Abstract
Background:
The current studies investigated associations between pain intensity and pain frequency with loneliness, hostility, and social functioning using cross-sectional, longitudinal, and within-person data from community-dwelling adults with varying levels of pain.
Methods:
Secondary analysis of preexisting data was conducted. Study 1 investigated cross-sectional (baseline data: n = 741) and longitudinal (follow-up data: n = 549; observed range between baseline and follow up: 6–53 months) associations. Study 2 tested within-person associations using daily diaries across 30 days from a subset of the participants in Study 1 (n=69).
Results:
Cross-sectionally, pain intensity and frequency were associated with higher loneliness (βintensity=0.16, βfrequency=0.17) and worse social functioning (βintensity=−0.40; βfrequency=−0.34). Intensity was also associated with higher hostility (β=0.11). Longitudinally, pain intensity at baseline predicted hostility (β=0.19) and social functioning (β=−0.20) at follow-up, whereas pain frequency only predicted social functioning (β=−0.21). Within people, participants reported higher hostility (γ=.002) and poorer social functioning (γ=−.013) on days with higher pain, and a significant average pain by daily pain interaction was found for loneliness. Pain intensity did not predict social variables on the following day.
Conclusion:
Pain intensity and frequency may interfere with social wellbeing, but it is more likely that social wellbeing influences pain in community-dwelling adults.
Keywords: Acute Pain, Biopsychosocial, Community-Dwelling Adults, Social Wellbeing
In both humans and animals, the social environment influences how pain is perceived and expressed [1–5]. For example, pain is rated less intensely when experienced in the presence of a close social partner than alone [1, 6] but more intensely when experienced in the context of a familiar dyad experiencing similar pain [7]. These studies provide evidence that the social context influences pain outcomes and are complemented by cross-sectional research showing that patients with chronic pain report worse social wellbeing than those without chronic pain [e.g., 8–9]. Yet, research has focused more on how social factors influences pain than on how pain influences social wellbeing. Furthermore, little work has examined the association between pain and social wellbeing in non-clinical or community-dwelling samples, and little is known about which aspects of pain (intensity or frequency) are associated with social outcomes in this population. The current paper aimed to investigate if pain frequency and intensity predicted social wellbeing in community-dwelling adults using cross-sectional, longitudinal, and within-person data.
Social wellbeing is a complex construct and refers to how people function in the social world. It can include both subjective and objective components [10]. Because subjective measures of social wellbeing are often better predictors of health outcomes than objective predictors [10–11], we chose three facets of social wellbeing to investigate in the current study. Two of them (loneliness and hostility) assess how people perceive their interpersonal relationships with others in their social network; one of them (social functioning) assesses how health and psychological functioning influence social activities. We chose to focus on these three subjective facets of social wellbeing because 1) of their potential to be influenced by pain in the moment and over time, 2) of literature supporting their role in influencing subsequent pain outcomes, and 3) data were available for each cross-sectionally, longitudinally, and within-people. The three facets will be referred to as “components of social wellbeing” hereafter, although they admittedly represent a small part of the broader social wellbeing construct.
Loneliness and Pain
Loneliness, or the discrepancy between desired and perceived connection with others [12], has been linked with higher pain intensity in fibromyalgia patients (r = .35; [13]) and in community-dwelling younger and older adults [14–16]. Among community-dwelling older adults with acute pain, loneliness predicted pain intensity above and beyond social network size or perceived affiliation, but no relationship existed between loneliness and pain intensity in adults with chronic pain [16]. Additionally, lonelier breast cancer survivors reported higher pain intensity than less lonely survivors [17]. Loneliness longitudinally predicted pain seven years later in older adults with chronic lower back pain [15], and social isolation predicted pain interference in patients with persistent pain, although pain did not predict social isolation [18]. In adults with fibromyalgia, within-person increases in loneliness predicted subsequent pain intensity [13, 19]. Cumulatively, cross-sectional, longitudinal, and within-person evidence suggests loneliness influences pain.
Hostility and Pain
Like loneliness, hostility has also been associated with higher pain intensity. Hostility - unfriendliness, antagonism, or opposition to others in a social group - has been positively correlated with pain in cross-sectional studies [20–21]. In pain-free undergraduates, a pain manipulation increased aggressive behavior for those high in state hostility, suggesting that the hostility-pain relationship may be bidirectional [22]. Individuals with musculoskeletal and vulvar pain reported higher pain intensity up to several hours after they perceived hostility from their spouses [23–24]. However, whether pain predicts hostility over longer periods of time remains untested.
Social Functioning and Pain
People may withdraw from social activities when in pain. Social functioning refers to how well people maintain social activities like visiting family, friends, neighbors, or other groups in the face of physical or emotional problems [25]. Cross-sectionally, an extensive body of literature suggests that chronic pain is negatively correlated with social functioning (e.g., r = −.48; [e.g., 9, 26–28]), but longitudinal data exploring the temporal nature of these associations remains scarce.
Summary of the Literature on Pain and Social Outcomes
In summary, evidence suggests that loneliness, hostility, and social functioning influence pain, but whether pain influences these components of social wellbeing remains an important yet understudied question in community-dwelling adults. Many community-dwelling adults do not have chronic pain, defined as pain that persists beyond the normal healing time (generally taken to be 3–6 months), but instead experience episodes of acute pain with known etiology. Examining relationships between pain and social wellbeing in community-dwelling adults is important because it can 1) increase the generalizability of results beyond chronic pain samples; 2) provide an unrestricted range in pain frequency/intensity in which to study their relationship with social wellbeing, and 3) reveal that the negative consequences of pain are more ubiquitous than previously imagined. In this population, there are two characteristics of pain that may be particularly important: intensity, which refers to how salient the pain is, and frequency, which refers to how often pain occurs. Frequency can be examined by the number of days pain occurs, or by comparing those with frequent pain from those with infrequent pain.
The Current Studies
The current studies had three aims. The first aim was to examine the cross-sectional associations between pain intensity and frequency with loneliness, hostility, and social functioning in community-dwelling midlife adults with varying levels of pain (Study 1). The second aim was to examine these relationships longitudinally (Study 1). The third aim was to examine whether within-person changes in pain intensity predicted concurrent and next-day levels of loneliness, hostility, and social functioning (Study 2). This third aim was included because between-person associations do not necessarily correspond to how people cope with increases in pain relative to their own baseline levels [29]. It was predicted that pain intensity and frequency would each be associated with higher loneliness, higher hostility, and worse social functioning cross-sectionally and longitudinally. It was also predicted that greater within-person deviations in pain would predict worse social outcomes on the same day and on the following day.
Study 1
Study 1 examined cross-sectional and longitudinal relationships between pain and loneliness, hostility, and social functioning over a period of 6 to 53 months.
Methods
Participants
The current study is a secondary analysis of data from a larger study of resilience in mid-life. An a priori power analysis was conducted for the parent study; for the current study, a post hoc power analysis using GPower revealed sufficient power to detect small effects in longitudinal analysis (r ≤ .12) and in correlational analyses (r ≤ .10).
