number (percentage) of patients unless otherwise indicated.
Included Asian, Hispanic, North American Native, unknown, and other (not broken down further due to small cell sizes and complying with the Centers for Medicare & Medicaid Services cell suppression policy).
Incidence of CIED Infection and Extraction
The cumulative incidence of CIED infection was 0.4% (3521 patients) at 1 year, 0.6% (5802 patients) at 2 years, and 1.2% (9564 patients) at 5 years. Infection occurred a mean (SD) of 3.7 (2.4) years after implantation, and 1-year survival was 68.3%. The frequency of device infection varied across device types, including 1.67% in patients with a CRTD, 0.89% in patients with a cardiac resynchronization therapy pacemaker (CRTP), 0.81% in patients with a pacemaker, and 1.77% in patients with an ICD.
As shown in Table 2, most patients (9202 [81.4%]) did not undergo extraction within 30 days of CIED infection, while 2102 (18.6%) underwent extraction within 30 days (1511 [13.4%] within 6 days of diagnosis and 591 [5.2%] on days 7 to 30). The cumulative 1-year incidence of extraction within the population with CIED infection was 23.2% (2610 patients). The incidence of extraction at 5 years increased to 25.4% (2787 patients). Female patients and patients who were Black or other race vs White were less likely to have extraction within 30 days of CIED infection (Table 2). Patients with diabetes, kidney disease, history of stroke or transient ischemic attack, and frailty were less likely to undergo extraction within 30 days of CIED infection. The observed cumulative incidence of extraction according to device type was as follows: 28.5% of patients with an ICD (942), 27.0% of patients with a CRTP or CRTD (466), and 19.4% of patients with a pacemaker (1202) (all P < .001). Patients with a pacemaker were also less likely to undergo extraction within 30 days, while patients with an ICD were more likely to undergo extraction within 30 days. Frailty was similar in those undergoing extraction at less than 7 days vs at 7 to 30 days. The use of extraction increased over time in the study cohort from 15.6% in 2007 to 20.0% in 2012 and 24.8% in 2019.
Table 2. Baseline Characteristics of Patients With CIED Infection According to Extraction.
| Characteristic | Patientsa | |||
|---|---|---|---|---|
| No extraction within 30 d (n = 9202) | Extraction at <7 d (n = 1511) | Extraction at 7-30 d (n = 591) | P value | |
| Age at infection, median (IQR), y | 75.0 (67.0-82.0) | 75.0 (68.0-81.0) | 75.0 (66.0-80.0) | .06 |
| Age group at infection, y | ||||
| <65 | 1941 (21.1) | 300 (19.9) | 139 (23.5) | <.001 |
| 65 to <75 | 2512 (27.3) | 447 (29.6) | 153 (25.9) | |
| 75 to <85 | 3227 (35.1) | 565 (37.4) | 229 (38.7) | |
| ≥85 | 1522 (16.5) | 199 (13.2) | 70 (11.8) | |
| Sex | ||||
| Female | 3852 (41.9) | 478 (31.6) | 175 (29.6) | <.001 |
| Male | 5350 (58.1) | 1033 (68.4) | 416 (70.4) | |
| Race | ||||
| Black | 1554 (16.9) | 159 (10.5) | 60 (10.2) | <.001 |
| White | 6941 (75.4) | 1278 (84.6) | 503 (85.1) | |
| Otherb | 707 (7.7) | 74 (4.9) | 28 (4.7) | |
| Comorbidities | ||||
| Dementia | 1213 (13.2) | 111 (7.3) | 48 (8.1) | <.001 |
| Diabetes | 6478 (70.4) | 889 (58.8) | 357 (60.4) | <001 |
| Ischemic heart disease | 8411 (91.4) | 1345 (89.0) | 524 (88.7) | .002 |
