Key Points
Question
What is the optimal prognostic measure of perineural invasion (PNI) for cutaneous squamous cell carcinoma (CSCC)?
Findings
In this cohort study of 140 patients with CSCC, PNI of 5 or more distinct nerves (extensive PNI [ePNI]) was independently associated with local recurrence and disease-specific death. Inclusion of ePNI in lieu of nerve caliber as a risk factor improved the prognostic ability of the Brigham and Women’s Hospital staging system.
Meaning
These findings suggest that ePNI is a strong prognostic feature in CSCC and should be considered for inclusion as a high-risk feature in CSCC guidelines and staging systems.
This cohort study compares prognostic assessments of perineural invasion in cutaneous squamous cell carcinoma and their associations with poor outcomes and implications for staging system revision.
Abstract
Importance
Perineural invasion (PNI) is an adverse risk feature in cutaneous squamous cell carcinoma (CSCC) that affects patient prognosis and disease management. However, research comparing different PNI patterns on patient outcomes is limited.
Objective
To compare 4 assessments of PNI in CSCC, their associations with poor outcomes, and implications for their inclusion in the Brigham and Women’s Hospital (BWH) staging system.
Design, Setting, and Participants
This retrospective cohort study was performed at a single tertiary care institution and compared 4 PNI assessments: nerve caliber, number of involved nerves per section, PNI maximal depth, and PNI location with respect to tumor. Patients with primary, localized, invasive CSCC with PNI diagnosed between January 1, 2000, and December 31, 2017, were identified via an electronic in-house database. Available pathology slides were secondarily reviewed by study authors. Relevant patient and tumor characteristics and outcomes were abstracted from the medical record. Data analysis was performed between September 6 and October 20, 2022.
Main Outcomes and Measures
Risks of recurrence, disease-specific death, and a composite end point (any poor outcome) were calculated via multivariable stepwise Fine and Gray competing-risks regression. Considered revisions to the BWH staging system were assessed via receiver operating characteristic curves and test characteristics.
Results
This study included 140 patients with CSCC, with a mean (SD) age of 75.1 (11.2) years. More than half of the patients were men (93 [66.4%]), and most identified as White (132 [94.3%]). Of the 4 PNI assessments studied, only involvement of multiple nerves was associated with poor outcomes. Perineural invasion of 5 or more distinct nerves (extensive PNI [ePNI]) was independently associated with local recurrence (subhazard ratio [SHR], 13.83 [95% CI, 3.50-54.62]; P < .001), disease-specific death (SHR, 6.20 [95% CI, 1.59-24.21]; P = .009), and any poor outcome (SHR, 10.21 [95% CI, 2.88-36.15]; P < .001). A revised BWH staging system with substitution of ePNI for large-caliber PNI resulted in improved area under the curve and test characteristics compared with current BWH staging criteria that use nerve caliber as the measure of PNI.
Conclusions and Relevance
The findings of this cohort study suggest that ePNI is the best prognostic measure of PNI. Because ePNI obviated the need for a micrometer and had superior prognostic capacity to nerve caliber in this cohort, ePNI should be considered for inclusion in CSCC tumor staging. Inclusion of ePNI as a high-risk factor in CSCC staging systems may optimize patient selection for primary treatment and adjuvant interventions.
Introduction
Perineural invasion (PNI) occurs in approximately 2.5% to 14% of tumors1 and is a well-recognized poor prognostic feature in cutaneous squamous cell carcinoma (CSCC)2,3,4 and a risk factor in both the Brigham and Women’s Hospital (BWH)5 and the American Joint Committee on Cancer (AJCC) staging systems.6 The National Comprehensive Cancer Network (NCCN) guidelines currently recognize large-caliber PNI (lcPNI), defined as involved nerves with a diameter of 0.1 mm or greater, and deep PNI (beyond the dermis) as very high-risk features and recommend peripheral and deep en face margin assessment (which includes Mohs surgery, Tübingen methods, and en face sectioning) for their management.7 The NCCN guidelines further recommend multidisciplinary consultation for consideration of adjuvant radiotherapy (ART) if PNI is extensive, is large caliber, or involves named nerves.7
Various attempts to quantify the risk associated with PNI have been reported, with differential patient outcomes observed within distinct subtypes. For example, worse patient outcomes are observed with invasion of large-caliber nerves than with small-caliber nerves.8,9 By extension, involvement of nerves deep to the dermis (which are generally large caliber) is considered an adverse histologic feature and, according to the AJCC, a factor for tumor upstaging. Involvement of named nerves and PNI that is clinically symptomatic has also been shown to confer unfavorable outcomes.10 Conversely, small-caliber PNI without additional CSCC risk factors has an excellent prognosis.11 The BWH and AJCC staging systems thus incorporate lcPNI (but not small-caliber PNI) as a risk factor to upstage patients with CSCC.5,6 However, lcPNI requires a micrometer and is time-consuming to measure, which is why the AJCC also allows for upstaging if PNI deep to the dermis is present.
