Abstract
Background and Objectives
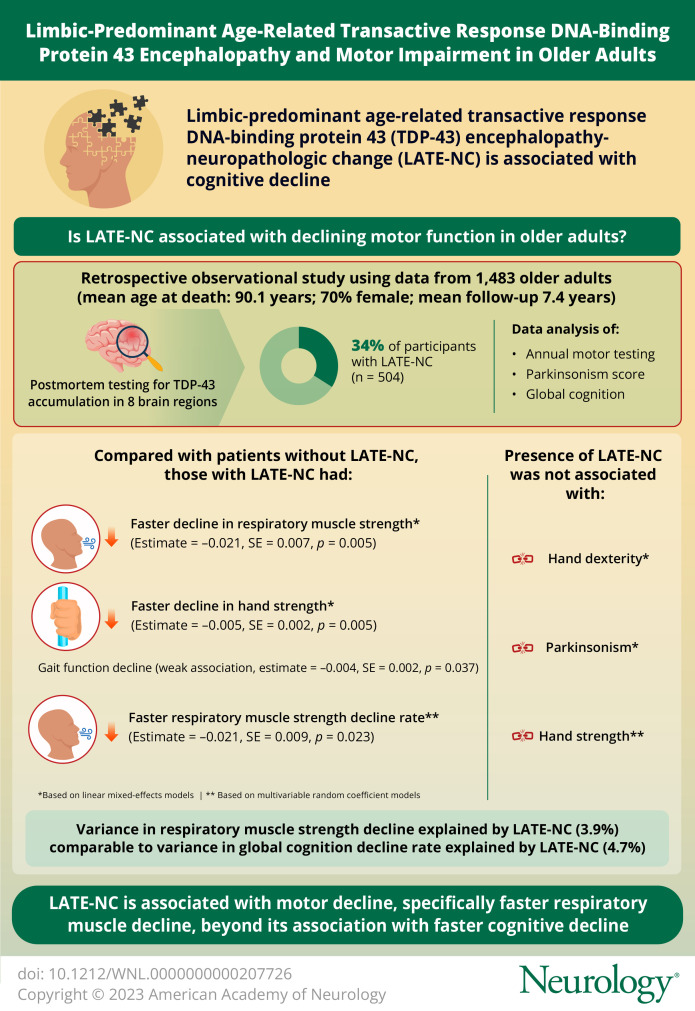
Limbic-predominant age-related transactive response DNA-binding protein 43 (TDP-43) encephalopathy neuropathologic change (LATE-NC) is common and is a major contributor to cognitive decline and Alzheimer dementia in older adults. The objective of the current study was to examine whether LATE-NC was also associated with declining motor function in older adults.
Methods
Participants were from 2 longitudinal clinical pathologic studies of aging who did not have dementia at the time of enrollment. Postmortem pathologic examination included immunohistochemical staining for TDP-43 in 8 brain regions, which was summarized as a dichotomous variable indicating advanced LATE-NC stages at which TDP-43 pathology had accumulated in the hippocampus, entorhinal, or neocortical regions. Annual motor testing included maximal inspiratory and expiratory pressures (summarized as respiratory muscle strength), grip and pinch strength (summarized as hand strength), finger tapping speed and the Purdue Pegboard Test (summarized as hand dexterity), and walking 8 feet and turning 360° (summarized as gait function). The severity of parkinsonism was also assessed and summarized as a global parkinsonism score. Global cognition was a summary of standardized scores of 19 neuropsychological tests. We used linear mixed-effect models to examine the associations of LATE-NC with longitudinal changes of motor decline and used multivariate random coefficient models to simultaneously examine the associations of LATE-NC with cognitive and motor decline.
Results
Among 1,483 participants (mean age at death 90.1 [SD = 6.4] years, 70% women, mean follow-up 7.4 [SD = 3.8] years), LATE-NC was present in 34.0% (n = 504). In separate linear mixed-effect models controlling for demographics and other brain pathologies, LATE-NC was associated with faster decline in respiratory muscle strength (estimate = −0.857, SE = 0.322, p = 0.008) and hand strength (estimate = −0.005, SE = 0.002, p = 0.005) but was not related to hand dexterity, gait function, or parkinsonism. In multivariate random coefficient models including respiratory muscle strength, hand strength, and global cognition as the outcomes, LATE-NC remained associated with a faster respiratory muscle strength decline rate (estimate = −0.021, SE = 0.009, p = 0.023), but the association with hand strength was no longer significant (estimate = −0.002, SE = 0.003, p = 0.390).
Discussion
Motor impairment, specifically respiratory muscle weakness, may be an unrecognized comorbidity of LATE-NC that highlights the potential association of TDP-43 proteinopathy with noncognitive phenotypes in aging adults.
Introduction
Limbic-predominant age-related TDP-43 encephalopathy (LATE) was first coined in 2019 by a working group to describe an age-related neurocognitive syndrome characterized by progressive cognitive decline yielding disability in activities of daily living and dementia.1 Neuropathologic changes of LATE (LATE-NCs) are present in more than one-third of adults older than 80 years2 and may contribute to 17% of dementia cases of Alzheimer types.1
Inclusions of transactive response DNA-binding protein 43 kDa (TDP-43), including round neuronal cytoplasmic inclusions and ropy neurites, are hallmarks of LATE-NC.3 Besides LATE-NC, TDP-43 inclusions are also seen in other brain diseases, most persons with amyotrophic lateral sclerosis (ALS),4 and a subset of patients with frontotemporal lobar degeneration (FTLD).5 The predominant deficit in a motor neuron disease such as ALS is muscle weakness, with respiratory muscle deficits a crucial determinant of survival.6 Nonetheless, impaired cognition has been reported in up to 50% of patients with ALS,7 and 10%–15% of patients with ALS develop dementia.7 Similarly, 10% of patients with frontotemporal dementia also have signs and symptoms of motor neuron disease.8 Although prior studies have examined both motor and cognitive dysfunctions in more familiar TDP-43–related neurologic syndromes, few studies have examined motor impairment in older adults with LATE-NC.9
The primary objective of the current study was to examine whether LATE-NC was associated with declining motor function in older adults. The secondary objective was examining whether LATE-NC was differentially associated with motor decline and cognitive decline, considering the strong correlation between the rate of change of cognitive and motor decline in older adults.
