Abstract
Diplopia is a relatively common chief complaint encountered in an outpatient neurology clinic and carries a broad differential diagnosis. In this case, a 67-year-old woman presented with new horizontal, binocular diplopia and ptosis of 8-month duration, which persisted without significant progression. This case highlights the need for a comprehensive list of differential diagnoses for patients with acquired ophthalmoplegia and ptosis. Key learning points include an illustration of the stepwise diagnostic approach to evaluate for common etiologies, the importance of interpreting test results in the appropriate clinical setting, and the significance of recognizing specific signs and symptoms in achieving the correct diagnosis.
Section 1
A 67-year-old woman presented with 8 months of horizontal, binocular diplopia. The diplopia was constant, without fluctuation or diurnal variation, present in primary gaze, and worsened when looking left. Associated symptoms included right upper eyelid drooping, pressure sensation behind the right orbit, redness of both eyes, and increased lacrimation. There was no associated vision loss, photophobia, pain with eye movement, tinnitus, vertigo, headache, dysarthria, dysphagia, and weakness or numbness.
Her medical history included hypertension, prediabetes, and remote endometrial adenocarcinoma status post hysterectomy and radiation. Neurologic examination showed a best-corrected visual acuity of 20/25 bilaterally, and equal, round, and reactive pupils. Humphrey visual fields (24-2 SITA Fast protocol) were unremarkable. There was no exophthalmos, orbital bruit, or papilledema. There was mild, right-sided, nonfatigable ptosis and limited abduction of the left eye with a left esotropia that ranged from 16 to 40 prism diopters, maximal in the left gaze. Notably, ophthalmic evaluation 8 months prior documented a right esotropia. There was no facial sensory loss or facial muscle weakness. The remainder of the neurologic examination was normal.
Questions for Consideration:
What is the localization for this presentation?
What are the differential diagnoses?
GO TO SECTION 2
Section 2
The limitation in abduction of the left eye noted in this case suggested a left abducens nerve palsy, within or outside the brainstem. Ptosis may result from an oculomotor nerve palsy or disruption of the oculosympathetic chain, leading to Horner syndrome. A single lesion in the cavernous sinus or at the superior orbital fissure, where several critical neurovascular structures controlling orbital function and cerebral blood flow are located, may explain the patient's full constellation of symptoms. Specific pathologies affecting the cavernous sinus include space-occupying lesions (e.g., meningioma), thrombosis, aneurysm, carotid cavernous fistula (CCF), and Tolosa-Hunt syndrome. Traumatic fracture, retro-orbital hematoma, neoplasm (e.g., lymphoma and rhabdomyosarcoma), orbital pseudotumor, systemic lupus erythematosus, and infection (e.g., syphilis and herpes zoster) are known to involve the superior orbital fissure. Outside of these specific locales, pathology involving multiple sites simultaneously, including sarcoidosis, ocular myasthenia gravis (OMG), and Miller-Fisher syndrome, may also be considered.
Question for Consideration:
Which investigations narrow the differential?
GO TO SECTION 3
Section 3
Serum testing at initial ophthalmic evaluation revealed a positive acetylcholine receptor (AChR) binding antibody (by radioimmunoassay [RIA]) at a titer of 0.63 nmol/L (normal <0.21 nmol/L). AChR blocking (RIA) and modulating (semiquantitative flow cytometry) antibodies were negative. This finding initially raised suspicion for OMG.
OMG is a disorder of the neuromuscular junction characterized by extraocular muscle weakness, leading to diplopia and ptosis, both of which were noted in this patient.1 Muscle weakness and ptosis in OMG typically fluctuate in severity, with more pronounced symptoms later in the day or after exercise.1,2 New onset diplopia and ptosis in an older individual with an elevated AChR binding antibody would normally be diagnostic of OMG. Despite these findings, the absence of fatigability and diurnal variation in her symptoms and the presence of local ocular symptoms, including eye pressure, redness, and tearing, argued against OMG. Treatment with pyridostigmine 60 mg 3 times daily was trialed and failed to lead to significant improvement.