| Peripheral vascular disease | 6215 (67.5) | 817 (54.1) | 327 (55.3) | <.001 |
| Congestive heart failure | 8081 (87.8) | 1261 (83.5) | 484 (81.9) | <.001 |
| Cerebrovascular disease | 4984 (54.2) | 662 (43.8) | 267 (45.2) | <.001 |
| Hypertension | 9072 (98.6) | 1471 (97.4) | 572 (96.8) | <.001 |
| COPD | 6616 (71.9) | 973 (64.4) | 400 (67.7) | <.001 |
| Kidney disease | 6869 (74.6) | 820 (54.3) | 311 (52.6) | <.001 |
| Stroke or TIA | 3468 (37.7) | 415 (27.5) | 170 (28.8) | <.001 |
| Myocardial infarction | 4310 (46.8) | 700 (46.3) | 292 (49.4) | .43 |
| Cancer | 2318 (25.2) | 347 (23.0) | 157 (26.6) | .12 |
| Valvular heart disease | 7565 (82.2) | 1147 (75.9) | 441 (74.6) | <.001 |
| Device type | ||||
| CRTD or CRTP | 1354 (14.7) | 277 (18.3) | 100 (16.9) | <.001 |
| Pacemaker | 5247 (57.0) | 723 (47.8) | 271 (45.9) | |
| ICD | 2601 (28.3) | 511 (33.8) | 220 (37.2) | |
| Geographic region | ||||
| Midwest | 2155 (23.4) | 389 (25.7) | 151 (25.5) | .03 |
| Northeast | 1719 (18.7) | 242 (16.0) | 85 (14.4) | |
| South | 3865 (42.0) | 650 (43.0) | 261 (44.2) | |
| West or other | 1463 (15.9) | 230 (15.2) | 94 (15.9) | |
| Dual Medicaid eligibility | 3522 (38.3) | 443 (29.3) | 182 (30.8) | <.001 |
| Time since device implantation, median (IQR), y | 2.9 (1.8-4.9) | 3.2 (1.8-5.6) | 3.5 (2.1-5.8) | <.001 |
| Claims-based frailty indicator | 3606 (39.2) | 411 (27.2) | 159 (26.9) | <.001 |
| Frailty index score, median (IQR) | 0.2 (0.1-0.4) | 0.2 (0.1-0.3) | 0.2 (0.1-0.3) | <.001 |
Abbreviations: CIED, cardiac implantable electronic device; COPD, chronic obstructive pulmonary disease; CRTD, cardiac resynchronization therapy defibrillator; CRTP, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter defibrillator; TIA, transient ischemic attack.
Data are presented as the number (percentage) of patients unless otherwise indicated.
Included Asian, Hispanic, North American Native, unknown, and other (not broken down further due to small cell sizes and complying with the Centers for Medicare & Medicaid Services cell suppression policy).
Outcomes With Extraction
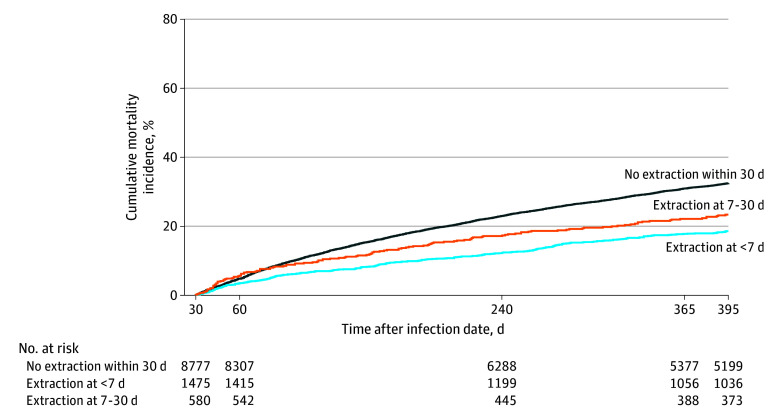
In the landmark analysis, the cumulative 1-year mortality was 32.5% for patients without extraction at 30 days, compared with 18.6% among patients with extraction within 6 days and 23.4% for patients with extraction on days 7 to 30 after CIED infection (P < .001) (Figure). Using a multivariable model with any extraction included as the time-dependent indicator, extraction compared with no extraction was associated with lower mortality (adjusted hazard ratio [AHR], 0.82; 95% CI, 0.74-0.90; P < .001). A separate multivariable time-varying model found that extraction within 6 days was associated with lower mortality (AHR, 0.69; 95% CI, 0.61-0.78; P < .001) compared with no extraction within 6 days, and there was no association between mortality and extraction on days 7 to 30 compared with no extraction within 7 to 30 days (AHR, 0.88; 95% CI, 0.73-1.04; P = .14) (Table 3). Both older age (AHR, 1.08 per 5 years; 95% CI, 1.07-1.10; P < .001) and female sex (AHR, 1.10; 95% CI, 1.02-1.18; P = .01) were independently associated with higher mortality. The proportional hazards assumption was violated, and these results represent the average hazard.
