Abstract
Background and Objectives
Ocular myasthenia gravis (OMG) is an autoimmune disorder resulting in ocular symptoms such as diplopia and ptosis. The proportion of patients who convert to secondary generalized myasthenia gravis (SGMG) reported in the literature has been varied. The aim of this systematic review was to determine the clinical characteristics of patients with OMG and the proportion of SGMG conversion.
Methods
We conducted an electronic database search for randomized controlled trials, prospective nonrandomized studies, observational studies, and retrospective studies in EMBASE, CENTRAL, MEDLINE, and Web of Science. We included studies with patients with OMG who initially presented with ocular symptoms and signs only and were seen in clinical practice, reporting on the characteristics and outcomes of SGMG. We excluded studies with pediatric and congenital myasthenia gravis populations. Eligible studies included articles written in any language and containing data on patients with OMG. The main outcome measured was the proportion of patients with OMG who converted to SGMG and risk factors associated with secondary generalization of OMG. Two independent reviewers screened titles and abstracts and extracted data from full texts, reporting findings according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The methodology was evaluated using the Joanna Briggs Institute critical appraisal forms. PROSPERO registration number: CRD2021285257.
Results
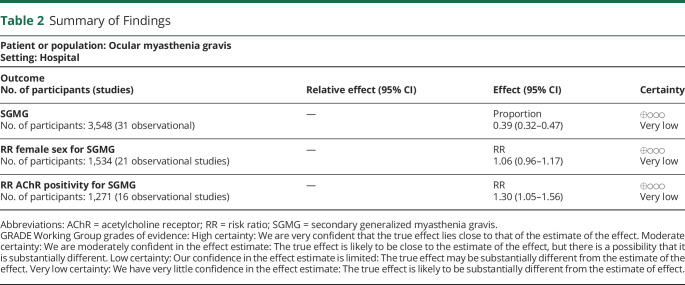
Thirty-one studies were included in the quantitative and qualitative analysis. The proportion of generalization ranged from 11% to 84%. The pooled proportion was 39% (95% CI 32%–47%, I2 = 95.86%, p < 0.001 unweighted, low certainty). The pooled risk ratio of female sex for conversion to SGMG was 1.06 (95% CI 0.96–1.17, I2 = 0% p = 0.614, 21 studies included, very low certainty), and the pooled risk ratio of acetylcholine receptor (AChR) positivity was 1.30 (95% CI 1.05–1.56, I2 = 0% p = 0.455, 16 studies included, very low certainty).
Discussion
Risk factors such as female sex and anti-AChR positivity have been identified to have possible associations with SGMG, but there are not enough quality observational studies. There is a need for a prospective global database of patients with OMG, including all countries with different populations.
Introduction
Ocular myasthenia gravis (OMG) is an autoimmune disorder affecting the neuromuscular junction, resulting in diplopia and ptosis. Secondary generalized myasthenia gravis (SGMG) occurs when patients with initial ocular symptoms develop muscle weakness in the bulbar muscles, limbs, or respiration.1 The literature results of this have been widespread and variable, with the proportion of patients with OMG converting to secondary generalization varying between 30% and 80%, frequently within the first 2 years of onset of ocular symptoms.2
The clinical course of OMG is characterized by exacerbations and remissions. The management involves treatments with pyridostigmine or corticosteroids and/or immunosuppressants. When managing patients with OMG, one of the main concerns for both the patient and the clinician is conversion to the generalized form. Adding on to this, it is hard to establish the natural history of OMG. Studying a true, population-based cohort is challenging because treatment with immunosuppression or the exclusion of patients treated with immunosuppression may alter the reported natural history of the disease. Understandably, most population-based cohorts are retrospective, and various risk factors have been associated accordingly with secondary generalization. An example of a reported risk factor is the presence of acetylcholine receptors (AChRs), muscle-specific kinase (MuSK), or low-density lipoprotein receptor-related protein 4 (LRP4).1,3 A percentage of patients with OMG are found to have thymic hyperplasia or thymoma on radiology imaging, and this has been shown to be associated with OMG.4,5
A growing number of articles have been published on the risk factors associated with SGMG.6-8 It is an opportune time for a systematic review to guide future clinical studies in this field.9 This review focuses on the clinical characteristics of patients with OMG and reports on the proportion who develop SGMG.
Methods
This systematic review was performed guided by the Joanna Briggs Institute methodology for systematic reviews of effectiveness evidence.10,11 The protocol of this systematic review was prospectively registered on the PROSPERO database (registration number: CRD2021285257).
Search Strategy
We conducted a systematic electronic database search for randomized controlled trials, prospective nonrandomized studies, and observational studies in EMBASE, CENTRAL, MEDLINE, and Web of Science. See eTable 1 (links.lww.com/WNL/D67) for the search strategy including index terms and keywords. The reference list of all studies selected was assessed for additional studies. The full-database search was last performed in December 2022.
Study Selection
All identified citations were gathered and uploaded to a reference management software (Endnote X9, Clarivate Analytics), and duplicate citations were removed. All eligible results were independently screened by 2 review authors by title, abstract, and then by full text. We reported outcomes guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12 All discrepancies between the authors were resolved by discussion. We documented the excluded study numbers with exclusion reasons in Figure 1 PRISMA flow diagram.12
Figure 1. Flowchart of Studies Included in This Systematic Review.
Flowchart summarizing included and excluded studies, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. MG = myasthenia gravis; OMG = ocular MG; SGMG = secondary generalized MG.
Inclusion Criteria
Types of Studies
Because there were few randomized controlled trials involving patients with OMG in the literature, we included a wide range of studies to have a comprehensive overview and preserve generalizability without reducing validity. These included nonrandomized studies and observational studies. We only included full articles and excluded case reports or small case series of less than 10 participants. We included studies in all languages. Where there were publications of duplicate data sets, we chose the publications with the most comprehensive reports of data sets.