Methods
Participants
To achieve the study objectives, we used data from participants of 2 ongoing clinical-autopsy cohort studies of aging, the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP).10 The ROS and MAP both recruit older adults without known dementia at the time of enrollment who consent to annual clinical evaluations and brain autopsy at the time of death. The ROS began enrollment in 1994, recruiting nuns, priests, and brothers across the United States. The MAP began enrollment in 1997, recruiting Illinoisans living in retirement centers and single-family dwellings across northeastern Illinois. Harmonized study protocols with identical clinical and postmortem data collection methods performed by the same staff facilitated joint analyses. Additional details about the design and instruments used in both studies are provided in prior publications.10
Because the main objective of the current study was to examine whether declining motor function in older adults was associated with LATE-NC, we excluded participants with a pathologic diagnosis of FTLD. At the time of the study analyses, 2,112 of 3,690 recruited participants had died, and 1,725 had undergone autopsy with completed postmortem examinations, of whom 1,483 composed the analytic sample of the current study because they did not have a pathologic diagnosis of FTLD and had 2 or more assessments of motor functions. Of note, 7.1% (n = 54) and 13.3% (n = 122) of participants with and without dementia before death, respectively, were excluded because of not having 2+ motor assessments including hand strength, which indicated that our analytic sample size was not biased by inadequate inclusion of participants with dementia.
Postmortem Assessment of Brain Pathologies
The median (interquartile range) of postmortem intervals was 6.8 (5.1–10.3) hours. After brain removal, one hemisphere was frozen for multiomics studies, and the other hemisphere was fixed in 4% formaldehyde in the phosphate buffer. The fixed hemisphere was cut into 1-cm-thick slabs, and tissue blocks and sections were prepared from predetermined regions for the pathologic assessments. Additional details of the autopsy procedures are provided elsewhere.11
LATE-NC
Sections of 8 brain regions were immunohistochemically examined using a phosphorylated monoclonal TAR5P-1D3 anti-TDP-43 antibody (pS409/410; 1:100). The examined brain regions were the amygdala, hippocampus (CA1 and subiculum), dentate gyrus, entorhinal cortex, and neocortices (orbital frontal, midfrontal, anterior temporal, and middle temporal cortices). Abnormally phosphorylated TDP-43 inclusions in the cytoplasm of the neurons and glia were detected and manually counted in a 0.25 mm2 area with the greatest density.12 We used a modified LATE-NC working group recommendation13,14 to summarize the burden of TDP-43 inclusions in 4 stages. Stage 0 indicated no TDP-43 inclusions; stage 1 indicating TDP-43 inclusions in the amygdala alone; stage 2 extension to the hippocampus, dentate gyrus, or entorhinal cortex; and stage 3 extension into the neocortices. In this study, we used a dichotomous variable, indicating that TDP-43 was in the entorhinal, hippocampus, or beyond (stages 2–3).
Alzheimer Disease
A modified silver Bielschowsky stain was used for visualizing diffuse plaques, neuritic plaques, and neurofibrillary tangles in sections of 5 brain regions.15 A board-certified neuropathologist blinded to clinical data adjudicated pathologic Alzheimer disease (AD) diagnosis.16
Hippocampal Sclerosis
Coronal sections of the mid hippocampus were examined for the presence of hippocampal sclerosis, which was summarized using a dichotomous variable indicating severe neuronal loss and gliosis in the CA1 and/or subiculum.17
Parkinson Disease
Antibodies against α-synuclein were used for the detection of Lewy bodies, which were summarized using a dichotomous variable indicating the presence of Lewy bodies. At the level of the third nerve exit root, sections of the midbrain containing the substantia nigra were stained with hematoxylin and eosin (H&E). Nigral neuronal loss was assessed using a semiquantitative scale (none, mild, moderate, and severe). Parkinson disease (PD) pathology was defined by a dichotomous variable indicating the presence of Lewy bodies and moderate to severe nigral neuronal loss.18
Macroinfarcts
Slabs of the fixed hemisphere and photographs of slabs of the frozen hemisphere were examined for the presence of macroinfarcts, which were confirmed microscopically.19 We included only chronic macroinfarcts, summarized by a dichotomous variable, because the pathologies affect progressive motor decline that occurs over years of follow-up.
Microinfarcts
A minimum of 9 brain regions was examined microscopically for the detection of microinfarcts using H&E-stained sections. Like macroinfarcts, only chronic microinfarcts were included, which were summarized using a dichotomous variable.19
Atherosclerosis
Circle of Willis vessels and their proximal branches were examined for atherosclerosis,20 which was summarized using a dichotomous variable indicating the presence of moderate to severe atherosclerosis.
Arteriolosclerosis
H&E sections of the anterior basal ganglia region were examined for arteriolosclerosis,19 which was summarized using a dichotomous variable indicating the presence of moderate to severe arteriolosclerosis.
Cerebral Amyloid Angiopathy
Immunohistochemical methods were used for the detection of mural β-amyloid in the meningeal and parenchymal vessels of 4 brain regions, which was summarized using a dichotomous variable indicating the presence of moderate to severe cerebral amyloid angiopathy (CAA).19
Assessment of Motor Function
Multiple motor performances were tested to capture the heterogeneity of late-life motor impairment in aging adults and considering the well-recognized distribution of weakness in ALS, another TDP-43–related syndrome. In prior publications, we developed composite measures of the diverse motor performances. Using composite measures was done to minimize random errors and floor and ceiling effects associated with the examination of individual motor performances and provided more power in examining associations between LATE-NC and declining motor function.18,21
Hand Strength
Grip and pinch strength were measured annually using a handheld dynamometer (Lafayette Instruments, Lafayette, IN). Participants were asked to perform each test with each hand twice. The average of the 4 trials for each test was calculated, representing grip strength and pinch strength in pounds of pressure. Stratified by sex, the grip and pinch strength were separately standardized using the baseline mean and SD of the tests in each sex. Then, the standardized grip and pinch strength scores were averaged to make a hand strength composite variable.
