Abstract
JC polyomavirus (JCV) establishes an asymptomatic latent and/or persistent infection in most of the adult population. However, in immunocompromised individuals, JCV can cause a symptomatic infection of the brain, foremost progressive multifocal leukoencephalopathy (PML). In the past 2 decades, there has been increasing concern among patients and the medical community because PML was observed as an adverse event in individuals treated with modern (selective) immune suppressive treatments for various immune-mediated diseases, especially multiple sclerosis. It became evident that this devastating complication also needs to be considered beyond the patient populations historically at risk, including those with hematologic malignancies or HIV-infected individuals. We review the clinical presentation of PML, its variants, pathogenesis, and current diagnostic approaches. We further discuss the need to validate JCV-directed interventions and highlight current management strategies based on early diagnosis and restoring JCV-specific cellular immunity, which is crucial for viral clearance and survival. Finally, we discuss the importance of biomarkers for diagnosis and response to therapy, instrumental in defining sensitive study end points for successful clinical trials of curative or preventive therapeutics. Advances in understanding PML pathophysiology, host and viral genetics, and diagnostics in conjunction with novel immunotherapeutic approaches indicate that the time is right to design and perform definitive trials to develop preventive options and curative therapy for JCV-associated diseases.
Introduction
Progressive multifocal leukoencephalopathy (PML) is a potentially life-threatening disease of the brain in the immunocompromised host caused by lytic infection of white and gray matter cells by JC polyomavirus (JCV), also referred to as JC virus or human polyomavirus type 2. The mortality rate can be high, ranging from 20% to 90%, depending on the underlying condition.1 Although no Class I evidence for JCV-specific therapy is yet available,2 the recent encouraging application of a variety of immunotherapeutic approaches, including virus-directed T-cell therapies and checkpoint inhibitors, creates the justified hope that the development of such therapies may advance to formal testing in clinical trials.3
This review provides a brief overview of the current understanding of PML and its clinical variants. It focuses on the pathogenesis, diagnostic tools, and potential biomarkers of response to therapy. Based on current literature, we conclude that candidate surrogate biomarkers need to be optimized and validated to facilitate diagnosis of PML, ideally at a presymptomatic or asymptomatic stage. These and additional biomarkers should further be evaluated as potential end points of response to therapy in studies aiming at approval of JCV-directed curative and preventive therapies. The biomarkers discussed include standardized brain MRI protocols, measures of JCV viral variant load in CSF, and biomarkers of humoral and cellular JCV-directed immune responses.
JCV-Associated Diseases
PML and Common Predisposing Conditions
A prerequisite for the development of PML is a weakening of JCV-directed cellular immunity.4 Accordingly, PML was first described in patients with hematologic malignancies, such as Hodgkin disease and chronic lymphocytic leukemia,5 which today still represent at least 10% of PML cases.6
From a historical perspective, 2 more consecutive epochs of PML can be identified based on the frequency of PML-related publications (Figure 1). First, the emergence of the HIV and AIDS pandemic in the early 1980s increased the number of publications. PML is listed among the AIDS-defining conditions, and infection with HIV to date remains the most prominent underlying condition for PML, accounting for 50%–80% of all PML cases.6
Figure 1. Number of Results per Year on PubMeda (as of December 2022) of Articles Reporting on “Progressive Multifocal Leukoencephalopathy”.
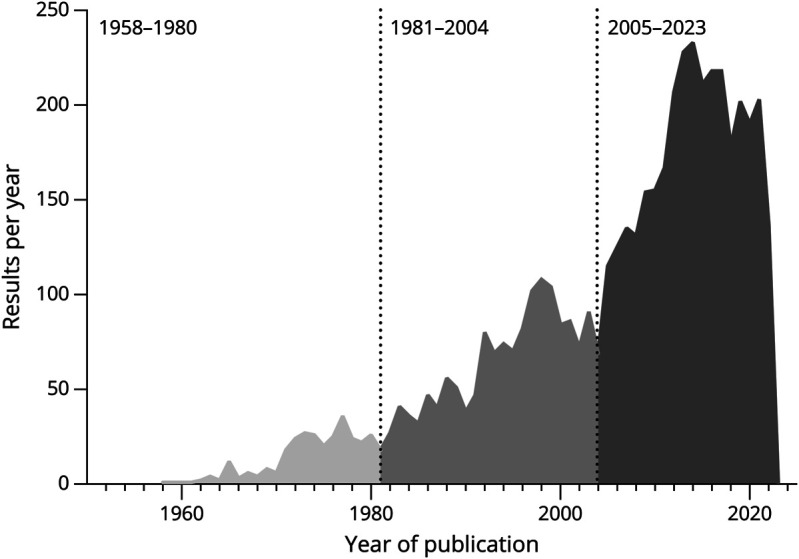
apubmed.ncbi.nlm.nih.gov. A first epoch of research can be noted between 1958 and 1980 (n = 298), a second between 1981 and 2004 (n = 1,544), and a third starting in 2005 (n = 2,849).
From 2005 on, the interest in PML-related research was triggered by the occurrence of PML in people with multiple sclerosis (MS) treated with the monoclonal antibody against α4integrin, natalizumab (Biogen, Cambridge, MA). Compared with other approved modern disease-modifying therapies (DMTs), natalizumab is associated with the highest risk for developing PML, with an overall incidence of 3.1 in 1,000 treated individuals. The risk of developing PML notably increases beyond the second year of therapy, in individuals previously treated with classical immunosuppressants (such as azathioprine, mitoxantrone, cyclophosphamide), and those with high JCV-specific serum antibody levels as determined by the Stratify-JCV serologic testing and reported as “index values.” Accordingly, the risk is lowest among seronegative individuals (around 1 per 10,000) and highest (around 1 per 100) among individuals treated for more than 5 years and with Stratify-JCV index values above 1.5.e81 Extended interval dosing (more than 4 weeks) of natalizumab may reduce but does not eliminate the risk of PML,7,8 and seroconversion rates (negative to positive) explain the recommendation for biannual retesting minimum in the JCV seronegative.
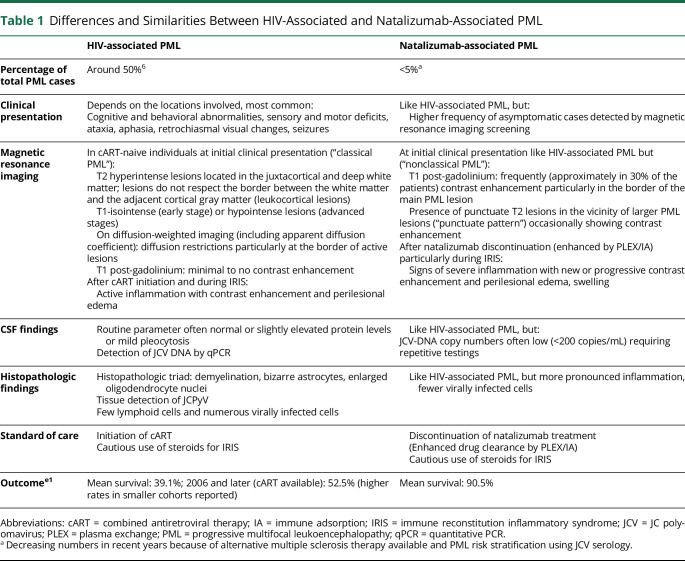
The LFA1-targeting monoclonal antibody efalizumab, previously used for the treatment of psoriasis, was withdrawn from markets in 2009 because of the occurrence of PML cases, indicating that integrin-targeting DMTs specifically increase PML risk.9 After this observation, PML was added as a possible complication of several DMTs in immune-mediated diseases, although with far lower overall risk estimates (<1 in 10,000). DMTs used in the field of neurology with labeled warnings of risk of PML include dimethylfumarate, fingolimod and other S1P modulators, ocrelizumab, rituximab (off-label), ofatumumab, and inebilizumab, although, as of December 2022, no confirmed cases of PML have been reported for ofatumumab (in MS) and inebilizumab (for neuromyelitis optica).e82-e87. Altogether, people with MS treated with DMTs, foremost natalizumab, are currently estimated to account for around 5% of all PML cases.6 As immune competence can be restored by drug discontinuation, DMT-associated PML may have a better prognosis for survival and disability as compared to PML in individuals with hematologic malignancies or in HIV patients before the availability of combined antiretroviral therapy (cART, for additional information comparing HIV-associated and natalizumab-associated PML see Table 1).
Table 1.
