Highlights
-
•
Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare but severe dermatologic immune-related adverse events (irAEs) characterized by the separation of the epidermal and dermal layers of the skin.
-
•
We present the case of a 33-year-old African American female with a pertinent past medical history of recurrent progressive metastatic squamous cell carcinoma cervical cancer treated with pembrolizumab presenting with a diffuse hyperpigmented maculopapular rash with blisters and oral/genital mucosal involvement proven by biopsy to be SJS/TEN.
-
•
Diagnosis of SJS/TEN was delayed three days, highlighting the need for all subspecialties to be familiar with this new class of immune-oncologic agents and the unique toxicity profile when compared to more classic chemotherapeutic agents.
-
•
Lastly, lack of educational material on the presentation of SJS/TEN in medium to dark skin tones (Fitzpatrick IV-VI) could have further delayed diagnosis and appropriate treatment of this patient, urging for a need of more diverse dermatologic imagery in the literature.
Keywords: SJS/TEN, irAE, Pembrolizumab, Immune checkpoint inhibitors
Abstract
Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare but severe dermatologic immune-related adverse events (irAEs) characterized by the separation of the epidermal and dermal layers of the skin. Less commonly documented, these adverse events have shown to be secondary to immune checkpoint inhibitors such as anti-PD-1 monoclonal antibody pembrolizumab. We present the case of a 33-year-old African American female with a pertinent past medical history of history of recurrent progressive metastatic squamous cell carcinoma cervical cancer treated with pembrolizumab. The patient presented with symptoms of SJS/TEN four weeks after treatment with pembrolizumab was initiated. Intervention was delayed because the definitive diagnosis of an irAE was difficult due to time from initiation of treatment and obfuscated by intervening urosepsis episode treated with meropenem, and lack of literature illustrating SJS/TEN in patients of darker skin. From this case, we can learn the importance of immediate intervention in cases of irAE secondary to immune complex inhibitors and demonstrate the presentation of such a severe-life threatening condition in a patient of a darker skin tone.
1. Introduction
Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare but severe and acute hypersensitivity reactions affecting the skin epithelium and mucosa, characterized by separation of the epidermal and dermal layers and manifested by water-filled lesions, and sloughing of mucous membranes (Perwitasari et al., 2021, Othman Almadfaa et al., 2022). A common etiology of these conditions is an adverse reaction to drug classes such as NSAIDs, anticonvulsants, barbiturates, and beta-lactams. Less commonly encountered historically, but increasing use of immunotherapies is highlighting the need to report and describe the association between the development of SJS/TEN with immune checkpoint inhibitors (ICIs) such as pembrolizumab, ipilimumab, and nivolumab that inhibit Programmed Death (PD-1), PD ligand-1 (PDL-1), and CTLA-4 T-cell receptors in order to block or down-regulate the suppression of the anti-tumor response (Robinson et al., 2020). Pembrolizumab is the most commonly used immunotherapy and is an anti-PD-1 monoclonal antibody that prohibits the binding of PD-1 on T cells and PD-L1 on tumor cells. This prevents the tumor cells from inactivating T cells and subsequent evasion of immune system detection, ultimately leading to immune related tumor cell death (Viscuse et al., 2021). Pembrolizumab is approved for use in the treatment of lymphomas, melanomas, and many other solid tumors including several gynecologic malignancies (Robinson et al., 2020). Related adverse events include immune-related events, with dermatologic toxicities being one of the most common. Often these immune-related adverse events (irAEs) include fatigue, pruritis, non-specific rash, and gastrointestinal irritation, but rare and more severe effects include bullous pemphigoid, SJS, and TEN. In a cohort study in which 153 patients who were treated with ICIs ipilimumab, pembrolizumab, nivolumab, or combination ipilimumab plus nivolumab, fifty percent of those patients developed dermatological irAEs. The most common adverse events included pruritis in 31 % of patients, maculopapular rash in 28 %, eczematous dermatitis in 7 %, vitiligo-like depigmentation in 7 %, and lichenoid mucositis in 5 % (Villa-Crespo et al., 2022). Typically, dermatologic adverse events present as grade 1 or grade 2, managed by topical corticosteroids or holding treatment until symptom resolution. However, in cases of more serious grade 3–4 maculopapular rash or pruritus, immunotherapy is withheld and high-dose steroids given (New, 2018).