Respiratory Muscle Strength
A handheld device that contained a pressure transducer sensor (MicroMouth Pressure Meter MP01; MicroMedical Ltd., Kent, United Kingdom) was used for respiratory muscle strength measurements, which was performed only in MAP. Participants were asked to take a deep breath, seal their lips around the mouthpiece of the device, maximally expire, and hold their maximal expiration for at least 1 second. This performance was done twice for the measurement of maximal expiratory pressure (MEP) in cm H2O. Similar testing was used to measure maximal inspiratory pressure (MIP). The 2 MEP and MIP performances were averaged separately. The initial review of our data showed that men had higher MEP and MIP scores. So, women's and men's MEP and MIP scores were divided by their corresponding sex-specific averages of baseline MEP and MIP, and respiratory muscle strength was the average of these fractions multiplied by 100. Therefore, an average woman or man had a score of 100 at baseline, with higher scores indicating higher respiratory muscle strength.
Other Motor Functions
Other motor function composite variables were hand dexterity (derived from finger tapping speed and the Purdue Pegboard Test), gait (derived from steps and the time to complete walking 8 feet and turning 360° twice), and parkinsonism severity score (assessed using the Unified Parkinson's Disease Rating Scale). Details of these motor function assessments are provided in the eMethods (links.lww.com/WNL/D63) and elsewhere.18,22-24
Assessment of Cognition and Diagnosis of Dementia
At annual assessments, a battery of 19 neuropsychological tests was administered whose scores were standardized using means and SDs of the tests at baseline. The standardized scores were averaged to make a global cognition score.25 Moreover, the tests' scores were reviewed and rated by a neuropsychologist blinded to clinical data. The neuropsychologist's ratings together with clinical and physical examination data were reviewed by a neurologist, and cognition status of the participants including the presence of dementia was determined.26
Covariates
Sex was determined by self-report. Age at death was calculated using reported dates of birth and death.
Statistical Analyses
Categorical and continuous data were compared between higher and lower stages of LATE-NC by the χ2 and t test, respectively. We used linear mixed-effect models to examine associations of LATE-NC with longitudinal changes of motor decline. The core model consisted of fixed effects for age at death, sex, time (slope of motor decline), and interactions of age and sex with time and random intercept and slope. The random effects account for the person-specific level of motor function and the person-specific rate of motor decline. Then, we added terms for LATE-NC and its interaction with time to examine whether LATE-NC was associated with the level of motor function and rate of motor decline, respectively. As most participants had multiple brain pathologies that might affect motor function, in subsequent models we controlled the associations of LATE-NC with motor decline for other brain pathologies.
Prior work has generally examined the longitudinal change of different phenotypes such as motor and cognitive function in separate models. However, an individual experiences the change of both motor and cognitive function. Therefore, to account for the correlations between changes of cognitive and motor functions in older adults, we used multivariate random coefficient models that simultaneously estimated levels of cognition and motor function and their rates of decline. For these analyses, we standardized respiratory muscle strength using its baseline mean and SD to make the 3 outcomes of the same measurement unit. Then, we added terms for LATE-NC to examine whether LATE-NC was differentially associated with global cognition and motor function. The analysis was controlled for age at death and sex and their interaction with time. Two-sided p values less than 0.05 were used for rejecting null hypotheses.
Standard Protocol Approvals, Registrations, and Patient Consents
Each study was approved by the Institutional Review Board at Rush University Medical Center. The institutional review board approval numbers are L91020181 (ROS) and L86121802 (MAP). All participants signed an Anatomic Gift Act and informed consent.
Data Availability
The application process to obtain the data is initiated by filling an application including a short study premise and a brief research plan. The application should be submitted at the Rush Alzheimer's Disease Center Research Resource Sharing Hub at radc.rush.edu. Almost all applications get approved.
Results
Clinical and Pathologic Characteristics of Participants
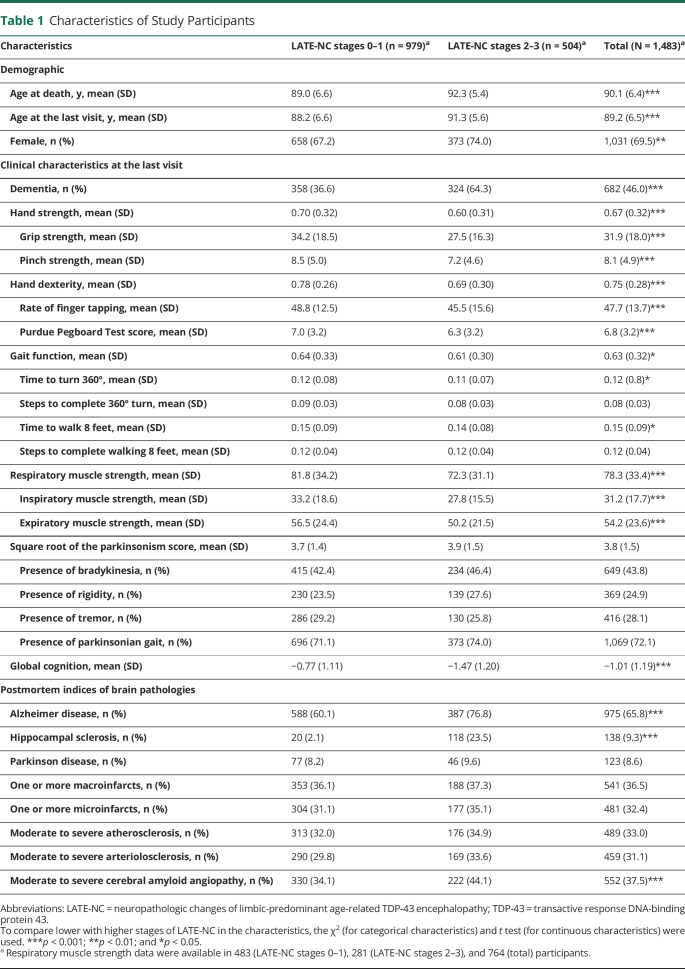
The characteristics of the 1,483 older adults, who were followed for an average 7.4 years (SD = 3.8) before death, are summarized in Table 1. The average age at death was 90 years. Participants with LATE-NC were on average 3 years older than participants without LATE-NC. AD, hippocampal sclerosis, and CAA pathologies were more frequent in adults with LATE-NC, but the 2 groups were not different in the frequency of other brain pathologies (Table 1).