AChR binding, blocking, and modulating antibody testing was repeated 4 months later and resulted negative. AChR antibodies detected by RIA are approximately 85% sensitive and 99% specific in generalized MG, but sensitivity falls to 50% in patients with OMG.3 False-positive AChR antibody testing has been observed in other disease states, including autoimmune liver disorders, multiple sclerosis, neuromyelitis optica spectrum disorder, celiac disease, systemic lupus erythematosus, amyotrophic lateral sclerosis, and as an idiopathic finding.4 Recent work suggests that diagnostic accuracy in MG may be improved in the right clinical setting with an AChR binding antibody titer exceeding 0.5 nmol/L and the addition of an abnormal AChR modulating antibody.4 No further evaluation for MG was pursued in this case because of the presence of atypical clinical features, negative repeat serologies, and lack of treatment response.
MRI of the brain and orbits without contrast was performed 4 months after symptom onset. This revealed symmetric STIR hyperintensity in the bilateral lateral rectus muscles with myotendinous junction sparing and normal muscle bulk. No abnormality was noted in the brainstem or remaining brain parenchyma. An MRI of the orbits with and without contrast 4 months later showed prominence of the inferior and medial rectus muscles bilaterally as well as the right superior and lateral rectus muscles and left superior oblique muscle, without contrast enhancement. These imaging results along with the patient's retro-orbital eye discomfort and lacrimation raised suspicion for thyroid eye disease, although there was no exophthalmos on examination and systemic findings suggesting hyperthyroidism or hypothyroidism were absent. Serum thyroid-stimulating hormone, T3, T4, thyroglobulin, thyroid-stimulating immunoglobulin antibodies, and creatine kinase were normal.
Thyroid eye disease is typically seen in patients with Graves' hyperthyroidism and rarely in hypothyroid or euthyroid patients.5 The most commonly affected muscles are the inferior and medial recti.1,5 Imaging shows enlargement of the muscle bellies with relative tendon sparing.1,5 The lateral recti and obliques are the least commonly involved muscles.5 The atypical pattern of extraocular muscle involvement and lack of serologic evidence of thyroid dysfunction argued against thyroid eye disease in this case.
Alternative diagnoses to consider that can present with ophthalmoplegia, ptosis, and local ocular symptoms include orbital pseudotumor and Tolosa-Hunt syndrome.1,6 Both involve idiopathic granulomatous inflammation of multiple tissue types in and around the orbit, and typically present with unilateral acute/subacute onset periorbital pain, cranial nerve palsy (III, IV, V1/2, and VI), and exophthalmos in the case of orbital pseudotumor.6 MRI may show inflamed, gadolinium-enhancing tissue that is T1 hypointense relative to fat and isointense with muscle and T2 isointense with fat.6 Mild retro-orbital pressure was reported in this case without significant pain. The lack of pain, exophthalmos, and typical MRI findings made both of these diagnoses unlikely.
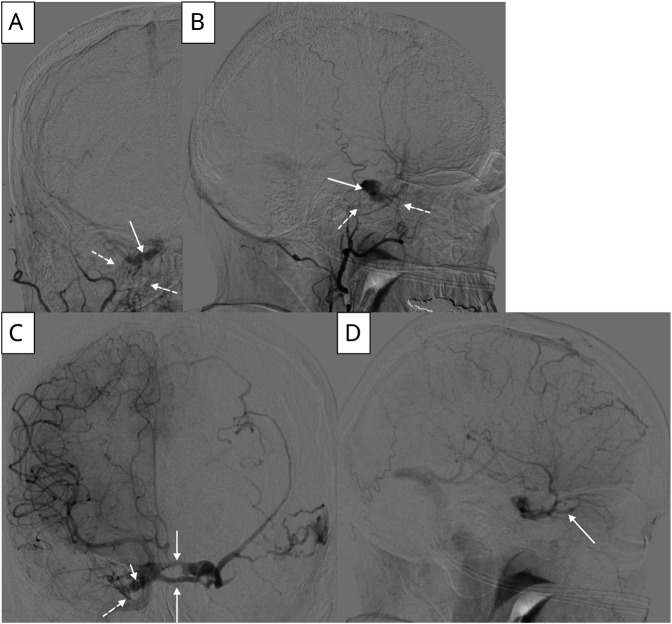
Neuro-ophthalmology subsequently assessed the patient and found prominent corkscrew vessels (Figure 1) bilaterally on slit lamp examination, suggestive of elevated episcleral venous pressure. Digital subtraction angiography was performed, revealing a direct, high-flow, right CCF with indirect external carotid artery dural branches on the right and small meningeal branches originating from the posterior wall of the ophthalmic internal carotid artery (ICA) segment on the left (mixed Barrow type A/D; Figure 2).7 Obliteration of the fistula was achieved with transvenous coiling and onyx-18 embolization. Follow-up at 4 months showed a significant decrease in residual corkscrew vessels (Figure 1) and improvement of her esotropia.