Figure. Observed 1-Year Cumulative Incidence of All-Cause Mortality in the Cohort With Infection Using the Landmark Approach.
Outcomes are restricted to patients with at least 30 days of follow-up after cardiac implantable electronic device infection.
Table 3. Association Between CIED Extraction and All-Cause Mortality.
| Parameter | Hazard ratio (95% CI)a | P value |
|---|---|---|
| Extraction within 30 d | ||
| No extraction | 1 [Reference] | NA |
| Extraction at 0-6 d, time dependent | 0.69 (0.61-0.78) | <.001 |
| Extraction at 7-30 d, time dependent | 0.88 (0.73-1.04) | .14 |
| Age at infection, per 5 y | 1.08 (1.07-1.10) | <.001 |
| Female sex | 1.10 (1.02-1.18) | .01 |
| Race | ||
| Black | 0.94 (0.86-1.04) | .23 |
| White | 1 [Reference] | NA |
| Other | 0.89 (0.78-1.02) | .09 |
| Medicaid dual eligibility | 1.15 (1.06-1.25) | <.001 |
| Geographic region | ||
| South | 1 [Reference] | NA |
| Midwest | 0.96 (0.88-1.05) | .36 |
| Northeast | 0.92 (0.84-1.00) | .06 |
| Other | 0.99 (0.39-2.52) | .99 |
| West | 0.85 (0.75-0.95) | .004 |
| Comorbidities | ||
| Myocardial infarction | 1.12 (1.04-1.21) | .003 |
| Peripheral vascular disease | 1.27 (1.18-1.37) | <.001 |
| Cerebrovascular disease | 0.96 (0.87-1.06) | .44 |
| Dementia | 1.50 (1.36-1.65) | <.001 |
| COPD | 1.25 (1.15-1.36) | <.001 |
| Kidney disease | 1.92 (1.74-2.13) | <.001 |
| Congestive heart failure | 1.50 (1.30-1.73) | <.001 |
| Stroke or TIA | 1.25 (1.13-1.39) | <.001 |
| Ischemic heart disease | 1.09 (0.92-1.28) | .32 |
| Diabetes | 1.14 (1.05-1.24) | .002 |
| Hypertension | 0.93 (0.63-1.36) | .70 |
| Valvular heart disease | 1.13 (1.04-1.24) | .007 |
| Cancer | 1.14 (1.05-1.23) | <.001 |
| Device type | ||
| Pacemaker | 1 [Reference] | NA |
| CRTD | 1.06 (0.95-1.18) | .30 |
| CRTP | 0.95 (0.75-1.21) | .69 |
| ICD | 1.14 (1.04-1.24) | .003 |
| Time from device implantation, y | 1.00 (0.99-1.02) | .72 |
| Year of infection, per 1 y | 0.99 (0.98-1.00) | .045 |
Abbreviations: CIED, cardiac implantable electronic device; COPD, chronic obstructive pulmonary disease; CRTD, cardiac resynchronization therapy defibrillator; CRTP, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter defibrillator; NA, not applicable; TIA, transient ischemic attack.
The time-dependent indicators of extraction at less than 7 days and extraction at 7 to 30 days were included in the model. The Cox proportional hazards regression model was adjusted for age, sex, race, Medicaid dual eligibility, baseline comorbidities, device type, geographic region, year of device infection, and years since device implantation.
Sensitivity Analyses
A total of 5128 of the patients with CIED infection (45.4%) had end-stage kidney disease and were younger than 65 years. After excluding these patients for a sensitivity analysis, the median age increased to 78 years (IQR, 74-84 years) (eTable 5 in Supplement 1). In the adjusted analysis, extraction compared with no extraction was associated with lower mortality, with results similar to those for the overall population (AHR, 0.82; 95% CI, 0.72-0.93; P = .002). The multivariable time-varying model findings for lower mortality with early extraction within 6 days were similar to those for the overall population (AHR, 0.68; 95% CI, 0.58-0.79; P < .001) (eTable 6 in Supplement 1).