Participants were 741 community-dwelling adults living in the Phoenix, Arizona metropolitan area who completed a baseline visit (described below). Age, gender, education, and relationship status of the sample are provided in Table 1. Ethnic composition of the total sample was 73.6% White, 3.1% African American, 2.9% Asian American, American Indian, or Alaska Native, 12% more than one race, and 8.4% missing.
Table 1.
Demographic Characteristic of Study Samples at Baseline
| Study 1 | Study 2 | |||
|---|---|---|---|---|
|
| ||||
| Full Sample (N = 741) |
Frequent Pain Subsample1 (n = 312) |
Follow-up Subsample (n = 549) |
Daily Diary Subsample (n = 69) |
|
|
| ||||
| % Female | 54.5 | 58.8 | 54.9 | 65.2 |
| Mean Age (SD) | 53.73 (7.25) | 54.05 (6.91) | 54.15 (7.29) | 54.23 (6.53) |
| Pain Intensity (SD) | 2.62 (1.39) | 3.64 (1.17) | 2.64 (1.38) | 3.74 (1.04) |
| Pain Frequency in days/month (SD) | 9.19 (12.22) | 21.07 (10.51) | 9.11 (12.16) | 20.75 (10.42) |
| Loneliness (SD) | 0.90 (1.20) | 1.13 (1.33) | 0.87 (1.18) | 1.00 (1.31) |
| Hostility (SD) | 1.35 (0.67) | 1.38 (0.73) | 1.32 (0.63) | 1.29 (0.57) |
| Social Functioning (SD) | 82.73 (24.60) | 74.40 (28.41) | 83.27 (24.61) | 75.54 (29.03) |
| Education | ||||
| Less than high school | 6.6% | 5.5% | 4.6% | 8.9% |
| High school or GED | 16.4% | 18.1% | 7.9% | 5.9% |
| Some college | 27.3% | 33.1% | 35.2% | 35.3% |
| Graduated college | 30.7% | 27.6% | 32.6% | 35.2% |
| Professional Degree | 18.1% | 15.6% | 19.7% | 14.7% |
| Relationship Status | ||||
| Married | 50.8% | 48.2% | 50.7% | 47.1% |
| Divorced or separated | 23.2% | 26.9% | 24.0% | 26.5% |
| Unmarried but in a committed relationship | 12.7% | 13.1% | 11.9% | 13.1% |
| Single and never married | 7.3% | 7.5% | 8.0% | 5.9% |
| Widowed | 5.3% | 4.3% | 5.4% | 7.4% |
Note: BL = Baseline, FU = Follow-up, SD = Standard Deviation.
Frequent pain subsample only includes participants who scored “1” on the dichotomous pain frequency variable (i.e., pain “every week,” “several times a week,” or “daily”).
Of the total sample, 549 participants also completed a follow-up phone interview over the phone approximately 6–53 months following their baseline visit. Table 1 provides demographic characteristics of the follow-up sample at baseline. Those who completed the follow-up data were slightly older than those who did not (M = 54.15 vs. 52.51 respectively, t(738) = 2.71, Cohen’s d = 0.23, p = .007), but did not differ on baseline pain intensity (t(739) = 0.66, d = 0.06, p = .51), pain frequency (t(739) = −0.31, d = 0.02, p = .76), loneliness (t(739) = 1.33, d = 0.10, p = .19), hostility (t(738) = 1.70, d = 0.13, p = .12), or social functioning (t(739) = 1.01. d = 0.08, p = .31).
Procedure
All procedures were approved by the Arizona State University Institutional Review Board, and all participants provided informed consent. Participants were required to be 40 to 65 years of age, English- or Spanish-speaking, and primarily residing in the Phoenix metropolitan area. Because the parent study aimed to examine the influence of community characteristics on resilience, purposive sampling was employed to ensure sampling from diverse neighborhoods. Upon study enrollment, participants completed questionnaires and a phone interview to assess pain and social wellbeing. Participants were compensated for agreeing to participate in the study ($10), completing questionnaires ($30), and completing the phone interview ($30). Data were collected from July 2007 to February 2012.
Over the course of the study, the follow-up interview was added to track social functioning over time. Consequently, the length of time from completion of the original study to the follow-up phone assessment varied substantially (M = 20 months, Median = 11 months; Observed range = 6–53 months). All participants who indicated interest in participating in future studies were contacted for this follow-up assessment. Of the 741 original participants, 192 declined to provide follow-up data (see Participants section). Individuals received $30 for participation in the follow-up phone assessment. Follow-up data were collected between March and December 2012.
Measures
Pain Location.
At baseline, participants reported the extent to which they experienced pain over the past four weeks in 22 distinct body locations on a 0 = “none” to 3 = “severe” scale. Mean pain score for each of the 22 body sites were computed for descriptive purposes only.
Pain Frequency.
At baseline, participants reported how often during the past four weeks they experienced physical pain or discomfort lasting for more than a few minutes. Item responses were: “never” (32.3% of the sample), “less than once a month” (7.0%), “once a month” (9.0%), “twice a month” (9.6%), “every week” (8.2%), “several times a week” (10.8%), and “daily” (23.1%). This ordinal variable, with unequal intervals between any two consecutive units on the scale (i.e., the difference between “never and “less than once a month” was unequal to the difference between “several times a week” and “daily”) was converted into a continuous variable by assigning numerical values representing number of days of pain as follows: “never” = 0 days; “less than once a month” = 0.5 days; “once a month” = 1 day; “twice a month” = 2 days; “every week” = 4 days; “several times a week” = 15 days; and “daily” = 30 days.
Additionally, a dichotomous pain frequency variable was created to differentiate those with frequent pain (“every week,” “several times a week,” and “daily”; n = 312; coded 1) from those with infrequent or no pain (“twice a month” or less; coded 0), as those with pain ≥ 2 days per week are more likely to have negative pain-related impact in daily activities than those with less frequent pain [30].
Pain Intensity.
A single item from the SF-36 was used at baseline and follow-up to assess pain intensity [25]. The item asked participants to rate the severity of their pain over the past four weeks using a scale of 1 = “none” to 6 = “severe.” The single item was chosen over the bodily pain subscale because the latter confounds pain intensity and pain interference.
Loneliness.
Loneliness was assessed at baseline and follow-up using a single item that asked participants to report “how much during the past four weeks have you felt lonely?” using a scale ranging from 1 = “all of the time” to 6 = “none of the time.” The item was reverse-scored so that higher values indicated more loneliness.
Hostility.
Hostility was assessed at baseline and follow-up using a single item from the Positive and Negative Affect Schedule [PANAS; 31]. Participants reported how much they felt “hostile” during the past 7 days using a scale from 1 = “very slightly/not at all” to 5 = “extremely.” Single-item hostility measures have previously been used to predict pain outcomes [23].
Social Functioning.
The SF-36 social functioning subscale was used at baseline and follow-up to assess social functioning [25]. The subscale consisted of two items that assessed how much physical or emotional problems interfered with social activities. Scores ranged from 0 – 100 and were scored such that higher scores indicated better social functioning. Reliability for the social functioning subscale in the current sample was α = .85 at baseline and α = .87 at follow-up.