Types of Participants
We considered studies or trials of patients with OMG, described consecutively through a clinical setting, or a population-based study. We excluded articles that selected patients based on interventions alone (e.g., an observational study of patients only treated with prednisolone). We excluded studies involving pediatric and congenital myasthenia gravis (MG) populations.
Based on the proposals for systematic review reporting on prevalence and incidence by Munn et al.,11 our inclusion criteria were established as:
Condition: OMG
Context: Data from a clinical setting or population-based registries
Population: Adults with OMG at onset
Outcomes
The primary outcome of this review was the proportion of patients with OMG who converted to SGMG. Secondary outcomes included reported risk factors associated with secondary generalization, age, sex, presenting features, and investigations (serologic, neurophysiologic, and thymic imaging)
Assessment of Methodologic Quality
Included studies were critically appraised by 2 independent assessors using standardized appraisal guidance from the Joanna Briggs Institute.10,13,14 Where necessary, we contacted corresponding authors of studies to request missing data or for clarification. Two reviewers assessed the risk of bias independently, and the results were reported in descriptive text and summarized in a table. The study methodology was assessed using the Ottawa Newcastle Scale.10,13,15 Any discrepancies were resolved through discussion.
Data Extraction
Two independent reviewers extracted data from included studies guided by the Joanna Briggs Institute standardized data extraction tools.10,13,14 To reduce errors, we used a data extraction form. Data extracted included16
Baseline characteristics such as country and sample size
Trial type
Patient characteristics such as age, sex, and ethnicity
Number of patients withdrawn from the study or lost to follow-up
The proportion of patients with OMG who converted to SGMG and time to generalization
Follow-up duration
Medical intervention such as immunosuppression
Antibody status (e.g., AChR, MuSK or LRP4 antibodies) or double seronegativity
Thymic abnormalities on imaging or thymic surgery
Electrophysiology tests including repetitive nerve stimulation (RNS) and single-fiber electromyography (sfEMG)
Data Synthesis
Studies included were pooled for statistical meta-analysis. Statistical analyses were completed using RevMan 5.3. We used the metaprop command in Stata 17 to pool the proportion of patients with SGMG. 95% CIs were computed, and Freeman-Turkey double arcsine was used for transformation of proportions. There was significant heterogeneity of the studies (χ2 and I2 tests), and best evidence synthesis was used to summarize the results. The risk ratios and their 95% CIs were calculated from data extracted from the studies where available. Where statistical pooling was not feasible, the findings were presented in descriptive text, including tables and figures.
Assessing Certainty in the Findings
We followed the Grading of Recommendations, Assessment, Development and Evaluation (GRADE)17 method for assessing the certainty of the findings, and a summary of findings table18 was made with GRADEpro software (McMaster University, Ontario, Canada). We graded the standard of evidence for the outcomes by appraising study limitations, consistency, and risk of reporting bias. We assigned 4 levels of quality of evidence: high, moderate, low, and very low accordingly.
Standard Protocol Approvals, Registrations, and Patient Consents
The institutional review board and ethics team stated that approval was not necessary for the review. The need for informed consent was waived because of the retrospective nature of the review. All research adhered to the principles of the Declaration of Helsinki.
Data Availability
Template data collection forms, data extracted from included studies, and data used for all analyses will be available on request by any qualified investigator.
Results
Figure 1 shows a flowchart summarizing the included and excluded studies in this systematic review. Of the 653 studies identified through database search, 48 were included after evaluating titles and abstracts. Thirty-one studies met the predefined inclusion criteria. The quantitative analysis included 31 studies with a total of 3,548 participants. Countries of studies included were from Argentina, Australia, Austria, China, France, Italy, Japan, Korea, Kuwait, Singapore, Slovakia, South Korea, Spain, Thailand, Turkey, the United Kingdom, and the United States. All countries included reported 1 study each, apart from the United States with 7 studies, China with 3 studies, the United Kingdom with 3 studies, Thailand with 3 studies, South Korea and Italy with 2 studies each. The sample size in each study ranged from 34 to 946; the mean age ranged from 40 to 83 years; and the percentage of female participants ranged from 26% to 72%. Characteristics of studies included are summarized in Table 1. There was 1 prospective study and 30 retrospective observational studies. See Table 2 for the outcomes reported in the summary of findings.
Table 1.
