Abstract
Background and Objectives
Brain frailty may impair the ability of acute stroke patients to cope with the injury, irrespective of their chronologic age, resulting in impaired recovery. We aim to investigate the impact of brain atrophy on functional outcome assessed at different time points after endovascular thrombectomy (EVT).
Methods
In this retrospective post hoc analysis of the ESCAPE-NA1 trial, we analyzed CT imaging data for cortical atrophy by using the GCA scale, including region-specific scales, and subcortical atrophy by using the intercaudate distance to inner table width (CC/IT) ratio. The primary outcome was 90-day mRS (ordinal shift analysis), and the secondary outcome was the mRS score over time. Adjustments were made for age, sex, baseline NIHSS, final infarct volume, stroke laterality, total Fazekas score, and nerinetide-alteplase interaction. Sensitivity analyses were additionally performed in only those patients for whom MRI data were available.
Results
Of 1,102 participants (mean age of 69.5 ± 13.7 years; 554 men), 818 (74%) had GCA = 0, 220 (20%) had GCA = 1, and 64 (6%) had GCA = 2/3. The median CC/IT ratio was 0.12 (IQR0.10-0.15). Cortical atrophy (GCA ≥ 1 vs GCA 0) was associated with worse 90-day mRS (acOR = 1.62 [95% CI 1.22–2.16]; p = 0.001), lower rates of 90-day mRS0-2 (aOR = 0.65 [95% CI 0.45–0.94]; p = 0.022), and higher mortality (aOR = 2.12 [95% CI 1.28–3.5]; p = 0.003), regardless of the region assessed. Subcortical atrophy was associated with worse 90-day mRS (acOR [per 0.01 increase in CC/IT ratio] = 1.07 [95% CI 1.04–1.11]; p < 0.001) and lower rates of 90-day mRS0-2 (aOR = 0.92 [95% CI 0.88–0.97]; p = 0.001). Furthermore, with various degrees of atrophy, we observed heterogeneity in mRS measurements during follow-up: worse mRS scores for higher atrophy grades (p < 0.001). Compared with participants with GCA = 0, the mRS for participants with GCA = 1 was higher at 30 days (adjusted difference = 0.41 [95% CI 0.18–0.65]) and remained worse at 90 days (adjusted difference = 0.72 [95% CI 0.49–0.95]). Similar effects were seen for participants with worse cortical atrophy, regardless of the region assessed, and worse subcortical atrophy. Furthermore, 26/63(41%) and 124/274(45%) patients with severe cortical/subcortical atrophy (GCA 2/3 and highest CC/IT ratio quartile, respectively) achieved good functional outcome (mRS0-2), compared with 539/812(66.4%) with no cortical atrophy and 209/274(76%) in the lowest CC/IT ratio quartile.
Discussion
In this large RCT-derived population, participants with brain atrophy, as visually assessed on acute noncontrast computed tomography imaging, showed less favorable stroke recovery after EVT and worse 90-day functional outcomes compared with participants without brain atrophy. This may support physicians with recovery expectations when planning post-EVT care with patients and their families.
Introduction
Endovascular thrombectomy (EVT) is the standard treatment for acute ischemic stroke (AIS) in the anterior circulation due to an intracranial large vessel occlusion (LVO) because it has proven efficacious in several randomized controlled clinical trials.1 However, patients undergoing EVT show large interindividual differences in functional recovery with a proportion of up to 50% not achieving functional independence, despite technically successful reperfusion.1,2
Selection of suitable candidates for EVT as well as patients' and proxies' decisions to pursue or forgo treatment heavily rely on predicted outcomes and how physicians frame individual prognoses and risks vs benefits of treatment.3 However, even when including the most comprehensive model (i.e., baseline + procedural + postprocedural variables), still one-fifth of patients were misclassified in post-EVT outcome.4 Thus, exploring additional influencing factors, assessable at baseline, is crucial to refine prognostication and, at the same time, to inform clinical decision-making.