SJS/TEN has a variety of possible skin presentations including decentralizing atypical targetoid macules resulting in dusky plaques and subsequent skin-sloughing, macular targetoid lesions, or flaccid bullous lesions (Diep et al., 2022). The percentage of body surface area (BSA) affected in SJS and TEN is what differentiates the two conditions; SJS affects < 10 % BSA, while TEN affects > 30 %BSA, and overlapping SJS/TEN covers between 10 and 30 % BSA (Barvaliya et al., 2011). Medical education resources demonstrate the different presentations of SJS through images, which is a helpful tool to enable trainees and physicians to identify such a severe condition, allowing them to intervene and treat before the condition is exacerbated. However, majority of the images in medical textbooks and google search images primarily showcase SJS presentations on light and white skin tones as opposed to medium to dark skin tones. Lack of exposure to images of SJS on darker skin tones in medical education can result in delays when caring for minority groups, which could translate to inferior outcomes, especially when associated with new drug classes, such as immune checkpoint inhibitors, like pembrolizumab (Diep et al., 2022).
2. Case report
2.1. The subject of this case report has given their written informed consent to publish their case (including publication of images
We present the case of a 33-year-old African American female with a history of recurrent progressive metastatic squamous cell carcinoma cervical cancer, initially diagnosed 4 years prior as FIGO Stage IIB. She had initial definitive external pelvic chemoradiation, chemotherapy weekly with cisplatin, and intracavitary high-dose rate brachytherapy. She experienced recurrent disease in her upper abdomen and iliac bone. Due to multiple admissions during prior cytotoxic chemotherapy including sepsis, and pancreatitis requiring small bowel resection and permanent ostomy formation, she was dispositioned to single agent pembrolizumab on approximately 4 weeks prior to presentation. The patient was treated by an out of state oncologist and initially presented to outside hospital with complaints of a painful, hyperpigmented maculopapular rash along with blister-like lesions which started on her lower back and feet. Subsequently, the maculopapular rash evolved to include her forehead, trunk, and thighs as well as painful blistering of her lips. Initial evaluation determined that she did have ocular involvement. A biopsy at the outside hospital was performed and confirmed the diagnosis of SJS/TEN with apoptotic keratinocytes with lymphocytic infiltration involving the dermis.
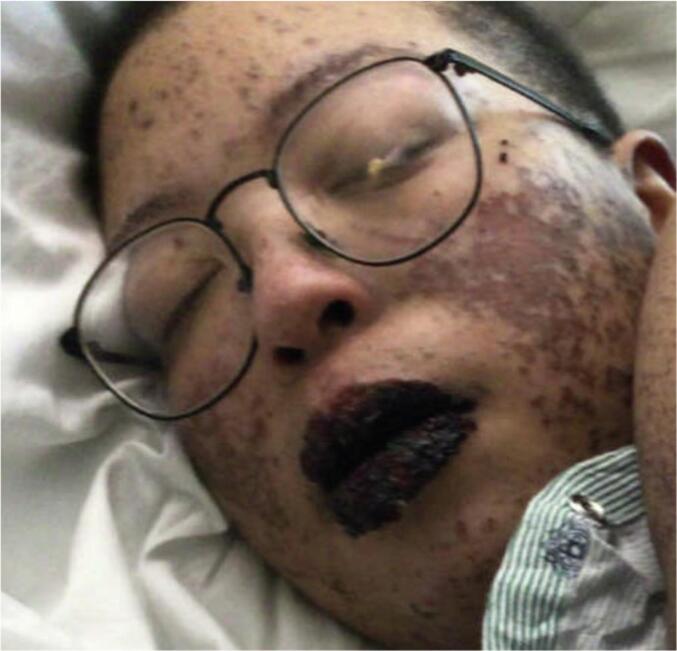
Shortly after transfer to the local University Burn Intensive Care Unit, the patient was assessed, and the initial diagnosis was attributed to recent IV antibiotic administration for treatment of complicated urosepsis with daptomycin and meropenem within days prior to initial presentation. However, the causative agent was obfuscated by the fact that patient was started on pembrolizumab roughly two weeks prior to her urosepsis event but was not clearly documented in her available medical records at time of transfer, but rather only self-reported by the patient. After the time of transfer, she was noted to be in moderate distress with an approximately 15 % BSA desquamative rash predominantly on buttocks (shown in Fig. 1.), sub mammary (shown