Baseline Characteristics of Studies Included in Quantitative Analysis
| First author, year | Type/design of study | Country | n | Proportion of conversion to SGMG (%) | Age (mean) | Gender percentage (%F) | Antibody (%) AChR+ | Antibody status of those converted to SGMG | Minimum duration of ocular symptoms (only) before inclusion in study | Mean follow-up duration (mo) |
| Aguirre, 2018 | Observational | Argentina | 61 | 73.7 | 46 | 43 | 72.8 | 81.4% (35/43) | All included | 103 |
| Allen, 2010 | Observational | USA | 39 | 31.0 | 76 | 39 | 88.6 | 100% (11/11) | 3 mo | 47 |
| Apinyawasisuk, 2020 | Observational | Thailand | 71 | 50.7 | 54 OMG 51 SGMG |
61 | 100% (inclusion criteria) | 100% (inclusion criteria) | 3 mo | 9 |
| Behbehani, 2022 | Observational | Kuwait | 47 | 51.0 | 41 | 51 | 98.0 | NR | All included | 42 |
| Diaz-Maroto, 2022 | Observational | Spain | 62 | 50.0 | 68 | 39 | 66.1 | NR | All included | 54 |
| Feng, 2020 | Observational | China | 127 | 83.5 | 46 | 57 | 78.9 | 89% (8/9) | All included | NR |
| Galassi, 2018 | Observational | Italy | 175 | 21.1 | 64 | 41 | 38.0 | 26.2% | All included | 93 |
| Guan, 2015 | Observational | China | 102 | 53.9 | 41 | 52 | 47.1 | 60.4% (29/55) | 2 mo | 60 |
| Gueguen, 2022 | Observational | France | 151 | 30.5 | 49 | 62 | 55.9 | NR | 1 mo | 68 |
| Hendricks, 2019 | Observationala | United States | 98 | 54.5 | 59 | 26 | 80.0 | 88% (28/33) | NR | 91 |
| Hong, 2008 | Observational | South Korea | 202 | 23.3 | 45 | 56 | 69.9 | 0.935 | 24 mo | 12 |
| Isshiki, 2020 | Observationala | Japan | 52 | 21.0 | 64 | 48 | 100% (inclusion criteria) | 100% (inclusion criteria) | 3 mo | NR |
| Kamarajah, 2018 | Observationala | United Kingdom | 93 | 52.0 | Categories of </>50 y | 33 | 69.0 | 86.4% (38/44) | All included | 38 |
| Kemchoknatee, 2021 | Observational | Thailand | 155 | 20.6 | 49 | 68 | 55.9 | 85.7% (12/14) | All included | NR |
| Kim, 2021 | Observational | South Korea | 112 | 21.4 | OMG 45 SGMG 52 |
58 | 70.0 | 83.3% (20/24) | All included | 38 |
| Kisabay, 2021 | Observational | Turkey | 148 | 38.8 | 54 | 47 | 50.0 | n = 39 | NR | 56 |
| Martinka, 2017 | Observational | Slovakia | 946 | 58.6 | 54 | 49 | 50.0 | 70.0% (388/554) | 3 mo | NR |
| Mazzoli, 2018 | Observational | Italy | 168 | 18.4 | 65 | 41 | 39.6 | NR | 24 mo | 47 |
| Mee, 2003 | Observational | Australia | 34 | 61.8 | 55 | 44 | 100% (inclusion criteria) | 100% (inclusion criteria) | All included | 50 |
| Mittal, 2011 | Observationala | United States | 101 | 16.7 | 58 | 50 | 33.7 | 69% (9/13) | NR | 36 |
| Nagia, 2015 | Observational | United States | 158 | 20.9 | 62 | 33 | 72.0 | NR | All included | 61 |
| Peeler, 2015 | Observational | United States | 223 | 20.2 | 59 | 38 | 70.9 | NR | NR | 60 |
| Rostedt, 2000 | Observational | United States | 50 | 52.0 | 46 | 28 | 50.0 | 28 | All included | NR |
| Sabre, 2019 | Observational | United Kingdom | 96 | 13.5 | 83 | 41 | 68.7 | 92 | All included | 47 |
| Teo, 2018 | Observational | Singapore | 155 | 10.6 | 59 | 48 | 48.4 | NR | All included | 41 |
| Wang, 2017 | Observationala | China | 40 | 85.0 | 40 | 65 | 100% (inclusion criteria) | 100% (inclusion criteria) | All included | 24 |
| Weinberg, 1999 | Prospective cohort | United States | 37 | 46.0 | 48 | 54 | 50.0 | 79% (11/x) | All included | 55 |
| Witthayaweerasak, 2021 | Observational | Thailand | 115 | 30.4 | 48 | 72 | NR | 1 mo | 35 | |
| Wong, 2016 | Observational | United Kingdom | 101 | 31.0 | 52 | 44 | 40.0 | NR | 3 mo | 101 |
| Zach, 2013 | Observational | Austria | 44 | 56.8 | 54 | 42 | 70.5 | 81.8% (9/11) | 24 mo | 24 |
| Zhao, 2022 | Observational | China | 51 | 49.0 | 73 | 46 | 86.7 | 82.6% (19/23) | NR | NR |
Abbreviations: AChR = acetylcholine receptor; NR = not reported; OMG = ocular myasthenia gravis; SGMG = secondary generalized myasthenia gravis.
Studies reported on all myasthenia gravis (nonocular) but also had relevant data on secondary generalization.
Table 2.
Summary of Findings
| Patient or population: Ocular myasthenia gravis Setting: Hospital | |||
| Outcome No. of participants (studies) |
Relative effect (95% CI) | Effect (95% CI) | Certainty |
| SGMG No. of participants: 3,548 (31 observational) |
— | Proportion 0.39 (0.32–0.47) |
⊕◯◯◯ Very low |
| RR female sex for SGMG No. of participants: 1,534 (21 observational studies) |
— | RR 1.06 (0.96–1.17) |
⊕◯◯◯ Very low |
| RR AChR positivity for SGMG No. of participants: 1,271 (16 observational studies) |
— | RR 1.30 (1.05–1.56) |
⊕◯◯◯ Very low |
Abbreviations: AChR = acetylcholine receptor; RR = risk ratio; SGMG = secondary generalized myasthenia gravis.
GRADE Working Group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Primary Outcome: Proportion of SGMG
The proportion of generalization ranged from 11% to 84% (Figure 2). The pooled proportion was 39% of those with OMG converted to SGMG (95% CI 32%–47%, I2 = 95.86%, p < 0.001 unweighted, low certainty). The studies were heterogeneous with different inclusion and exclusion criteria. In addition, there was heterogeneity of the minimum duration of ocular symptoms (only) for the inclusion criteria. This is where patients were considered to have SGMG if they had ocular symptoms only for a specified duration of time before generalizing. This ranged from 1 month to 2 years. Twenty-seven studies included patients with a stated minimum duration of ocular symptoms before conversion to SGMG as part of the inclusion criteria, whereas 4 studies did not report this datum. Twenty-six studies were of consecutive unselected populations, with the inclusion of patients with OMG only and with the primary outcome of SGMG. Five included studies reported on all types of MG (i.e., ocular MG, primary GMG, and SGMG) but also had relevant data on secondary generalization and were, therefore, included (Table 1).
Figure 2. Proportion of Reported Generalization in Patients With Ocular Myasthenia Gravis.
Heterogeneity I2 = 95.86% p < 0.001, all 31 studies included. Heterogeneity χ2 = 724.30 (df = 30) p < 0.001, 31 studies included.