Brain reserve refers to the ability of the brain to compensate for acquired dysfunction, for example, due to ischemic injury,5 and could represent such an additional variable that is available at baseline. The concept of brain reserve may be attributable to various mechanisms, such as neuroplasticity,6 neurogenesis,7,8 cortical integrity,9 and synaptic density,10 and has been linked to the previously described conceptual difference between a neuroimaging-predicted age (biologic age) and a chronologic age.11,12 Brain atrophy is a key component of brain reserve and is easily obtainable in the acute stroke setting using conventional brain imaging, such as noncontrast computed tomography (NCCT).11
Previous research has explored the influence of brain atrophy on clinical outcome after EVT.11,13,14 Most of the studies, however, included small sample sizes11,13 and/or performed (semi)quantitative analyses.14 In addition, the role of brain atrophy on trajectories of functional recovery, as determined by repeated assessments of the modified Rankin Scale (mRS) poststroke, has not been studied to date.
We aim to investigate the impact of brain atrophy on functional outcome assessed at 90 days and multiple time points between discharge and 90 days in a large RCT-derived population of AIS patients who underwent EVT within 12 hours of stroke onset.
Methods
Patient Sample
This is a retrospective analysis of the Safety and Efficacy of Nerinetide (NA-1) in Subjects Undergoing Endovascular Thrombectomy for Stroke (ESCAPE-NA1])15 trial (ClinicalTrials.gov: NCT01778335). This trial investigated the efficacy and safety of a neuroprotectant drug—nerinetide—after endovascular thrombectomy for acute ischemic stroke. In brief, the inclusion criteria are (1) 18 years or older, (2) a baseline NIHSS of 6 or higher, (3) patients should be functional independent prestroke (i.e., Barthel index score >90), and (4) included patients were last seen normal within 12 hours from symptom onset. Imaging criteria for this trial included (1) the presence of a large vessel occlusion in the anterior circulation as detected on baseline computed tomography angiography, (2) moderate-to-good collaterals, and (3) a baseline ASPECTS score equal to or higher than 5, assessed on NCCT. For this study, participants should have at least 1 plane available according to the atrophy scale that is used (e.g., coronal plane for the MTA scale, axial plane for the GCA scale, and sagittal plane for the Koedam scale), and no important artefacts should be present (e.g., motion or beam hardening). Previously published studies that have reported findings from ESCAPE-NA1 are summarized in eTable 1 (links.lww.com/WNL/D54). No studies are published to date about the impact of atrophy on outcome.
Image Analysis
All available data were independently reviewed by a core laboratory (F. Benali, F. Bala, J.F., N.S. with 4–7 years of experience; eTable 2, links.lww.com/WNL/D54), blinded to any clinical or outcome data. In case of any disagreement between the readers, a senior reader (A.G. with 10 years of experience) provided consensus. Quality parameters of acquired NCCT include a minimum power of 120–140 kV, 170–200 mA; 2-second scanning time; 5-mm section thickness; appropriate algorithms reducing bone artefacts and rendering high SNR for gray-white matter differentiation; a good quality stroke protocol defined by criteria on the unaffected side (e.g., lateral margin of the lentiform nucleus should be well discriminated in the absence of previous infarction; insular ribbon is well defined in the absence of previous infarction); and helical acquisition. Patients with follow-up MRI should have at least an axial diffusion weighted imaging, gradient recovery echo, and fluid-attenuated inversion recovery (FLAIR) at 24 ± 12 hours postrandomization. Further details on image acquisition can be found in the protocol of the ESCAPE-NA1 trial.15 Final infarct volumes (FIV) were manually segmented on follow-up imaging (NCCT or MRI, wherever available), by using open-source software ITK-SNAP, version 3.8.0 (itksnap.org), without any atlas template. The global cortical atrophy (GCA) scale16 (eFigure 1A) was used to assess cortical atrophy, a pragmatic qualitative scale that evaluates the width of the sulci and volume of the gyri ranging from 0-3. This was assessed for each lobe separately (regional GCA) in both hemispheres. In case of any asymmetry, the score of the most affected side was chosen. The Medial Temporal Atrophy (MTA; grade 0–4)17,18 scale (eFigure 1B) was used to assess hippocampal atrophy, and the KOEDAM scale (grade 0–3)19 was used to assess cortical atrophy in the parietal lobe (eFigure 1C).