Differences and Similarities Between HIV-Associated and Natalizumab-Associated PML
| HIV-associated PML | Natalizumab-associated PML | |
| Percentage of total PML cases | Around 50%6 | <5%a |
| Clinical presentation | Depends on the locations involved, most common: Cognitive and behavioral abnormalities, sensory and motor deficits, ataxia, aphasia, retrochiasmal visual changes, seizures |
Like HIV-associated PML, but: Higher frequency of asymptomatic cases detected by magnetic resonance imaging screening |
| Magnetic resonance imaging | In cART-naive individuals at initial clinical presentation (“classical PML”): T2 hyperintense lesions located in the juxtacortical and deep white matter; lesions do not respect the border between the white matter and the adjacent cortical gray matter (leukocortical lesions) T1-isointense (early stage) or hypointense lesions (advanced stages) On diffusion-weighted imaging (including apparent diffusion coefficient): diffusion restrictions particularly at the border of active lesions T1 post-gadolinium: minimal to no contrast enhancement After cART initiation and during IRIS: Active inflammation with contrast enhancement and perilesional edema |
At initial clinical presentation like HIV-associated PML but (“nonclassical PML”): T1 post-gadolinium: frequently (approximately in 30% of the patients) contrast enhancement particularly in the border of the main PML lesion Presence of punctuate T2 lesions in the vicinity of larger PML lesions (“punctuate pattern”) occasionally showing contrast enhancement After natalizumab discontinuation (enhanced by PLEX/IA) particularly during IRIS: Signs of severe inflammation with new or progressive contrast enhancement and perilesional edema, swelling |
| CSF findings | Routine parameter often normal or slightly elevated protein levels or mild pleocytosis Detection of JCV DNA by qPCR |
Like HIV-associated PML, but: JCV-DNA copy numbers often low (<200 copies/mL) requiring repetitive testings |
| Histopathologic findings | Histopathologic triad: demyelination, bizarre astrocytes, enlarged oligodendrocyte nuclei Tissue detection of JCPyV Few lymphoid cells and numerous virally infected cells |
Like HIV-associated PML, but more pronounced inflammation, fewer virally infected cells |
| Standard of care | Initiation of cART Cautious use of steroids for IRIS |
Discontinuation of natalizumab treatment (Enhanced drug clearance by PLEX/IA) Cautious use of steroids for IRIS |
| Outcomee1 | Mean survival: 39.1%; 2006 and later (cART available): 52.5% (higher rates in smaller cohorts reported) | Mean survival: 90.5% |
Abbreviations: cART = combined antiretroviral therapy; IA = immune adsorption; IRIS = immune reconstitution inflammatory syndrome; JCV = JC polyomavirus; PLEX = plasma exchange; PML = progressive multifocal leukoencephalopathy; qPCR = quantitative PCR.
Decreasing numbers in recent years because of alternative multiple sclerosis therapy available and PML risk stratification using JCV serology.
Rare genetic disorders resulting in inborn errors of immunity, commonly grouped as primary immune deficiencies, can also lead to an increased risk of PML.10 Moreover, PML is seen in posttransplant patients, individuals with sarcoidosis and rheumatological diseases, or patients with cancer treated with targeted therapy (including rituximab, brentuximab, ofatumumab, alemtuzumab, obinutuzumab, ibrutinib, belimumab, idelalisib, and CAR T-cell therapy) and has also been rarely noted in higher-aged individuals without apparent immunosuppression.11-15
Granule Cell Neuronopathy and Rare Variants of JCV-Associated Disease
Granule cell neuronopathy (JCV-GCN) defines a distinct disease entity of JCV-associated disease with an infection primarily of granule cells of the cerebellum. Clinical and neuroradiologic signs include ataxia and progressive cerebellar atrophy.16 Specific viral genetic changes may explain the tropism for cerebellar granule cells.17 JCV-GCN can occur distinct from PML but also simultaneously with PML, as observed in around 5% of PML cases.18 Other variants of JCV-associated disease may exist but are not well defined, including JCV-associated encephalopathy (with predominant involvement of the cortical gray matter and infection of pyramidal neuronal cells), meningitis, or nephropathy.6
Pathogenesis of PML
Transmission and Reservoir
JCV establishes a persistent asymptomatic infection in 50%–80% of the adult population, showing increasing seroprevalence with age. Illustrating this lifetime exposure risk in people with MS, anti-JCV antibody seroprevalence was reported at 47% among individuals aged 15–29 years, 59% among 40–49 years, and 64% for individuals older than 60 years.19 JCV transmission and the route of primary infection are not well understood; however, this most likely occurs through a fecal-oral route during childhood.1,4,6 It has been assumed that following a period of asymptomatic viremia, JCV establishes latent and/or persistent infection in the kidney, lymphoid organs, bone marrow, and possibly the brain and elsewhere in the body. In association with immunosuppression, JCV can reactivate from sites of latency or persistency, undergoing viral genetic changes to become a pathogenic neurotropic virus. Thus far, it is unclear where the disease-associated JCV reservoir is located and how JCV acquires the relevant genetic changes. Several potential mechanisms of viral trafficking into the CNS have been proposed, with conclusive evidence still lacking.6
JCV DNA excretion in urine is commonly observed in around 30% of healthy individuals. However, PML-type JCV genetic variants (see below) have not been found in urine, and excretion of JCV in urine does not appear to increase the likelihood of developing PML in HIV-infected individuals, arguing against uroepithelial cells as a relevant reservoir for JCV from which PML development is initiated.20,21 In the context of natalizumab-associated PML, the bone marrow has been proposed as a potentially relevant reservoir because hematopoietic progenitor and B-cell numbers increase in peripheral blood during natalizumab therapy, and the B-cell DNA recombination machinery might allow the virus to acquire pathogenic viral genetic changes.22,23 However, no further evidence has been generated supporting this hypothesis.24
Besides entering the brain as a free virus or within B cells, JCV might use extracellular vesicles (EVs) to relocate to the brain. Viral particles can be released by infected cells within or attached to EVs, thereby influencing viral tropism by allowing viral receptor-independent cell entry and permitting immune escape of virus hiding within protective EV membranes. A clear role of EVs in JCV spread and PML pathogenesis has not yet been established. However, JCV virions have been found in vitro associated with EVs when infecting human fetal glia-derived SVG-A cells and immortalized human choroid plexus epithelial cells.25,26 More recently, JCV DNA has been detected in EVs in the plasma of HIV-infected human subjects.27
Viral Genetics
The JCV genome is a double-stranded, circular DNA molecule of ∼5,100 bp in size, encoding 6 proteins, which can be divided into 3 segments: early and late genes and a noncoding control region (NCCR). Proteins encoded by the early genes, comprising the small and the large T-antigen, facilitate efficient viral replication and the transcription of late genes. These are the capsid proteins viral protein 1 (VP1), VP2, and VP3, as well as the regulatory agnoprotein. NCCR includes the origin of replication and sequences that control the transcription of both early and late genes. Different JCV genotypes can be determined by the length and sequence of the viral genome based on the NCCR or analysis of sequence variations within the major capsid protein gene, VP1, and the T antigen.28
Rearrangements of the JCV genome in the NCCR have been identified as a prerequisite for establishing PML. The wild-type virus detected in the urine of healthy, asymptomatic individuals lacks rearrangements in the NCCR region. Thus, the rearranged NCCR sequences define the PML-type JCV, detected in CSF, blood, and cerebral tissue of patients with PML. Rearrangements occur because of partial duplications, deletions, and combinations of both and are believed to increase replicative ability.29
JCV-PML genetic variants frequently have point mutations in the VP1 region.30 Point mutations in the VP1 gene cause conformational changes of the VP1 protein on the viral capsid and can alter binding ability and cellular tropism, possibly impeding viral neutralization and facilitating the infection of brain cells.20,21,31
Host Genetics
While predisposing conditions causing profound immune suppression are essential for developing symptomatic JCV-associated disease in most PML cases, only a small proportion of individuals at risk will develop it. It is, therefore, hypothesized that host genetics influence susceptibility to PML. In support of this, JCV infection is strongly associated with human leukocyte antigen (HLA) Class II variants.32 Among non-HLA genes that might regulate susceptibility to PML, blood group genes appear to be relevant candidates.33 Nineteen rare germline PML risk variants that affect 19 genes related to immune function have been identified using whole-exome sequencing.34 Furthermore, polymorphisms of the tumor suppressor protein p53, possibly able to modulate viral gene expression, could also increase PML susceptibility.35
Diagnosing PML
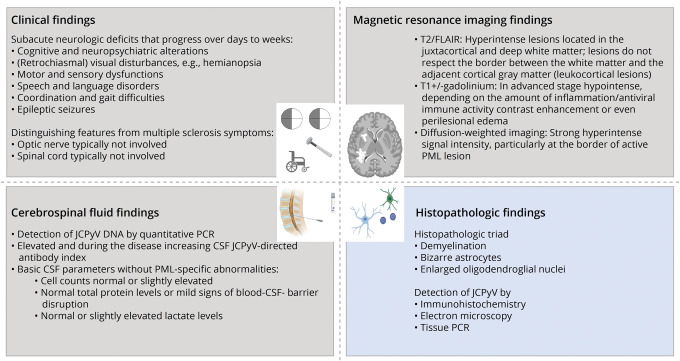
In 2013, a consensus statement for PML diagnostic criteria was published by members of the Neuroinfectious Disease Section of the American Academy of Neurology (AAN). Diagnostic classification distinguishes between definite, probable, and possible PML based on compatible clinical presentation, demonstration of the lesion(s) suggestive of PML on brain MRI, and detection of JCV. The direct detection of JCV is the prerequisite for a definite diagnosis of PML and can be achieved either by JCV DNA in CSF or histologically (Figure 2).36
Figure 2. Summary of Findings in Progressive Multifocal Leukoencephalopathy.