When grouped into countries with predominant European descent (eFigure 1, links.lww.com/WNL/D67), the predominantly European descent group had a pooled proportion of generalization of 38% (95% CI 29%–48%, I2 = 95.94% p < 0.001) and the predominantly non-European/Asian descent group had a pooled proportion of generalization of 41% (95% CI 24%–56%, I2 = 96.48% p < 0.001). The heterogeneity was very high.
Secondary Outcome: Associated Risk Factors of Secondary Generalization
The secondary outcome of this systematic review includes the reported risk factors associated with secondary generalization such as demographics, presenting features, and investigations (serologic, neurophysiologic, and thymic imaging). The results were as follows:
Presenting Signs and Symptoms
All studies mentioned presenting signs or symptoms as part of the diagnostic criteria of MG. Four studies reported ocular symptoms that were associated with a risk of generalization. Galassi et al.7 found that diplopia at onset, but not ptosis, was associated with generalization. Another study reported that bilateral ptosis was associated with earlier generalization.19 Kemchoknatee et al.20 reported that combined ptosis and diplopia at onset was associated with SGMG. Kisabay et al.21 noted that bilateral ptosis was common in secondary generalization, but this did not reach statistical significance.
Sex
All studies reported on this. The average percentage of SGMG in female patients ranged from 28% to 72% (Table 1). The pooled risk ratio of female sex to SGMG was 1.06 (95% CI 0.96–1.17, I2 = 0% p = 0.614, 21 studies included) (Figure 3). Of the 31 studies, only 5 studies found an association of female sex with risk of generalization.7,22-25 Only 1 study demonstrated male sex to have an association with generalization.26 Martinka et al. reported a significant risk ratio of 1.23 of male sex with generalization. However, they also reported that female sex was associated with more severe OMG symptoms (risk ratio 1.2).
Figure 3. RR of Female Sex With Secondary Generalization.
Overall RR was 1.06 (95% CI 0.96–1.17, I2 = 0% p = 0.614), 21 studies included. Data to calculate RR not reported by 10 studies could not be included in analysis. RR = risk ratio.
Age of Onset
All studies reported on this. Most of the studies (19/31) found no association with age of onset with generalization. Six studies found an association of SGMG with older age,22,25-29 whereas 3 studies found an association with younger age.19,30,31 Two studies included only older populations, such as 70 years or older32 and 65 years or older.33
AChR and Other Antibodies
All studies reported on the AChR serology. The average percentage of AChR seropositivity in all patients with OMG at the time of recruitment into studies ranged from 33% to 100%. The average percentage of AChR seropositivity in those who converted to SGMG was 26%–100% (Table 1). Of note, 4 studies only included patients with positive AChR as part of their inclusion criteria and their definition of OMG.23,27,31,34 In the analysis, removing the 4 studies that included 100% AChR—the pooled proportion of secondary generalization was 0.37 (95% CI 0.27–0.46), meaning 37% instead of 39% (all studies included) converted to SGMG.
Nine of 31 studies reported on the frequency of anti-MuSK antibodies,7,9,21,22,25,26,28,35,36 and only 1 study reported on the frequency of LRP4 antibodies.28 Double seronegativity (DSN) was reported in 4 of 31 studies.7,21,22,28
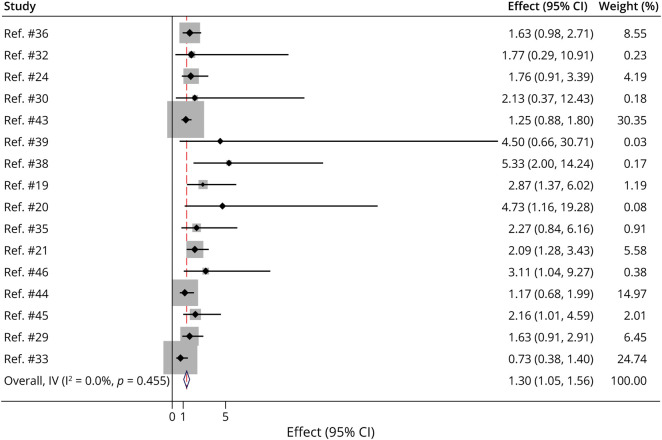
Fifteen of 31 studies found an association with positive AChR as a risk factor of generalization.9,19-22,24,25,28,29,34,36-40 Eight studies reported no association with AChR antibodies and secondary generalization.7,9,32,33,35,41-44 The pooled risk ratio of AChR positivity to SGMG was 1.30 (95% CI 1.05–1.56, I2 = 0% p = 0.455, 16 studies included) (Figure 4).
Figure 4. RR for AChR Seropositivity to Secondary Generalization.
Overall RR was 1.30 (95% CI 1.05–1.56, I2 = 0% p = 0.455), 16 studies included. Four studies only included patients with positive AChR because part of their inclusion criteria could not be included in the analysis. Data to calculate RR not reported by 11 studies could not be included in analysis. AChR = acetylcholine receptor; RR = risk ratio.
AChR antibody titers were found to be significantly higher in SGMG.29,36,40 Isshiki et al.34 reported an association with GMG with higher levels of AChR, although all the patients included in the study were AChR-positive. The authors concluded that patients with AChR antibody levels of 48.0 nmol/L and above, particularly those above 100.0 nmol/L at the first test, may progress to GMG. Likewise, Teo et al.37 found that the mean AChR antibody titer level was significantly higher in patients who subsequently converted to GMG (15.51 ± 15.41 nmol/L) compared with those who remained ocular (9.38 ± 10.941 nmol/L). Similarly, Behbani et al.29 reported a significantly higher AChR antibody titer level of 2.0 ± 1.1 nmol/L in patients with SGMG compared with 1.27 ± 0.65 nmol/L in those who remained ocular.