Figure 1. Preoperative and Postoperative Slit Lamp Photographs of Corkscrew Vessels.

(A) Picture from slit lamp examination of the tortuous conjunctival vessels (corkscrew vessels) in the right eye of the patient presented in this case before surgical intervention. (B) Picture from slit lamp examination of the tortuous conjunctival vessels (corkscrew vessels) in the left eye of the patient presented in this case before surgical intervention. (C) Improvement in tortuous conjunctival vessels on slit lamp examination in the right eye 4 months after surgical intervention. (D) Improvement in tortuous conjunctival vessels on slit lamp examination in the left eye 4 months after surgical intervention.
Figure 2. Digital Subtraction Angiography of Carotid Cavernous Fistula.
(A) Anterior view of right external carotid artery (ECA) injection, dashed lines pointing to arterial feeders from ECA and solid arrows pointing to the carotid cavernous fistula (CCF). (B) Lateral view of right ECA injection, dashed lines pointing to arterial feeders from ECA and solid arrow pointing to CCF. (C) Anterior view of right internal carotid artery (ICA) injection, dashed line pointing to arterial feeders from ICA, solid arrow pointing to cross filling of contralateral cavernous sinus through anterior and posterior channels of the intercavernous sinus, and short solid arrow pointing to CCF. (D) Lateral view of right ECA injection, solid arrow pointing to venous drainage through the superior ophthalmic vein.
Discussion
CCF is an abnormal communication between the carotid arterial system and the cavernous sinus. CCFs can be classified as direct (Barrow type A) and indirect, or dural, fistulas (Barrow types B, C, and D).7,8 Direct fistulas involve a connection between the ICA and the cavernous sinus and are typically of high flow.7 Causes include trauma, rupture of an ICA aneurysm within the cavernous sinus, or are iatrogenic from prior invasive procedures.8 Indirect CCFs are abnormal connections between the cavernous sinus and cavernous arterial branches, are usually of low flow, and may be caused by hypertension, connective tissue disorders, ICA dissection, or occur spontaneously.7,8 The CCF in this case was classified as mixed Barrow type A/D because of the presence of both direct and indirect fistulas.
Typical clinical signs and symptoms of CCF include ophthalmoplegia, proptosis, lagophthalmos, ptosis, lacrimation, headache, increased intraocular pressure, and injection (arterialization of conjunctival and episcleral blood vessels from elevated episcleral venous pressure, leading to the development of corkscrew vessels).8-10 Anteriorly draining fistulas are more likely to cause ocular symptoms, whereas posteriorly draining fistulas often result in isolated ocular motor nerve paresis and ensuing diplopia.8 Corkscrew vessels may be observed in other pathologies that elevate episcleral venous pressure, including thyroid eye disease, Sturge-Weber syndrome, orbital tumor or varices, and venous sinus thrombosis.11 In a retrospective study of 80 patients with CCF, corkscrew vessels were found in 93% of cases.12 Ophthalmoplegia in CCF may be myogenic due to engorged, enlarged extraocular muscles or neurogenic from ocular motor cranial nerve dysfunction.2 Abducens nerve involvement occurs more frequently because of its central location adjacent to the ICA within the cavernous sinus.2,8
Symptoms can be unilateral or bilateral, despite a CCF on one side, due to the communicating nature of the cavernous sinus through the anterior and posterior intercavernous sinuses. Changes in the flow rate through the CCF, pattern of venous drainage, and pressure variation within this communicating system likely explain the observed change in laterality of the patient's esotropia over months, from right to left. Although rare, regression of symptoms ipsilateral to the side of the CCF and development of contralateral symptoms has been described previously.13 Right-sided ptosis may be accounted for by the involvement of the right oculomotor nerve within the cavernous sinus. The lack of pupillary abnormality argues against a Horner phenomenon.