Involvement of multiple nerves, also referred to as extensive PNI (ePNI), has similarly been associated with poor outcomes in CSCC.8,12,13,14 However, the literature on this topic is heterogeneous, with various numbers of involved nerves used as thresholds for poorer CSCC outcomes (including >2,12 ≥3,8 and >513,14 nerves) and inconsistent methodologies employed for assessment of involved nerve counts. Indeed, no definition for what constitutes ePNI is provided by the NCCN.
Despite recognition of the importance of PNI in the prognosis and management of CSCC, limited research has comprehensively compared different methods of measuring and quantifying PNI with regard to prognostic prediction. To improve risk stratification within the subset of patients with CSCC with PNI, this study was undertaken to compare 4 different PNI assessments: nerve caliber, number of nerves involved, depth of PNI involvement, and location of PNI with respect to tumor. Associations between these 4 characteristics and outcomes were measured, while adjusting for patient and tumor variables. If a PNI pattern was independently associated with poor outcomes, a secondary aim was to determine whether its inclusion in the BWH staging system enhanced prognostic discrimination.
Methods
Cohort
This retrospective cohort study was approved by the Massachusetts General Brigham Human Research Committee and Institutional Review Board. Informed consent was waived due to minimal risk to participants. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patients with CSCC tumors with PNI diagnosed on either pathology or Mohs operative reports at BWH between January 1, 2000, and December 31, 2017, were identified via an electronic in-house database. Medical records were abstracted for patient and tumor characteristics, including self-reported patient race and ethnicity, as well as outcomes of interest.2 Race and ethnicity data were collected along with other key demographic characteristics such as age and sex to provide information about the study population. For statistical analysis, adverse outcomes were classified as local recurrence, metastasis (nodal or distant), disease-specific death, or a composite end point (ie, any poor outcome). All pathology slides pertaining to each tumor, including biopsies, excisions, re-excisions, and Mohs micrographic surgery excisions, were requested from a central pathology storehouse. Slides were manually reviewed by a study author (P.R.M., P.M., E.R.-G., W.M.L., or A.P.) for the following selected features of interest: largest PNI diameter, deepest level of PNI invasion, greatest number of distinct nerves involved per section, and location of PNI relative to the tumor. Tumors were excluded from the study if slides could not be retrieved or if PNI was not in fact identified by the study authors on review. Any noncutaneous, anogenital, in situ, metastatic at presentation, and recurrent tumors were excluded from analysis.
PNI Classification
Tumor cells within any of the 3 layers of the nerve sheath (epineurium, perineurium, or endoneurium) or in close association with at least one-third of the nerve circumference were required for slide reviewers to confirm a diagnosis of PNI, in keeping with the definition put forth by Liebig et al.15 For the measure of nerve caliber, the largest involved nerve in slides reviewed was measured with a micrometer and recorded. Involvement of nerves with a diameter of 0.1 mm or greater was defined as lcPNI. Three a priori categories were established for assessment of the number of nerves: focal (1 distinct involved nerve per section), moderate (2-4 distinct involved nerves per section), or extensive (≥5 distinct involved nerves per section). These categories were assigned according to the section with the greatest number of distinct nerves involved. It is important to note that the numbers of involved nerves in separate sections were not added together (eg, 3 foci of PNI in one section and 2 in another section did not result in a categorization of extensive). For the measure of location of PNI relative to the tumor, the choices were intratumoral (PNI occurring within the main tumor body), advancing edge (PNI at the tumor periphery), extratumoral (PNI discontinuous with tumor), or not applicable if the tumor was too poorly differentiated to allow for identification of a tumor body. Depth of invasion was determined by the deepest histologic level at which PNI was noted (dermis, fat, muscle, fascia, etc); PNI depth of invasion is a distinct entity from tumor depth of invasion.
If features were absent, equivocal, or differed from the initial formal pathology report, a senior study author (A.P., E.S.R., or C.D.S.) served as an ultimate arbiter. For all categories, pathology reviewers were asked to grade the most advanced element of PNI in each case encountered, regardless of whether it was encountered on biopsy, excision, or re-excision. For example, if both small-caliber PNI and lcPNI were noted, lcPNI was recorded as the pattern of record for nerve caliber.
Assessment of Distinct Nerves
Care was taken to ensure that counting of involved nerves did not result in duplication. An understanding of cutaneous histologic anatomy is needed for this assessment. Nerves follow a predictable course in the skin, running parallel to the skin surface alongside vascular plexuses in the papillary dermis and fat, with perpendicular branches (relative to the epithelium) found in the reticular dermis.16 Experienced pathologists and Mohs surgeons used this knowledge and used reasonable judgment to ensure that involved nerves were counted only when representing plausibly distinct branches (eFigure in Supplement 1). For example, if perpendicularly oriented involved dermal nerves were noted on either side of a pilosebaceous unit, these were counted separately. Conversely, if multiple involved foci were noted near one another in the same histologic plane, this was counted as single nerve involvement.