Of the 8 of 31 studies that reported data on anti-MuSK antibodies, the proportion of conversion to SGMG ranged from 0% to 100%. None of the patients were anti-MuSK+ in 2 studies.26,35 On the opposite spectrum, 100% of the MuSK+ patients in 2 studies,21,36 with 4 of 148 and 3 of 11 MuSK+ patients with OMG, reported to have converted to SGMG after an average of 4.7 years’ and 8.5 years’ duration of follow-up, respectively. Other studies had a MuSK+ OMG to SGMG conversion rate between 12.5% and 75%; in the study by Sabre et al.28 where 8 of 83 patients with OMG were MuSK+, 1 (12.5%) converted to SGMG after 47.4 months; and the study by Galassi et al.7 had 8 of 175 patients with OMG positive for MuSK, of which 6 (75%) converted to SGMG.
A handful of studies had explicitly stated that they tested all patients for AChR and MuSK, that is, the double seronegative patients. In the study by Galassi et al.,7 of 175 patients, 98 were double seronegative, of which 14 (14%) converted to SGMG within 15 years compared with 17 of 65 (26%) AChR+ and 6 of 8 (75%) MuSK+. Sabre et al. reported 16 of 83 (19.3%) and Mazzoli et al. reported 99 of 164 (60.3%) patients with OMG to have tested double seronegative, although the proportion of those who generalized was not mentioned.22,28
Neurophysiology: sfEMG
sfEMG was reported in only 14 of 31 studies, with varied locations of muscles tested. In these studies, the abnormality on sfEMG was found in 24%–92% of patients tested in total (eTable 2, links.lww.com/WNL/D67).7,9,20-22,24,29,31,32,39,42-45
Of the 14 studies with sfEMG data, 2 studies found an association of abnormal sfEMG with risk of SGMG; Kisabay et al.21 tested the orbicularis oculi muscle and reported that 28 of 51 patients (55%) with abnormal sfEMG converted to SGMG; and Hendricks et al.39 reported that an abnormal sfEMG (location of testing not specified in the study) was found in 10 of 13 patients (77%) who converted to SGMG. Conversely, Weinberg et al.,45 testing the extensor digitorum communis muscles, found that normal sfEMG was associated with remaining OMG and that an abnormal sfEMG was not predictive of subsequent development of SGMG. The other 10 studies did not report an association of an abnormal sfEMG result with SGMG (tested at orbicularis oculi, frontalis, extensor digitorum communis, trapezius, or masseter).7,9,22,29,31,32,42-44 Two studies reported the results of sfEMG testing, but did not analyze for association with SGMG.20,24
Neurophysiology: RNS
RNS was reported in 16 of 31 studies.20-24,29,32,33,35,37,38,41,43,45-47 Of these, 8 studies reported on both sfEMG and RNS data (eTable 3, links.lww.com/WNL/D67).20-22,24,29,32,43,45 The association of an abnormal RNS with risk of SGMG was also mixed: 6 of 16 studies (pooled n = 638)21,33,35,37,38,47 reported that an abnormal RNS (tested in facial nerve, axillary nerve, ulnar nerve, accessory nerve, orbicularis oculi, proximal/distal limb muscles, abductor digiti minimi, flexor carpi ulnaris, nasalis, trapezius, and deltoid muscles) were associated with risk of SGMG.
By contrast, 7 of 16 studies (pooled n = 386)22,23,29,32,41,43,45 did not find this association of abnormal RNS (tested in facial nerve, axillary nerve, accessory nerve, ulnar nerve, orbicularis oculi, masseter, or deltoid muscles) with SGMG risk. The details of muscles tested and study size are provided in eTable 3 (links.lww.com/WNL/D67), with a similar spread of muscles tested in the positive and negative studies.
Thymus Imaging
Thymus imaging was reported in 27 of 31 studies. Thymic abnormalities were reported as thymic hyperplasia (based on radiology imaging) or thymoma. Thymic abnormalities of thymoma or hyperplasia were reported to be associated with risk of SGMG in 12 studies: 6 studies detailed this as thymoma,25,29,34,37,38,43 1 study as thymic hyperplasia,9 and 5 studies combined thymoma or hyperplasia or did not specify details of the abnormality.21,23,27,36,47 Nine studies found no association with secondary generalization.7,20,22,24,33,35,39,41,42 Six studies did not report on an analysis of association with SGMG.19,26,32,44-46 Four studies did not report on the presence or absence of thymic abnormalities.28,30,31,40 Details are provided in eTable 4 (links.lww.com/WNL/D67).
Treatments/Intervention
Twenty-nine of 31 studies reported on treatment or medical intervention received. All these reports on treatment or medication were from retrospective or uncontrolled studies. In addition to pyridostigmine, a wide range of immunosuppression treatments were reported, detailed in eTable 5 (links.lww.com/WNL/D67). The effect of immunosuppression on the SGMG risk was mixed. Six of 29 studies (21%, pooled n = 441) showed a lower risk of SGMG in the immunosuppressed group.23,31-33,38,41 One study (n = 158) found a trend of lower proportion of immunosuppressed patients with SGMG, but this did not reach statistical significance.42 Seven of 29 studies (24%, pooled n = 754) showed no effect of immunosuppression on SGMG risk.20,21,24,27,28,37,39
Risk of Bias and Publication Bias
Ottawa Newcastle Score was assessed for all studies included (eTable 6, links.lww.com/WNL/D67). Few studies reported on loss of follow-up (attrition bias). We avoided the risk of bias of studies selecting patients based on treatment received, by selecting studies that had consecutive recruitment of patients. There is likely to be publication bias because authors tend not to report or publish if there was no association with a particular risk factor. We assessed 17 studies using the adherence assessment form for measuring adherence to the TRIPOD prognostic and development model for a score out of 30.48 The studies assessed7,9,19-25,27-30,33,35,39,47 each scored 29/30 with 1-point reduction for missing data.