in Fig. 4.), and pressure areas (shown in Fig. 3.), but also extending to the upper and lower extremities (shown in Fig. 2.). She also reported increasing odynophagia and worsening mouth ulcers (shown in Fig. 5.). The presumptive diagnosis of SJS/TEN due to meropenem was made, but possible recent immunotherapy was noted as well. Both meropenem and pembrolizumab had been held since presentation and the patient was started on high-dose patient-controlled analgesics (PCA). Routine cultures noted Staphylococcus epidermidis sepsis for which she was treated with IV vancomycin 1500 mg every twelve hours for an expected trough of 15–20 mcg/mL and started on solumedrol 125 mg every 24 h for edema. Patient reported improvement in cutaneous symptoms.
Fig. 1.
Desquamating rash on left flank and buttock.
Fig. 4.
Desquamating rash and maculopapular rash in sub-mammary region.
Fig. 3.
Desquamating rash on sacral region.
Fig. 2.
Hyperpigmented maculo-papular rash on bilateral lower extremities.
Fig. 5.
Crusted lesion on lips and desquamating rash on cheek.
During her admission, dermatology, ophthalmology, burn, and gynecologic oncology were consulted for routine care of SJS/TEN, including wound care, prophylactic antibiotics, pain management, and nutritional management. Dermatology started the patient on a topical antibiotic ointment and cyclosporine 5 mg/kg/day by mouth twice a day, with minimal improvement. Although the patient had no ocular involvement, ophthalmology started the patient on artificial tears 4–6 times per day and erythromycin ointment in both eyes at night. The burn team dressed the wounds with silverlon, dampened 10-ply gauze, and a burn net. Gauze changes were set to take place daily or as needed by the primary nursing team for the following week. The burn team also recommended aggressive nutritional supplementation with protein for a goal of 2 mg/kg/day, micronutrient supplementation with vitamin C, and completion of weekly nutrition labs to monitor the patient’s nutrition status. Initially only benign gynecology was consulted due to vulvovaginal involvement, and intravaginal betamethasone therapy and estrogen due to her increased risk of labial agglutination and introital stenosis was initiated three times weekly. Gynecologic oncology was later consulted per gynecology recommendations. After a thorough review of her cancer treatment history was performed by the gyn oncology team, it was determined that as pembrolizumab was given approximately four weeks prior, a grade 4 immune-related dermatologic SJS/TEN-like reaction was the leading differential diagnosis and it was treated accordingly.
Thus, cyclosporine was discontinued in favor of prednisone 1.5 mg/kg/day per NCCN guidelines for management of immune-related adverse events (New, 2018). The patient’s symptoms improved over the next few days, and her dose of corticosteroid was tapered to 1.25 mg/kg/day. She continued to progressively improve, and discharge was initially planned to inpatient rehab. However, due to a lapse in insurance, she remained inpatient as her skin lesions remained too extensive for self-care at home. She subsequently had an episode of fever and hypotension for which she was placed on empiric meropenem and vancomycin and did well. Blood cultures grew Klebsiella pansensitive to Ciprofloxacin. She remained clinically stable and was discharged to home on ciprofloxacin and the remaining Prednisone steroid taper.