Statistical Analysis
Descriptive statistics were used to analyze the risk factors, including means (SDs) for continuous variables and proportions or percentages for categorical variables. Univariate competing-risks regression was first performed on all characteristics (including BWH T stage; Table 1) to identify the PNI categories (defined in PNI Classification) potentially associated with each outcome, and then to select tumor and patient factors representing potential confounders. Variables with P < .10 on univariable analysis were tested in the multivariable model. Stepwise Fine and Gray competing-risks regression analysis was then used to determine multivariable associations between selected PNI risk factors and outcomes of interest and to plot 5-year cumulative incidence curves. Models with the lowest Akaike information criterion were selected.
Table 1. Cohort Characteristicsa.
| Characteristic | No poor outcomes (n = 123) | Poor outcome (n = 17)b | P value |
|---|---|---|---|
| Follow-up, mean (SD), mo | 49.3 (48.9) | 34.7 (28.8) | .23c |
| Age, mean (SD), y | 75.7 (11.1) | 70.8 (11.3) | .09c |
| Sex | |||
| Female | 45 (95.7) | 2 (4.3) | .04d |
| Male | 78 (83.9) | 15 (16.1) | |
| Location | |||
| Head/neck | 82 (86.3) | 13 (13.7) | .71 |
| Extremities | 20 (95.2) | 1 (4.8) | |
| Trunk | 20 (87.0) | 3 (13.0) | |
| Other | 1 (100) | 0 | |
| Size, mean (SD), mm | 21.3 (15.3) | 29.2 (20.1) | .07c |
| Differentiation | |||
| Well | 48 (94.1) | 3 (5.9) | .17 |
| Moderate | 33 (89.2) | 4 (10.8) | |
| Poor | 39 (79.6) | 10 (20.4) | |
| Unknown | 1 (100) | 0 | |
| Tumor depth | |||
| Dermis | 43 (97.7) | 1 (2.3) | .02 |
| Subcutaneous fat | 47 (87.0) | 7 (13.0) | |
| Fascia | 19 (86.4) | 3 (13.6) | |
| Muscle | 3 (50.0) | 3 (50.0) | |
| Bone | 6 (100) | 0 | |
| Other | 3 (75.0) | 1 (25.0) | |
| PNI | |||
| Caliber, mm | |||
| <0.1 | 60 (90.9) | 6 (9.1) | .30d |
| ≥0.1 | 63 (85.1) | 11 (14.9) | |
| No. of involved nerves | |||
| Focal | 72 (93.5) | 5 (6.5) | .004 |
| Moderate | 38 (88.4) | 5 (11.6) | |
| Extensive | 12 (63.2) | 7 (36.8) | |
| Location | |||
| Intratumoral | 25 (92.6) | 2 (7.4) | .34 |
| Advancing edge | 31 (79.5) | 8 (20.5) | |
| Extratumoral | 50 (89.3) | 6 (10.7) | |
| Unable to determine | 17 (94.4) | 1 (5.6) | |
| Depth | |||
| Dermis | 56 (86.2) | 9 (13.9) | .57d |
| Beyond dermis | 67 (89.3) | 8 (10.7) | |
| BWH stage | |||
| T1 | 23 (95.8) | 1 (4.2) | .46 |
| T2a | 28 (87.5) | 4 (12.5) | |
| T2b | 52 (82.5) | 11 (17.5) | |
| T3 | 9 (90.0) | 1 (10.0) |
Abbreviations: BWH, Brigham and Women’s Hospital; PNI, perineural invasion.
Unless indicated otherwise, values are presented as No. (%) of tumors, and P values are based on the Fisher exact test.
Poor outcomes include local recurrence, metastasis, or death from disease.
t Test.
χ2 Test.
The current BWH staging system and comparators were analyzed via receiver operating characteristic (ROC) curves. Test characteristics (sensitivity, specificity, positive predictive value, and negative predictive value) were determined by comparing the presence or absence of a poor outcome with tumor identification as either high (T2b/T3) or low (T1/T2a) BWH T stage. Statistical analyses were conducted using Stata, version 16.1 (StataCorp). Statistical significance was set at P < .05 (2-tailed test). Data analysis was performed between September 6 and October 20, 2022.