Discussion
This is a systematic review on the risk of SGMG after the onset of OMG, with a comprehensive global representation, including patients from Argentina, Australia, Austria, China, France, Italy, Japan, Korea, Kuwait, Singapore, Slovakia, South Korea, Spain, Thailand, Turkey, the United Kingdom, and the United States, and a cumulative total of 4,014 patients. This systematic review has highlighted a few key points. The first important observation is the wide range of risk of SGMG, from 11% to 85%. This wide range reflects the heterogeneity of study populations, and the need for further research to elucidate differences and their relevance. Another significant finding is that the studies investigating the risk of SGMG after OMG that were available to date were predominantly retrospective and observational (30/31 studies). Only 1 study was prospective, reporting on consecutive patients presenting with OMG.45
In addition, the study inclusion criteria regarding the ocular symptoms were varied in the stated different minimum duration of ocular symptoms. This could range from none to 2 years, and we had to exclude studies that did not explicitly state whether GMG was “primary” or “secondary” because of the lack of clarity whether patients presented with initial ocular symptoms (only). The different duration of ocular symptoms (only) before study inclusion to assess the risk of SGMG will likely affect the data because the risk of SGMG likely reduces with longer intervals from time of onset.49
We had limited our study to adults only. It is likely that the risk of SGMG in adults and in pediatric populations differs.49 Because of limiting our systematic review to adults only, we were, therefore, unable to include data sets from institutions and influential researchers in our understanding of MG.49,50,e1-e9 For those studies that did not meet our systematic review inclusion criteria, we note the range of SGMG to be between 10% and 75%.e1-e9 Besides that, some studies only selected patients based on treatment and those studies were excluded.e6,e10,e11 Based on our inclusion criteria, we have included some data sets from less represented countries, but unfortunately, some large data sets were also excluded.
We set out to assess the risk factors associated with SGMG, but the results have been conflicting, likely a reflection of the heterogeneity of the populations for reasons outlined above and the drawbacks of relying on retrospective studies. While there is no consensus on age of onset with secondary generalization, female sex was a possible association, although many studies found this not to be significant. We were unable to comment directly on ethnicities because these data were unavailable, but further research to include may show these to be important. Not all risk factors of interest were reported in all studies. For example, neurophysiology reports on sfEMG and RNS have been varied. In addition, these tests are operator-dependent, and therefore, the results may vary depending on experience or expertise. Serologic reports on anti-MuSK and anti-LRP4 antibodies have also been limited. For example, Mazzoli et al.22 found no anti-MuSK–positive patients at diagnosis, although 1 double seronegative patient later tested positive for anti-MuSK.
The effect of treatment on the risk of SGMG was inconclusive. Although there were suggestions of immunosuppression reducing the risk of SGMG,e12 this has not been shown in other studies21,27,28,37,39 and may be biased in various ways, including retrospective bias, selection of “lower risk” patients receiving immunosuppression.9,e13 In the included studies, it was not clear whether the patients who generalized had immunosuppressive treatment, and therefore, this could not be evaluated in this systematic review. Patients with AChR-positive GMG benefit from thymectomy,e14 but it is not yet known whether thymectomy can influence the risk of SGMG and, if so, whether this is only in the seropositive patients or in all patients with OMG. Again, we cannot conclude from the included studies whether this is the case because an equivalent number of studies found an association between thymoma or thymic hyperplasia (based on radiology imaging) and secondary generalization.
Another potential bias on the risk of SGMG is the different duration of follow-up and potential loss to follow-up. Different follow-up times or long-term follow-up would coincidentally mean more percentage generalization. The wide range of SGMG risks may reflect this. Different methods of statistics were used to analyze the association of risk factors with SGMG in the studies. Too heterogeneous to pool together, risk ratio was a simple way to demonstrate the data, although not all studies could be included because of missing data. We need to be cautious about interpreting the data because these come from a heterogeneous population, again highlighting the need for a well-designed trial. We were not able to perform combined regression analysis because of this heterogeneity of studies. The studies reported different statistical analyses, for example, regression model, Cox model, hazard ratio, or odds ratio. Therefore, we were unable to perform a pooled analysis for time to SGMG.
The Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines48 for prognostic studies were published in 2015; therefore, we scored studies published after this year against the TRIPOD guidelines. Of those that we assessed, methods of handling missing data was the section that was not adequately reported on. The TRIPOD guidelines are a valuable resource and gold standard for future studies reporting on the risk of SGMG.
The search was run on January 2023, and no new studies meeting the inclusion criteria were identified.