3. Discussion
Immune checkpoint inhibitors such as pembrolizumab are effective and exciting new tools that are widely used by contemporary oncologists. However, patients on these immunotherapies are at risk of a variety of autoimmune-like or inflammatory side effects, also known as immune-related adverse events (irAEs), which can affect one or more organ systems. Prospective observational studies have identified several biomarkers that may serve as an indicator for potential cutaneous irAEs. Patients with rheumatoid factor (RF) above 15 IU/mL and/or specific HLA-DRB1 subtypes show an increased risk of development of cutaneous irAEs, indicating that a genetic workup prior to administration of these immunotherapies may be a helpful preventative measure in patients with a complicated dermatologic history or history of drug-related cutaneous adverse events (Chen et al., 2022). In addition, a thorough review of medical records is paramount in the setting of any suspected drug related reaction to ensure that proper treatment is initiated immediately. According to the NCCN, management of SJS/TEN secondary to immune-related dermatologic toxicity includes permanently discontinuing immunotherapy, initiating a high-dose corticosteroid taper, and requesting urgent dermatology, ophthalmology, and urology consultation (New, 2018). The first call to action in a suspected SJS/TEN case is to rule out an immunotherapy-caused reaction, as it is a rare but life-threatening condition that requires immediate intervention. There are several factors that influenced this patient’s hospital course. A three-day delay between the initial incidence of the blisters and patient admission to the hospital prevented immediate treatment for dermatologic related irAE according to the NCCN guidelines. This once again highlights, the need for all subspecialties to be familiar with this new class of immune-oncologic agents and the unique toxicity profile when compared to more classic chemotherapeutic agents and to have a high suspicion and urgency in understanding that irAE are a diagnosis of exclusion. It also illustrates the importance of including oncology subspecialty services in the management of complex patients. According to meta-analyses, average incidence for irAE secondary to immunomodulatory agents includes CTLA-4 inhibitor ipilimumab and PD-1 inhibitors pembrolizumab and nivolumab at 24.3 %, 16.7 %, 14.3 %, respectively. (Muntyanu et al., 2021) For this patient specifically, the duration between her first pembrolizumab treatment and initial drug-reaction symptoms along with an intervening urosepsis episode treated with meropenem made definitive diagnosis of an irAE difficult, though the usual time lapse between IV immunotherapy administration and manifestation of irAEs ranges from 2 to 8 weeks. Lastly, lack of educational material on the presentation of SJS/TEN in medium to dark skin tones (Fitzpatrick IV-VI) could have further delayed diagnosis and appropriate treatment of this patient. (Diep et al., 2022) A predominance of Fitzpatrick I-III skin types, or fair skin that easily burns with ultraviolet radiation exposure, is found in the current dermatologic literature, making diagnosis of SJS/TEN in skin of color more challenging. In our patient, the initial presentation differed from the typical clinical description in the literature, presenting as a dark-brown, hyperpigmented maculopapular rash lacking the erythema or violaceous hues commonly described in Fitzpatrick I-III patients. In contrast, the presentation in oral and genital mucosal surfaces presented similarly to those in fair skinned patients, indicating the importance of examining these areas on initial presentation to aid in diagnosis.
Statement of Ethics.
Consent to publish statement: Written informed consent was obtained from the subject of this report for publication of the details of their medical case and any accompanying images.
Author Jernigan reports that she has received honoraria as a member of the Speaker’s Beaurau for AstraZeneca.
Funding Sources.
This was an unfunded study.
