Abstract
Several known mammalian ribonucleotide reductase inhibitors featuring a polyhydroxyphenyl and/or hydroxamate moiety as the active group were screened for potency in inhibiting growth of the malaria parasite Plasmodium falciparum. Compounds containing a 2,3- or 3,4-dihydroxyphenyl group as well as benzohydroxamate appear to be the most effective inhibitors of the malaria parasite.
There is an urgent need to develop antimalarials targeted against new metabolic targets as the drugs available to fight the disease are rapidly losing their efficacy. Ribonucleotide reductase (RNR) catalyzes the reduction of ribonucleotides to deoxyribonucleotides, the first and rate-limiting step for de novo synthesis of 2′-deoxyribonucleoside 5′-triphosphates (16). RNR activity has been shown to be closely correlated to the rate of tumor growth (5, 19); consequently, inhibitors directed against RNR have been used for many years in cancer chemotherapy (7, 11, 12). The central role of the ubiquitous enzyme RNR in DNA metabolism also makes this enzyme an excellent target for chemotherapy of malaria. We have previously reported that an oligodeoxynucleotide phosphorothioate complementary to RNR small subunit sequences showed antimalarial activity (2). We have now initiated a systematic investigation to test known RNR inhibitors for possible antimalarial activity. Several antitumor and antiviral compounds exist which are specific inhibitors of RNR (15). The hydroxamate groups of RNR inhibitors such as hydroxyurea are electron reductants and destroy the tyrosyl radical (6). This paper reports evaluation of hydroxamic acid and polyhydroxyphenyl derivatives as potential antimalarials.
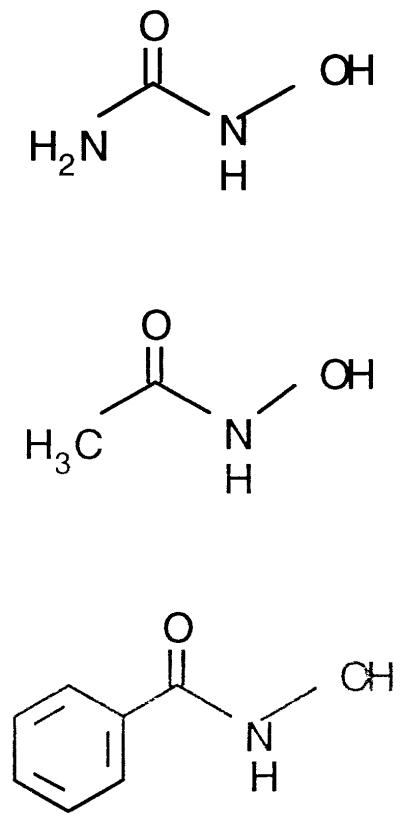
We evaluated three known RNR inhibitors, hydroxyurea, acetohydroxamate, and benzohydroxamate, for potential antimalarial activities in synchronous Plasmodium falciparum Dd2-infected erythrocyte culture according to the method of Desjardins et al. (3). Ring-stage cultures were incubated with [3H]hypoxanthine (1 μCi/μl; 1 μCi = 37 kBq) and various concentrations of inhibitors for 24 h at 37°C. The radiolabeled genomic DNA was isolated by sodium dodecyl sulfate-proteinase K treatment (14) and counted with a liquid scintillation spectrometer. The data were analyzed by logistic regression (11) since experimental variance is not constant for all drug concentrations, which makes the standard least squares technique inappropriate. This type of analysis was conducted for each drug evaluated to produce curves from which the drug concentration inhibiting 50% of the parasite growth (IC50) was calculated.