Risk factors such as female sex and presence of anti-AChR antibodies have been identified as possible associations with generalization of OMG, but there were not enough quality observational studies. The presentation is widespread, as seen in the studies included in this systematic review. There is a need for a prospective global database for OMG, including all countries with different populations. Such a project is one that could be delivered by a global OMG consortium.e15
Glossary
- AChR
acetylcholine receptor
- DSN
double seronegativity
- GMG
generalized myasthenia gravis
- GRADE
Grading of Recommendations, Assessment, Development and Evaluation
- LRP4
low-density lipoprotein receptor-related protein 4
- MG
myasthenia gravis
- MuSK
muscle-specific kinase
- OMG
ocular myasthenia gravis
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RNS
repetitive nerve stimulation
- sfEMG
single-fiber electromyography
- SGMG
secondary generalized myasthenia gravis
- TRIPOD
Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis
Appendix. Authors

| Name | Location | Contribution |
| Clarissa Ern Hui Fang, MB BCh BAO, MSc, MRCPI | Department of Ophthalmology, Manchester Royal Eye Hospital, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Desta Bokre, MA | Joint Library of Ophthalmology, Moorfields Eye Hospital and University College London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Sui Hsien Wong, MBBS, MD, FRCP | Moorfields Eye Hospital & Guys & St Thomas' Hospitals, London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
No targeted funding reported.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Wong SH, Huda S, Vincent A, Plant GT. Ocular myasthenia gravis: controversies and updates. Curr Neurol Neurosci Rep. 2014;14(1):421. doi: 10.1007/s11910-013-0421-9 [DOI] [PubMed] [Google Scholar]
- 2.Wong SH, Plant GT, Cornblath W. Does treatment of ocular myasthenia gravis with early immunosuppressive therapy prevent secondarily generalization and should it be offered to all such patients? J Neuroophthalmol. 2016;36(1):98-102. doi: 10.1097/WNO.0000000000000270 [DOI] [PubMed] [Google Scholar]
- 3.Lazaridis K, Tzartos SJ. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front Immunol. 2020;11:212. doi: 10.3389/fimmu.2020.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berrih-Aknin S, Le Panse R. Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun. 2014;52:90-100. doi: 10.1016/j.jaut.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 5.Luo H, Xie S, Ma C, et al. Correlation between thymus radiology and myasthenia gravis in clinical practice. Front Neurol. 2018;9:1173. doi: 10.3389/fneur.2018.01173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anil R, Kumar A, Alaparthi S, et al. Exploring outcomes and characteristics of myasthenia gravis: rationale, aims and design of registry: the EXPLORE-MG registry. J Neurol Sci. 2020;414:116830. doi: 10.1016/j.jns.2020.116830 [DOI] [PubMed] [Google Scholar]
- 7.Galassi G, Mazzoli M, Ariatti A, Kaleci S, Valzania F, Nichelli PF. Antibody profile may predict outcome in ocular myasthenia gravis. Acta Neurol Belg. 2018;118(3):435-443. doi: 10.1007/s13760-018-0943-7 [DOI] [PubMed] [Google Scholar]
- 8.Cea G, Martinez D, Salinas R, Vidal C, Hoffmeister L, Stuardo A. Clinical and epidemiological features of myasthenia gravis in Chilean population. Acta Neurol Scand. 2018;138(4):338-343. doi: 10.1111/ane.12967 [DOI] [PubMed] [Google Scholar]
- 9.Wong SH, Petrie A, Plant GT. Ocular myasthenia gravis: toward a risk of generalization score and sample size calculation for a randomized controlled trial of disease modification. J Neuroophthalmol. 2016;36(3):252-258. doi: 10.1097/WNO.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 10.Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-146. doi: 10.1097/xeb.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 11.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147-153. doi: 10.1097/xeb.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: systematic reviews of effectiveness. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. The Joanna Briggs Institute; 2020. [Google Scholar]
- 14.Aromataris E,Fernandez R, Godfrey C, Holly C, Kahlil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132-140. doi: 10.1097/XEB.0000000000000055 [DOI] [PubMed] [Google Scholar]
- 15.Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. The Joanna Briggs Institute; 2020. [Google Scholar]
- 16.Fang CEH, Bokre D, Wong SH. Clinical characteristics of ocular myasthenia gravis and outcomes of secondary generalisation: a systematic review protocol. BMJ Open. 2022;12(9):e060259. doi: 10.1136/bmjopen-2021-060259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamarajah SK, Sadalage G, Palmer J, Carley H, Maddison P, Sivaguru A. Ocular presentation of myasthenia gravis: a natural history cohort. Muscle Nerve. 2018;57(4):622-627. doi: 10.1002/mus.25971 [DOI] [PubMed] [Google Scholar]
- 20.Kemchoknatee P, Arepagorn A, Srisombut T. Ocular manifestation and generalization after ocular onset in ocular myasthenia gravis: a 5-year analysis. Asian Pac J Allergy Immunol. 2021. doi: 10.12932/AP-260521-1141 [DOI] [PubMed] [Google Scholar]
- 21.Kisabay A, Ozdemir HN, Gokcay F, Celebisoy N. Risk for generalization in ocular onset myasthenia gravis: experience from a neuro-ophthalmology clinic. Acta Neurol Belg. 2021;122(2):337-344. doi: 10.1007/s13760-020-01582-1 [DOI] [PubMed] [Google Scholar]
- 22.Mazzoli M, Ariatti A, Valzania F, et al. Factors affecting outcome in ocular myasthenia gravis. Int J Neurosci. 2018;128(1):15-24. doi: 10.1080/00207454.2017.1344237 [DOI] [PubMed] [Google Scholar]
- 23.Apinyawasisuk S, Chongpison Y, Thitisaksakul C, Jariyakosol S. Factors affecting generalization of ocular myasthenia gravis in patients with positive acetylcholine receptor antibody. Am J Ophthalmol. 2020;209:10-17. doi: 10.1016/j.ajo.2019.09.019 [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Maroto I, Garcia-Garcia J, Sanchez-Ayaso PA, et al. Ocular myasthenia gravis and risk factors for developing a secondary generalisation: description of a Spanish series. Neurologia (Engl Ed). 2023;38(4):229-235. doi: 10.1016/j.nrleng.2020.09.004 [DOI] [PubMed] [Google Scholar]