Data Analysis
Prior to data analysis, potential outliers on all study variables were flagged using a criterion of +/− 4 SD [32]. Three outliers were found for the baseline hostility variable; no outliers were found for other variables. Exclusion of outliers did not substantively change results, and outliers represented plausible values of hostility, so subsequent models include all data points. Missing data analysis revealed that one participant did not provide baseline or follow-up hostility data; all other variables had complete data available. Descriptive statistics and bivariate correlations were computed for all variables.
The first aim of Study 1 was to test whether pain intensity or frequency were cross-sectionally associated with components of social wellbeing. To do this, pain intensity was used as the single explanatory variable for loneliness, hostility, and social functioning in separate linear regressions. Pain intensity models were run twice; once with the full sample and a second time only including participants with frequent pain (i.e., 1 on the dichotomous pain frequency variable). Models were then repeated with continuous pain frequency as the single explanatory variable, and independent samples t-tests were used to compare components of social wellbeing between those with frequent pain and those without.
The second aim of Study 1 was to examine whether pain intensity or frequency were longitudinally associated with components of social wellbeing. Using hierarchical regression, the first step contained the social variable at baseline as the single explanatory variable of the social variable at follow-up. The second step added pain intensity at baseline. This two-step approach was used to compute the additional variance explained by adding the pain variable. Separate pain intensity models were run for the full sample and for the frequent pain subsample. Models were repeated with continuous pain frequency as the explanatory variable and again with dichotomous pain frequency as the explanatory variable. Time between baseline and follow-up was entered as a covariate in all models. Its inclusion did not substantively change any results so only unadjusted models are presented below. Analyses were conducted using SPSS version 22 (IBM Corp., 2015).
Results
Descriptive Pain Outcomes
Table 2 describes the sites of pain and average pain per site of the sample. The most frequently endorsed pain sites were lower back, neck, and knees. The sites with the highest pain intensity were ankles, buttocks, and hips. One-hundred-seventy-nine (24.2%) participants did not report pain at any site, 83 (11.2%) reported pain at one site, 93 (12.6%) reported pain at two sites, and the remainder (52%) reported pain at three or more sites.
Table 2.
Pain Sites of the Full Sample
| Area | n (%) of Full Sample Endorsing Pain in Area | Mean Pain of Those who Endorsed Pain in Area (SD, Observed Range) |
|---|---|---|
|
| ||
| Neck | 232 (31.3) | 1.66 (0.71, 1–3) |
| Chest | 43 (6.6) | 1.58 (0.72, 1–3) |
| Upper back | 150 (20.4) | 1.73 (0.72, 1–3) |
| Lower back | 318 (42.9) | 1.74 (0.75, 1–3) |
| Buttocks | 51 (6.9) | 1.88 (0.76, 1–3) |
| Abdomen/stomach | 72 (9.7) | 1.72 (0.77, 1–3) |
| Right shoulder | 122 (16.6) | 1.66 (0.72, 1–3) |
| Left shoulder | 121 (16.3) | 1.61 (0.71, 1–3) |
| Right upper arm | 54 (7.6) | 1.70 (0.78, 1–3) |
| Left upper arm | 46 (6.5) | 1.74 (0.79. 1–3) |
| Right elbow | 58 (7.8) | 1.69 (0.70, 1–3) |
| Left elbow | 49 (6.6) | 1.59 (0.75, 1–3) |
| Right hand | 94 (12.7) | 1.71 (0.72, 1–3) |
| Left hand | 89 (12.1) | 1.67 (0.68, 1–3) |
| Right hip | 99 (13.5) | 1.82 (0.69, 1–3) |
| Left hip | 102 (14.0) | 1.82 (0.83, 1–3) |
| Right knee | 168 (22.8) | 0.38 (0.82, 1–3) |
| Left knee | 164 (22.1) | 0.37 (0.78, 1–3) |
| Right ankle | 63 (8.5) | 1.69 (0.75, 1–3) |
| Left ankle | 58 (8.0) | 1.69 (0.73, 1–3) |
| Right foot | 72 (10.0) | 1.76 (0.74, 1–3) |
| Left foot | 75 (10.1) | 1.81 (0.79, 1–3) |
Descriptive Statistics and Cross-Sectional Associations
Tables 1 and 3 provide descriptive statistics, bivariate correlations, and test-retest correlations among study variables for the full sample and frequent pain subsample. Cross-sectionally, pain intensity was associated with higher loneliness (β = 0.16, t(740) = 4.35, p <.001, adjusted R2 = .03), higher hostility (β = 0.11, t(739) = 2.98, p = .003, adjusted R2 = .011), and worse social functioning (β = −0.40, t(740) = −11.68, p <.001, adjusted R2 = .16) in the full sample. Similar results were observed for pain intensity predicting higher loneliness (β = 0.11, t(311) = 2.01, p = .045, adjusted R2 = .01), hostility (β = 0.13, t(311) = 2.29, p = .02, adjusted R2 = .01), and worse social functioning (β = −0.41, t(311) = −7.90, p <.001, adjusted R2 = .17) in the subsample with frequent pain.
Table 3.
Bivariate Correlations among Study Variables in the Full Sample and Frequent Pain Subsample (Shown in Parentheses)
| 1 | 2a | 2b | 3a | 3b | 4a | 4b | 5a | 5b | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1. BL Pain Frequency-Continuous | 1 | .63* (.31*) | .48* (.27*) | .17* (.08) | .18* (.18*) | .07 (.08) | .12* (.01) | −.34* (−.23*) | −.36* (−.27*) |
| 2a. BL Pain Intensity | 1 | .52* (.48*) | .16* (.11^) | .17* (.14^) | .11* (.13^) | .22* (.22*) | −.40* (−.41*) | −.37* (−.41*) | |
| 2b. FU Pain Intensity | 1 | .25* (.29*) | .28* (.33*) | .11* (.17^) | .23* (.28*) | −.44* (−.44*) | −.57* (−.61*) | ||
| 3a. BL Loneliness | 1 | .58* (.61*) | .26* (.25*) | .21* (.30*) | −.42* (−.41*) | −.31* (−.32*) | |||
| 3b. FU Loneliness | 1 | .20* (.22*) | .24* (.32*) | −.33* (−.31*) | −.44* (−.49*) | ||||
| 4a. BL Hostility | 1 | .36* (.31*) | −.23* (−.25*) | −.13* (−.16^) | |||||
| 4b. FU Hostility | 1 | −.22* (−.25*) | −.28* (−.33*) | ||||||
| 5a. BL Social Functioning | 1 | .51* (.49*) | |||||||
| 5b. FU Social Functioning | 1 | ||||||||
|
| |||||||||
| n | - | - | 549 | - | 547 | - | 548 | - | 549 |
| Mean | - | - | 2.77 | - | 0.86 | - | 1.33 | - | 82.47 |
| SD | - | - | 1.36 | - | 1.17 | - | 0.67 | - | 25.33 |
Note: Values in parenthesis are from the frequent pain subsample.