Table 1.
Characteristics of Study Participants
Upper Extremity Motor Function
Hand Strength
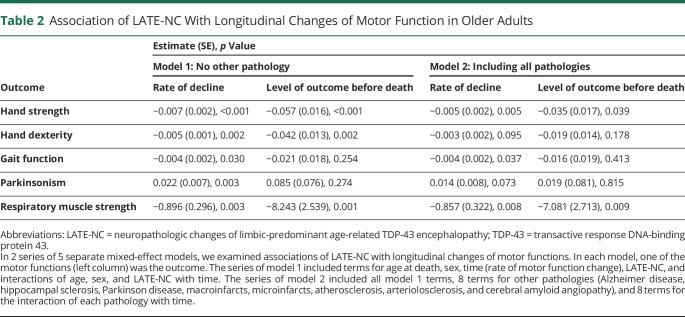
Examining longitudinal assessments of hand strength over years of follow-up indicated that hand strength on average declined (estimate = −0.041, SE = 0.001, p < 0.001). LATE-NC was associated with a faster rate of hand strength decline and a lower level of hand strength at death (Table 2, model 1; Figure 1). To contextualize the effect size, we used the model-derived estimates (eTable 1, model 1, links.lww.com/WNL/D63). In an average 90-year-old woman, LATE-NC was associated with an 18.4% faster hand strength decline rate. Moreover, estimating the variance of person-specific rates of hand strength decline indicated that LATE-NC explained 2.7% of the variance in the rate of hand strength decline.
Table 2.
Association of LATE-NC With Longitudinal Changes of Motor Function in Older Adults
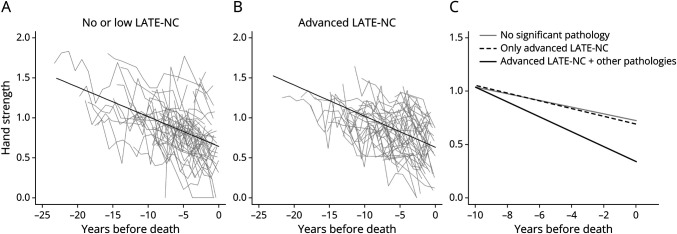
Figure 1. Association of LATE-NC With Hand Strength Decline.
Panels A and B illustrate raw trajectories of hand strength assessments in 50 randomly selected participants without (A) and with (B) LATE-NC stages 2–3. The overlaid black line shows the average trajectory of hand strength decline in these participants derived from mixed-effect models. Panel C illustrates the trajectories of hand strength decline in 3 average 90-year-old women derived from a mixed-effect model: a woman without significant brain pathologies (the solid gray line), a woman with only LATE-NC stages 2–3 (the dotted black line), and a woman with all the brain pathologies (the solid black line). LATE-NC = neuropathologic changes of limbic-predominant age-related TDP-43 encephalopathy; TDP-43 = transactive response DNA-binding protein 43.
We examined whether the association of LATE-NC with hand strength decline was independent of other brain pathologies. In a linear mixed-effect model including terms for the examined pathologies and their interaction with time, LATE-NC remained associated with both faster hand strength decline and a lower level of hand strength at death (Table 2, model 2; Figure 1). As LATE-NC co-occurs with AD pathology in many older adults27 and motor impairment in older adults is also a manifestation of AD,28 we examined whether the presence of AD modified the association of LATE-NC with hand strength decline. In a linear mixed-effect model including terms for LATE-NC, AD, and their interactions with each other and with time, neither the LATE-NC × AD (estimate = −0.019, SE = 0.035, p = 0.591) nor LATE-NC × AD × time (estimate = 0.0003, SE = 0.004, p = 0.932) was significant, indicating that comorbid AD pathology in an individual with LATE-NC was not synergistically associated with hand weakness.
Hand Dexterity
Hand dexterity on average declined during follow-ups (estimate = −0.025, SE = 0.001, p < 0.001). LATE-NC was associated with a faster rate of hand dexterity decline and a lower level of hand dexterity at death (Table 2, model 1). However, when we controlled for other pathologies, LATE-NC was not associated anymore with either the rate of decline or the level of hand dexterity at death (Table 2, model 2).
Gait
Gait function also on average declined during follow-ups (estimate = −0.042, SE = 0.001, p < 0.001). LATE-NC was associated with a faster rate of gait function decline (Table 2, model 1). However, the association of LATE-NC with a gait function decline rate, which persisted after controlling for other pathologies (Table 2, model 2), was not as strong as the association with hand strength. Moreover, estimating the variance of person-specific rates of gait decline indicated that LATE-NC did not explain any percentage of the variance in the rate of gait decline. These findings suggested that LATE-NC was not significantly related to gait impairment in older adults.
Parkinsonism
Examining longitudinal assessments of parkinsonism indicated progression of parkinsonism severity during the study (estimate = 0.120, SE = 0.004, p < 0.001). LATE-NC was associated with a faster parkinsonism progression (Table 2, model 1). However, the association of LATE-NC with a faster rate of parkinsonism progression was attenuated and not significant anymore after controlling for other pathologies (Table 2, model 2).
Respiratory Muscle Strength
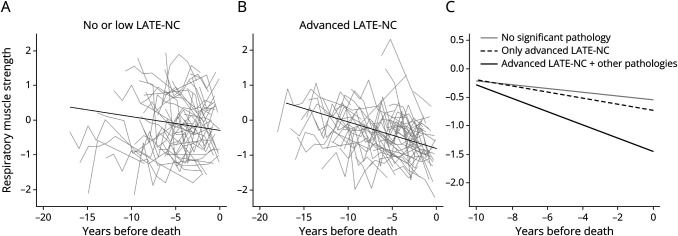
A total of 764 participants had respiratory muscle strength assessments. Respiratory muscle strength on average declined during the study (estimate = −2.703, SE = 0.175, p < 0.001). LATE-NC was associated with both a faster rate of respiratory muscle strength decline and a lower level of respiratory muscle strength at death (Table 2, model 1; Figure 2). In a 90-year-old woman, LATE-NC was associated with a 37.8% faster rate of respiratory muscle strength decline, an effect size larger than the association of LATE-NC with hand strength decline (eTable 1, model 2, links.lww.com/WNL/D63).