According to current PML diagnostic criteria, for a definitive PML diagnosis, consistent clinical and imaging findings not better explained otherwise in combination with the detection of JCV DNA by PCR in CSF are needed (gray).e79 Alternatively, biopsy-based diagnosis (blue) requires the histopathologic triad coupled with a technique showing the presence of JCV in tissue.e80 The figure was designed using biorender. JCV = JC polyomavirus; PML = progressive multifocal leukoencephalopathy.
Clinical Findings Compatible With PML
PML might commence as a focal leukocortical disease or as an early multifocal disease. Oligodendrocytes and astrocytes are typical target cells of symptomatic JCV infection, but growing evidence suggests early infection of granule cells of the cerebellum during GCN or cortical neurons contributing to encephalopathy.37 Accordingly, PML can have a variable initial clinical presentation, ranging from subacute cognitive and neuropsychiatric alterations (frequency 36%–54%, range reflects different patient cohorts studied), visual disturbances (e.g., hemianopsia, frequency 19%–41%), motor (frequency 33%–45%) or sensory (frequency 7%–19%) dysfunctions, coordination and gait difficulties (frequency 13%–35%), to epileptic seizures (frequency 5%–14%).36 Unlike in MS, the optic nerve and the spinal cord are typically not involved (Figure 2). Differences in frequencies of symptoms reported at presentation in relation to underlying diseases (e.g., HIV vs natalizumab-associated) or patient outcome cannot be reliably compared across studies, considering the variability of study quality and design. Systematic studies that associate symptoms at presentation with prognosis are lacking, but the clinical expert impression is that early infratentorial involvement might associate with worse prognosis.
Magnetic Resonance Imaging Findings in PML
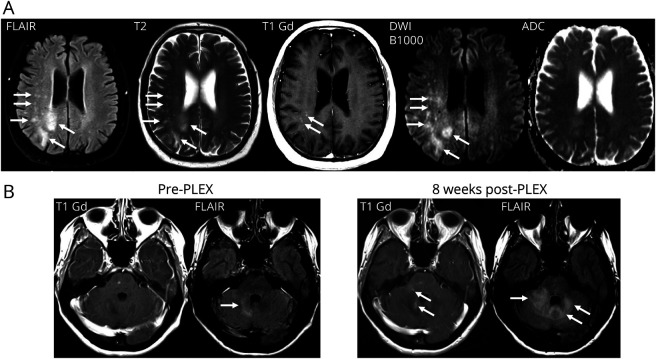
Changes on MRI highly suggestive of PML have been typically described as T2 hyperintense lesions located in the juxtacortical and deep white matter. The lesions do not respect the border between the white matter and the adjacent cortical gray matter and, therefore, may present as leukocortical lesions. Depending on the progression of the disease, the lesion may appear as T1 isointense lesions (early in course) or hypointense lesions (advanced disease), due to irreversible demyelination, compared with the nonaffected healthy appearing white matter. In PML lesions with active viral replication, areas with hyperintense signal intensity on diffusion-weighted imaging, particularly at the border of the PML lesion(s), can be observed, reflecting swelling of oligodendrocytes due to lytic infection. These areas can show various apparent diffusion coefficient (ADC) values (low, normal, or high values).38,39 Multiple small T2 hyperintense lesions (with or without contrast enhancement) in the vicinity of the primary PML lesions can be frequently identified, an imaging pattern labeled as a “punctuate pattern” or “milky way appearance”40,41 (Figure 3).
Figure 3. Magnetic Resonance Imaging Findings in PML.
(A) Axial(FLAIR), conventional T2-weighted, gadolinium-enhanced T1-weighted and diffusion-weighted imaging (including ADC mapping) typically used in clinical routine care for detection of PML. Multifocal leukocortical PML lesions involving both hemispheres with a more extensive lesion in the right parietal lobe involving the cortical gray matter, as well as the juxtacortical and deep white matter with multiple punctuate T2 lesions (arrows on the FLAIR and T2-weighted image). Some punctuate lesions show contrast enhancement. Focal areas show already a hypointense signal intensity in the T1-weighted sequence. Areas of high signal intensity on B1000 diffusion-weighted imaging with heterogenous ADC values indicate oligodendrocyte swelling suggestive of active viral replication (arrows). (B) Gadolinium-enhanced T1-weighted and axial FLAIR imaging before and 8 weeks after plasma exchange. Infratentorial PML lesions display more pronounced contrast enhancement on the gadolinium-enhanced T1-weighted image (arrows) and progressive hyperintense T2-FLAIR changes (arrows) partly due to perilesional edema and swelling after accelerated removal of natalizumab using plasma exchange (PLEX) in an individual who developed PML during therapy with natalizumab for MS. In a setting of a proposed reconstitution of the cellular immunity due to enhanced natalizumab drug clearance after PLEX and a more pronounced cerebellar syndrome indicating clinical deterioration, these imaging findings fit with a diagnosis of PML-IRIS. ADC = apparent diffusion coefficient; FLAIR = fluid-attenuated inversion recovery; IRIS = immune reconstitution inflammatory syndrome; MS = multiple sclerosis; PML = progressive multifocal leukoencephalopathy.
Depending on the amount of inflammatory activity or antiviral immune activity, initial imaging findings in PML differ from case to case. On a group level, HIV-associated PML in cART-naive individuals or cases of PML due to hematologic malignancies with minimal JCV-directed cellular immune function are unlikely to demonstrate contrast enhancement at first presentation, termed as classical PML in the literature. By contrast, in HIV-associated PML treated with cART or DMT-associated PML, best documented for natalizumab-associated PML in people with MS, the immune system likely remains partially functional, and even the earliest MRI may show evidence of active inflammation with gadolinium enhancement and perilesional edema.40,42
CSF Findings in PML
Along the current diagnostic criteria, detection of JCV DNA by PCR in CSF establishes the diagnosis of definite PML in individuals with consistent clinical and imaging findings not better explained by other disorders.36 However, particularly in cases of PML related to DMT use, CSF JCV DNA load is often below 100 copies/mL CSF, resulting in false-negative results by many commercially available quantitative molecular assays, which commonly have lower limits of detection above this threshold (often about 100–200 copies/mL CSF).43,44 Owing to a wide variability of sensitivity and specificity of available JCV DNA PCR assays, interpretation of accuracy of PML diagnosis needs to account for the method used. AAN diagnostic criteria claim that the sensitivity of newer ultrasensitive techniques that detect up to 10 copies/mL CSF is >95%.36 However, other publication in smaller cohorts of HIV-associated PML observed a drop in sensitivity from 89.5% in the pre-cART era to 57.5% in the cART era, likely related to assays with a higher lower limit of detection, but also related to cohorts with low copy numbers of JCV DNA in CSF.45 Therefore, undetected JCV DNA in CSF does not preclude the diagnosis of PML, and repeat and reference laboratory testing or brain biopsy may be needed to definitively establish diagnosis.
In the future, improvements and new developments in PCR techniques might help overcome the problem of limited PCR sensitivity, including more sensitive digital droplet PCR techniques.46 The detection of elevated anti-JCV–specific immunoglobulin G antibodies in CSF with evidence of intrathecal production has been observed in cases of PML, suggesting the so-called CSF JCV-specific antibody index that corrects for disturbances of the blood-CSF barrier function may be a promising future added tool for diagnosis of PML, still requiring independent and ideally prospective validation.47
Standard clinical CSF parameters are most often within the normal range in individuals with PML. Abnormalities are most commonly related to the underlying condition predisposing to PML, e.g., explaining slightly elevated leukocyte cell count levels and blood-CSF barrier dysfunction in individuals with AIDS or the detection of oligoclonal bands in individuals with MS (Figure 2).