Patients with CIED infection were more likely to be classified as having frailty. Frailty was associated with an increased risk of mortality (AHR, 1.29; 95% CI, 1.18-1.41) in those with CIED infection. After additional adjustment for frailty in the model, extraction within 0 to 6 days (AHR, 0.69; 95% CI, 0.61-0.78; P < .001) remained associated with lower risk of mortality compared with no extraction within 6 days (eTable 7 in Supplement 1).
Finally, we conducted a sensitivity analysis restricted to patients with a diagnosis of endocarditis. The rate of extraction at 1 year in those with a diagnosis of endocarditis was 16.7% (696 of 4215). The observed cumulative incidence of all-cause mortality at 1 year restricted to patients with endocarditis was 31.4% (1040 of 3710) in those who did not undergo extraction, 24.9% (68 of 303) in those undergoing extraction at less than 7 days, and 27.7% (52 of 202) in those undergoing extraction between 7 and 30 days (P = .053).
Discussion
Cardiac implantable electronic device infections are life threatening. Given the increasing prevalence of CIED infections,2 it is critical that they are appropriately recognized and managed to optimize patient outcomes. In this study, rates of guideline-recommended extraction among patients with CIED infections were low, such that only 18.6% of patients underwent extraction within 30 days of diagnosis. Mortality was lower with CIED extraction. As with outcomes in other infections such as sepsis, time to treatment matters. Earlier CIED extraction was associated with lower mortality.
Despite efforts to minimize risk at the time of device implantation, CIED device infection rates have increased over time.19,20,21 An analysis of CIED implantation between 2010 and 2014 found that infection rates were 2.4% at 3 years postimplantation.22 Our analysis identified a lower CIED infection incidence of 1.1% at 5 years; however, there are several reasons for this. First, given our focus on extraction, we excluded infections within 1 year of implantation. Second, we selected for higher-acuity infections, including infection of implanted devices or grafts and endocarditis with documented IV antibiotic therapy. Regardless of the incidence, CIED infection rates increased over time. Data from Olmstead County, Minnesota, identified CIED infection rates of 6.2% at 15 years and 11.7% at 25 years after initial implantation.3
The rates of extraction among patients with CIED infection in clinical practice remain relatively unknown. The current study found that only 1 in 5 patients underwent extraction within 30 days of being identified as having a CIED infection with an indication for extraction. The management of CIED infection is multifaceted and includes the diagnosis and evaluation of infection, initiation of antibiotic therapy, appropriate referral, and the decision to extract.9 In addition to competent lead extraction, appropriate and timely referral is key to improving outcomes. Therefore, there are multiple points in the care pathway where potential barriers or gaps exist, leading to poor guideline adherence. A previous survey was performed to better understand where gaps existed within clinicians’ knowledge and skill across all stages of CIED care.14 Specific areas that can be targeted within the patient care pathway include (1) perception of extraction risk, (2) diagnostic pathways for patient identification, and (3) patient referral to a lead management specialist or center.
Periprocedural mortality with lead extraction has been reported to be as low as 0.3% to 0.5% in multicenter analyses, including the prospective European Lead Extraction Controlled Registry (ELECTRa).23,24 Multiple small studies of fewer than 200 patients each have demonstrated that the risk of relapse of infection without complete hardware removal is high (between 50% and 100%).25,26,27,28 In the current analysis, there was a 32.5% mortality rate at 1 year without extraction within 30 days and an 18.6% mortality rate for patients who underwent extraction within 6 days. This was a nearly 14% absolute difference in 1-year mortality, which was similar to the 18.2% lower rate of all-cause mortality that was seen within 1 year with extraction within a multinational endocarditis registry.29
Two previously published analyses7,30 also evaluated early vs late CIED extraction. The first included 416 patients from 1991 to 2008 and found that immediate removal was associated with lower 1-year mortality (HR, 0.35; 95% CI, 0.16-0.75).7 The second analysis included 12 999 patients with CIED infection between 2016 and 2018 and found that in-hospital mortality was higher when extraction was delayed more than 7 days (HR, 1.55; 95% CI, 1.29-1.87).30 In addition to these 2 studies, Sciria and colleagues31 conducted an analysis of 25 303 admissions for patients with CIED infection and endocarditis between 2016 and 2019. Notably, only 11.5% of patients were treated with extraction, and extraction was associated with lower adjusted odds of death (odds ratio, 0.51; 95% CI, 0.40-0.66).