= significant at the .05 level (2-tailed)
= significant at the .01 level (2-tailed), BL = Baseline, FU = Follow-up, SD = Standard Deviation. N, mean, and SD are presented only for the full sample at follow up. These values for the full sample at baseline are found in Table 1
Pain frequency (measured continuously) was also associated with higher loneliness (β = 0.17, t(740) = 4.60, p <.001, adjusted R2 = .027) and worse social functioning (β = −0.34, t(740) = −9.78, p <.001, adjusted R2 = .11), but not higher hostility (β = 0.068, t(739) = 1.85, p = .065, adjusted R2 =.003). Similarly, compared to participants without frequent pain, those with frequent pain reported higher loneliness (t(739) = −4.39, p < .001, Cohen’s d = 0.32) and worse social functioning (t(739) = 8.20, p < .001, Cohen’s d = 0.59), but did not report higher hostility (t(739) = −1.16, p = .23, Cohen’s d = 0.09).
Longitudinal Associations of Pain Intensity and Frequency with Components of Social Wellbeing
Pain intensity at baseline was significantly associated with higher hostility and worse social functioning at follow-up after controlling for baseline hostility and social functioning (see Table 4). The effects of pain intensity on change in loneliness were small (ΔR2 = .003) and not likely to be of clinical significance, as the unstandardized betas revealed that a for every one-point increase in pain intensity, loneliness at follow-up increased by only .06 points. Similar results were found in the subsample with frequent pain.
Table 4.
Longitudinal Associations of Pain at Baseline (BL) Predicting Social Outcomes at Follow-up in the Full and Frequent Pain Samples
| Outcome | Sample | Predictor | β | t | p | Adj. R2 | Δ R2 | Model F (df) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Follow-up Loneliness | Full Sample | BL Loneliness | 0.57 | 15.94 | <.001 | .331 | - | - |
| BL Pain Intensity | 0.067 | 1.88 | .061 | .334 | .003 | 138.22 (2, 546) | ||
|
|
||||||||
| BL Loneliness | 0.56 | 15.90 | <.001 | .331 | - | - | ||
| BL Pain Freq- Cont. | 0.079 | 2.25 | .025 | .336 | .005 | 139.36 (2, 546) | ||
|
|
||||||||
| BL Loneliness | 0.57 | 16.08 | <.001 | .330 | - | - | ||
| BL Pain Freq- Dichot. | 0.032 | 0.91 | .36 | .331 | .001 | 136.20 (2, 546) | ||
|
|
||||||||
| Frequent Pain1 | BL Loneliness | 0.60 | 11.30 | <.001 | .364 | - | - | |
| BL Pain Intensity | 0.053 | 0.99 | .32 | .367 | .003 | 67.48 (2, 229) | ||
|
|
||||||||
| Follow-up Hostility | Full Sample | BL Hostility | 0.34 | 8.60 | <.001 | .126 | - | - |
| BL Pain Intensity | 0.19 | 4.73 | <.001 | .159 | .030 | 52.42 (2, 546) | ||
|
|
||||||||
| BL Hostility | 0.35 | 8.73 | <.001 | .126 | - | - | ||
| BL Pain Freq- Cont. | 0.093 | 2.32 | .021 | .133 | .007 | 42.70 (2, 546) | ||
|
|
||||||||
| BL Hostility | 0.35 | 8.84 | <.001 | .124 | - | - | ||
| BL Pain Freq- Dichot. | 0.12 | 3.04 | .002 | .139 | .015 | 44.92 (2, 546) | ||
|
|
||||||||
| Frequent Pain1 | BL Hostility | 0.29 | 4.61 | <.001 | .094 | - | - | |
| BL Pain Intensity | 0.18 | 2.92 | .004 | .123 | .033 | 16.92 (2, 228) | ||
|
|
||||||||
| Follow-up Soc. Funct. | Full Sample | BL Social Functioning | 0.43 | 10.69 | <.001 | .255 | - | - |
| BL Pain Intensity | -0.20 | -4.93 | <.001 | .285 | .030 | 110.46 (2, 548) | ||
|
|
||||||||
| BL Social Functioning | 0.43 | 11.35 | <.001 | .255 | - | - | ||
| BL Pain Freq- Cont. | -0.21 | -5.58 | <.001 | .294 | .039 | 115.07 (2, 548) | ||
|
|
||||||||
| BL Social Functioning | 0.46 | 12.15 | <.001 | .254 | - | - | ||
| BL Pain Freq- Dichot. | -0.17 | -4.45 | <.001 | .280 | .026 | 107.44 (2, 548) | ||
|
|
||||||||
| Frequent Pain1 | BL Social Functioning | 0.39 | 5.99 | <.001 | .238 | - | - | |
| BL Pain Intensity | -0.22 | -3.35 | .001 | .273 | .035 | 44.27 (2, 230) | ||
Notes: BL loneliness, BL hostility, and BL social functioning were entered in step 1 of all 12 models. Pain intensity or frequency variables were entered in Step 2 to examine the change in adjusted R squared from addition of the pain variable. All 12 models were run separately.
Abbreviations: BL = baseline; Soc. Funct. = social functioning; Freq- Cont. = Pain frequency using the continuous variable; Freq- Dichot. = pain frequency using the dichotomous variable ( 0 = not frequent pain; 1 = frequent pain).
Frequent pain subsample only includes participants who scored “1” on the dichotomous pain frequency variable (i.e., pain “every week,” “several times a week,” or “daily”).
Similarly, the continuous baseline pain frequency variable predicted worse social functioning at follow-up, even after controlling for baseline levels of social functioning. Effects were statistically significant but of limited clinical significance for pain frequency predicting future loneliness or hostility (see Tables 4 and 5), as unstandardized betas revealed that for every one-day increase in pain frequency, loneliness at follow-up increased by 0.008 points and hostility at follow-up increased by 0.005 points. Similar results were obtained when the dichotomous pain frequency variable was used as a predictor with one exception: the relationship between pain frequency and hostility was slightly stronger.
Table 5.
Summary Results of Studies
| Evidence for Relationship?* | ||||
|---|---|---|---|---|
|
| ||||
| Cross-sectional | Predictor: | Outcome: | ||
|
| ||||
| Loneliness | Hostility | Social Functioning | ||
|
| ||||
| Pain Intensity | Yes | Yes | Yes | |
|
| ||||
| Pain Frequency-Continuous | Yes | No | Yes | |
|
| ||||
| Pain Frequency-Dichotomous | Yes | No | Yes | |
|
| ||||
| Longitudinal | Pain Intensity | Negligible | Yes | Yes |
|
| ||||
| Pain Frequency | Negligible | Negligible | Yes | |
|
| ||||
| Pain Frequency-Dichotomous | No | Yes | Yes | |
|
| ||||
| Within-Person; Same Day | Pain Intensity | No | Yes | Yes |
|
| ||||
| Average Pain by Daily Pain Interaction | Yes | No | No | |
|
| ||||
| Within-Person; Lagged | Pain Intensity | No | No | No |
Note:
Evidence for relationship means that predictor explained 1% or more of variance in the outcome, and that the statistical inferential test used to model the relationship was statistically significant. Negligible relationships are those in which the predictor explained less than 1% of variance in the outcome, although the test was statistically significant. No relationship means that the test was not statistically significant. Results from all tests are presented in the results section.