Histopathology Findings in PML
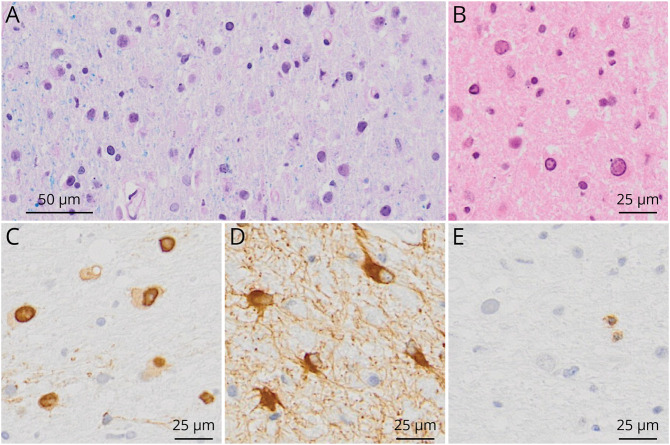
Definite diagnosis of PML can be established by neuropathologic demonstration of the typical histopathologic triad, which include demyelination, bizarre astrocytes, and enlarged oligodendrocyte nuclei, together with tissue detection of JCV (Figure 4). The latter can either be achieved by immunohistochemistry, in situ hybridization, electron microscopy,36 or by tissue PCR detecting JCV DNA (Figure 2). In classical PML, few lymphoid cells and numerous virally infected cells are found. By contrast, in inflammatory PML, as e.g., observed in natalizumab-treated patients, pronounced inflammation is noted and often only few virally infected cells with enlarged oligodendrocyte nuclei are present.48
Figure 4. Typical PML Lesion Characteristics With Demyelination.
(A) Luxol Fast Blue/Periodic Acid-Schiff staining, myelin stained in blue is missing. Only some residual myelin sheaths are present on the left side of the picture. (B) Hematoxylin and eosin staining, enlarged oligodendrocytic nuclei. (C) Anti-VP1, within lesions, numerous virus-replicating glial cells are found. (D) Anti-Glial Fibrillary Acidic Protein, bizarre astrocytes. (E) Anti-CD3, typical for classic PML, only a few T cells are evident. PML = progressive multifocal leukoencephalopathy; VP1 = viral protein 1.
Challenges in Diagnosing PML Early in Disease Course
The AAN consensus PML diagnostic criteria are widely in use in clinical routine. Nevertheless, PML diagnosis is still often delayed,12,43 negatively affecting the outcome. Several reasons contribute to this diagnostic delay.
To begin with, PML is a rare disease, such that awareness among physicians, even among neurologists and general radiologists, is limited. Furthermore, PML-specific symptoms do not exist. Nevertheless, in the context of patient history and preexisting risk factors, specific symptoms should raise a strong suspicion of PML. For example, new-onset retrochiasmal visual disturbance, such as hemianopsia, in younger individuals with an increased risk of developing PML (i.e., HIV-infected or JCV-seropositive patients with MS treated with natalizumab) must lead to appropriate diagnostic workup using MRI and CSF studies. Therefore, discussing PML in educational materials and management guidelines for certain conditions, such as AIDS or MS, is essential to increase awareness.
A more prominent role of MRI in diagnosing PML could potentially allow earlier diagnosis of PML in some patients. The knowledge of an increased risk of developing PML during therapy with natalizumab has led to risk mitigation strategies, including MRI screening for PML using sensitive protocols and imaging every 6 months or even more frequently. These recommendations were based on the observation that PML can be detected in a subgroup of patients with natalizumab-associated PML before developing clinical symptoms, termed asymptomatic or presymptomatic PML.43,49 Furthermore, early detection on MRI at an asymptomatic stage may lead to a better outcome and survival compared with patients already symptomatic at the time of diagnosis.50 One study retrospectively applied the current PML diagnostic criteria to a population of natalizumab-associated PML frequently screened for PML using MRI and compared the results with an alternatively proposed case definition, exposing limited sensitivity of both in this setting.51
As discussed above, current PML diagnostic criteria would possibly be more sensitive in a real-world clinical setting if highly sensitive JCV PCR protocols with optimized preanalytic procedures were available for all individuals suspected of PML. This highlights the need for improved molecular assays for JCV in biological fluids and broad access to these already at the time of first testing in local laboratories to avoid an unnecessary delay in the diagnosis of PML.
Taken together, these considerations drive an effort to revise the current PML diagnostic criteria and expanding them to include the less common JCV-associated entities mentioned above.e88
PML Immune Reconstitution Inflammatory Syndrome
The term “PML immune reconstitution inflammatory syndrome” (PML-IRIS) was first introduced in HIV-positive individuals to describe the unmasking of PML with new-onset neurologic symptoms after the commencement of cART or to describe clinical worsening after the initiation of cART in those already diagnosed with PML. PML-IRIS is reported in around 20% of PML cases in HIV-infected individuals.52 PML-IRIS is also observed in natalizumab-associated PML, particularly after discontinuation of the causative DMT, and may be aggravated by accelerated removal of natalizumab using plasma exchange or immune adsorption.53 PML-IRIS explains why a proportion of individuals with PML will become symptomatic after the treatment of an underlying condition has been initiated: A reconstituted cellular immune response against JCV-infected brain cells can result in overshooting inflammation, structural damage, and new symptoms and may require specific anti-inflammatory therapy to prevent added harm.
As introduced above, individuals with PML can display gadolinium enhancement and perilesional edema on MRI, mainly observed at the lesion borders. This can be noted already at initial presentation or during the clinical course of PML, reflecting the amount of inflammatory activity or antiviral immune activity.40,42 Histopathologically, this may correlate with pronounced lymphoid infiltrates in PML lesions and the surrounding white matter.48,54 Marked evidence of inflammation on MRI (T1 gadolinium-enhancing lesions, swelling on T2/fluid-attenuated inversion recovery [FLAIR]), or histopathologically, particularly when perilesional edema is present, may raise the paraclinical suspicion of PML-IRIS (Figure 3). Nevertheless, we suggest using the term PML-IRIS only when referring to the extreme with clinically relevant CNS inflammation because a continuum of inflammation is noted in PML, and a consensus definition of the term PML-IRIS based on MRI imaging or histopathologic features has not (yet) been established.
PML Prevention
Thus far, preventive measures for patients at increased risk of PML have exclusively been implemented for patients before or during natalizumab treatment. The risk mitigation strategy in place is based on the results of the Stratify-JCV test, treatment history (prior use of classical immunosuppressants), and treatment duration and may also include extended interval dosing in high-risk individuals who opt not to switch to alternative MS treatments available. The consequent application of such strategy, in particular the use of alternative therapy in JCV seropositives, is likely to reduce the overall PML risk in MS populations.
Additional promising biomarkers for PML prediction may include neurofilament light chain levels in blood, host genetic tests, monitoring of JCV genetic variants in various tissues, and prophylactic vaccine development e.g., based on JCV VP1 virus-like particles in populations at risk.34,55,56 These strategies were mainly proposed for and studied in MS populations and did not exceed the experimental level. Considering PML being a rare disease, further validation and clinical implementation particularly outside the MS field may prove to be challenging.
Treatment of PML
Standard of Care for PML
Restoring JCV-specific immunity remains key in clinical practice for treating PML. The approach depends on the underlying condition. Initiation of cART in HIV-infected individuals and the discontinuation of immunosuppressive therapy that caused PML are at the core of current management strategies.6 A clinical study also demonstrated benefit on the PML survival rate after the optimization of a cART regimen in HIV-positive individuals.57 Added value of the use of plasma exchange or immune adsorption for enhanced drug clearance in natalizumab-associated PML is not confirmed58 and needs to be weighed with the potential risk of triggering PML-IRIS on a case-by-case basis.
PML Therapy Studies Listed on ClinicalTrials.gov
As of January 2023, 13 interventional studies had been listed on ClinicalTrials.gov for PML or JCV infection, excluding a study that tested an optimized cART protocol for HIV-PML (see above) and 2 studies that assessed therapies for PML-IRIS. Six of the 13 studies listed are ongoing or remain in planning stage (Table 2).
Table 2.