Results
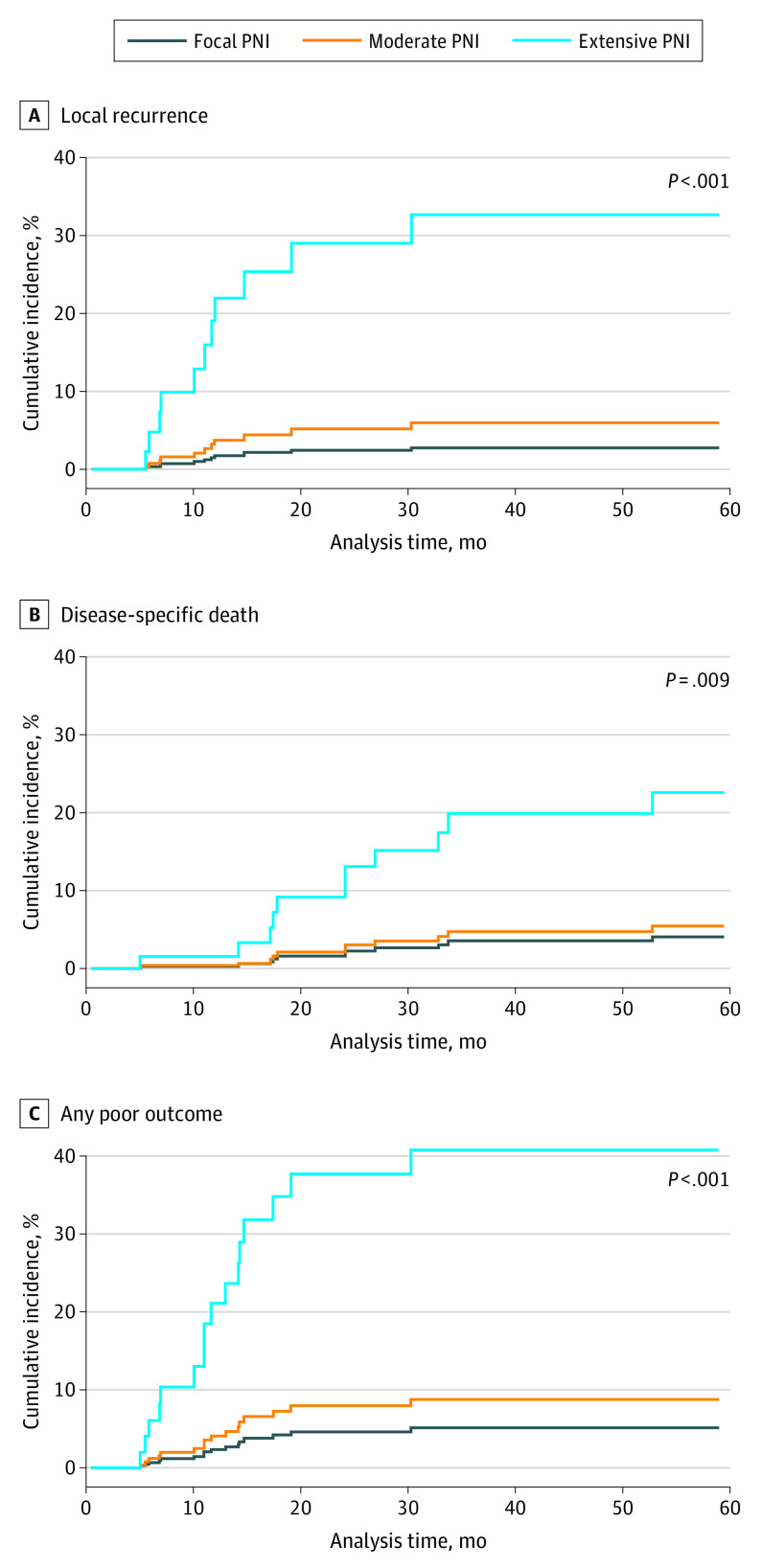
This study included 140 patients with CSCC, with a mean (SD) age of 75.1 (11.2) years. There were 93 men (66.4%) and 47 women (33.6%). Two patients (1.4%) identified as Black or African American, 1 (0.7%) identified as Hispanic or Latino, 132 (94.3%) identified as White, and 5 (3.6%) were of unknown race or ethnicity. Baseline patient, tumor, and PNI characteristics are presented in Table 1. Of the 4 PNI assessments studied, only involvement of multiple nerves was associated with poor outcomes. For primary treatment, 54 patients (38.6%) were treated with Mohs surgery, 77 (55.0%) with wide local excision, 7 (5.0%) with a combination of Mohs surgery and wide local excision, and 1 (0.7%) with radiation monotherapy; 1 (0.7%) was not treated after biopsy. A total of 41 patients (29.9%) received ART and 6 (4.4%) adjuvant chemotherapy. Overall, 17 of 140 patients (12.1%) had poor outcomes, including local recurrence (n = 12), nodal or distant metastasis (n = 10), or disease-specific death (n = 11). Of the assessed PNI characteristics, the number of nerves involved differed between tumors that developed a poor outcome, whereas lcPNI, PNI location relative to tumor, and PNI depth of involvement did not. Extensive PNI (defined a priori as involvement of ≥5 nerves) was found in 19 patients (13.6%), whereas lcPNI was found in 74 (52.9%). In multivariable analysis after adjustment for relevant patient and tumor characteristics (Table 2), involvement of 5 or more distinct nerves was independently associated with local recurrence (subhazard ratio [SHR], 13.83 [95% CI, 3.50-54.62]; P < .001), disease-specific death (SHR, 6.20 [95% CI, 1.59-24.21]; P = .009), and any poor outcome (SHR, 10.21 [95% CI, 2.88-36.15]; P < .001). Adjusted cumulative incidence functions are displayed in Figure 1.