Discussion
The cross-sectional associations between pain intensity and loneliness, hostility, and social functioning found in Study 1 replicate associations found in chronic pain samples [8, 9, 13, 19, 21, 23–24]. The magnitude of the associations varied widely, with pain intensity accounting for 1% to 16% of variance in components of social wellbeing. The largest association, between pain intensity and social functioning, suggested that as pain becomes more intense, it may disrupt people’s ability to maintain their usual engagement in social and recreational activities. Alternatively, changes in social and recreational activities may make pain more intense. The magnitude of the cross-sectional association between pain intensity and social functioning in Study 1 is similar to what has previously been found between pain intensity and physical health (r = −.55, [33]), pain-related interference with daily activities (r = .55 [32]), and pain-related disability (r = .35; [34]) in patients with chronic pain, suggesting that pain intensity may be similarly associated with social and physical dysfunction.
Surprisingly, pain intensity was similarly associated with components of social wellbeing in the full sample as in the subsample with frequent pain, even though the subsample with frequent pain reported higher pain intensity, loneliness, and social functioning than the full sample. This result stands in contrast to findings that suggest loneliness is cross-sectionally associated with greater pain intensity and disability in older adults with acute but not with chronic pain [16]. This discrepancy may be related to differences in sample characteristics. The prior study’s sample was composed of older adults, whereas the current study’s sample was composed of middle-aged adults (age 40–65 at baseline), suggesting that the relationship between loneliness and pain intensity may become stronger as people age. Second, the presence of chronic pain could not be confirmed in the current sample. Although pain chronicity is associated with a higher number of pain sites and more frequent pain [30], it is likely that the frequent pain subsample was relatively high functioning given that they were recruited from the community and were willing to participate in research.
Longitudinally, pain intensity at baseline predicted hostility and social functioning (but not loneliness) after controlling for baseline levels of these components. The magnitude of these associations was small, with most of the variance in the social components at follow-up being explained by baseline levels. In light of extant literature these small effects suggest that increases in pain intensity may contribute to hostility and social dysfunction, but given the extant literature the directionality appears to be stronger for hostility and social dysfunction contributing to increases in pain intensity. This is consistent with prior work finding that social isolation predicted pain interference but that pain interference did not predict social isolation in a large sample of patients with persistent pain [18]. The small longitudinal effects in the current study may also be related to the nature of the community-dwelling sample. Stronger effects might be seen in clinical samples solely comprised of individuals with chronic pain. The small effects also suggest the presence of possible moderating influences. Pain intensity has been linked with negative mood, depression, fatigue, and anger, among other variables – each of which may serve to moderate the longitudinal relationships between pain intensity and social wellbeing [35–37]. The potential moderating influences of these variables should be tested in future research.
Longitudinally, pain frequency and pain intensity were similarly associated with components of social wellbeing, with one difference: an association between pain frequency and hostility was not found when pain frequency was measured continuously and was very small when pain frequency was measured dichotomously. Frequent pain may not make people hostile as much as it may cause them to disengage from social environments and activities.
Cross-sectional effect sizes for pain frequency were similar to those of pain intensity, with frequency accounting for 3% to 11% of the variance in components of social wellbeing. The similarity in the magnitudes of correlations of pain frequency and intensity with components of social wellbeing is particularly surprising given that intensity and frequency were only moderately correlated with one another (r = .63). The findings suggest that pain intensity and frequency each explain unique variance (albeit in small amounts) in social wellbeing.
Similar to pain intensity, pain frequency prospectively accounted for worse social functioning. In chronic pain samples, there is a restriction of range in pain frequency, as patients often have constant pain. In community samples with varying degrees of pain, how often one has pain matters. Frequently-experienced pain may be more damaging to social functioning in the long term than infrequently-experienced pain. The mechanisms by which pain frequency interferes with future social functioning merit further exploration. People who experience frequent pain may limit social activities for fear that another painful episode might occur [38]. Alternatively, frequent pain may be perceived as uncontrollable and may negatively influence social activities. A third possibility is that people with frequent pain may have higher disability, fatigue, or poorer sleep, impairing their social functioning over time [21, 27, 32]. These possibilities are not mutually exclusive, and other mechanisms may also exist.
Several findings in the longitudinal analysis of the current study were surprising. First, controlling for days between the initial appointment and follow-up did not influence the results; if indeed components of social wellbeing become progressively worse over time as a function of pain, time should have been associated with worse social outcomes. One possibility is that components of social wellbeing change after 1 to 12 months following pain onset and then plateau, and that our follow-up window (M = 20 months after the initial appointment; SD = 11 months) was unable to capture this change in slope. Additionally, participants in the current study were not recruited at pain onset, so it may be that the effects of pain on social wellbeing were already established by the time they were recruited. Longitudinal data with more consistently-spaced follow-up assessments and with the ability to track people in the transition from acute to chronic pain are needed to understand how components of social wellbeing change as a function of pain.
Study 1 has important limitations. Cross-sectional analysis cannot be used to speculate about causal processes. The sample in Study 1 was highly educated and only included people in mid-life (40–65); as such, results may not generalize to all adults in the U.S population. The follow-up data in Study 1 did not assess pain, so the influence of components of wellbeing on future pain could not be examined.
Another significant limitation in Study 1 is that the results were based on retrospective aggregates. Whereas aggregates are useful in describing how people generally respond to pain, they do not describe how people respond to pain that differs from their own usual level of pain [39]. For a person who on average experiences pain intensity of 8 out of 10, that level of pain may not interfere with daily activities. In another person who typically experiences no pain, pain intensity of 8 out of 10 would likely be distressing and disruptive. Thus, examinations of within-person fluctuations in pain (rather than aggregate levels) are required to examine how changes in pain predict social wellbeing.
Study 2: Within-Person Associations
Study 2 used a subset of participants from Study 1 to examine within-person fluctuations in pain intensity. Relationships at the between-person level do not necessarily correspond to the within-person level. For instance, in community-dwelling older adults, within-person fluctuations in pain interact with fluctuations in executive functioning to predict future health, but between-person levels of pain and executive functioning do not [39]. Studying within-person associations is important because they provide insight into how people respond to changes in pain relative to their own usual levels. Study 2 sought to examine how between-person means and within-person deviations in pain intensity predicted loneliness, hostility, and social functioning on both the same day and the next day using daily diary data across 30 days. It was hypothesized that on days when pain was higher than usual, participants would report worse social wellbeing on the same day and on the following day. Reverse-directionality and cross-level interactions were also examined in Study 2 in exploratory analysis.
Method
Participants
One quarter of the Study 1 sample (n = 200) was randomly selected to participate in a study of daily life. Of these participants, 184 provided daily diary data, and 69 reported having recurrent pain. The sample for Study 2 consisted of these 69 participants. Table 1 provides demographic characteristics of Study 2 participants at baseline. The Study 2 sample reported significantly worse baseline social functioning (t(182) = 3.58, d = 0.51, p = .001) and marginally more baseline loneliness (t(182) = 1.93, d = 0.28, p = .055) than the remainder of the daily diary sample who did not report recurrent pain. The two subsamples did not differ on baseline hostility (t(182) = 0.15, d = 0.019, p = .88). Completion rate of the diary dataset was 79.1%.