Figure 2. Association of LATE-NC With Respiratory Muscle Strength Decline.
Panels A and B illustrate raw trajectories of respiratory muscle strength assessments in 50 randomly selected participants without (A) and with (B) LATE-NC stages 2–3. The overlaid black line shows the average trajectory of respiratory muscle strength decline in these participants derived from mixed-effect models. Panel C illustrates the trajectories of respiratory muscle strength decline in 3 average 90-year-old women derived from a mixed-effect model: a woman without significant brain pathologies (the solid gray line), a woman with only LATE-NC stages 2–3 (the dotted black line), and a woman with all the brain pathologies (the solid black line). LATE-NC = neuropathologic changes of limbic-predominant age-related TDP-43 encephalopathy; TDP-43 = transactive response DNA-binding protein 43.
The association of LATE-NC with faster respiratory muscle strength decline did not change when the model was further controlled for other pathologies (Table 2, model 2; Figure 2). Comorbid AD pathology in an individual with LATE-NC was not synergistically associated with a faster respiratory muscle strength decline rate (LATE-NC × AD × time: estimate = 0.316, SE = 0.646, p = 0.624) or with a lower level of respiratory muscle strength at death (LATE-NC × AD: estimate = −1.939, SE = 5.455, p = 0.722).
Sensitivity Analyses
LATE-NC is more common in older adults (Table 1).1 We examined whether the association between LATE-NC and faster hand strength and respiratory muscle strength decline was modified by age at death. In mixed-effect models that examined the associations of LATE-NC with hand strength and respiratory muscle strength, we added a 2-way interaction between LATE-NC and age and a 3-way interaction between LATE-NC, age, and time. The analyses indicated that none of the interactions were significant (eTable 2, links.lww.com/WNL/D63), which suggested that age did not modify the association of LATE-NC with faster motor decline in older adults.
We treated LATE-NC as a dichotomous variable by combining stages 0–1 into one group and 2–3 into another group because of parsimony and because of prior studies' findings that had examined the associations of LATE-NC stages with dementia.27 In a sensitivity analysis, we examined whether the use of the LATE-NC dichotomous variable in the association with motor decline was supported by the data. In 2 separate mixed-effect models, we examined the association of LATE-NC with hand strength and respiratory muscle strength decline using 3 dummy variables, representing LATE-NC stages 1–3, and their interactions with time. The analyses indicated that stage 1 was not different from stage 0 and stage 3 not different from stage 2, in the associations with hand strength and respiratory muscle strength decline, which supported the use of the dichotomous LATE-NC variable (eTable 3, links.lww.com/WNL/D63).
Motor and Cognitive Function
Cognitive and motor decline in older adults are related,29 and cognitive decline is a known manifestation of LATE-NC.1,27 Therefore, after showing that LATE-NC was associated with declining respiratory muscle strength and hand strength in separate models (Table 2), we tested a hypothesis that LATE-NC was differentially associated with motor decline and cognitive decline. To test this hypothesis, we first set up a model to simultaneously estimate the change in respiratory muscle strength, hand strength, and global cognition while accounting for the correlations between the 3 outcomes. Next, we examined the association of LATE-NC and other pathologies with the 3 outcomes in a single model.
All participants with repeated measures of respiratory muscle strengths had also repeated measures of global cognition and hand strength. We used multivariate random coefficient models including repeated measures of respiratory muscle strength, hand strength, and global cognition to estimate the correlation structure between these 3 outcomes. In a model including age at death, sex, and their associations with the rates of decline and levels of respiratory muscle strength, hand strength, and global cognition, the estimated person-specific rates of decline in the 3 outcomes were correlated (eFigure 1, eTable 4, links.lww.com/WNL/D63). The highest correlation was between the rate of decline in the motor outcomes (r = 0.83), followed by the correlation between respiratory muscle strength and global cognition (r = 0.68) and hand strength and global cognition (r = 0.53) decline rates.
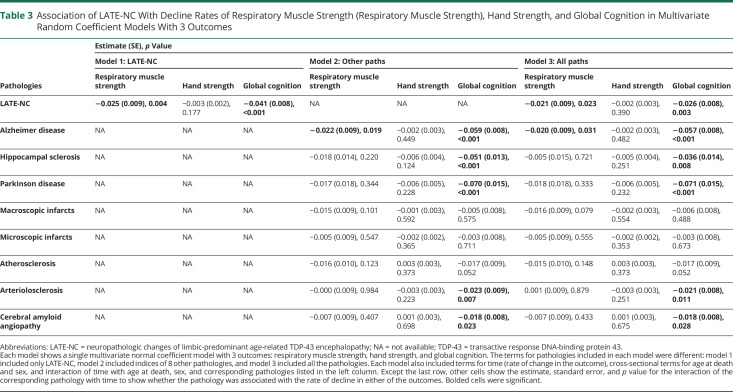
Next, we simultaneously examined the association of LATE-NC (Table 3, model 1), other brain pathologies (Table 3, model 2), and all pathologies (Table 3, model 3) with longitudinal changes of respiratory muscle strength, hand strength, and global cognition. In the first model that included only LATE-NC, LATE-NC remained associated with faster rates of decline and lower levels of respiratory muscle strength and global cognition, but the association of LATE-NC with hand strength was no longer significant (Table 3, model 1, eTable 5, model 1, links.lww.com/WNL/D63). The associations of LATE-NC with faster decline in the respiratory muscle strength and global cognition persisted after controlling for other pathologies (Table 3, model 3, eTable 5, model 3). Then, we compared the estimates of the associations of LATE-NC with respiratory muscle strength and global cognition decline rates, derived from the model that controlled for the other brain pathologies (Table 3, model 3). The estimates were different (estimate = 0.023, SE = 0.008, p = 0.004), indicating that LATE-NC was differentially associated with cognitive and motor decline.
Table 3.
Association of LATE-NC With Decline Rates of Respiratory Muscle Strength (Respiratory Muscle Strength), Hand Strength, and Global Cognition in Multivariate Random Coefficient Models With 3 Outcomes
We also estimated how much of the variance in the respiratory muscle strength and global cognition decline rates was explained by LATE-NC. LATE-NC explained 3.9% of the variance in the respiratory muscle strength decline rate and 4.7% of the variance in the global cognition decline rate (eFigure 2, links.lww.com/WNL/D63).