Subcortical atrophy was assessed by calculating the ratio of the intercaudate distance (ICD) to the inner table width, CC/IT ratio.20 To avoid any error because of potential infarct-associated edema, the ICD was measured on the contralateral side (i.e., hemi-ICD) and then multiplied by 2 (eFigure 1D, links.lww.com/WNL/D54). The hemi-ICD is defined as the minimum distance between the caudate head and the septum pellucidum at the level of Monro and has been used previously21,22 to assess subcortical atrophy in stroke patients.
Old infarctions were defined as any area of tissue loss exceeding an axial diameter of 15 mm. White matter lesions were rated through visual assessment using the Fazekas scale,23 adopted to NCCT, which has been performed previously.24-26 Both periventricular and deep Fazekas scores were combined to generate a “total white matter disease (WMD)-burden score.” To avoid interference with acute ischemic lesions, assessments were performed on the contralateral hemisphere.
Baseline characteristics, such as demographics, medical history, blood work, and stroke severity (NIHSS) at baseline, were collected in all participants. Assessments of the functional outcome (mRS) were performed with the patient and/or proxies by a trained personnel during clinical visits or telephone interviews, including 3 different time points poststroke, that is, day 5 or discharge, day 30, and day 90.
Standard Protocol Approvals, Registrations, and Patient Consents
All participants or their legally authorized representatives provided informed consent before study enrolment. This study was reviewed and approved by the local regulatory authorities and the respective ethics board at each center. More detailed information on the study protocol has been previously published.15
Outcomes of Interest
The primary outcome was a shift in 90-day mRS score on an ordinal scale (0–6). Secondary outcomes were (1) functional independence and mortality at 90 days and (2) the mRS trajectory over time (prestroke, day 5, day 30, and day 90).
Statistical Analyses
Baseline characteristics were summarized by using descriptive statistics. Categorical variables are reported as counts/percentages and continuous variables as medians/interquartile ranges (IQR). For statistical comparison, chi-square was used for categorical variables and Kruskal-Wallis was applied for continuous variables. For single 90-day time point regression analyses, we treated the GCA scale and the CC/IT ratio as ordinal variables. We categorized the CC/IT ratios into quartiles (i.e., <0.10; 0.10–0.12; 0.12–0.15, and >0.15). The GCA scale was trichotomized, as appropriate, given the distribution of the data (GCA 0 – 1 – 2/3).
For the primary outcome, multivariable ordinal logistic regression analyses were performed to assess the relationship between cortical or subcortical atrophy and functional outcome (mRS; ordinal shift analysis). The assumption of proportional odds was checked using the Brant test. Statistical models were adjusted for age, sex, FIV, total WMD-burden, the interaction between alteplase and nerinetide, NIHSS at baseline, stroke laterality, and presence of chronic infarctions. The nerinetide/alteplase interaction was included based on an observed statistical interaction between both drugs regarding 90-day outcome in the main paper.15 For secondary outcomes, 90-day mRS 0–2 and 90-day mortality were included as dependent variables. Adjustments were like the primary outcome analyses. As part of feature scaling for logistic regression modeling, we multiplied the CC/IT ratio by 100 to simplify the resulting a/acOR (i.e., per unit increase). To evaluate the association of cortical/subcortical atrophy with mRS changes over time, a repeated-measures mixed regression model was applied. Our independent variable was an interaction term for atrophy and the timing of assessment. Differences in the predicted mean mRS at each time point were presented as adjusted differences with covariates including age, sex, FIV, total WMD-burden, alteplase/nerinetide interaction, NIHSS at baseline, stroke laterality, and chronic infarctions.