As can be seen in Table 1, both hydroxyurea and acetohydroxamate were weak inhibitors of malaria parasite growth. However, the IC50 for benzohydroxamic acid was about 50-fold lower than that for either hydroxyurea or acetohydroxamate. Some trends emerge from these data which encourage us in thinking that RNR could be a promising antimalarial target. Hydroxyurea and benzohydroxamate inhibit mammalian RNR to similar extents with IC50s of 500 and 400 μM, respectively (7). We found benzohydroxamate to be a much more effective inhibitor of malaria parasite growth than hydroxyurea, requiring a 20-fold lower concentration to effect the same level of inhibition as that of the human enzyme. The fact that benzohydroxamic acid proved to be a much more potent inhibitor of P. falciparum than the human system is noteworthy for two reasons. Not only does this identify benzohydroxamate as a potential antimalarial, but in addition and perhaps more importantly, it provides evidence of a difference between P. falciparum and human RNRs. This evidence supports that of Klayman et al. (9, 10), who cured Plasmodium berghei malaria in mice with RNR inhibitors, suggesting a possible difference between the P. berghei and mouse forms of reductase. Unlike the P. berghei data, the P. falciparum result presented here could not be explained by a difference in drug permeability, since benzohydroxamate was more effective in inhibiting the P. falciparum reductase enclosed within the parasite than the human enzyme, which was free in solution.
TABLE 1.
Antimalarial activities of hydroxamic acidsa
| Compound and structure | IC50 (μM) | 95% upper bound (μM) | 95% lower bound (μM) | No. of tests |
|---|---|---|---|---|
| Aminohydroxamate (hydroxyurea) | 792 | >10,000 | 97 | 58 |
| Acetohydroxamate | 1,032 | 3,912 | 26.4 | 60 |
| Benzohydroxamate | 17.1 | 78.5 | 2.2 | 76 |
Hydroxyurea (Sigma), acetohydroxamate (National Cancer Institute), and benzohydroxamate (National Cancer Institute) were tested in an in vitro culture system for potential antimalarial activities according to the method of Desjardins et al. (3).
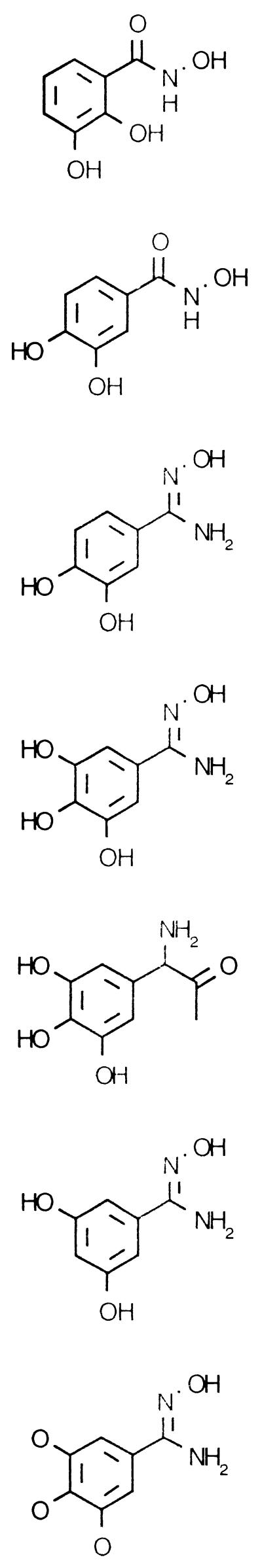
The results suggesting a difference between P. falciparum and human RNRs prompted us to test several substituted or modified forms of benzohydroxamate, such as vicinal polyhydroxyphenyl-containing compounds. This family of compounds has demonstrated antitumor activity, presumably due to inhibition of RNR activity (6–8, 17). We focused on vicinal di- and trihydroxyphenyls both with and without a hydroxamate moiety. The positions of the hydroxyl groups were varied; in addition, one drug (VF268) had nonadjacent hydroxyls and on another (VF282) the hydroxyl hydrogens were replaced with a methyl group.