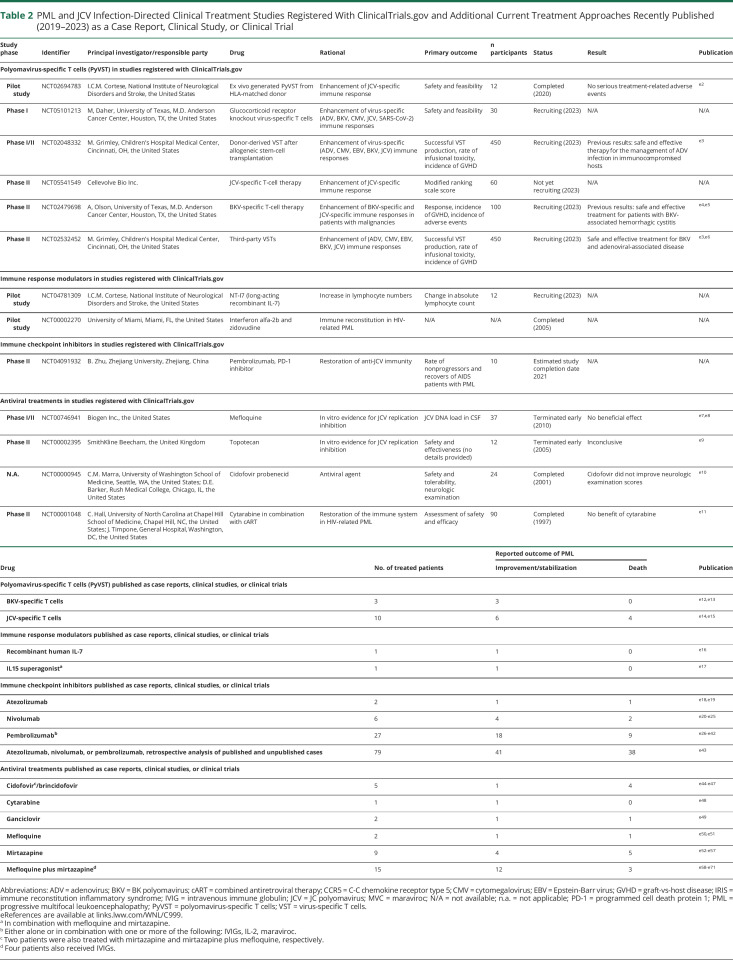
PML and JCV Infection-Directed Clinical Treatment Studies Registered With ClinicalTrials.gov and Additional Current Treatment Approaches Recently Published (2019–2023) as a Case Report, Clinical Study, or Clinical Trial
| Study phase | Identifier | Principal investigator/responsible party | Drug | Rational | Primary outcome | n participants | Status | Result | Publication |
| Polyomavirus-specific T cells (PyVST) in studies registered with ClinicalTrials.gov | |||||||||
| Pilot study | NCT02694783 | I.C.M. Cortese, National Institute of Neurological Disorders and Stroke, the United States | Ex vivo generated PyVST from HLA-matched donor | Enhancement of JCV-specific immune response | Safety and feasibility | 12 | Completed (2020) | No serious treatment-related adverse events | e2 |
| Phase I | NCT05101213 | M, Daher, University of Texas, M.D. Anderson Cancer Center, Houston, TX, the United States | Glucocorticoid receptor knockout virus-specific T cells | Enhancement of virus-specific (ADV, BKV, CMV, JCV, SARS-CoV-2) immune responses | Safety and feasibility | 30 | Recruiting (2023) | N/A | N/A |
| Phase I/II | NCT02048332 | M. Grimley, Children's Hospital Medical Center, Cincinnati, OH, the United States | Donor-derived VST after allogeneic stem-cell transplantation | Enhancement of virus-specific (ADV, CMV, EBV, BKV, JCV) immune responses | Successful VST production, rate of infusional toxicity, incidence of GVHD | 450 | Recruiting (2023) | Previous results: safe and effective therapy for the management of ADV infection in immunocompromised hosts | e3 |
| Phase II | NCT05541549 | Cellevolve Bio Inc. | JCV-specific T-cell therapy | Enhancement of JCV-specific immune response | Modified ranking scale score | 60 | Not yet recruiting (2023) | N/A | N/A |
| Phase II | NCT02479698 | A, Olson, University of Texas, M.D. Anderson Cancer Center, Houston, TX, the United States | BKV-specific T-cell therapy | Enhancement of BKV-specific and JCV-specific immune responses in patients with malignancies | Response, incidence of GVHD, incidence of adverse events | 100 | Recruiting (2023) | Previous results: safe and effective treatment for patients with BKV-associated hemorrhagic cystitis | e4,e5 |
| Phase II | NCT02532452 | M. Grimley, Children's Hospital Medical Center, Cincinnati, OH, the United States | Third-party VSTs | Enhancement of (ADV, CMV, EBV, BKV, JCV) immune responses | Successful VST production, rate of infusional toxicity, incidence of GVHD | 450 | Recruiting (2023) | Safe and effective treatment for BKV and adenoviral-associated disease | e3,e6 |
| Immune response modulators in studies registered with ClinicalTrials.gov | |||||||||
| Pilot study | NCT04781309 | I.C.M. Cortese, National Institute of Neurological Disorders and Stroke, the United States | NT-I7 (long-acting recombinant IL-7) | Increase in lymphocyte numbers | Change in absolute lymphocyte count | 12 | Recruiting (2023) | N/A | N/A |
| Pilot study | NCT00002270 | University of Miami, Miami, FL, the United States | Interferon alfa-2b and zidovudine | Immune reconstitution in HIV-related PML | N/A | N/A | Completed (2005) | N/A | N/A |
| Immune checkpoint inhibitors in studies registered with ClinicalTrials.gov | |||||||||
| Phase II | NCT04091932 | B. Zhu, Zhejiang University, Zhejiang, China | Pembrolizumab, PD-1 inhibitor | Restoration of anti-JCV immunity | Rate of nonprogressors and recovers of AIDS patients with PML | 10 | Estimated study completion date 2021 | N/A | N/A |
| Antiviral treatments in studies registered with ClinicalTrials.gov | |||||||||
| Phase I/II | NCT00746941 | Biogen Inc., the United States | Mefloquine | In vitro evidence for JCV replication inhibition | JCV DNA load in CSF | 37 | Terminated early (2010) | No beneficial effect | e7,e8 |
| Phase II | NCT00002395 | SmithKline Beecham, the United Kingdom | Topotecan | In vitro evidence for JCV replication inhibition | Safety and effectiveness (no details provided) | 12 | Terminated early (2005) | Inconclusive | e9 |
| N.A. | NCT00000945 | C.M. Marra, University of Washington School of Medicine, Seattle, WA, the United States; D.E. Barker, Rush Medical College, Chicago, IL, the United States | Cidofovir probenecid | Antiviral agent | Safety and tolerability, neurologic examination | 24 | Completed (2001) | Cidofovir did not improve neurologic examination scores | e10 |
| Phase II | NCT00001048 | C. Hall, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, the United States; J. Timpone, General Hospital, Washington, DC, the United States | Cytarabine in combination with cART | Restoration of the immune system in HIV-related PML | Assessment of safety and efficacy | 90 | Completed (1997) | No benefit of cytarabine | e11 |
| Drug | No. of treated patients | Reported outcome of PML | Publication | |
| Improvement/stabilization | Death | |||
| Polyomavirus-specific T cells (PyVST) published as case reports, clinical studies, or clinical trials | ||||
| BKV-specific T cells | 3 | 3 | 0 | e12,e13 |
| JCV-specific T cells | 10 | 6 | 4 | e14,e15 |
| Immune response modulators published as case reports, clinical studies, or clinical trials | ||||
| Recombinant human IL-7 | 1 | 1 | 0 | e16 |
| IL15 superagonista | 1 | 1 | 0 | e17 |
| Immune checkpoint inhibitors published as case reports, clinical studies, or clinical trials | ||||
| Atezolizumab | 2 | 1 | 1 | e18,e19 |
| Nivolumab | 6 | 4 | 2 | e20-e25 |
| Pembrolizumabb | 27 | 18 | 9 | e26-e42 |
| Atezolizumab, nivolumab, or pembrolizumab, retrospective analysis of published and unpublished cases | 79 | 41 | 38 | e43 |
| Antiviral treatments published as case reports, clinical studies, or clinical trials | ||||
| Cidofovirc/brincidofovir | 5 | 1 | 4 | e44-e47 |
| Cytarabine | 1 | 1 | 0 | e48 |
| Ganciclovir | 2 | 1 | 1 | e49 |
| Mefloquine | 2 | 1 | 1 | e50,e51 |
| Mirtazapine | 9 | 4 | 5 | e52-e57 |
| Mefloquine plus mirtazapined | 15 | 12 | 3 | e58-e71 |
Abbreviations: ADV = adenovirus; BKV = BK polyomavirus; cART = combined antiretroviral therapy; CCR5 = C-C chemokine receptor type 5; CMV = cytomegalovirus; EBV = Epstein-Barr virus; GVHD = graft-vs-host disease; IRIS = immune reconstitution inflammatory syndrome; IVIG = intravenous immune globulin; JCV = JC polyomavirus; MVC = maraviroc; N/A = not available; n.a. = not applicable; PD-1 = programmed cell death protein 1; PML = progressive multifocal leukoencephalopathy; PyVST = polyomavirus-specific T cells; VST = virus-specific T cells.
eReferences are available at links.lww.com/WNL/C999.
In combination with mefloquine and mirtazapine.
Either alone or in combination with one or more of the following: IVIGs, IL-2, maraviroc.
Two patients were also treated with mirtazapine and mirtazapine plus mefloquine, respectively.
Four patients also received IVIGs.
