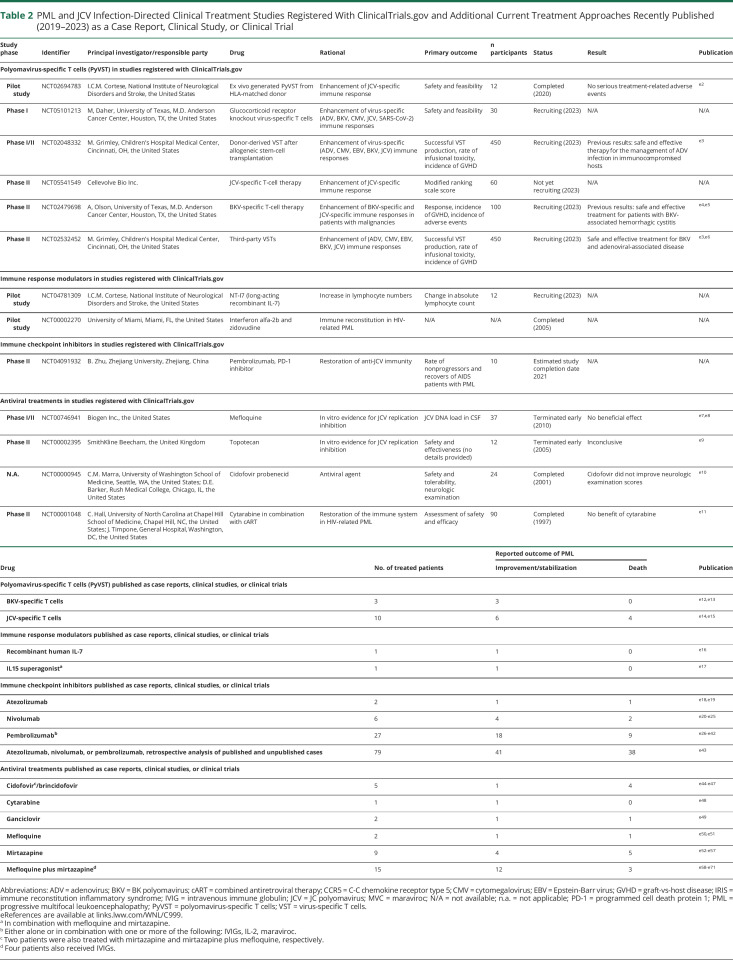
Table 2.
PML and JCV Infection-Directed Clinical Treatment Studies Registered With ClinicalTrials.gov and Additional Current Treatment Approaches Recently Published (2019–2023) as a Case Report, Clinical Study, or Clinical Trial
| Study phase | Identifier | Principal investigator/responsible party | Drug | Rational | Primary outcome | n participants | Status | Result | Publication |
| Polyomavirus-specific T cells (PyVST) in studies registered with ClinicalTrials.gov | |||||||||
| Pilot study | NCT02694783 | I.C.M. Cortese, National Institute of Neurological Disorders and Stroke, the United States | Ex vivo generated PyVST from HLA-matched donor | Enhancement of JCV-specific immune response | Safety and feasibility | 12 | Completed (2020) | No serious treatment-related adverse events | e2 |
| Phase I | NCT05101213 | M, Daher, University of Texas, M.D. Anderson Cancer Center, Houston, TX, the United States | Glucocorticoid receptor knockout virus-specific T cells | Enhancement of virus-specific (ADV, BKV, CMV, JCV, SARS-CoV-2) immune responses | Safety and feasibility | 30 | Recruiting (2023) | N/A | N/A |
| Phase I/II | NCT02048332 | M. Grimley, Children's Hospital Medical Center, Cincinnati, OH, the United States | Donor-derived VST after allogeneic stem-cell transplantation | Enhancement of virus-specific (ADV, CMV, EBV, BKV, JCV) immune responses | Successful VST production, rate of infusional toxicity, incidence of GVHD | 450 | Recruiting (2023) | Previous results: safe and effective therapy for the management of ADV infection in immunocompromised hosts | e3 |
| Phase II | NCT05541549 | Cellevolve Bio Inc. | JCV-specific T-cell therapy | Enhancement of JCV-specific immune response | Modified ranking scale score | 60 | Not yet recruiting (2023) | N/A | N/A |
| Phase II | NCT02479698 | A, Olson, University of Texas, M.D. Anderson Cancer Center, Houston, TX, the United States | BKV-specific T-cell therapy | Enhancement of BKV-specific and JCV-specific immune responses in patients with malignancies | Response, incidence of GVHD, incidence of adverse events | 100 | Recruiting (2023) | Previous results: safe and effective treatment for patients with BKV-associated hemorrhagic cystitis | e4,e5 |
| Phase II | NCT02532452 | M. Grimley, Children's Hospital Medical Center, Cincinnati, OH, the United States | Third-party VSTs | Enhancement of (ADV, CMV, EBV, BKV, JCV) immune responses | Successful VST production, rate of infusional toxicity, incidence of GVHD | 450 | Recruiting (2023) | Safe and effective treatment for BKV and adenoviral-associated disease | e3,e6 |
| Immune response modulators in studies registered with ClinicalTrials.gov | |||||||||
| Pilot study | NCT04781309 | I.C.M. Cortese, National Institute of Neurological Disorders and Stroke, the United States | NT-I7 (long-acting recombinant IL-7) | Increase in lymphocyte numbers | Change in absolute lymphocyte count | 12 | Recruiting (2023) | N/A | N/A |
| Pilot study | NCT00002270 | University of Miami, Miami, FL, the United States | Interferon alfa-2b and zidovudine | Immune reconstitution in HIV-related PML | N/A | N/A | Completed (2005) | N/A | N/A |
| Immune checkpoint inhibitors in studies registered with ClinicalTrials.gov | |||||||||
| Phase II | NCT04091932 | B. Zhu, Zhejiang University, Zhejiang, China | Pembrolizumab, PD-1 inhibitor | Restoration of anti-JCV immunity | Rate of nonprogressors and recovers of AIDS patients with PML | 10 | Estimated study completion date 2021 | N/A | N/A |