The findings of the current study of Medicare patients are consistent with these earlier studies and extend the findings to a nationwide cohort of individuals older than 65 years. In this nationwide cohort of 1 065 549 patients with a device, including 11 304 patients with infection more than a year after implantation, we found that mortality rates were lower with earlier extraction. We used a fairly strict definition of infection that required a diagnosis code for an infected implant or endocarditis and use of IV antibiotics. Thus, it is likely that we included more severe cases than prior studies of device infection. Our findings were robust across several sensitivity analyses. Our results are also consistent with outcomes observed in other types of infection, including those focused on the “golden hour” of sepsis (ie, timely initiation of antibiotic treatment for improved outcomes) and the surgical treatment of infective endocarditis.32,33 It should also be pointed out that the time from development of infection to extraction is likely longer than the time from diagnosis to extraction. Nonetheless, these nationwide data raise and provide support for the hypothesis that earlier extraction can potentially reduce mortality. Prospective quality improvement studies should test this hypothesis.
Limitations
This study has limitations. It was a large, retrospective, and claims-based analysis in a Medicare patient population. Such analyses are subject to the sensitivity and specificity of the coding for comorbidities and events. Prior analyses of diagnostic codes for infective endocarditis have identified that they have moderate sensitivity but high specificity (>94%).34 As with any observational study, residual measured and unmeasured confounding may have influenced the outcomes despite adjusted modeling. However, the observed associations remained robust to a number of sensitivity analyses, including those accounting for extreme phenotypes, such as patients with end-stage kidney disease and frailty. Additionally, we did not have any information on several variables that influence prognosis during lead management procedures, including patient characteristics, such as body mass index and dependency on pacing; lead characteristics, such as the number of coils for defibrillator leads; and procedural characteristics, such as tools used during the extraction or procedural success. It is important to note that we excluded patients with early infection (within 1 year of implantation). The mechanism of early infections and late infections or delayed detection of device infection are different, may affect the mortality rate, and may not only be related to a more complex extraction procedure. The microbiologic causes of these infections were also not available. Finally, we did not evaluate rates of CIED reimplantation and downstream consequences. Given the strict nature of the inclusion criteria, these results likely underestimate the gap in care.
This analysis was limited to FFS Medicare patients, so the results may not apply to non-Medicare patients or to Medicare managed care patients. However, this patient population is important to study because the decision-making is often more complex based on comorbidities and life expectancy as well as the high event rates in this population.
Conclusions
In this cohort study, a minority of patients with CIED infection underwent extraction. Notably, female patients and Black patients were less likely to undergo extraction compared with male patients and White patients. Lead extraction, and particularly early lead extraction, was associated with a significantly lower risk of death compared with no extraction. The findings suggest that among patients with CIED infection, there is an important unmet need to improve adherence to guideline-directed care. Quality improvement initiatives appear to be needed to improve treatment of CIED infections and complete hardware removal across health systems.