Procedure
Participants completed daily diaries on a tablet computer for 30 consecutive days. They were trained to use the equipment and were instructed to notify staff immediately if a problem occurred; malfunctioning equipment was replaced as needed. The dates of each entry were verified with built-in software to ensure entries were not retrospectively entered. After the 30-day period, participants were compensated $3 for every entry for up to $90 in total.
Measures
Pain.
Each night, participants reported “what number between 0 and 100 best describes [his/her] average level of physical pain,” with anchors of 0 = “no pain” to 100 = “pain as bad as it can be”. The intraclass correlation (ICC) for pain across all days was .65, indicating that about 2/3 of the variance in pain was stable individual differences, and 1/3 was fluctuations within people over time.
Loneliness.
Each night, participants reported the extent to which they felt lonely that day using a single-item scale, with response options ranging from 1 = “not at all” to 5 = “very much”. The ICC for loneliness across all days was .64.
Hostility.
Each night, participants reported how much they felt “hostile” that day on a single-item scale from ranging from 1 = “very slightly/not at all” to 5 = “extremely”. The ICC for hostility across all days was .55.
Social Functioning.
Each night, participants reported “to what extent have [their] physical health or emotional problems interfered with [their] normal social activities with family, friends, neighbors, or groups” with anchors of 1 = “not at all” to 5 = “extremely”. The variable was reverse-coded so that higher scores represent better functioning. The ICC for social functioning across all days was .56.
Data Analysis
All analyses used the linear mixed modeling package LME4 in R, and r-squared estimates were computed using the r2glmm package. This analytic approach controls for clustering of observations in hierarchical datasets (e.g., daily diaries), thereby allowing for analysis of daily-level variables without biasing p values. For all analyses, daily predictors were centered using the cluster (person) mean and person-level predictors (e.g., average levels of daily pain aggregated across the 30-day period) were centered on the grand mean. For daily variables, a value of 0 indicated a typical level of pain, positive numbers indicated more pain than usual for that person, and negative numbers indicated less pain than usual for that person. These centered predictors were entered as simultaneous predictors of loneliness, hostility, and social function on both the same day (equation 1) and on the following day, while controlling for the current day’s pain (equation 2), while also controlling for average levels of pain intensity across the 30-day period (a person-level variable):
| (1) |
| (2) |
Random slopes were estimated for the effects of daily pain on each outcome (allowing variability between individuals in estimating the effects of daily pain), along with random intercepts, as likelihood ratio tests revealed that random effects models were a better fit for the data than fixed effects models. Cross-level interaction terms were estimated between average pain levels over the 30-day period with daily pain levels to determine whether these daily relationships varied significantly between participants who reported different levels of pain intensity on average across the 30-day period (equation 3) [40].
| (3) |
In cases of significant interaction terms, simple slopes were estimated for grand mean (level-2) scores in 30-day average pain for the entire sample, as well as average daily pain scores 1 standard deviation above and below the grand mean.
As a secondary step, and as a means of comparing our results to the results of Study 1, we also examined the relationships between these variables at the individual mean level using aggregated daily variables for each participant (equation 4):
| (4) |
For significant daily effects, r2 variance estimates were computed to address the relative size of each estimated effect [41].
Results
Results of the hierarchical models revealed that there were no lagged associations between pain intensity on one day and ratings of loneliness, hostility, or overall social functioning on the next day (p > .05 in all cases). When these paths were reversed, no social variable was found to predict pain intensity on the next day (p > .05 in all cases).
However, same-day associations revealed that on days when pain was higher than people’s personal average, participants reported higher hostility (γ = .003, SE = .001, p = .007, r2 = .005) and poorer levels of social functioning (γ = −.015, SE = .002, p < .001, r2 = .068), but not loneliness (p = .23, see Table 6). Regarding the effects of aggregated (i.e., between-person) scores, individuals with higher average levels of daily pain intensity reported worse social functioning (γ = .031, SE = .003, p < .001, r2 = .35) and higher average levels of hostility (γ = .006, SE = .003, p = .039, r2 = .033), but pain intensity was not significantly related to average levels of loneliness (γ = .008, SE = .005, p = .087, r2 = .025).
Table 6.
Summary Table of Same-Day Associations between Pain Intensity, Loneliness, Hostility, and Social Functioning
| Loneliness | Hostility | Social Function | |
|---|---|---|---|
|
| |||
| Level 1 | |||
| Intercept | 1.62 (.104)** | 1.21 (.062)** | 1.76 (.065)** |
| Daily Pain Intensity | .003 (.002) | .003 (.001)* | .016 (.002)** |
| Level 2 | |||
| Average Pain Intensity | .009 (.005) | .006 (.002)* | .031 (.003)** |
| Interaction | −.0002 (.00008)* | .00001 (.00006) | .000 (.000) |
| Variance Components | |||
| Level-1 Slope | .00004 | .00003 | .0001 |
| Level-2 Intercept | .713 | .249 | .251 |
| Residual | .390 | .190 | .494 |
| Number of Parameters | 8 | 8 | 8 |
| R2 | .034 | .039 | .393 |
| −2 Log Likelihood | −1640.5 | −1068.7 | −1781.2 |
Note:
= p < .01
= p < .05
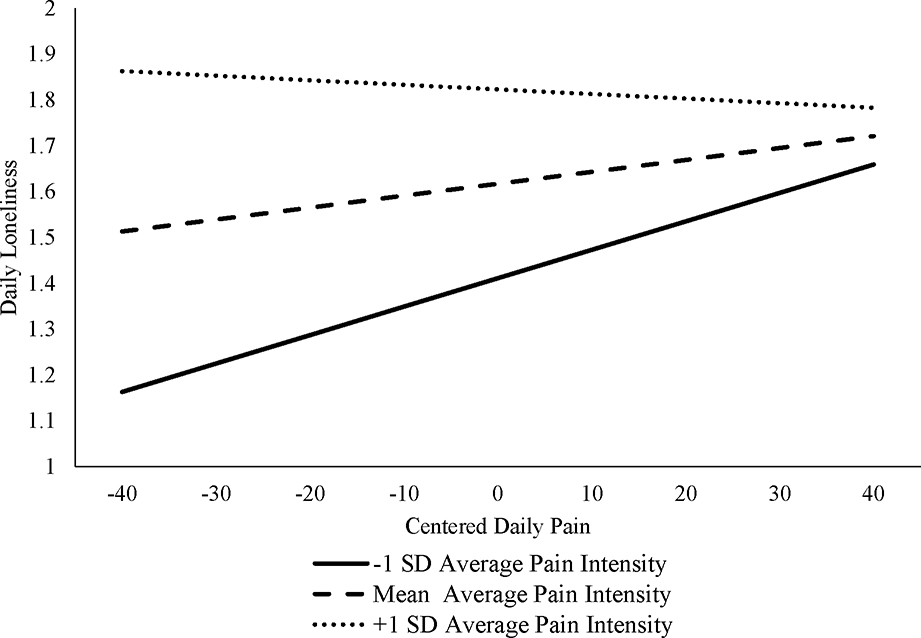
Of note, a significant average pain-by-daily pain interaction was found for daily levels of loneliness (interaction γ = −.0002, SE = .00007, p = .047), suggesting that the magnitude of the relationship between daily pain intensity and loneliness decreased as average pain increased. The relationship of daily pain and daily loneliness was strongest at lower levels of average pain (see Figure 1). The interactions of daily and average pain levels were not significant for hostility (interaction γ = .00001, SE = .00005, p = .81) or social functioning (interaction γ = .0002, SE = .00001, p = .056).