Experimental Direct Antiviral Therapy
Several antiviral treatments selected based on in vitro evidence have been tested in small clinical trials or case series (Table 2), targeting different aspects of the JCV replication cycle, including DNA replication, retrograde transport, and viral entry. These treatments would be expected to work in all individuals affected by PML, regardless of the underlying disease and immune function. However, none of the drugs tested to date have shown beneficial effects on survival or neurologic disability.2 Among proposed antiviral compounds, mefloquine was the most recently proposed agent, studied in a phase II clinical PML trial that was terminated early.59
Experimental Treatments Leveraging the Immune Response Against JCV
The success of treatments that aim at increasing the host's JCV-directed immune response is anticipated to be variable depending on the underlying condition, genetic factors (such as HLA genes), and the phase of disease (early vs advanced in PML).
Strategies used to restore JCV-specific T-cell responses have included recombinant interleukin-2 (IL-2) and IL-7 that stimulate T-cell growth, proliferation, and survival. While data thus far has been presented by anecdotal evidence from single cases or cases series, a prospective pilot study investigating a long-acting recombinant IL-7 molecule is currently underway (Table 2).
Another approach described in clinical case reports and case series is the use of checkpoint inhibitors targeting programmed cell death protein 1, thus far showing variable results. A study using pembrolizumab in HIV-associated PML listed on ClinicalTrials.gov, with an estimated study completion date in 2021, provides no publicly available data yet (Table 2).
Probably the most promising in clinical development is passive immunization using JCV or BK polyomavirus (BKV)–specific T cells, each tested in several small retrospective cohort studies, suggesting potential beneficial effects60 (Table 2). A first open-label, pilot study using BKV-specific T cells3 supported safety and feasibility. Definite demonstration of efficacy, however, remains to be investigated.
Treatment for PML-IRIS
For the treatment of PML-IRIS, the standard of care is the cautious use of corticosteroids to minimize the damage resulting from a florid immune response, although higher and prolonged doses might interfere with effective viral clearance, possibly leading to negative outcomes. To date, standards for the type, dose, or duration of corticosteroid treatment are lacking. Data on the added use of CCR5-blocking maraviroc did not convincingly demonstrate a benefit, including one prospective study in patients with HIV-associated PML (Table 3).
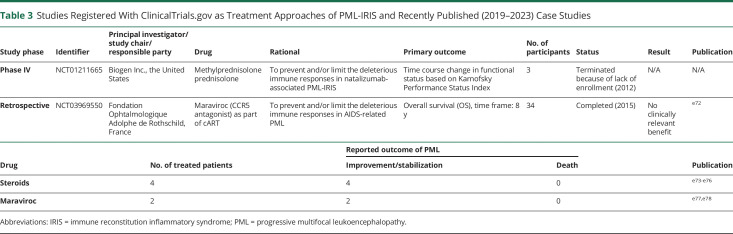
Table 3.
Studies Registered With ClinicalTrials.gov as Treatment Approaches of PML-IRIS and Recently Published (2019–2023) Case Studies
| Study phase | Identifier | Principal investigator/study chair/responsible party | Drug | Rational | Primary outcome | No. of participants | Status | Result | Publication |
| Phase IV | NCT01211665 | Biogen Inc., the United States | Methylprednisolone prednisolone | To prevent and/or limit the deleterious immune responses in natalizumab-associated PML-IRIS | Time course change in functional status based on Karnofsky Performance Status Index | 3 | Terminated because of lack of enrollment (2012) | N/A | N/A |
| Retrospective | NCT03969550 | Fondation Ophtalmologique Adolphe de Rothschild, France | Maraviroc (CCR5 antagonist) as part of cART | To prevent and/or limit the deleterious immune responses in AIDS-related PML | Overall survival (OS), time frame: 8 y | 34 | Completed (2015) | No clinically relevant benefit | e72 |
| Drug | No. of treated patients | Reported outcome of PML | Publication | |
| Improvement/stabilization | Death | |||
| Steroids | 4 | 4 | 0 | e73-e76 |
| Maraviroc | 2 | 2 | 0 | e77,e78 |
Abbreviations: IRIS = immune reconstitution inflammatory syndrome; PML = progressive multifocal leukoencephalopathy.
Perspective and Conclusions–Toward PML Becoming a Treatable Disease
Weighing the currently available possible treatment options, BKV-specific or JCV-specific T cells and enhancement of T-cell responses using checkpoint inhibitors or recombinant cytokines appear the most promising experimental strategies and warrant testing in prospective clinical trials. Furthermore, recent advances in gene therapy and the speed with which SARS-CoV-2–directed novel treatment options were advanced create justified hope for the future development also of effective direct antivirals.
The development of PML-specific treatment, however, faces several methodological and ethical obstacles because of its rarity and severity as well as the heterogeneity of affected patients.e89
PML is a rare disease. Therefore, limited clinical data and few clinically established biomarkers are available. Recruiting large numbers of patients will also be challenging.
PML being a relatively rapid and often fatal disease with no validated treatment so far, the recruitment, acceptability, and feasibility of complex study protocols might prove to be difficult.
The heterogeneity of underlying diseases in PML individuals makes the selection of trial end points and interventional approaches challenging. The prognosis of PML depends on the underlying condition and the ability to reconstitute the immune system. Thus, large numbers of patients or more homogenous subcohorts are needed to determine which type of patient can benefit from which type of treatment strategy.
Outcome measures for treatment response need to be sensitive, robust, and generally accepted. Ethical considerations may hinder use of robust clinical end points such as survival without offering rescue therapy to individuals that show deterioration, e.g., clinically or by MRI.
Considering the challenges mentioned above and aiming at a successful phase II/III clinical trial for PML, a study design using robust surrogate markers of response to therapy is needed. Possible surrogate biomarkers, still requiring validation, may include the following:
Standardized MRI protocols, e.g., the time to stable MRI40
Fold change in JCV DNA load in CSF
Robust measures of anti-JCV–directed humoral and T-cell responses, including PML type variant-specific responses and CSF measures.
To improve PML diagnostics and identify surrogate markers of treatment response, at the German National Reference Center for Papillomavirus and Polyomavirus, we currently work on developing more sensitive molecular assays for JCV DNA detection. These efforts comprise preanalytic steps, including the enrichment of EV-associated JCV DNA and the distinct quantification of different viral variants.e90 In addition, we work on high-throughput techniques, based on flow cytometry or microsphere hybridization assays, which might allow us to robustly study JCV viral variant-specific cellular and humoral immunity.e91 Efforts at the National Institute of Neurological Disorders and Stroke (NIH) include an ongoing natural history study of PML (NCT01730131) to identify robust surrogate outcomes and the application of these to early-phase prospective interventional studies (Table 2). To raise awareness of this severe disease and to document the natural history of the disease as a basis for conducting therapy studies, the CurePML Registry was established in Hannover, Germany.
Sufficient funding and research efforts to overcome the remaining hurdles are warranted, considering PML is a devastating disease and a possible iatrogenic complication in a highly effective class of biologicals. As variable treatment responses can be expected, particularly for compounds that aim at increasing JCV-directed immunity of the host, the success of definitive trials that aim at demonstrating efficacy will critically depend on careful selection of study participants, e.g., based on the underlying condition, the stage of the disease, and possibly also genetic factors. Nevertheless, considering the recent advances in understanding JCV biology and in biomarker development including imaging, blood, and CSF surrogates, as well as in immune therapeutics, we believe that PML may soon become a condition with more specific treatment options available.
Glossary
- AAN
American Academy of Neurology
- ADC
apparent diffusion coefficient
- BKV
BK polyomavirus
- cART
combined antiretroviral therapy
- DMT
disease-modifying treatments
- EV
extracellular vesicle
- FLAIR
fluid-attenuated inversion recovery
- GCN
granule cell neuronopathy
- IL
interleukin
- IRIS
immune reconstitution inflammatory syndrome
- JCV
JC polyomavirus
- MS
multiple sclerosis
- NCCR
noncoding control region
- PML
progressive multifocal leukoencephalopathy
- VP1
viral protein 1
Appendix. Authors
| Name | Location | Contribution |
| Finja Schweitzer, PhD | Department of Neurology, Facuty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Sarah Laurent, MD, PhD | Department of Neurology, Facuty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Irene Cortese, MD | Experimental Immunotherapeutics Unit, NIH, Bethesda, MD | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Gereon R. Fink, MD | Department of Neurology, Facuty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Cognitive Neuroscience, Institute of Neuroscience and Medicine (INM-3), Research Centre Jülich, Jülich, Germany | Drafting/revision of the manuscript for content, including medical writing for content |
| Steffi Silling, PhD | Institute of Virology, National Reference Center for Papilloma- and Polyomaviruses, Faculty of Medicine, University Hospital Cologne, Cologne Germany | Drafting/revision of the manuscript for content, including medical writing for content |
| Thomas Skripuletz, MD | Department of Neurology, Hannover Medical School, Hannover, Germany | Drafting/revision of the manuscript for content, including medical writing for content |
| Imke Metz, MD | Institute of Neuropathology, University Medical Center Göttingen, Göttingen, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Mike P. Wattjes, MD, PhD | Department of Neuroradiology, Hannover Medical School, Hannover, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Clemens Warnke, MD | Department of Neurology, Facuty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Footnotes
CME Course: NPub.org/cmelist
Study Funding
F. Schweitzer and C. Warnke are supported by the German Research Foundation (501362249). I. Cortese is supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program. S. Silling is supported by the German Federal Ministry of Health (German National Reference Center for Papilloma- and Polyomaviruses; Grant No. 1369-401).