eFigure. Graphical Demonstration of the Study Design
eTable 1. Codes Used to Determine De Novo Pacemaker or ICD Implantation Procedure
eTable 2. Codes Used to Determine CIED Infection
eTable 3. Antibiotic Therapy
eTable 4. Extraction Codes
eTable 5. Baseline Characteristics of Infection Cohort, Excluding ESKD and Patients Aged Younger Than 65 Years
eTable 6. Association Between Extraction and All-Cause Mortality, Excluding ESKD and Patients Aged Younger Than 65 Years
eTable 7. Association Between Extraction and All-Cause Mortality, Including Adjustment for Frailty Indicator
Data Sharing Statement
References
- 1.Baddour LM, Cha YM, Wilson WR. Clinical practice: infections of cardiovascular implantable electronic devices. N Engl J Med. 2012;367(9):842-849. doi: 10.1056/NEJMcp1107675 [DOI] [PubMed] [Google Scholar]
- 2.Greenspon AJ, Patel JD, Lau E, et al. 16-Year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58(10):1001-1006. doi: 10.1016/j.jacc.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 3.Dai M, Cai C, Vaibhav V, et al. Trends of cardiovascular implantable electronic device infection in 3 decades: a population-based study. JACC Clin Electrophysiol. 2019;5(9):1071-1080. doi: 10.1016/j.jacep.2019.06.016 [DOI] [PubMed] [Google Scholar]
- 4.Wilkoff BL, Boriani G, Mittal S, et al. ; WRAP-IT Investigators . Impact of cardiac implantable electronic device infection: a clinical and economic analysis of the WRAP-IT Trial. Circ Arrhythm Electrophysiol. 2020;13(5):e008280. doi: 10.1161/CIRCEP.119.008280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshmukh A, Patel N, Noseworthy PA, et al. Trends in use and adverse outcomes associated with transvenous lead removal in the United States. Circulation. 2015;132(25):2363-2371. doi: 10.1161/CIRCULATIONAHA.114.013801 [DOI] [PubMed] [Google Scholar]
- 6.del Río A, Anguera I, Miró JM, et al. ; Hospital Clínic Endocarditis Study Group . Surgical treatment of pacemaker and defibrillator lead endocarditis: the impact of electrode lead extraction on outcome. Chest. 2003;124(4):1451-1459. doi: 10.1378/chest.124.4.1451 [DOI] [PubMed] [Google Scholar]
- 7.Le KY, Sohail MR, Friedman PA, et al. ; Mayo Cardiovascular Infections Study Group . Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm. 2011;8(11):1678-1685. doi: 10.1016/j.hrthm.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 8.Baddour LM, Epstein AE, Erickson CC, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association . Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121(3):458-477. doi: 10.1161/CIRCULATIONAHA.109.192665 [DOI] [PubMed] [Google Scholar]
- 9.Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14(12):e503-e551. doi: 10.1016/j.hrthm.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 10.Blomström-Lundqvist C, Traykov V, Erba PA, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020;41(21):2012-2032. doi: 10.1093/eurheartj/ehaa010 [DOI] [PubMed] [Google Scholar]
- 11.Sohail MR, Eby EL, Ryan MP, Gunnarsson C, Wright LA, Greenspon AJ. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large US payer database 1-year post implantation. Circ Arrhythm Electrophysiol. 2016;9(8):e003929. doi: 10.1161/CIRCEP.116.003929 [DOI] [PubMed] [Google Scholar]
- 12.Greenspon AJ, Eby EL, Petrilla AA, Sohail MR. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. Pacing Clin Electrophysiol. 2018;41(5):495-503. doi: 10.1111/pace.13300 [DOI] [PubMed] [Google Scholar]
- 13.Traykov V, Bongiorni MG, Boriani G, et al. Clinical practice and implementation of guidelines for the prevention, diagnosis and management of cardiac implantable electronic device infections: results of a worldwide survey under the auspices of the European Heart Rhythm Association. Europace. 2019;21(8):1270-1279. doi: 10.1093/europace/euz137 [DOI] [PubMed] [Google Scholar]
- 14.Rao A, Garner D, Starck C, et al. Knowledge gaps, lack of confidence, and system barriers to guideline implementation among European physicians managing patients with CIED lead or infection complications: a European Heart Rhythm Association/European Society of Cardiology educational needs assessment survey. Europace. 2020;22(11):1743-1753. doi: 10.1093/europace/euaa218 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services . CCW Virtual Research Data Center (VRDC). Accessed September 4, 2023. https://resdac.org/cms-virtual-research-data-center-vrdc