Discussion
In a cohort of approximately 1,500 participants, we found that LATE-NC was associated not only with cognitive decline but also with faster motor decline, specifically respiratory muscle strength. Moreover, the association of LATE-NC with faster motor decline was different from its association with faster cognitive decline. These findings suggest that LATE-NC, like other neurodegenerative pathologies including AD and PD, may have negative effects on not only cognitive but also noncognitive phenotypes. Moreover, considering that LATE-NC was observed in a 1/3 of our participants, the current study suggests that TDP-43 accumulating in aging brains may have a heretofore unrecognized role in the heterogeneity of late-life motor decline. Future studies are needed to explore the mechanisms underlying LATE-NC so that targeted treatments can be developed.
Since the first reports of the associations of TDP-43 inclusions with cognitive impairment in older adults in 200730 and development of LATE as a neurodegenerative disease of cognitive impairment in older adults,1 most studies have focused on the association of LATE-NC with cognitive impairment.2,31 Other investigated phenotypes included behavioral manifestations of LATE-NC such as psychosis.32 In our prior reports, we had included TDP-43 among other common age-related brain pathologies in association with 1 or 2 phenotypes of motor decline in older adults, such as parkinsonism33 and impaired global motor function.22 This study extends prior studies by focusing on LATE-NC and examining several phenotypes of motor impairment in older adults. Moreover, by using multivariate random coefficient models, we untangled cognitive from motor decline and found that the association of LATE-NC with motor decline was different from its association with cognitive decline. Therefore, the current study findings advance the field by introducing new clinical correlates of LATE-NC that are also important from the public health view, as incident motor impairment is very common29 and associated with morbidity and mortality.34,35 Moreover, the current findings suggest the addition of LATE to AD and PD that are multisystem disorders affecting both cognitive and motor systems.
LATE-NC was associated with a faster respiratory muscle strength decline that was different from the association of LATE-NC with cognitive decline. Although motor and sensory cortices together with the insula are also involved in respiratory control,36 the respiratory network is mainly located in the medullary pontine junction including pre-Bötzinger complex,37,38 which lies close to the inferior olive.39 Furthermore, studies that examined the distribution of TDP-43 inclusions across cerebral hemispheres and brainstem found that TDP-43 inclusions were also observed in the inferior olive.40 Therefore, it is possible that LATE-NC also affects the respiratory network in the medullary pontine junction, which underlies respiratory muscle strength decline. Moreover, as neurons in the medullary pontine junction are less involved in cognition, this hypothesis can explain differential associations of LATE-NC with faster respiratory muscle strength decline vs global cognition decline that is possibly caused by LATE-NC involvement of cortical regions in the brain hemispheres. Further studies are needed to examine this hypothesis.
Faster respiratory muscle strength and hand strength decline were the 2 motor impairments associated with LATE-NC. However, the effect size of the association of LATE-NC with the rate of hand strength decline was half of the effect size of the association with the rate of respiratory muscle strength decline. In addition, the association of LATE-NC with hand strength decline was attenuated in the models that also included global cognition and respiratory muscle strength as the outcomes. These findings suggest that although having weak hands is a comorbidity of LATE, LATE-NC has a preferential association with weak respiratory muscles as the motor correlate. We previously showed that weak hand strength was a risk factor for incident cognitive impairment and AD dementia41 to which LATE-NC contributes.31 Neuronal networks in brain regions including frontal, temporal, and insular cortices contribute to both hand strength42 and cognitive function and are vulnerable for the development of LATE-NC,1 which can explain the association of LATE-NC with both hand strength and cognitive decline.
Weakness of respiratory muscle strength43 and hand strength44 are also manifestations of ALS, another phenotype of TDP-43 proteinopathy. Moreover, patients with ALS may have cognitive impairment in addition to their prominent motor impairment.45 Similarly, patients with FTLD may present with cognitive and motor impairments even during prodromal phases.5 Therefore, TDP-43 proteinopathy may be considered as a spectrum of diseases with motor and cognitive impairments, with prominent motor impairment in one disease (ALS) and cognitive impairment in others (LATE and FTLD). In fact, studies have reported that limbic-predominant TDP-43 depositions are more frequent with advancing age in patients with ALS46 or FTLD,47 which supports TDP-43 spectrum disorders because LATE-NC is also more frequent in the oldest old. Further studies are required to uncover structures of the pathologic TDP-43 aggregates across these diseases.48 If further evidences support this spectrum, risk factors and treatments of one of the diseases in the spectrum may be beneficial in the other disease. Of interest, diabetes mellitus has been reported as a protective factor for ALS, and we previously reported an inverse association between higher hemoglobin A1c and more severe LATE-NC.12
We did not find consistent associations between LATE-NC and hand dexterity, gait, or parkinsonism. These null findings together with the associations of hand and respiratory muscle strengths with LATE-NC may be illustrative of the heterogeneity of motor decline in older adults. An individual may lose the ability to walk but have strong grip strength compared with another adult who walks independently but manifests hand weakness. In the current study, LATE-NC was most commonly assessed in cognitive-related brain regions. Neural systems underlying appendicular or axial motor performances extend beyond the brain to the spinal cord and muscles that may also be vulnerable to the accumulation of TDP-43 inclusions as reported in ALS.49 The current results highlight the need for further studies with larger sample sizes that examine motor-related sites for TDP-43 inclusions within and outside the brain to determine the full extent to which TDP-43 may contribute to late-life motor impairment.
The current study has several limitations. Participants were volunteers, mostly Whites, with high educational levels. Thus, these data require replication in other more diverse populations. Although the number of brain regions assessed for the presence of TDP-43 inclusions was more than the current recommendations,1 motor-related brain regions underlying the distributed motor pathways within and outside the brain, including motor cortex, spinal cord, and skeletal muscles, were not examined and could account for the lack of associations of LATE-NC with some of the motor performances examined in this study. Biomarkers of LATE-NC are not available yet, and levels of LATE-NC could not be determined in life before decline in cognitive and motor functions. Therefore, the temporal ordering of the accumulation of LATE-NC and the onset of motor decline cannot be determined. The findings are exploratory, not corrected for multiple comparisons, and will need to be confirmed in further studies.