Table 2. Multivariable Analysis for Poor Outcomes.
| Factor | SHR (95% CI) | P value |
|---|---|---|
| Local recurrence (n = 12) | ||
| Sex | ||
| Female | 1 [Reference] | |
| Male | 9.82 (1.48-65.34) | .02a |
| Differentiation | ||
| Well or moderate | 1 [Reference] | |
| Poor differentiation | 2.81 (0.88-9.00) | .08 |
| PNI extent | ||
| Focal | 1 [Reference] | |
| Moderate | 2.15 (0.42-10.90) | .36 |
| Extensive | 13.83 (3.50-54.62) | <.001a |
| Any metastasis (n = 10) | ||
| Depth | ||
| Limited to dermis/subcutaneous fat | 1 [Reference] | |
| Beyond subcutaneous fat | 2.62 (0.74-9.30) | .14 |
| Differentiation | ||
| Well or moderate | 1 [Reference] | |
| Poor | 5.04 (1.28-19.84) | .02a |
| Disease-specific death (n = 11) | ||
| Age, y | 0.94 (0.88-1.01) | .12 |
| Sex | ||
| Female | 1 [Reference] | |
| Male | 8.39 (0.92-76.69) | .06 |
| PNI extent | ||
| Focal | 1 [Reference] | |
| Moderate | 1.35 (0.32-5.73) | .68 |
| Extensive | 6.20 (1.59-24.21) | .009a |
| Any poor outcome (n = 17) | ||
| Depth | ||
| Limited to dermis/subcutaneous fat | 1 [Reference] | |
| Beyond subcutaneous fat | 1.91 (0.69-5.29) | .21 |
| Sex | ||
| Female | 1 [Reference] | |
| Male | 6.86 (1.45-32.39) | .02a |
| Differentiation | ||
| Well or moderate | 1 [Reference] | |
| Poor | 3.22 (1.22-8.47) | .02a |
| PNI extent | ||
| Focal | 1 [Reference] | |
| Moderate | 1.78 (0.47-6.74) | .40 |
| Extensive | 10.21 (2.88-36.15) | <.001a |
Abbreviations: PNI, perineural invasion; SHR, subhazard ratio.
Statistically significant.
Figure 1. Adjusted Cumulative Incidence Functions for Local Recurrence, Disease-Specific Death, and Any Poor Outcome.
Competing-risks regression analyses were adjusted for sex and differentiation (A); sex, invasion beyond fat, and differentiation (B); and age and sex (C). P values are based on comparison of extensive perineural invasion (PNI) to focal PNI curves.
Associations between the PNI characteristics studied and established BWH CSCC risk factors are presented in eTable 1 in Supplement 1. Number of nerves involved was associated with tumor depth beyond fat (Cochran-Armitage trend test statistic, 0.11; P = .04) and lcPNI (Cochran-Armitage trend test statistic, 0.24; P < .001). Of 19 tumors with 5 or more involved nerves, 16 (84.2%) also had lcPNI. Large-caliber PNI was associated with large tumor diameter (Cochran-Armitage trend test statistic, 0.30; P = .001). Overall rates of local recurrence, metastasis, disease-specific death, or any poor outcome for tumors with 5 or more involved nerves were 26.3%, 10.5%, 21.1%, and 36.8% compared with 9.5%, 8.1%, 10.8%, and 14.9% for those with lcPNI, respectively (eTable 2 in Supplement 1).
The salience of ePNI as an independent variable for risk of local recurrence, disease-specific death, and any poor outcome was compared with current BWH staging (model A), a BWH staging model substituting ePNI in lieu of lcPNI (model B), and a BWH staging model allowing either ePNI or lcPNI (model C) (Figure 2). In ROC analysis, model B demonstrated a superior area under the curve (69.0% [95% CI, 56.2%-81.7%]) compared with models A and C (58.5% [95% CI, 46.8%-70.2%] and 64.4% [95% CI, 53.2%-75.7%], respectively). Likewise, specificity, positive predictive values, and negative predictive values were superior when ePNI was substituted for lcPNI as a risk factor (eTable 3 in Supplement 1).
Figure 2. Receiver Operating Characteristic (ROC) Curve Analysis for Current and Modified Brigham and Women’s Hospital (BWH) Staging Systems.