Polyhydroxyphenyl and hydroxamic acid compounds are effective metal chelators. Since a ferric iron center plays a key role in RNR activity, the metal-chelating capacity of these compounds could explain their ability to inhibit RNR. Although it has been shown that changing hydroxyl group positions on the benzene ring has little effect on Fe3+-chelating activity if hydroxamic acid is present (7), such changes have large and correlative effects on RNR inhibition and free radical quenching potency (4). Hence, the mechanism by which polyhydroxyphenyls inhibit RNR is now believed to be free radical scavenging. Table 2 provides the structure and a summary of test results for each drug tested. VF149 and VF147, the two vicinal dihydroxybenzohydroxamates tested, outperformed the other drugs as inhibitors of P. falciparum growth. Trihydroxyphenyl-containing compounds are more effective mammalian RNR inhibitors than are compounds which contain one fewer hydroxyl group but are otherwise identical (4, 7, 8, 17). Yet we found the reverse to be true when these drugs were tested as antimalarials. It could be argued that the trihydroxyphenyls were at a disadvantage in our test program since they are not associated with the hydroxamate group. But testing on mammalian systems demonstrated that the hydroxamate functional group is relatively unimportant for antitumor activity and that the polyhydroxyphenyl group is the primary source of activity (6). This appears to be further evidence of a difference between mammalian and malarial forms of RNR, since hydroxamate-containing agents were the best antimalarials. Of the drugs tested, vicinal dihydroxyphenyl-substituted hydroxamic acids are the most effective antimalarials. The inhibitory effect though was reversible at the IC50. At four times the IC50 the effects of these inhibitors were found to be irreversible (data not shown). Didox (VF147, 3,4-dihydroxybenzohydroxamate) has been in clinical trials as an anticancer agent since 1988 (18). It exhibits low toxicity to the extent that steady-state concentrations in plasma during treatment (involving 36 h of infusion) are typically near the malaria parasite IC50 of 15 μM (1). Didox also stimulated hemoglobin F production in an anemic baboon and transgenic mice (13), an added benefit for malaria patients with anemia. Efforts are under way to characterize the mechanisms of action of these inhibitors in in vitro assays with recombinant malarial RNR.
TABLE 2.
Antimalarial activities of substituted benzohydroxamic acidsa
| Compound | Structure | IC50 (μM) | 95% upper bound (μM) | 95% lower bound (μM) | No. of tests |
|---|---|---|---|---|---|
| VF 149 | 6.4 | 25.7 | <1 | 76 | |
| VF 147 | 15.5 | 35.5 | 3.0 | 68 | |
| VF 236 | 23 | 80 | 3.5 | 52 | |
| VF 233 | 38 | 185 | 9.2 | 51 | |
| VF 368 | 301 | >2,000 | 46 | 51 | |
| VF 268 | 589 | >2,000 | 91 | 51 | |
| VF 282 | 2,010 | >10,000 | 520 | 52 | |
RNR inhibitors synthesized in one of our laboratories (H.L.E.) were tested on P. falciparum cultures.
Acknowledgments
We thank Michelle Fluegge and Cherie DelVecchio for technical assistance.
This investigation was supported by a grant (AI40692) from the National Institutes of Health.