| Antiviral treatments in studies registered with ClinicalTrials.gov | |||||||||
| Phase I/II | NCT00746941 | Biogen Inc., the United States | Mefloquine | In vitro evidence for JCV replication inhibition | JCV DNA load in CSF | 37 | Terminated early (2010) | No beneficial effect | e7,e8 |
| Phase II | NCT00002395 | SmithKline Beecham, the United Kingdom | Topotecan | In vitro evidence for JCV replication inhibition | Safety and effectiveness (no details provided) | 12 | Terminated early (2005) | Inconclusive | e9 |
| N.A. | NCT00000945 | C.M. Marra, University of Washington School of Medicine, Seattle, WA, the United States; D.E. Barker, Rush Medical College, Chicago, IL, the United States | Cidofovir probenecid | Antiviral agent | Safety and tolerability, neurologic examination | 24 | Completed (2001) | Cidofovir did not improve neurologic examination scores | e10 |
| Phase II | NCT00001048 | C. Hall, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, the United States; J. Timpone, General Hospital, Washington, DC, the United States | Cytarabine in combination with cART | Restoration of the immune system in HIV-related PML | Assessment of safety and efficacy | 90 | Completed (1997) | No benefit of cytarabine | e11 |
| Drug | No. of treated patients | Reported outcome of PML | Publication | |
| Improvement/stabilization | Death | |||
| Polyomavirus-specific T cells (PyVST) published as case reports, clinical studies, or clinical trials | ||||
| BKV-specific T cells | 3 | 3 | 0 | e12,e13 |
| JCV-specific T cells | 10 | 6 | 4 | e14,e15 |
| Immune response modulators published as case reports, clinical studies, or clinical trials | ||||
| Recombinant human IL-7 | 1 | 1 | 0 | e16 |
| IL15 superagonista | 1 | 1 | 0 | e17 |
| Immune checkpoint inhibitors published as case reports, clinical studies, or clinical trials | ||||
| Atezolizumab | 2 | 1 | 1 | e18,e19 |
| Nivolumab | 6 | 4 | 2 | e20-e25 |
| Pembrolizumabb | 27 | 18 | 9 | e26-e42 |
| Atezolizumab, nivolumab, or pembrolizumab, retrospective analysis of published and unpublished cases | 79 | 41 | 38 | e43 |
| Antiviral treatments published as case reports, clinical studies, or clinical trials | ||||
| Cidofovirc/brincidofovir | 5 | 1 | 4 | e44-e47 |
| Cytarabine | 1 | 1 | 0 | e48 |
| Ganciclovir | 2 | 1 | 1 | e49 |
| Mefloquine | 2 | 1 | 1 | e50,e51 |
| Mirtazapine | 9 | 4 | 5 | e52-e57 |
| Mefloquine plus mirtazapined | 15 | 12 | 3 | e58-e71 |
Abbreviations: ADV = adenovirus; BKV = BK polyomavirus; cART = combined antiretroviral therapy; CCR5 = C-C chemokine receptor type 5; CMV = cytomegalovirus; EBV = Epstein-Barr virus; GVHD = graft-vs-host disease; IRIS = immune reconstitution inflammatory syndrome; IVIG = intravenous immune globulin; JCV = JC polyomavirus; MVC = maraviroc; N/A = not available; n.a. = not applicable; PD-1 = programmed cell death protein 1; PML = progressive multifocal leukoencephalopathy; PyVST = polyomavirus-specific T cells; VST = virus-specific T cells.
eReferences are available at links.lww.com/WNL/C999.
In combination with mefloquine and mirtazapine.
Either alone or in combination with one or more of the following: IVIGs, IL-2, maraviroc.
Two patients were also treated with mirtazapine and mirtazapine plus mefloquine, respectively.
Four patients also received IVIGs.