The study has several important strengths. Approximately 1,500 older adults were annually followed for an average 7 years before death with objective repeated metrics of multiple motor performances. Moreover, we used a novel analytic approach that examined the simultaneous changes of both cognition and different motor performances and their association with LATE-NC in the same individuals. Other study strengths were high autopsy rates, pathologic assessments blinded to clinical data, and the availability of diverse indices of brain pathologies to account for in examining the associations of LATE-NC with motor and cognitive phenotypes.
Acknowledgment
The authors thank the study participants and the staff of the Rush Alzheimer's Disease Center.
Glossary
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- CAA
cerebral amyloid angiopathy
- FTLD
frontotemporal lobar degeneration
- H&E
hematoxylin and eosin
- LATE
limbic-predominant age-related TDP-43 encephalopathy
- LATE-NC
neuropathologic change of LATE
- MAP
Rush Memory and Aging Project
- MEP
maximal expiratory pressure
- MIP
maximal inspiratory pressure
- PD
Parkinson disease
- ROS
Religious Orders Study
- TDP
transactive response DNA-binding protein 43 kDa
Appendix. Authors
Footnotes
Infographic: links.lww.com/WNL/D113
Study Funding
The study was supported by NIH grants R01AG067482, P30AG10161, P30AG72975, R01AG15819, R01AG17917, R01AG075728, and R01AG056352.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142(6):1503-1527. doi: 10.1093/brain/awz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson PT, Brayne C, Flanagan ME, et al. Frequency of LATE neuropathologic change across the spectrum of Alzheimer's disease neuropathology: combined data from 13 community-based or population-based autopsy cohorts. Acta Neuropathol. 2022;144(1):27-44. doi: 10.1007/s00401-022-02444-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josephs KA, Murray ME, Tosakulwong N, et al. Pathological, imaging and genetic characteristics support the existence of distinct TDP-43 types in non-FTLD brains. Acta Neuropathol. 2019;137(2):227-238. doi: 10.1007/s00401-018-1951-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goutman SA, Hardiman O, Al-Chalabi A, et al. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol. 2022;21(5):465-479. doi: 10.1016/s1474-4422(21)00414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeve BF, Boxer AL, Kumfor F, Pijnenburg Y, Rohrer JD. Advances and controversies in frontotemporal dementia: diagnosis, biomarkers, and therapeutic considerations. Lancet Neurol. 2022;21(3):258-272. doi: 10.1016/s1474-4422(21)00341-0 [DOI] [PubMed] [Google Scholar]
- 6.Polkey MI, Lyall RA, Yang K, Johnson E, Leigh PN, Moxham J. Respiratory muscle strength as a predictive biomarker for survival in amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 2017;195(1):86-95. doi: 10.1164/rccm.201604-0848oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005;65(4):586-590. doi: 10.1212/01.wnl.0000172911.39167.b6 [DOI] [PubMed] [Google Scholar]
- 8.Seelaar H, Schelhaas HJ, Azmani A, et al. TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain. 2007;130(5):1375-1385. doi: 10.1093/brain/awm024 [DOI] [PubMed] [Google Scholar]
- 9.Besser LM, Teylan MA, Nelson PT. Limbic predominant age-related TDP-43 encephalopathy (LATE): clinical and neuropathological associations. J Neuropathol Exp Neurol. 2020;79(3):305-313. doi: 10.1093/jnen/nlz126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(suppl 1):S161-S189. doi: 10.3233/jad-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer's disease and common neuropathologies of aging. Acta Neuropathol. 2018;136(6):887-900. doi: 10.1007/s00401-018-1920-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oveisgharan S, Capuano AW, Nag S, et al. Association of hemoglobin A1C with TDP-43 pathology in community-based elders. Neurology. 2021;96(22):e2694-e2703. doi: 10.1212/wnl.0000000000012025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer's disease. Acta Neuropathol Commun. 2018;6(1):33. doi: 10.1186/s40478-018-0531-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal S, Yu L, Kapasi A, et al. Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change and microvascular pathologies in community-dwelling older persons. Brain Pathol. 2021;31(3):e12939. doi: 10.1111/bpa.12939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DA, Wilson RS, Schneider JA, et al. Apolipoprotein E ε4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology. 2003;60(2):246-252. doi: 10.1212/01.wnl.0000042478.08543.f7 [DOI] [PubMed] [Google Scholar]
- 16.Hyman BT, Trojanowski JQ. Editorial on consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease: J Neuropathol Exp Neurol. 1997;56(10):1095-1097. doi: 10.1097/00005072-199710000-00002 [DOI] [PubMed] [Google Scholar]
- 17.Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol. 2015;77(6):942-952. doi: 10.1002/ana.24388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oveisgharan S, Yu L, Wang T, Schneider JA, Bennett DA, Buchman AS. Neurodegenerative and cerebrovascular brain pathologies are differentially associated with declining grip strength and gait in older adults. J Gerontol A Biol Sci Med Sci. 2022;78(3):504-513. doi: 10.1093/gerona/glac128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oveisgharan S, Yu L, Capuano A, et al. Late-life vascular risk score in association with postmortem cerebrovascular disease brain pathologies. Stroke. 2021;52(6):2060-2067. doi: 10.1161/strokeaha.120.030226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oveisgharan S, Yu L, Barnes LL, et al. Association of statins with cerebral atherosclerosis and incident parkinsonism in older adults. Neurology. 2022;98(19):e1976-e1984. doi: 10.1212/wnl.0000000000200182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Oertzen T, Hertzog C, Lindenberger U, Ghisletta P. The effect of multiple indicators on the power to detect inter-individual differences in change. Br J Math Stat Psychol. 2010;63(3):627-646. doi: 10.1348/000711010x486633 [DOI] [PubMed] [Google Scholar]
- 22.Buchman AS, Wang T, Yu L, Leurgans SE, Schneider JA, Bennett DA. Brain pathologies are associated with both the rate and variability of declining motor function in older adults. Acta Neuropathol. 2020;140(4):587-589. doi: 10.1007/s00401-020-02212-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle PA, Wilson RS, Buchman AS, et al. Lower extremity motor function and disability in mild cognitive impairment. Exp Aging Res. 2007;33(3):355-371. doi: 10.1080/03610730701319210 [DOI] [PubMed] [Google Scholar]