- 25.Gueguen A, Hijazi B, Zuber K, et al. Generalization of ocular myasthenia gravis 10 years after onset. J Neurol. 2022;269(12):6597-6604. doi: 10.1007/s00415-022-11316-3 [DOI] [PubMed] [Google Scholar]
- 26.Martinka I, Cibulcik F, Bednarik J, Spalek P. Ocular myasthenia gravis in Slovak Republic. Cesk Slov Neurol Neurochir. 2017;80(2):190-196. doi: 10.14735/amcsnn2017190 [DOI] [Google Scholar]
- 27.Wang LL, Zhang Y, He ML. Clinical predictors for the prognosis of myasthenia gravis. BMC Neurol. 2017;17(1):77. doi: 10.1186/s12883-017-0857-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabre L, Maddison P, Wong SH, et al. miR-30e-5p as predictor of generalization in ocular myasthenia gravis. Ann Clin Transl Neurol. 2019;6(2):243-251. doi: 10.1002/acn3.692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behbehani R, Ali A, Al-Moosa A. Ocular myasthenia: clinical course and the diagnostic utility of assaying acetylcholine receptor antibodies. Neuroophthalmology. 2022;46(4):220-226. doi: 10.1080/01658107.2022.2037662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng X, Huan X, Yan C, et al. Adult ocular myasthenia gravis conversion: a single-center retrospective analysis in China. Eur Neurol. 2020;83(2):182-188. doi: 10.1159/000507853 [DOI] [PubMed] [Google Scholar]
- 31.Mee J, Paine M, Byrne E, King J, Reardon K, O'Day J. Immunotherapy of ocular myasthenia gravis reduces conversion to generalized myasthenia gravis. J Neuroophthalmol. 2003;23(4):251-255. [DOI] [PubMed] [Google Scholar]
- 32.Allen JA, Scala S, Jones HR. Ocular myasthenia gravis in a senior population: diagnosis, therapy, and prognosis. Muscle Nerve. 2010;41(3):379-384. doi: 10.1002/mus.21555 [DOI] [PubMed] [Google Scholar]
- 33.Zhao S, Yan X, Ding J, et al. Lack of immunotherapy as the only predictor of secondary generalization in very-late-onset myasthenia gravis with pure ocular onset. Front Neurol. 2022;13:857402. doi: 10.3389/fneur.2022.857402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isshiki Y, Mimura O, Gomi F. Clinical features and treatment status of antiacetylcholine receptor antibody-positive ocular myasthenia gravis. Jpn J Ophthalmol. 2020;64(6):628-634. doi: 10.1007/s10384-020-00770-z [DOI] [PubMed] [Google Scholar]
- 35.Kim KH, Kim SW, Shin HY. Initial repetitive nerve stimulation test predicts conversion of ocular myasthenia gravis to generalized myasthenia gravis. J Clin Neurol. 2021;17(2):265-272. doi: 10.3988/jcn.2021.17.2.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aguirre F, Villa AM. Prognosis of ocular myasthenia gravis in an Argentinian population. Eur Neurol. 2018;79(3-4):113-117. doi: 10.1159/000487132 [DOI] [PubMed] [Google Scholar]
- 37.Teo KY, Tow SL, Haaland B, et al. Low conversion rate of ocular to generalized myasthenia gravis in Singapore. Muscle Nerve. 2018;57(5):756-760. doi: 10.1002/mus.25983 [DOI] [PubMed] [Google Scholar]
- 38.Hong YH, Kwon SB, Kim BJ, et al. Prognosis of ocular myasthenia in Korea: a retrospective multicenter analysis of 202 patients. J Neurol Sci. 2008;273(1-2):10-14. doi: 10.1016/j.jns.2008.05.023 [DOI] [PubMed] [Google Scholar]
- 39.Hendricks TM, Bhatti MT, Hodge DO, Chen JJ. Incidence, epidemiology, and transformation of ocular myasthenia gravis: a population-based study. Am J Ophthalmol. 2019;205:99-105. doi: 10.1016/j.ajo.2019.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peeler CE, De Lott LB, Nagia L, Lemos J, Eggenberger ER, Cornblath WT. Clinical utility of acetylcholine receptor antibody testing in ocular myasthenia gravis. JAMA Neurol. 2015;72(10):1170-1174. doi: 10.1001/jamaneurol.2015.1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zach H, Cetin H, Hilger E, et al. The effect of early prednisolone treatment on the generalization rate in ocular myasthenia gravis. Eur J Neurol. 2013;20(4):708-713. doi: 10.1111/ene.12057 [DOI] [PubMed] [Google Scholar]
- 42.Nagia L, Lemos J, Abusamra K, Cornblath WT, Eggenberger ER. Prognosis of ocular myasthenia gravis: retrospective multicenter analysis. Ophthalmology. 2015;122(7):1517-1521. doi: 10.1016/j.ophtha.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 43.Guan YZ, Cui LY, Liu MS, Niu JW. Single-fiber electromyography in the extensor digitorum communis for the predictive prognosis of ocular myasthenia gravis: a retrospective study of 102 cases. Chin Med J. 2015;128(20):2783-2786. doi: 10.4103/0366-6999.167354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rostedt A, Saders LL, Edards LJ, Massey JM, Sanders DB, Stalberg EV. Predictive value of single-fiber electromyography in the extensor digitorum communis muscle of patients with ocular myasthenia gravis: a retrospective study. J Clin Neuromuscul Dis. 2000;2(1):6-9. doi: 10.1097/00131402-200009000-00003 [DOI] [PubMed] [Google Scholar]
- 45.Weinberg DH, Rizzo JF III, Hayes MT, Kneeland MD, Kelly JJ Jr. Ocular myasthenia gravis: predictive value of single-fiber electromyography. Muscle Nerve. 1999;22(9):1222-1227. doi: [DOI] [PubMed] [Google Scholar]
- 46.Mittal MK, Barohn RJ, Pasnoor M, et al. Ocular myasthenia gravis in an academic neuro-ophthalmology clinic: clinical features and therapeutic response. J Clin Neuromuscul Dis. 2011;13(1):46-52. doi: 10.1097/CND.0b013e31821c5634 [DOI] [PubMed] [Google Scholar]
- 47.Witthayaweerasak J, Rattanalert N, Aui-Aree N. Prognostic factors for conversion to generalization in ocular myasthenia gravis. Medicine (Baltimore). 2021;100(19):e25899. doi: 10.1097/MD.0000000000025899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. doi: 10.1136/bmj.g7594 [DOI] [PubMed] [Google Scholar]
- 49.Bever CT Jr, Aquino AV, Penn AS, Lovelace RE, Rowland LP. Prognosis of ocular myasthenia. Ann Neurol. 1983;14(5):516-519. doi: 10.1002/ana.410140504 [DOI] [PubMed] [Google Scholar]
- 50.Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141-149. doi: 10.1002/mus.20950 [DOI] [PubMed] [Google Scholar]
- Access eReferences at; links.lww.com/WNL/D66.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Template data collection forms, data extracted from included studies, and data used for all analyses will be available on request by any qualified investigator.