Disclosure
F. Schweitzer and S. Laurent report no disclosures relevant to the manuscript. I. Cortese is a shareholder of Nouscom AG, Keires AG, and PDC*line pharma (outside the submitted work) and sits on the scientific advisory board for Cellevolve. G.R. Fink and S. Silling report no disclosures relevant to the manuscript. T. Skripuletz reports research support from Alnylam Pharmaceuticals, Bristol-Myers Squibb Foundation for Immuno-Oncology, CSL Behring, Novartis, Sanofi Genzyme, VHV Stiftung and honoraria for lectures and travel grants from Alexion, Alnylam Pharmaceuticals, Argenx, Bayer Vital, Biogen, Celgene, Centogene, CSL Behring, Euroimmun, Janssen, Merck Serono, Novartis, Pfizer, Roche, Sanofi, Siemens, Sobi, Teva. I. Metz reports personal fees from Biogen Idec, Bayer Healthcare, TEVA, Serono, Novartis Pharma GmbH, Genzyme, Roche, grants from Biogen Idec, Genzyme, and Novartis Pharma GmbH, outside the submitted work. M.P. Wattjes received speaker or consultancy honoraria from Bayer Healthcare, Biogen, Biologix, Celgene, Genilac, Imcyse, IXICO, Medison, Merck-Serono, Novartis, Roche, Sanofi-Genzyme. C. Warnke has received institutional support from Novartis, Alexion, Sanofi Genzyme, Biogen, Merck, and Roche. Go to Neurology.org/N for full disclosures.
References
- 1.Joly M, Conte C, Cazanave C, et al. Progressive multifocal leukoencephalopathy: epidemiology and spectrum of predisposing conditions. Brain. 2023;146(1):349-358. doi. 10.1093/brain/awac237 [DOI] [PubMed] [Google Scholar]
- 2.Pavlovic D, Patera AC, Nyberg F, Gerber M, Liu M, Progressive Multifocal Leukeoncephalopathy Consortium. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord. 2015;8(6):255-273. doi. 10.1177/1756285615602832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortese I, Beck ES, Al-Louzi O, et al. BK virus-specific T cells for immunotherapy of progressive multifocal leukoencephalopathy: an open-label, single-cohort pilot study. Lancet Neurol. 2021;20(8):639-652. doi. 10.1016/S1474-4422(21)00174-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warnke C, Olsson T, Hartung HP. PML: the dark side of immunotherapy in multiple sclerosis. Trends Pharmacol Sci. 2015;36(12):799-801. doi. 10.1016/j.tips.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 5.Astrom KE, Mancall EL, Richardson EP. Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain J Neurol. 1958;81(1):93-111. doi. 10.1093/brain/81.1.93 [DOI] [PubMed] [Google Scholar]
- 6.Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. 2021;17(1):37-51. doi. 10.1038/s41582-020-00427-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryerson LZ, Foley J, Chang I, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurol. 2019;93(15):e1452-e1462. doi. 10.1212/WNL.0000000000008243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley JF, Defer G, Ryerson LZ, et al. Comparison of switching to 6-week dosing of natalizumab versus continuing with 4-week dosing in patients with relapsing-remitting multiple sclerosis (NOVA): a randomised, controlled, open-label, phase 3b trial. Lancet Neurol. 2022;21(7):608-619. doi. 10.1016/S1474-4422(22)00143-0 [DOI] [PubMed] [Google Scholar]
- 9.Warnke C, Menge T, Hartung HP, et al. Natalizumab and progressive multifocal leukoencephalopathy: what are the causal factors? Can it be avoided? Arch Neurol. 2010;67(8):923-930. doi. 10.1001/archneurol.2010.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerbe CS, Marciano BE, Katial RK, et al. Progressive multifocal leukoencephalopathy in primary immune deficiencies: stat1 gain of function and review of the literature. Clin Infect Dis. 2016;62(8):986-994. doi. 10.1093/cid/civ1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113(20):4834-4840. doi. 10.1182/blood-2008-10-186999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goereci Y, Schweitzer F, Wellstein A, et al. Clearance of JC polyomavirus from cerebrospinal fluid following treatment with interleukin-2 and pembrolizumab in an individual with progressive multifocal leukoencephalopathy and no underlying immune deficiency syndrome. Eur J Neurol. 2020;27(11):2375-2377. doi. 10.1111/ene.14435 [DOI] [PubMed] [Google Scholar]
- 13.Raisch DW, Rafi JA, Chen C, Bennett CL. Detection of cases of progressive multifocal leukoencephalopathy associated with new biologicals and targeted cancer therapies from the FDA's adverse event reporting system. Expert Opin Drug Saf. 2016;15(8):1003-1011. doi. 10.1080/14740338.2016.1198775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenkranz SC, Häußler V, Kolster M, et al. Treating sarcoidosis-associated progressive multifocal leukoencephalopathy with infliximab. Brain Commun. 2022;4(1):fcab292. doi. 10.1093/braincomms/fcab292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sdrimas K, Diaz-Paez M, Camargo JF, Lekakis LJ. Progressive multifocal leukoencephalopathy after CAR T therapy. Int J Hematol. 2020;112(1):118-121. doi. 10.1007/s12185-020-02840-x [DOI] [PubMed] [Google Scholar]
- 16.Koralnik IJ, Wüthrich C, Dang X, et al. JC virus granule cell neuronopathy: a novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol. 2005;57(4):576-580. doi. 10.1002/ana.20431 [DOI] [PubMed] [Google Scholar]
- 17.Agnihotri SP, Dang X, Carter JL, et al. JCV GCN in a natalizumab-treated MS patient is associated with mutations of the VP1 capsid gene. Neurology. 2014;83(8):727-732. doi. 10.1212/WNL.0000000000000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wüthrich C, Cheng YM, Joseph JT, et al. Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 2009;68(1):15-25. doi. 10.1097/NEN.0b013e3181912570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozic C, Subramanyam M, Richman S, Plavina T, Zhang A, Ticho B. Anti-JC virus (JCV) antibody prevalence in the JCV Epidemiology in MS (JEMS) trial. Eur J Neurol. 2014;21(2):299-304. doi. 10.1111/ene.12304 [DOI] [PubMed] [Google Scholar]
- 20.Reid CE, Li H, Sur G, et al. Sequencing and analysis of JC virus DNA from natalizumab-treated PML patients. J Infect Dis. 2011;204(2):237-244. doi. 10.1093/infdis/jir256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorelik L, Reid C, Testa M, et al. Progressive multifocal leukoencephalopathy (PML) development is associated with mutations in JC virus capsid protein VP1 that change its receptor specificity. J Infect Dis. 2011;204(1):103-114. doi. 10.1093/infdis/jir198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Major EO, Frohman E, Douek D. JC viremia in natalizumab-treated patients with multiple sclerosis. N Engl J Med. 2013;368(23):2240-2241. doi. 10.1056/NEJMc1214233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meira M, Sievers C, Hoffmann F, et al. Natalizumab-induced POU2AF1/Spi-B upregulation: a possible route for PML development. Neurol Neuroimmunol Neuroinflammation. 2016;3(3):e223. doi. 10.1212/NXI.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warnke C, Smolianov V, Dehmel T, et al. CD34+ progenitor cells mobilized by natalizumab are not a relevant reservoir for JC virus. Mult Scler Houndmills Basingstoke Engl. 2011;17(2):151-156. doi. 10.1177/1352458510385834 [DOI] [PubMed] [Google Scholar]
- 25.Morris-Love J, Gee GV, O'Hara BA, et al. JC polyomavirus uses extracellular vesicles to infect target cells. mBio. 2019;10(2):e00379-19. doi. 10.1128/mBio.00379-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Hara BA, Morris-Love J, Gee GV, Haley SA, Atwood WJ. JC Virus infected choroid plexus epithelial cells produce extracellular vesicles that infect glial cells independently of the virus attachment receptor. PLoS Pathog. 2020;16(3):e1008371. doi. 10.1371/journal.ppat.1008371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scribano S, Guerrini M, Arvia R, et al. Archetype JC polyomavirus DNA associated with extracellular vesicles circulates in human plasma samples. J Clin Virol. 2020;128:104435. doi. 10.1016/j.jcv.2020.104435 [DOI] [PubMed] [Google Scholar]