- 24.Buchman AS, Yu L, Wilson RS, et al. Progressive parkinsonism in older adults is related to the burden of mixed brain pathologies. Neurology. 2019;92(16):e1821-e1830. doi: 10.1212/wnl.0000000000007315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oveisgharan S, Buchman AS, Yu L, et al. APOE ε2ε4 genotype, incident AD and MCI, cognitive decline, and AD pathology in older adults. Neurology. 2018;90(24):e2127-e2134. doi: 10.1212/wnl.0000000000005677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer's-type dementia. Brain. 2016;139(11):2983-2993. doi: 10.1093/brain/aww224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement. 2015;11(1):70-98. doi: 10.1016/j.jalz.2014.04.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L, Boyle PA, Leurgans SE, Wilson RS, Bennett DA, Buchman AS. Incident mobility disability, mild cognitive impairment, and mortality in community-dwelling older adults. Neuroepidemiology. 2019;53(1-2):55-62. doi: 10.1159/000499334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amador-Ortiz C, Lin W-L, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61(5):435-445. doi: 10.1002/ana.21154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agrawal S, Yu L, Nag S, et al. The association of Lewy bodies with limbic-predominant age-related TDP-43 encephalopathy neuropathologic changes and their role in cognition and Alzheimer's dementia in older persons. Acta Neuropathol Commun. 2021;9(1):156. doi: 10.1186/s40478-021-01260-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teylan MA, Mock C, Gauthreaux K, et al. Differences in symptomatic presentation and cognitive performance among participants with LATE-NC compared to FTLD-TDP. J Neuropathol Exp Neurol. 2021;80(11):1024-1032. doi: 10.1093/jnen/nlab098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchman AS, Yu L, Oveisgharan S, Farfel JM, Schneider JA, Bennett DA. Person-specific contributions of brain pathologies to progressive parkinsonism in older adults. J Gerontol A Biol Sci Med Sci. 2021;76(4):615-621. doi: 10.1093/gerona/glaa176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the rush memory and aging project, a community-based cohort study. BMC Med. 2011;9(1):42. doi: 10.1186/1741-7015-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchman AS, Dawe RJ, Leurgans SE, et al. Different combinations of mobility metrics derived from a wearable sensor are associated with distinct health outcomes in older adults. J Gerontol A Biol Sci Med Sci. 2020;75(6):1176-1183. doi: 10.1093/gerona/glz160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu L, De Mazancourt M, Hess A, et al. Functional connectivity and information flow of the respiratory neural network in chronic obstructive pulmonary disease. Hum Brain Mapp. 2016;37(8):2736-2754. doi: 10.1002/hbm.23205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci. 2018;19(6):351-367. doi: 10.1038/s41583-018-0003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhingra RR, Dick TE, Furuya WI, Galán RF, Dutschmann M. Volumetric mapping of the functional neuroanatomy of the respiratory network in the perfused brainstem preparation of rats. J Physiol. 2020;598(11):2061-2079. doi: 10.1113/jp279605 [DOI] [PubMed] [Google Scholar]
- 39.Schwarzacher SW, Rüb U, Deller T. Neuroanatomical characteristics of the human pre-Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain. 2011;134(1):24-35. doi: 10.1093/brain/awq327 [DOI] [PubMed] [Google Scholar]
- 40.Josephs KA, Murray ME, Whitwell JL, et al. Updated TDP-43 in Alzheimer's disease staging scheme. Acta Neuropathol. 2016;131(4):571-585. doi: 10.1007/s00401-016-1537-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Grip strength and the risk of incident Alzheimer's disease. Neuroepidemiology. 2007;29(1-2):66-73. doi: 10.1159/000109498 [DOI] [PubMed] [Google Scholar]
- 42.Ward NS, Frackowiak RSJ. Age-related changes in the neural correlates of motor performance. Brain. 2003;126(4):873-888. doi: 10.1093/brain/awg071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto S, Gromicho M, Oliveira Santos MO, Swash M, De Carvalho M. Respiratory onset in amyotrophic lateral sclerosis: clinical features and spreading pattern. Amyotroph Lateral Scler Frontotemporal Degener. 2022;24(1-2):40-44. doi: 10.1080/21678421.2022.2067777 [DOI] [PubMed] [Google Scholar]
- 44.Hannaford A, Higashihara M, Pavey N, et al. Split-hand index: a diagnostic and prognostic marker in amyotrophic lateral sclerosis across varying regions of onset. Clin Neurophysiol. 2021;132(9):2130-2135. doi: 10.1016/j.clinph.2021.06.008 [DOI] [PubMed] [Google Scholar]
- 45.Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013;12(4):368-380. doi: 10.1016/s1474-4422(13)70026-7 [DOI] [PubMed] [Google Scholar]
- 46.Murakami A, Koga S, Sekiya H, et al. Old age amyotrophic lateral sclerosis and limbic TDP-43 pathology. Brain Pathol. 2022;32(6):e13100. doi: 10.1111/bpa.13100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buciuc M, Whitwell JL, Baker MC, Rademakers R, Dickson DW, Josephs KA. Old age genetically confirmed frontotemporal lobar degeneration with TDP-43 has limbic predominant TDP-43 deposition. Neuropathol Appl Neurobiol. 2021;47(7):1050-1059. doi: 10.1111/nan.12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arseni D, Hasegawa M, Murzin AG, et al. Structure of pathological TDP-43 filaments from ALS with FTLD. Nature. 2022;601(7891):139-143. doi: 10.1038/s41586-021-04199-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cykowski MD, Powell SZ, Appel JW, Arumanayagam AS, Rivera AL, Appel SH. Phosphorylated TDP-43 (pTDP-43) aggregates in the axial skeletal muscle of patients with sporadic and familial amyotrophic lateral sclerosis. Acta Neuropathol Commun. 2018;6(1):28. doi: 10.1186/s40478-018-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The application process to obtain the data is initiated by filling an application including a short study premise and a brief research plan. The application should be submitted at the Rush Alzheimer's Disease Center Research Resource Sharing Hub at radc.rush.edu. Almost all applications get approved.