As a sensitivity analysis, we reperformed all regression analyses among those participants with available post-treatment MRI. In addition, NCCT analyses were performed for each brain region separately (i.e., frontal, temporal, parietal, occipital, and hippocampal) and including the final TICI score as confounding variable. Applied statistical tests were 2-sided, and p values ≤0.05 were considered significant. All statistical analyses were conducted using Stata/MP 16.1 (StataCorp, College Station, TX). In addition, we performed the likelihood ratio test (lrtest) to compare the goodness of fit of a nested model with and without brain atrophy (i.e., cortical and subcortical atrophy) with respect to the predicted outcomes.
Data Availability
The ESCAPE-NA1 data are not currently publicly available for distribution, but a future public data set may be made available. Researchers interested in the data are asked to contact the corresponding author with a proposal.
Results
Patient Characteristics
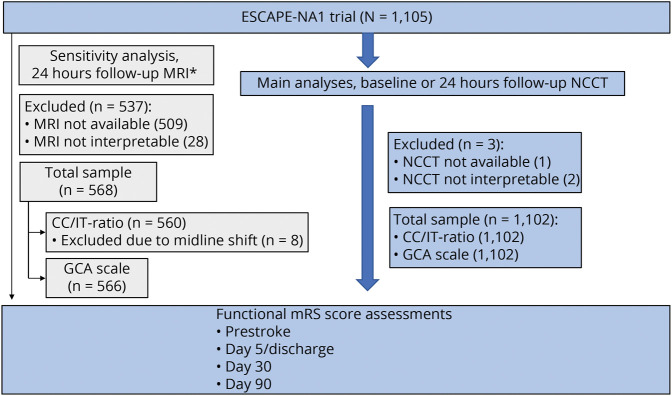
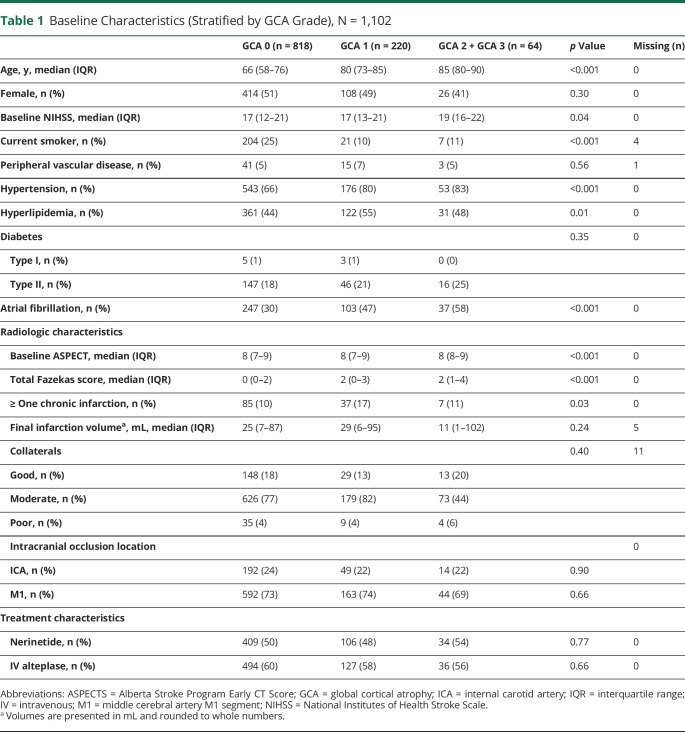
Among 1,105 ESCAPE-NA1 participants, NCCT was unavailable or of insufficient quality in 3 participants, who were excluded (Figure 1). Of the remaining 1,102 participants, the mean age ± standard deviation was 69.5 years ± 13.7; 554 were men. Brain atrophy assessments were performed on baseline NCCT in 1,088 (99%) and follow-up NCCT in 14 (1%). Of 1,102 participants, 818 (74%) had no cortical atrophy (GCA = 0), 220 (20%) had mild atrophy (GCA = 1), and 64 (6%) had moderate to severe atrophy (GCA = 2/3). The median (IQR) CC/IT ratio was 0.12 (0.10–0.15). Participants with worse atrophy (GCA 2/3 vs 1 vs 0) were older (median age, 85 [IQR 80–90] vs 80 [IQR 73–85] vs 66 [IQR 58–76] years, p < 0.001), had higher baseline NIHSS (median, 19 [IQR 16–22] vs 17 [IQR 13–21] vs 17 [IQR 12–21], p = 0.04), and had more comorbidities (hypertension, n = 53 [83%] vs n = 176 [80%] vs 543 [66%], p < 0.001; atrial fibrillation, n = 37 [58%] vs n = 103 [47%] vs 247 [30%], p < 0.001). The total WMD-burden (median total Fazekas score) increased (0 [IQR 0–2] vs 2 [IQR 0–3] vs 2 [IQR 1–4], p < 0.001), whereas the median FIV did not differ significantly (25 [IQR 7–87] vs 29 [IQR 6–95] vs 11 [IQR 1–102] mL, p = 0.24) with worse atrophy grades (GCA 0 vs 1 vs 2/3). Table 1 presents all clinical, radiologic, and treatment-related characteristics. For the predicted outcomes, the model including the features of cortical and subcortical brain atrophy provided a significantly better goodness of fit than a restricted model without these features (p = 0.0047).