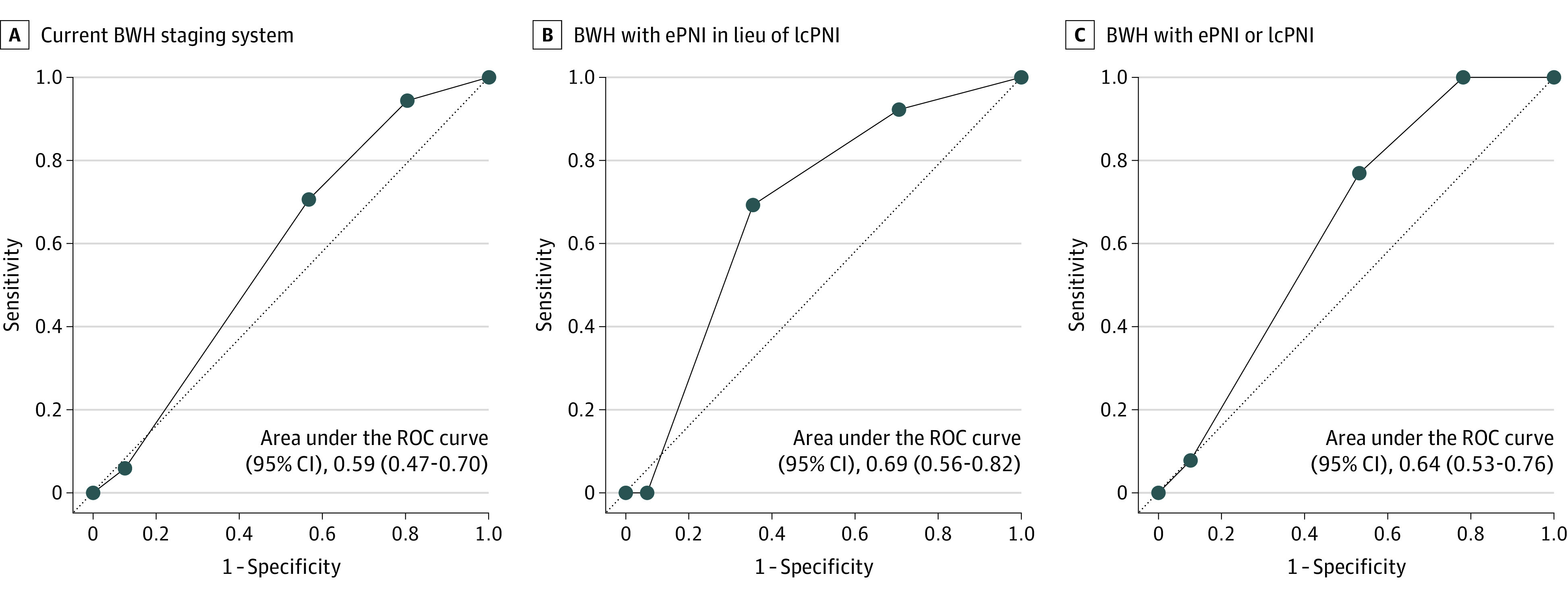
A, Current BWH staging system. B, BWH staging system with extensive perineural invasion (ePNI) in lieu of large-caliber PNI (lcPNI) as a factor. C, BWH staging system with ePNI or lcPNI as a risk factor.
Changes in cohort BWH T stages comparing current BWH staging to BWH staging with ePNI in lieu of lcPNI are presented in Table 3. Using this revised BWH staging system, 30.0% of tumors were restaged, resulting in 36 tumors downstaged and 0 upstaged. Of the 36 tumors downstaged, only 3 experienced a poor outcome. A total of 22 restaged tumors (61.1%) were downstaged from high (BWH T2b) to low (BWH T2a) T stage when using ePNI vs lcPNI as a risk factor.
Table 3. Current and Revised BWH Staging System T Stagesa.
| Current BWH (model A) | Revised BWH (model B) | ||||
|---|---|---|---|---|---|
| T1 | T2a | T2b | T3 | Total | |
| T1 | 23 | 0 | 0 | 0 | 23 |
| T2a | 9/10b | 21 | 0 | 0 | 31 |
| T2b | 0 | 21/22b | 34 | 0 | 56 |
| T3 | 0 | 0 | 3/4b | 6 | 10 |
| Total | 33 | 43 | 38 | 6 | 120 |
Abbreviation: BWH, Brigham and Women’s Hospital.
Tumors with complete staging information displayed. No upstaging occurred.
Fractions represent the portion of patients with an appropriate downstage, indicating that no poor outcomes occurred.
Discussion
In this retrospective cohort study, ePNI was independently associated with local recurrence, disease-specific death, and a composite end point (any poor outcome), and substituting ePNI for lcPNI improved the prognostic ability of the BWH staging system. Other assessed PNI patterns, including nerve caliber, location, and depth of involvement of PNI, were not associated with poor outcomes.
These results are consistent with prior literature demonstrating inferior patient outcomes with multifocal or extensive PNI. The 36.8% overall poor outcome rate observed in patients with involvement of 5 or more nerves is similar to the 35.7% rate reported by Totonchy et al13 in a recent cohort of CSCC with PNI, indicating the extremely high-risk nature of this degree of PNI involvement. Conde-Ferreirós et al8 reported that ePNI, defined as 3 or more involved nerves, was an independent poor prognostic factor for local recurrence but not for lymphatic progression or disease-specific death. In the current study, involvement of 5 or more nerves was independently associated with poor outcomes, whereas involvement of 2 to 4 nerves was not. However, the association of nerve involvement with patient outcomes is likely a continuum, and this study may not have been powered to detect the impact of lower numbers of involved nerves.