- 28.Ferenczy MW, Marshall LJ, Nelson CDS, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 2012;25(3):471-506. doi. 10.1128/CMR.05031-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gosert R, Kardas P, Major EO, Hirsch HH. Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate. J Virol. 2010;84(20):10448-10456. doi. 10.1128/JVI.00614-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauver MD, Lukacher AE. JCPyV VP1 mutations in progressive multifocal leukoencephalopathy: altering tropism or mediating immune evasion? Viruses. 2020;12(10):1156. doi. 10.3390/v12101156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geoghegan EM, Pastrana DV, Schowalter RM, et al. Infectious entry and neutralization of pathogenic JC Polyomaviruses. Cell Rep. 2017;21(5):1169-1179. doi. 10.1016/j.celrep.2017.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundqvist E, Buck D, Warnke C, et al. JC polyomavirus infection is strongly controlled by human leucocyte antigen class II variants. PLoS Pathog. 2014;10(4):e1004084. doi. 10.1371/journal.ppat.1004084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frenken P, Hartung HP, Olsson T, Adams O, Warnke C. Type O blood group associates with higher anti-JC polyomavirus antibody levels. Brain Behav. 2021;11(8):e2298. doi. 10.1002/brb3.2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatchwell E, Smith EB, Jalilzadeh S, et al. Progressive multifocal leukoencephalopathy genetic risk variants for pharmacovigilance of immunosuppressant therapies. Front Neurol. 2022;13:1016377. 10.3389/fneur.2022.1016377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Power C, Gladden JG, Halliday W, et al. AIDS- and non-AIDS-related PML association with distinct p53 polymorphism. Neurology. 2000;54(3):743-746. doi. 10.1212/wnl.54.3.743 [DOI] [PubMed] [Google Scholar]
- 36.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430-1438. doi. 10.1212/WNL.0b013e31828c2fa1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miskin DP, Koralnik IJ. Novel syndromes associated with JC virus infection of neurons and meningeal cells: no longer a gray area. Curr Opin Neurol. 2015;28(3):288-294. doi. 10.1097/wco.0000000000000201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergui M, Bradac GB, Oguz KK, et al. Progressive multifocal leukoencephalopathy: diffusion-weighted imaging and pathological correlations. Neuroradiology. 2004;46(1):22-25. doi. 10.1007/s00234-003-1115-9 [DOI] [PubMed] [Google Scholar]
- 39.Wattjes MP, Barkhof F. Diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy using MRI. Curr Opin Neurol. 2014;27(3):260-270. doi. 10.1097/WCO.0000000000000099 [DOI] [PubMed] [Google Scholar]
- 40.Baldassari LE, Wattjes MP, Cortese ICM, et al. The neuroradiology of progressive multifocal leukoencephalopathy: a clinical trial perspective. Brain. 2022;145(2):426-440. doi. 10.1093/brain/awab419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wijburg MT, Witte BI, Vennegoor A, et al. MRI criteria differentiating asymptomatic PML from new MS lesions during natalizumab pharmacovigilance. J Neurol Neurosurg Psychiatry. 2016;87(10):1138-1145. doi. 10.1136/jnnp-2016-313772 [DOI] [PubMed] [Google Scholar]
- 42.Wattjes MP, Wijburg MT, van Eijk J, et al. Inflammatory natalizumab-associated PML: baseline characteristics, lesion evolution and relation with PML-IRIS. J Neurol Neurosurg Psychiatry. 2018;89(5):535-541. doi. 10.1136/jnnp-2017-316886 [DOI] [PubMed] [Google Scholar]
- 43.Blankenbach K, Schwab N, Hofner B, Adams O, Keller-Stanislawski B, Warnke C. Natalizumab-associated progressive multifocal leukoencephalopathy in Germany. Neurology. 2019;92(19):e2232-e2239. doi. 10.1212/WNL.0000000000007451 [DOI] [PubMed] [Google Scholar]
- 44.Maas RPPWM, Muller-Hansma AHG, Esselink RAJ, et al. Drug-associated progressive multifocal leukoencephalopathy: a clinical, radiological, and cerebrospinal fluid analysis of 326 cases. J Neurol. 2016;263(10):2004-2021. doi. 10.1007/s00415-016-8217-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzocchetti A, Di Giambenedetto S, Cingolani A, Ammassari A, Cauda R, De Luca A. Reduced rate of diagnostic positive detection of JC virus DNA in cerebrospinal fluid in cases of suspected progressive multifocal leukoencephalopathy in the era of potent antiretroviral therapy. J Clin Microbiol. 2005;43(8):4175-4177. doi. 10.1128/JCM.43.8.4175-4177.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ngouth N, Monaco MC, Walker L, et al. Comparison of qPCR with ddPCR for the quantification of JC polyomavirus in CSF from patients with progressive multifocal leukoencephalopathy. Viruses. 2022;14(6):1246. doi. 10.3390/v14061246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warnke C, von Geldern G, Markwerth P, et al. Cerebrospinal fluid JC virus antibody index for diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76(6):792-801. doi. 10.1002/ana.24153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metz I, Radue EW, Oterino A, et al. Pathology of immune reconstitution inflammatory syndrome in multiple sclerosis with natalizumab-associated progressive multifocal leukoencephalopathy. Acta Neuropathol (Berl). 2012;123(2):235-245. doi. 10.1007/s00401-011-0900-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wattjes MP, Vennegoor A, Steenwijk MD, et al. MRI pattern in asymptomatic natalizumab-associated PML. J Neurol Neurosurg Psychiatry. 2015;86(7):793-798. doi. 10.1136/jnnp-2014-308630 [DOI] [PubMed] [Google Scholar]
- 50.Dong-Si T, Richman S, Wattjes MP, et al. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Ann Clin Transl Neurol. 2014;1(10):755-764. doi. 10.1002/acn3.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wijburg MT, Warnke C, Killestein J, Wattjes MP. Application of “Mentzer's PML case definition” to natalizumab-treated patients in the setting of strict MRI-based pharmacovigilance. J Neurol. 2020;267(9):2599-2602. doi. 10.1007/s00415-020-09880-7 [DOI] [PubMed] [Google Scholar]
- 52.Cinque P, Koralnik IJ, Gerevini S, Miro JM, Price RW. Progressive multifocal leukoencephalopathy complicating HIV-1 infection. Lancet Infect Dis. 2009;9(10):625-636. doi. 10.1016/S1473-3099(09)70226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan IL, McArthur JC, Clifford DB, Major EO, Nath A. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology. 2011;77(11):1061-1067. doi. 10.1212/WNL.0b013e31822e55e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vendrely A, Bienvenu B, Gasnault J, Thiebault JB, Salmon D, Gray F. Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol (Berl). 2005;109(4):449-455. doi. 10.1007/s00401-005-0983-y [DOI] [PubMed] [Google Scholar]
- 55.Fissolo N, Pignolet B, Rio J, et al. Serum neurofilament levels and PML risk in patients with multiple sclerosis treated with natalizumab. Neurol Neuroimmunol Neuroinflammation. 2021;8(4):e1003. doi. 10.1212/NXI.0000000000001003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jelcic I, Combaluzier B, Jelcic I, Sospedra M, Grimm J, Martin R. Prevention and therapy of JC polyomavirus-mediated progressive multifocal leukoencephalopathy–a realistic possibility? Swiss Med Wkly. 2017;147:w14520. doi. 10.4414/smw.2017.14520 [DOI] [PubMed] [Google Scholar]
- 57.Gasnault J, Costagliola D, Hendel-Chavez H, et al. Improved survival of HIV-1-infected patients with progressive multifocal leukoencephalopathy receiving early 5-drug combination antiretroviral therapy. PLoS One. 2011;6(6):e20967. doi. 10.1371/journal.pone.0020967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scarpazza C, Prosperini L, De Rossi N, et al. To do or not to do? plasma exchange and timing of steroid administration in progressive multifocal leukoencephalopathy. Ann Neurol. 2017;82(5):697-705. doi. 10.1002/ana.25070 [DOI] [PubMed] [Google Scholar]
- 59.Clifford DB, Nath A, Cinque P, et al. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol. 2013;19(4):351-358. doi. 10.1007/s13365-013-0173-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Möhn N, Grote-Levi L, Hopfner F, et al. Innovative therapeutic concepts of progressive multifocal leukoencephalopathy. J Neurol. 2022;269(5):2403-2413. doi. 10.1007/s00415-021-10952-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- eReferences are available at links.lww.com/WNL/C999.