Figure 1. Patient Selection Flowchart, N = 1,102.
*At least FLAIR MRI and at least axial plane available. FLAIR = fluid-attenuated inversion recovery; FU = follow-up; GCA = global cortical atrophy; ICD = intercaudate distance; ITW = inner table width; NCCT = noncontrast CT.
Table 1.
Baseline Characteristics (Stratified by GCA Grade), N = 1,102
| GCA 0 (n = 818) | GCA 1 (n = 220) | GCA 2 + GCA 3 (n = 64) | p Value | Missing (n) | |
| Age, y, median (IQR) | 66 (58–76) | 80 (73–85) | 85 (80–90) | <0.001 | 0 |
| Female, n (%) | 414 (51) | 108 (49) | 26 (41) | 0.30 | 0 |
| Baseline NIHSS, median (IQR) | 17 (12–21) | 17 (13–21) | 19 (16–22) | 0.04 | 0 |
| Current smoker, n (%) | 204 (25) | 21 (10) | 7 (11) | <0.001 | 4 |
| Peripheral vascular disease, n (%) | 41 (5) | 15 (7) | 3 (5) | 0.56 | 1 |
| Hypertension, n (%) | 543 (66) | 176 (80) | 53 (83) | <0.001 | 0 |
| Hyperlipidemia, n (%) | 361 (44) | 122 (55) | 31 (48) | 0.01 | 0 |
| Diabetes | 0.35 | 0 | |||
| Type I, n (%) | 5 (1) | 3 (1) | 0 (0) | ||
| Type II, n (%) | 147 (18) | 46 (21) | 16 (25) | ||
| Atrial fibrillation, n (%) | 247 (30) | 103 (47) | 37 (58) | <0.001 | 0 |
| Radiologic characteristics | |||||
| Baseline ASPECT, median (IQR) | 8 (7–9) | 8 (7–9) | 8 (8–9) | <0.001 | 0 |
| Total Fazekas score, median (IQR) | 0 (0–2) | 2 (0–3) | 2 (1–4) | <0.001 | 0 |
| ≥ One chronic infarction, n (%) | 85 (10) | 37 (17) | 7 (11) | 0.03 | 0 |
| Final infarction volumea, mL, median (IQR) | 25 (7–87) | 29 (6–95) | 11 (1–102) | 0.24 | 5 |
| Collaterals | 0.40 | 11 | |||
| Good, n (%) | 148 (18) | 29 (13) | 13 (20) | ||
| Moderate, n (%) | 626 (77) | 179 (82) | 73 (44) | ||
| Poor, n (%) | 35 (4) | 9 (4) | 4 (6) | ||
| Intracranial occlusion location | 0 | ||||
| ICA, n (%) | 192 (24) | 49 (22) | 14 (22) | 0.90 | |
| M1, n (%) | 592 (73) | 163 (74) | 44 (69) | 0.66 | |
| Treatment characteristics | |||||
| Nerinetide, n (%) | 409 (50) | 106 (48) | 34 (54) | 0.77 | 0 |
| IV alteplase, n (%) | 494 (60) | 127 (58) | 36 (56) | 0.66 | 0 |
Abbreviations: ASPECTS = Alberta Stroke Program Early CT Score; GCA = global cortical atrophy; ICA = internal carotid artery; IQR = interquartile range; IV = intravenous; M1 = middle cerebral artery M1 segment; NIHSS = National Institutes of Health Stroke Scale.