CRediT authorship contribution statement
A.B. Pierre: . A.M. Jernigan: Supervision. T. Castellano: Conceptualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Barvaliya M., Sanmukhani J., Patel T., Paliwal N., Shah H., Tripathi C. Drug-induced stevens-johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study. Journal of Postgraduate Medicine. 2011;57(2):115–119. doi: 10.4103/0022-3859.81865. https://ezproxy.lsuhsc.edu/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=83373488&site=eds-live&scope=site [DOI] [PubMed] [Google Scholar]
- Chen C., Yu H., Yu S. Cutaneous adverse events associated with immune checkpoint inhibitors: A review article. Current Oncology. 2022;29(4):2871–2886. doi: 10.3390/curroncol29040234. https://ezproxy.lsuhsc.edu/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=ccm&AN=156533384&site=eds-live&scope=site [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep D., Aluri B., Crane A., Miao K., Kannan K.S., Goldsteen R. Stevens-johnson syndrome in a patient of color: A case report and an assessment of diversity in medical education resources. Cureus. 2022;14(2) doi: 10.7759/cureus.22245. Accessed Oct 2,2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep D., Aluri B., Crane A., Miao K., Kannan K.S., Goldsteen R. Stevens-johnson syndrome in a patient of color: A case report and an assessment of diversity in medical education resources. Cureus. 2022;14(2):e22245. doi: 10.7759/cureus.22245. https://ezproxy.lsuhsc.edu/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=35340465&site=eds-live&scope=site [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntyanu A., Netchiporouk E., Gerstein W., Gniadecki R., Litvinov I.V. Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: A dermatology perspective on management [formula: See text] Journal of Cutaneous Medicine and Surgery. 2021;25(1):59–76. doi: 10.1177/1203475420943260. Accessed Sep 6, 2022. [DOI] [PubMed] [Google Scholar]
- Othman Almadfaa A., Khalid Alattas M., Aleissa A.I., et al. An overview on the evaluation and management of stevens-johnson syndrome/toxic epidermal necrolysis. International Journal of Pharmaceutical Research & Allied Sciences. 2022;11(1):29–34. doi: 10.51847/K833tMZjQP. https://ezproxy.lsuhsc.edu/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=156309691&site=eds-live&scope=site [DOI] [Google Scholar]
- Perwitasari D.A., Febriana S.A., Tristiana R.S. Quality of life of drug reaction with eosinophilia and systemic symptom (DRESS) and stevens-johnson syndrome (SJS) and/or toxic epidermal necrolysis (TEN) patients. Patient Preference & Adherence. 2021;15:329–335. doi: 10.2147/PPA.S285256. https://ezproxy.lsuhsc.edu/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=148966259&site=eds-live&scope=site [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S., Saleh J., Curry J., Mudaliar K. Pembrolizumab-induced stevens-johnson syndrome/toxic epidermal necrolysis in a patient with metastatic cervical squamous cell carcinoma: A case report. The American Journal of Dermatopathology. 2020;42(4):292–296. doi: 10.1097/DAD.0000000000001527. Accessed Jul 23, 2022. [DOI] [PubMed] [Google Scholar]
- Thompson J.A. New NCCN guidelines: Recognition and management of immunotherapy-related toxicity. Journal of the National Comprehensive Cancer Network. 2018;16(5S):594–596. doi: 10.6004/jnccn.2018.0047. Accessed Sep 6, 2022. [DOI] [PubMed] [Google Scholar]
- Villa-Crespo L., Podlipnik S., Anglada N., et al. Timeline of adverse events during immune checkpoint inhibitors for advanced melanoma and their impacts on survival. Cancers (basel) 2022;14(5) doi: 10.3390/cancers14051237. Accessed Sep 6, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscuse P.V., Marques-Piubelli M.L., Heberton M.M., et al. Case report: Enfortumab vedotin for metastatic urothelial carcinoma: A case series on the clinical and histopathologic spectrum of adverse cutaneous reactions from fatal stevens-johnson syndrome/toxic epidermal necrolysis to dermal hypersensitivity reaction. Frontiers in Oncology. 2021;0 doi: 10.3389/fonc.2021.621591. Accessed Jul 23, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]