Although previous PNI studies have assessed multifocal PNI and lcPNI together in statistical models,8,11,13 this study is the first, to our knowledge, to compare ePNI vs lcPNI as risk factors within the framework of the BWH staging system. The improved performance of the BWH staging system in an ROC analysis upon substitution of ePNI for lcPNI suggests that ePNI is a more powerful estimator of poor CSCC outcomes and thus may capture distinct tumor biology. For example, in this study cohort, 2 of the 3 patients with ePNI alone experienced poor outcomes, including metastasis and disease-specific death. Conversely, in the PNI cohort reported by Carter et al,11 no patients with lcPNI alone experienced a poor outcome. Furthermore, the restaging analysis herein of ePNI as a risk factor in lieu of lcPNI downstaged 30.0% of the cohort (36 of 120), with only 3 of the 36 downstaged patients experiencing a poor outcome. Of the 36 patients who were downstaged, the majority (22 [61.1%]) were restaged from BWH T2b to T2a. This is clinically meaningful because BWH T2b is often used as a threshold for adjuvant interventions, including surveillance imaging,17 sentinel lymph node biopsy,18 or radiotherapy.19
These findings have implications for CSCC guidelines. First, this study contributes a plausible definition of what constitutes ePNI to the CSCC literature. Second, given the high overall rate of poor outcomes and the independent association with both local recurrence and disease-specific death, ePNI may merit inclusion alongside deep PNI and lcPNI in the very high-risk CSCC entity recently delineated by the NCCN7 and validated in a large multi-institutional cohort study.20 In our cohort, the 36.8% overall poor outcome rate for tumors with ePNI was more than twice the rate of those with lcPNI (14.9%) and more than 3 times the rate of patients with deep PNI (10.7%).
The NCCN guidelines currently recommend consideration of ART if PNI is extensive.7 In our cohort, 38.9% of patients with ePNI received ART compared with 28.8% of patients without ePNI. The relatively low utilization in our study among those with ePNI as defined here underscores the need for more precise definitions for ePNI and better estimates of prognosis. The NCCN guidelines are supported by a study by Sapir et al,12 in which ART resulted in a 2-year disease-free survival rate of 73% in patients with extensive microscopic PNI (defined as >2 involved nerves in resection specimens) compared with 40% in patients who were observed. Although our study was not designed to assess the association between ART and ePNI prognosis, an individualized postoperative risk assessment for poor outcomes is needed to determine potential benefit from ART. In a recent study by Ruiz et al,19 ART halved the rate of local and locoregional recurrence in patients with CSCC at risk for poor outcomes (high BWH T stage, recurrent or large diameter) after negative surgical margin resection.
Limitations and Strengths
This study has some limitations, including restaging analyses being performed post hoc. However, this allowed for treatment to be unaltered and thus provide realistic estimations of risks associated with various tumor stages and PNI categorizations under current treatment patterns. Another limitation is the single-center setting, and these results should be validated in other cohorts. In addition, the relatively low event rate reported herein limited the statistical power and the number of covariates that could be included in study models. Finally, the assessment of PNI requires additional time. Our experience is that the time involved to identify ePNI in clinical practice approximates that needed to identify lcPNI, which may involve identifying multiple candidate nerves for lcPNI measurement and has the benefit of precluding the use of a micrometer or permanent section histology to verify nerve diameter. We recommend identification of potential ePNI in the most tumor-involved sections of a tissue block, with use of a glass-slide marking pen to facilitate rapid counting of nerves.
Strengths of this study include the manual review of slides from biopsy through definitive surgery to ensure that all PNI characteristics were captured. Multiple reviewers, including board-certified dermatopathologists, participated in the slide review. In addition, the study benefited from strict definitions of PNI characteristics.
Conclusions
In this cohort study, tumor invasion of 5 or more distinct nerves was independently associated with poor outcomes in CSCC and was a better prognostic estimator in this cohort than nerve caliber. Although these findings require further validation in other cohorts, inclusion of ePNI as a high-risk factor in CSCC staging may optimize recurrence risk assessment and patient selection for surgical treatment modality and postoperative adjuvant interventions.