- 16.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480-485. doi: 10.1097/01.mlr.0000160417.39497.a9 [DOI] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 18.Segal JB, Chang HY, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55(7):716-722. doi: 10.1097/MLR.0000000000000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol. 2010;33(4):414-419. doi: 10.1111/j.1540-8159.2009.02569.x [DOI] [PubMed] [Google Scholar]
- 20.Greenspon AJ, Patel JD, Lau E, et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60(16):1540-1545. doi: 10.1016/j.jacc.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 21.Joy PS, Kumar G, Poole JE, London B, Olshansky B. Cardiac implantable electronic device infections: who is at greatest risk? Heart Rhythm. 2017;14(6):839-845. doi: 10.1016/j.hrthm.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 22.Cantillon DJ, Exner DV, Badie N, et al. Complications and health care costs associated with transvenous cardiac pacemakers in a nationwide assessment. JACC Clin Electrophysiol. 2017;3(11):1296-1305. doi: 10.1016/j.jacep.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 23.Wazni O, Epstein LM, Carrillo RG, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010;55(6):579-586. doi: 10.1016/j.jacc.2009.08.070 [DOI] [PubMed] [Google Scholar]
- 24.Bongiorni MG, Kennergren C, Butter C, et al. ; ELECTRa Investigators . The European Lead Extraction Controlled (ELECTRa) study: a European Heart Rhythm Association (EHRA) registry of transvenous lead extraction outcomes. Eur Heart J. 2017;38(40):2995-3005. doi: 10.1093/eurheartj/ehx080 [DOI] [PubMed] [Google Scholar]
- 25.Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000;133(8):604-608. doi: 10.7326/0003-4819-133-8-200010170-00011 [DOI] [PubMed] [Google Scholar]
- 26.Klug D, Wallet F, Lacroix D, et al. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart. 2004;90(8):882-886. doi: 10.1136/hrt.2003.010595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49(18):1851-1859. doi: 10.1016/j.jacc.2007.01.072 [DOI] [PubMed] [Google Scholar]
- 28.Margey R, McCann H, Blake G, et al. Contemporary management of and outcomes from cardiac device related infections. Europace. 2010;12(1):64-70. doi: 10.1093/europace/eup362 [DOI] [PubMed] [Google Scholar]
- 29.Athan E, Chu VH, Tattevin P, et al. ; ICE-PCS Investigators . Clinical characteristics and outcome of infective endocarditis involving implantable cardiac devices. JAMA. 2012;307(16):1727-1735. doi: 10.1001/jama.2012.497 [DOI] [PubMed] [Google Scholar]
- 30.Lee JZ, Majmundar M, Kumar A, et al. Impact of timing of transvenous lead removal on outcomes in infected cardiac implantable electronic devices. Heart Rhythm. 2022;19(5):768-775. doi: 10.1016/j.hrthm.2021.12.023 [DOI] [PubMed] [Google Scholar]
- 31.Sciria CT, Kogan EV, Mandler AG, et al. Low utilization of lead extraction among patients with infective endocarditis and implanted cardiac electronic devices. J Am Coll Cardiol. 2023;81(17):1714-1725. doi: 10.1016/j.jacc.2023.02.042 [DOI] [PubMed] [Google Scholar]
- 32.Kodan LR, Verschueren KJC, Kanhai HHH, van Roosmalen JJM, Bloemenkamp KWM, Rijken MJ. The golden hour of sepsis: an in-depth analysis of sepsis-related maternal mortality in middle-income country Suriname. PLoS One. 2018;13(7):e0200281. doi: 10.1371/journal.pone.0200281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang D-H, Kim Y-J, Kim S-H, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366(26):2466-2473. doi: 10.1056/NEJMoa1112843 [DOI] [PubMed] [Google Scholar]
- 34.Kim HN, Gupta A, Lan K, Stewart J, Dhanireddy S, Corcorran MA. Diagnostic accuracy of ICD code versus discharge summary-based query for endocarditis cohort identification. Medicine (Baltimore). 2021;100(51):e28354. doi: 10.1097/MD.0000000000028354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Graphical Demonstration of the Study Design
eTable 1. Codes Used to Determine De Novo Pacemaker or ICD Implantation Procedure
eTable 2. Codes Used to Determine CIED Infection
eTable 3. Antibiotic Therapy
eTable 4. Extraction Codes
eTable 5. Baseline Characteristics of Infection Cohort, Excluding ESKD and Patients Aged Younger Than 65 Years
eTable 6. Association Between Extraction and All-Cause Mortality, Excluding ESKD and Patients Aged Younger Than 65 Years
eTable 7. Association Between Extraction and All-Cause Mortality, Including Adjustment for Frailty Indicator
Data Sharing Statement