Figure 1:
Interactions of Average Pain by Daily Pain Predicting Daily Loneliness
Discussion
Within-person associations allow for the examination of how people respond to pain when it is higher or lower than their usual levels. When daily pain was higher than usual, people reported being more hostile and having worse social functioning. Exploratory analysis revealed that for loneliness, daily pain interacted with average pain such that the relationship of daily pain and daily loneliness was strongest at lower levels of average pain (see Figure 1). For those with higher average pain intensity, there appeared to be a ceiling effect on loneliness. This complements results from Study 1 and previous work that finds loneliness to be cross-sectionally associated with greater pain intensity and disability in those with acute but not with chronic pain [16]. In cross-sectional studies pain intensity may be unrelated to loneliness because those with chronic pain are already at high levels of loneliness. These results suggest that interventions aimed at promoting social wellbeing should target individuals before changes in loneliness have taken place. The effectiveness of such early interventions presents an important area for future research.
Importantly, there was no evidence of lagged effects; that is, pain intensity did not appear to affect levels of social wellbeing, nor did these relationships emerge when the models were tested in reverse. The lack of support for lagged effects suggests that pain-related disruption in components of social wellbeing may be brief, lasting only for perhaps minutes or hours but not days. Alternatively, the effects of pain intensity on components of social wellbeing from one day to the next may be insignificant in and of themselves but may, over time, accumulate to produce significant changes. The longitudinal analyses in Study 1 provide only partial support for this hypothesis, as longitudinal effects were found for social functioning but not for loneliness or hostility. These findings suggest that pain may change how people function in their social networks before it changes perceptions of interpersonal functioning. Future work should continue to explore the time course over which pain may (or may not) shape individual components of social wellbeing.
Study 2 had several important limitations that should be noted. First, daily diaries were only examined in the frequent pain subsample and as such the within-person associations between pain frequency and the components of social wellbeing were not examined. Second, the daily diaries utilized single-item measures to assess the constructs of interest. Third, because pain chronicity was not directly assessed or verified, results cannot be expected to generalize to chronic pain samples. Despite these shortcomings, there are notable aspects of study 2 that add to the literature. Results highlight the importance of assessing within-person deviations in pain and average levels of pain, as each may uniquely contribute to components of social wellbeing at the moment. Additional strengths of Study 2 include the relatively long assessment period (30 days of data) and the ability to test cross-level interactions between daily and average levels of pain.
General Discussion
Higher pain intensity was associated with worse social wellbeing in both cross-sectional data and aggregated daily-diary data. However, contrary to the study hypotheses, support for pain intensity influencing later social wellbeing was limited. The longitudinal associations between pain intensity and social wellbeing were small or not statistically significant, and the lagged associations between daily pain intensity and next-day social wellbeing were nonsignificant. Results were largely similar for pain frequency. Overall, comparing results from both of our studies with those in the literature, it appears that that social variables may be stronger predictors of pain than vice-versa in community-dwelling adults, although more work is needed to definitively answer this question in light of the current studies’ limitations.
Nevertheless, the results from both studies contribute to the literature on pain and social wellbeing in several important ways. They are among the first studies to examine the role of pain as a predictor of social wellbeing in community-dwelling adults. The sample was demographically diverse, with good generalizability to middle-aged community-dwelling adults in the general population. Additionally, these were the first studies to examine three social outcomes simultaneously, and the first to examine pain as a longitudinal and within-person predictor of components of social wellbeing. Further, these were the first studies to examine the effects of pain frequency on components of social wellbeing in a community-dwelling sample. Unique relationships between pain intensity and frequency were found for some outcomes while similar relationships were found for others, suggesting that they each should be examined when assessing the interrelationships between pain and social wellbeing.
Compliance with Ethical Standards
This research was funded by the National Institute on Aging of the National Institutes of Health under Award Numbers F31AG048692, R01AG026006, and K02AG033629, and by the National Multiple Sclerosis Society under Award Number MB0026. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Ian Boggero declares that he has no conflict of interest. John Sturgeon declares that he has no conflict of interest. Anne Arewasikporn declares that she has no conflict of interest. Saul Castro declares that he has no conflict of interest. Christopher King declares that he has no conflict of interest. Suzanne Segerstrom declares that she has no conflict of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- 1.Master SL, Eisenberger NI, Taylor SE, Naliboff BD, Shirinyan D, Lieberman MD. A picture’s worth: Partner photographs reduce experimentally induced pain. Psychol Sci. 2009; 20(11): 1316–1318. Doi: 10.1111/j.1467-9280.2009.02444.x [DOI] [PubMed] [Google Scholar]
- 2.Eisenberger NI. Social pain and the brain: Controversies, questions, and where to go from here. Ann Rev Psychol. 2015; 66: 601–629. Doi: 10.1146/annurev-psych-010213-115146 [DOI] [PubMed] [Google Scholar]
- 3.Craig KD. Social communication model of pain. Pain. 2015;156(7):1198–1199. Doi: 10.1097/j.pain.0000000000000185 [DOI] [PubMed] [Google Scholar]
- 4.Sullivan MJ. The communal coping model of pain catastrophising: Clinical and research implications. Canadian Psychol. 2012;53(1): 32–41. Doi: 10.1037/a0026726 [DOI] [Google Scholar]
- 5.Martin LJ, Tuttle AH, Mogil, JS. The interaction between pain and social behavior in humans and rodents. In Behavioral Neurobiology of Chronic Pain, pp. 233–250. Springer, Berlin, Heidelberg, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Brown JL, Sheffield D, Leary MR, Robinson ME. Social support and experimental pain. Psychosom Med. 2003;65(2):276–283. Doi: 10.1097/01.PSY.0000030388.62434.46 [DOI] [PubMed] [Google Scholar]
- 7.Martin LJ, Hathaway G, Isbester K, Mirali S, Acland EL, Niederstrasser N, … Sternberg WF. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Current Biolo. 2015;25(3):326–332. Doi: 10.1016/j.cub.2014.11.028 [DOI] [PubMed] [Google Scholar]
- 8.Stewart AL, Greenfield S, Hays RD, Wells K, Rogers WH, Berry SD, … Ware JE. Functional status and well-being of patients with chronic conditions: results from the Medical Outcomes Study. JAMA. 1989;262(7):907–913. [PubMed] [Google Scholar]