eTable 1. Association Between Assessed Perineural Invasion (PNI) Factors of Interest and Brigham and Women’s Hospital (BWH) Risk Factors
eTable 2. Poor Outcomes by Assessed Perineural Invasion (PNI) Characteristic
eTable 3. Test Characteristics of Models Under Study to Detect Any Poor Outcome
eFigure. Example of Perineural Invasion in at Least 3 Distinct Nerves of a Hematoxylin and Eosin–Stained Mohs Section
Data Sharing Statement
References
- 1.Leibovitch I, Huilgol SC, Selva D, Hill D, Richards S, Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. Perineural invasion. J Am Acad Dermatol. 2005;53(2):261-266. doi: 10.1016/j.jaad.2005.03.048 [DOI] [PubMed] [Google Scholar]
- 2.Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol. 2013;149(5):541-547. doi: 10.1001/jamadermatol.2013.2139 [DOI] [PubMed] [Google Scholar]
- 3.Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152(4):419-428. doi: 10.1001/jamadermatol.2015.4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149(4):402-410. doi: 10.1001/jamadermatol.2013.2456 [DOI] [PubMed] [Google Scholar]
- 5.Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32(4):327-334. doi: 10.1200/JCO.2012.48.5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin MB, Edge SB, Greene FL, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017. doi: 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 7.National Comprehensive Cancer Network . Squamous CSC. Updated March 10, 2023. Accessed June 16, 2023. https://www.nccn.org
- 8.Conde-Ferreirós A, Corchete LA, Jaka A, et al. Patterns of incidental perineural invasion and prognosis in cutaneous squamous cell carcinoma: a multicenter, retrospective cohort study. J Am Acad Dermatol. 2021;84(6):1708-1712. doi: 10.1016/j.jaad.2020.08.017 [DOI] [PubMed] [Google Scholar]
- 9.Ross AS, Whalen FM, Elenitsas R, Xu X, Troxel AB, Schmults CD. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35(12):1859-1866. doi: 10.1111/j.1524-4725.2009.01354.x [DOI] [PubMed] [Google Scholar]
- 10.Karia PS, Morgan FC, Ruiz ES, Schmults CD. Clinical and incidental perineural invasion of cutaneous squamous cell carcinoma: a systematic review and pooled analysis of outcomes data. JAMA Dermatol. 2017;153(8):781-788. doi: 10.1001/jamadermatol.2017.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter JB, Johnson MM, Chua TL, Karia PS, Schmults CD. Outcomes of primary cutaneous squamous cell carcinoma with perineural invasion: an 11-year cohort study. JAMA Dermatol. 2013;149(1):35-41. doi: 10.1001/jamadermatol.2013.746 [DOI] [PubMed] [Google Scholar]
- 12.Sapir E, Tolpadi A, McHugh J, et al. Skin cancer of the head and neck with gross or microscopic perineural involvement: patterns of failure. Radiother Oncol. 2016;120(1):81-86. doi: 10.1016/j.radonc.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 13.Totonchy MB, McNiff JM, Suozzi KC, Leffell DJ, Christensen SR. A histopathologic scoring system for perineural invasion correlates with adverse outcomes in patients with cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47(4):445-451. doi: 10.1097/DSS.0000000000002923 [DOI] [PubMed] [Google Scholar]
- 14.Cohen ER, Misztal C, Dable C, et al. Redefining perineural invasion in head and neck cutaneous squamous cell carcinoma. Otolaryngol Head Neck Surg. 2022;167(4):705-715. doi: 10.1177/01945998221076110 [DOI] [PubMed] [Google Scholar]
- 15.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115(15):3379-3391. doi: 10.1002/cncr.24396 [DOI] [PubMed] [Google Scholar]
- 16.Glatte P, Buchmann SJ, Hijazi MM, Illigens BM, Siepmann T. Architecture of the cutaneous autonomic nervous system. Front Neurol. 2019;10:970. doi: 10.3389/fneur.2019.00970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox M, Brown M, Golda N, et al. ; High Risk Squamous Cell Carcinoma Workgroup; Dermatologic Surgery Section of the Association of Professors of Dermatology . Nodal staging of high-risk cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2019;81(2):548-557. doi: 10.1016/j.jaad.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pride RLD, Lopez JJ, Brewer JD, et al. Outcomes of sentinel lymph node biopsy for primary cutaneous squamous cell carcinoma of the head and neck. Dermatol Surg. 2022;48(2):157-161. doi: 10.1097/DSS.0000000000003304 [DOI] [PubMed] [Google Scholar]
- 19.Ruiz ES, Kus KJB, Smile TD, et al. Adjuvant radiation following clear margin resection of high T-stage cutaneous squamous cell carcinoma halves the risk of local and locoregional recurrence: a dual-center retrospective study. J Am Acad Dermatol. 2022;87(1):87-94. doi: 10.1016/j.jaad.2022.03.044 [DOI] [PubMed] [Google Scholar]
- 20.Stevens JS, Murad F, Smile TD, et al. Validation of the 2022 National Comprehensive Cancer Network risk stratification for cutaneous squamous cell carcinoma. JAMA Dermatol. 2023;159(7):728-735. doi: 10.1001/jamadermatol.2023.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Association Between Assessed Perineural Invasion (PNI) Factors of Interest and Brigham and Women’s Hospital (BWH) Risk Factors
eTable 2. Poor Outcomes by Assessed Perineural Invasion (PNI) Characteristic
eTable 3. Test Characteristics of Models Under Study to Detect Any Poor Outcome
eFigure. Example of Perineural Invasion in at Least 3 Distinct Nerves of a Hematoxylin and Eosin–Stained Mohs Section
Data Sharing Statement