Volumes are presented in mL and rounded to whole numbers.
Primary Outcome Measures
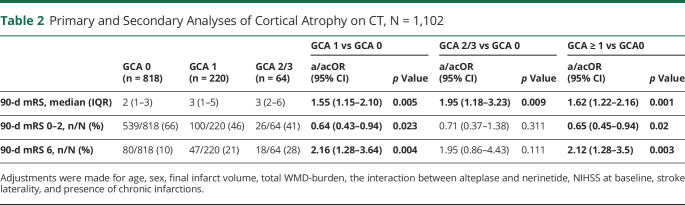
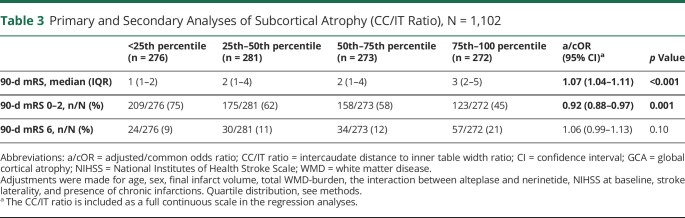
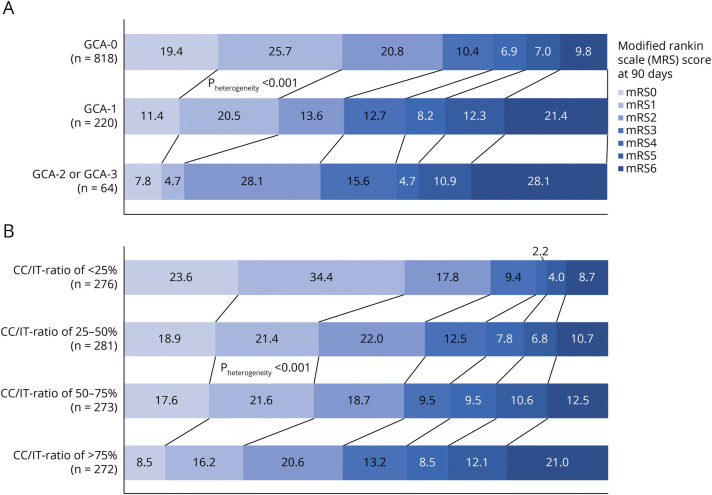
Higher grades of cortical atrophy were associated with worse 90-day mRS (adjusted common OR [acOR] for GCA1 vs GCA0: 1.55 [95% CI 1.15–2.10] and acOR for GCA2/3 vs GCA0: 1.95 [95% CI 1.18–3.23]), after correcting for age, sex, final infarct volume (FIV), total WMD-burden, alteplase/nerinetide interaction, NIHSS at baseline, stroke laterality, and presence of chronic infarctions. Similarly, increasing subcortical atrophy, as reflected by the CC/IT ratio, was significantly associated with worse 90-day mRS (acOR 1.07 [95% CI 1.04–1.11]; p < 0.001) (Tables 2 and 3, Figure 2, A and B). Of 63 patients with a GCA 2 or GCA 3, 26 (41%) had good 90-day functional outcome (mRS0-2), and of 274 patients with the highest quartile of CC/IT ratio, 124 (45%) had good 90-day functional outcome (mRS0-2).
Table 2.