Despite the presenting symptoms and results of AChR binding antibody testing, this case illustrates the importance of considering the entire clinical picture and broadening the differential when atypical features are present. Recognition of unique physical examination findings, such as corkscrew vessels, can alter the diagnostic approach, facilitate timely diagnosis, and allow early treatment.
Appendix. Authors

| Name | Location | Contribution |
| John Daniel Bireley, MD | Department of Neurology, Neurological Institute, Cleveland Clinic, OH | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Joshua Santucci, MD | Department of Neurology, Neurological Institute, Cleveland Clinic, OH | Major role in the acquisition of data; additional contributions: contribution to figures |
| Yuebing Li, MD, PhD | Department of Neurology, Neurological Institute, Cleveland Clinic, OH | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Devon A. Cohen, MD | Department of Ophthalmology, Cole Eye Institute, Cleveland Clinic, OH | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
No targeted funding reported.
Disclosure
J.D. Bireley and J. Santucci report no disclosures relevant to the manuscript. Y. Li has consulted for argenx, Catalysis, Immunovant, and UCB Pharma and has received grant support from argenx. D. Cohen reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Roper-Hall G. Acquired ophthalmoplegia in older children and adults. J Binocul Vis Ocul Motil. 2018;68(1):10-19. doi: 10.1080/2576117X.2017.1421820 [DOI] [PubMed] [Google Scholar]
- 2.Merrill KS, Lee MS, McClelland CM. Red flags in the assessment of adult ophthalmoplegia. J Binocul Vis Ocul Motil. 2018;68(1):20-23. doi: 10.1080/2576117X.2017.1420134 [DOI] [PubMed] [Google Scholar]
- 3.Lazaridis K, Tzartos SJ. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front Immunol. 2020;11:212. doi: 10.3389/fimmu.2020.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun F, Tavella-Burka S, Li J, Li Y. Positive acetylcholine receptor antibody in nonmyasthenic patients. Muscle Nerve. 2022;65(5):508-512. doi: 10.1002/mus.27500 [DOI] [PubMed] [Google Scholar]
- 5.Weiler DL. Thyroid eye disease: a review. Clin Exp Optom. 2017;100(1):20-25. doi: 10.1111/cxo.12472 [DOI] [PubMed] [Google Scholar]
- 6.Wasmeier C, Pfadenhauer K, Rösler A. Idiopathic inflammatory pseudotumor of the orbit and Tolosa-Hunt syndrome: are they the same disease? J Neurol. 2002;249(9):1237-1241. doi: 10.1007/s00415-002-0818-x [DOI] [PubMed] [Google Scholar]
- 7.Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62(2):248-256. doi: 10.3171/jns.1985.62.2.0248 [DOI] [PubMed] [Google Scholar]
- 8.Henderson AD, Miller NR. Carotid-cavernous fistula: current concepts in aetiology, investigation, and management. Eye (Lond). 2018;32(2):164-172. doi: 10.1038/eye.2017.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cmelo J. Carotid-cavernous fistula from the perspective of an ophthalmologist: a review. Cesk Slov Oftalmol. 2020;1:1-8. English. doi: 10.31348/2020/8 [DOI] [PubMed] [Google Scholar]
- 10.Miller NR. Dural carotid-cavernous fistulas: epidemiology, clinical presentation, and management. Neurosurg Clin N Am. 2012;23(1):179-192. doi: 10.1016/j.nec.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 11.Madeira C, Alves G, Godinho G, et al. Bilateral episcleral corkscrew vessels: expedition into the unknown: case report. Case Rep Ophthalmol. 2022;13(1):109-115. doi: 10.1159/000515971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preechawat P, Narmkerd P, Jiarakongmun P, Poonyathalang A, Pongpech SM. Dural carotid cavernous sinus fistula: ocular characteristics, endovascular management and clinical outcome. J Med Assoc Thai. 2008;91(6):852-858. [PubMed] [Google Scholar]
- 13.Dandy WE, Follis RH. On the pathology of carotid-cavernous aneurysms (pulsating exophthalmos). Am J Ophthalmol. 1941;24(4):365-385. doi: 10.1016/S0002-9394(41)94125-9 [DOI] [Google Scholar]