REFERENCES
- 1.Carmichael J, Cantwell B M J, Mannix K A, Veale D, Elford H L, Blackie R, Kerr D J, Kaye S B, Harris A L. A phase I pharmacokinetic study of didox administered by 36 hr infusion. Br J Cancer. 1990;61:447–450. doi: 10.1038/bjc.1990.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti D, Schuster S M, Chakrabarti R. Cloning and characterization of subunit genes of ribonucleotide reductase, a cell-cycle-regulated enzyme, from Plasmodium falciparum. Proc Natl Acad Sci USA. 1993;90:12020–12024. doi: 10.1073/pnas.90.24.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desjardins R E, Canfield C J, Haynes J D, Chulay J D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elford H L, Elford R M, Wampler G L, van’t Riet B. New cancer chemotherapeutic agents that inhibit ribonucleotide reductase. In: Bardos T, Kalman T, editors. New approaches to the design of antineoplastic agents. New York, N.Y: Elsevier Science; 1982. [Google Scholar]
- 5.Elford H L, Freese M, Passamani E, Morris H P. Ribonucleotide reductase and cell proliferation. I. Variations of ribonucleotide reductase activity with tumor growth rate in a series of rat hepatomas. J Biol Chem. 1970;245:5228–5233. [PubMed] [Google Scholar]
- 6.Elford H L, van’t Riet B. The inhibition of nucleoside diphosphate reductase by hydroxybenzohydroxamic acid derivatives. In: Cory J G, Cory A H, editors. Inhibitors of ribonucleoside diphosphate reductase activity. Oxford, United Kingdom: Pergamon Press; 1989. [Google Scholar]
- 7.Elford H L, van’t Riet B, Wampler G L, Lin A L, Elford R M. Regulation of ribonucleotide reductase in mammalian cells by chemotherapeutic agents. Adv Enzyme Regul. 1981;19:151–168. doi: 10.1016/0065-2571(81)90014-5. [DOI] [PubMed] [Google Scholar]
- 8.Elford H L, Wampler G L, van’t Riet B. New ribonucleotide reductase inhibitors with antineoplastic activity. Cancer Res. 1979;39:844–851. [PubMed] [Google Scholar]
- 9.Klayman D L, Acton N, Scovill J P. 2-Acetylpyridine thiosemicarbazones. 12. Derivatives of 3-acetylisoquinoline as potential antimalarial agents. Arzneim-Forsch. 1986;36:10–13. doi: 10.1002/chin.198619236. [DOI] [PubMed] [Google Scholar]
- 10.Klayman D L, Mason C J, Scovill J P. 2-Acetylpyridine thiosemicarbazones. 10. 2-Propionyl-, 2-butryl-, and 2-(2-methylpropionyl)pyridine thiosemicarbazones as potential antimalarial agents. Arzneim-Forsch. 1984;34:1701–1703. [PubMed] [Google Scholar]
- 11.McCullagh P, Nelder J A. Generalized linear models. 2nd ed. London, United Kingdom: Chapman and Hall; 1989. [Google Scholar]
- 12.Moore E C, Sartorelli A C. The inhibition of ribonucleotide reductase by a-(N)-heterocyclic carboxaldehyde thiosemicarbazones. In: Cory J G, Cory A H, editors. Inhibitors of ribonucleoside diphosphate reductase activity. Oxford, United Kingdom: Pergamon Press; 1989. [Google Scholar]
- 13.Pace B S, Elford H L, Stamatoyannopoulos G. Transgenic mouse model of pharmacologic induction of fetal hemoglobin: studies using a new ribonucleotide reductase inhibitor, Didox. Am J Hematol. 1994;45:136–141. doi: 10.1002/ajh.2830450208. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 15.Stubbe J. Ribonucleotide reductases. Adv Enzymol Relat Areas Mol Biol. 1990;63:349–419. doi: 10.1002/9780470123096.ch6. [DOI] [PubMed] [Google Scholar]
- 16.Thelander L, Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- 17.van’t Riet B, Wampler G L, Elford H L. Synthesis of hydroxy- and amino-substituted benzohydroxamic acids: inhibition of ribonucleotide reductase and antitumor activity. J Med Chem. 1979;22:589–592. doi: 10.1021/jm00191a027. [DOI] [PubMed] [Google Scholar]
- 18.Veale D, Carmichael J, Cantwell B M J, Elford H L, Blackie R, Kerr D J, Kaye S B, Harris A L. A phase I and pharmacokinetic study of didox: a ribonucleotide reductase inhibitor. Br J Cancer. 1988;58:70–72. doi: 10.1038/bjc.1988.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber G. Enzymology of cancer cells. N Engl J Med. 1977;296:486–493. doi: 10.1056/NEJM197703032960905. [DOI] [PubMed] [Google Scholar]