- 9.Forgeron PA, King S, Stinson JN, McGrath PJ, MacDonald AJ, Chambers CT. Social functioning and peer relationships in children and adolescents with chronic pain: A systematic review. Pain Res Manag. 2010; 15(1): 27–41. Doi: 10.1155/2010/820407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golden J, Conroy RM, Bruce I, Denihan A, Greene E, Kirby M, Lawlor BA. Loneliness, social support networks, mood and wellbeing in community-dwelling elderly. Internatl J Geriatric Psychiat. 2009;24(7):694–700. Doi: 10.1002/gps.2181. [DOI] [PubMed] [Google Scholar]
- 11.McDowell TL, Serovich JM. The effect of perceived and actual social support on the mental health of HIV-positive persons. AIDS Care. 2007;19(10):1223–1229. Doi: 10.1080/0954012070140283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peplau LA. Loneliness: A sourcebook of current theory, research, and therapy (Vol. 36). Hoboken, NJ: John Wiley & Sons Inc; 1982. [Google Scholar]
- 13.Wolf LD, Davis MC. Loneliness, daily pain, and perceptions of interpersonal events in adults with fibromyalgia. Health Psycholo. 2014; 33(9): 929–937. Doi: 10.1037/hea0000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emerson K, Boggero I, Ostir G, Jayawardhana J. Pain as a risk factor for loneliness among older adults. J Aging Health. 2017. Doi: 10.1177/0898264317721348 [DOI] [PubMed] [Google Scholar]
- 15.Jacobs JM, Hammerman-Rozenberg R, Cohen A, Stessman J. Chronic back pain among the elderly: prevalence, associations, and predictors. Spine. 2006; 31(7): 203–207. Doi: 10.1097/01.brs.0000206367.57918.3c [DOI] [PubMed] [Google Scholar]
- 16.Hanssen DJ, Naarding P, Collard RM, Comijs HC, Voshaar RCO. Physical, lifestyle, psychological, and social determinants of pain intensity, pain disability, and the number of pain locations in depressed older adults. Pain. 2014;155(10):2088–2096. Doi: 10.1016/j.pain.2014.07.019 [DOI] [PubMed] [Google Scholar]
- 17.Jaremka LM, Fagundes CP, Glaser R, Bennett JM, Malarkey WB, Kiecolt-Glaser JK. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrin. 2013;38(8):1310–1317. Doi: 10.1016/j.psyneuen.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karayannis NV, Baumann I, Sturgeon JA, Melloh M, Mackey SC. The impact of social isolation on pain interference: A longitudinal study. Annals Behav Med. 2019;53,65–74. Doi: 10.1093/abm/kay017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf LD, Davis MC, Yeung EW, Tennen HA. The within-day relation between lonely episodes and subsequent clinical pain in individuals with fibromyalgia: mediating role of pain cognitions. J Psychosomat Res. 2015; 79(3): 202–206. Doi: 10.1016/j.jpsychores.2014.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns JW. Anger management style and hostility: Predicting symptom-specific physiological reactivity among chronic low back pain patients. J Behav Med. 1997;20(6):505–522. [DOI] [PubMed] [Google Scholar]
- 21.Burke AL, Mathias JL, Denson LA. Psychological functioning of people living with chronic pain: A meta-analytic review. Brit J Clin Psychol. 2015; 54(3): 345–360. Doi: 10.1111/bjc.12078 [DOI] [PubMed] [Google Scholar]
- 22.Anderson KB, Anderson CA, Dill KE. Hostility, pain, and aggressive thoughts. Aggressive Behav. 1998; 24: 161–171. [Google Scholar]
- 23.Burns JW, Gerhart JI, Post KM, Smith DA, Porter LS, Schuster E, … Keefe FJ. The communal coping model of pain catastrophizing in daily life: A within-couples daily diary study. J Pain. 2015; 16(11): 1163–1175. Doi: 10.1016/j.jpain.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desrosiers M, Bergeron S, Meana M, Leclerc B, Binik YM, Khalifé S. Psychosexual characteristics of vestibulodynia couples: Partner solicitousness and hostility are associated with pain. J Sex Med. 2008; 5(2): 418–427. Doi: 10.1111/j.1743-6109.2007.00705.x [DOI] [PubMed] [Google Scholar]
- 25.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992; 473–483. [PubMed] [Google Scholar]
- 26.Brazier JE, Harper R, Jones NM, O’cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Brit Med J. 1992; 305(6846): 160–164. Doi: 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osborne TL, Jensen MP, Ehde DM, Hanley MA, Kraft G. (2007). Psychosocial factors associated with pain intensity, pain-related interference, and psychological functioning in persons with multiple sclerosis and pain. Pain. 2007; 127(1): 52–62. Doi: 10.1016/j.pain.2006.07.017 [DOI] [PubMed] [Google Scholar]
- 28.Kashikar-Zuck S, Lynch AM, Graham TB, Swain NF, Mullen SM, Noll RB. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis Care Res. 2007;57(3):474–480. Doi: 10.1002/art.22615 [DOI] [PubMed] [Google Scholar]
- 29.Kievit R, Frankenhuis WE, Waldorp L, Borsboom D. Simpson’s paradox in psychological science: A practical guide. Frontiers Psychol. 2013; 4: 513–527. Doi: 10.3389/fpsyg.2013.00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230–1239. Doi: 10.1016/j.jpain.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 31.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988; 54(6): 1063–1070. Doi: 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- 32.Boggero IA, Rojas-Ramirez MV, Carlson CR. All fatigue is not created equal: The association of fatigue and its subtypes on pain interference in orofacial pain. Clin J Pain. 2017; 33(3): 231–237. Doi: 10.1097/AJP.0000000000000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alschuler KN, Kratz AL, Ehde DM. Resilience and vulnerability in individuals with chronic pain and physical disability. RehabPsychol. 2016;61(1):7–18. Doi: 10.1037/rep0000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimitriadis Z, Kapreli E, Strimpakos N, Oldham J. Do psychological states associate with pain and disability in chronic neck pain patients? J Back Musculoskelet Rehab. 2015;28(4);797–802. Doi: 10.3233/BMR-150587 [DOI] [PubMed] [Google Scholar]
- 35.Boggero IA, Kniffin TC, de Leeuw R., Carlson CR. Fatigue mediates the relationship between pain interference and distress in patients with persistent orofacial pain. J Oral Facial Pain Headache. 2014; 28(1): 38–45. doi: 10.11607/jop.1204 [DOI] [PubMed] [Google Scholar]
- 36.Bruehl S, Liu X, Burns JW, Chont M, Jamison RN. Associations between daily chronic pain intensity, daily anger expression, and trait anger expressiveness: An ecological momentary assessment study. Pain. 2012; 153(12): 2352–2358. Doi: 10.1016/j.pain.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilderink PH, Burger H, Deeg DJ, Beekman AT, Voshaar RCO. The temporal relation between pain and depression: results from the longitudinal aging study Amsterdam. Psychosom Med. 2012; 74(9): 945–951. Doi: 10.1097/PSY.0b013e3182733fdd [DOI] [PubMed] [Google Scholar]
- 38.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000; 85(3): 317–332. Doi: 10.1016/S0304-3959(99)00242-0 [DOI] [PubMed] [Google Scholar]
- 39.Boggero IA, Eisenlohr-Moul T, Segerstrom SC. Task-switching ability protects against the adverse effects of pain on health: A longitudinal study of older adults. Brit J Health Psychol. 2016; 21(2): 434–450. Doi: 10.1111/bjhp.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psycholo Method. 2007; 12: 121–138. Doi: 10.1037/1082-989X.12.2.121 [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed effects models. Methods Ecol Evol. 2013; 4(2), 133–142. Doi: 10.1111/j.2041-210x.2012.00261.x [DOI] [Google Scholar]