Primary and Secondary Analyses of Cortical Atrophy on CT, N = 1,102
| GCA 0 (n = 818) | GCA 1 (n = 220) | GCA 2/3 (n = 64) | GCA 1 vs GCA 0 | GCA 2/3 vs GCA 0 | GCA ≥ 1 vs GCA0 | ||||
| a/acOR (95% CI) | p Value | a/acOR (95% CI) | p Value | a/acOR (95% CI) | p Value | ||||
| 90-d mRS, median (IQR) | 2 (1–3) | 3 (1–5) | 3 (2–6) | 1.55 (1.15–2.10) | 0.005 | 1.95 (1.18–3.23) | 0.009 | 1.62 (1.22–2.16) | 0.001 |
| 90-d mRS 0–2, n/N (%) | 539/818 (66) | 100/220 (46) | 26/64 (41) | 0.64 (0.43–0.94) | 0.023 | 0.71 (0.37–1.38) | 0.311 | 0.65 (0.45–0.94) | 0.02 |
| 90-d mRS 6, n/N (%) | 80/818 (10) | 47/220 (21) | 18/64 (28) | 2.16 (1.28–3.64) | 0.004 | 1.95 (0.86–4.43) | 0.111 | 2.12 (1.28–3.5) | 0.003 |
Adjustments were made for age, sex, final infarct volume, total WMD-burden, the interaction between alteplase and nerinetide, NIHSS at baseline, stroke laterality, and presence of chronic infarctions.
Table 3.
Primary and Secondary Analyses of Subcortical Atrophy (CC/IT Ratio), N = 1,102
| <25th percentile (n = 276) | 25th–50th percentile (n = 281) | 50th–75th percentile (n = 273) | 75th–100 percentile (n = 272) | a/cOR (95% CI)a | p Value | |
| 90-d mRS, median (IQR) | 1 (1–2) | 2 (1–4) | 2 (1–4) | 3 (2–5) | 1.07 (1.04–1.11) | <0.001 |
| 90-d mRS 0–2, n/N (%) | 209/276 (75) | 175/281 (62) | 158/273 (58) | 123/272 (45) | 0.92 (0.88–0.97) | 0.001 |
| 90-d mRS 6, n/N (%) | 24/276 (9) | 30/281 (11) | 34/273 (12) | 57/272 (21) | 1.06 (0.99–1.13) | 0.10 |
Abbreviations: a/cOR = adjusted/common odds ratio; CC/IT ratio = intercaudate distance to inner table width ratio; CI = confidence interval; GCA = global cortical atrophy; NIHSS = National Institutes of Health Stroke Scale; WMD = white matter disease.
Adjustments were made for age, sex, final infarct volume, total WMD-burden, the interaction between alteplase and nerinetide, NIHSS at baseline, stroke laterality, and presence of chronic infarctions. Quartile distribution, see methods.
The CC/IT ratio is included as a full continuous scale in the regression analyses.
Figure 2. Distribution of 90-Day mRS for Each Category of (A) Global Cortical Atrophy (GCA-Scale) and (B) Subcortical Atrophy (CC/IT-Ratio).
Numbers in bars represent percentages. p Values are derived from unadjusted ordinal regression model (CC/IT-ratio included as a full continuous variable). Abbreviations: CC/IT-ratio = intercaudate-distance-to-inner-table-width ratio; GCA = global cortical atrophy; mRS = modified Rankin Scale.
Secondary Outcome Measures
Cortical atrophy was associated with lower rates of 90-day functional independence (aOR for GCA ≥ 1 vs GCA0 = 0.65 [95% CI of 0.45–0.94]; p = 0.02) and higher mortality (aOR for GCA ≥ 1 vs GCA0 = 2.12 [95% CI 1.28–3.5]; p = 0.003. Increased subcortical atrophy (per 0.01 increase in CC/IT ratio) was associated with lower rates of 90-day functional independence (aOR 0.92 [95% CI 0.88–0.97]; p = 0.001) (Tables 2 and 3).
Unadjusted regression analyses are shown in the supplemental materials (eTables 3 and 4, links.lww.com/WNL/D54) and show significant results for both the primary and secondary outcomes. Sensitivity analyses showed similar results, irrespective of the region examined, the used imaging modality (CT vs MRI), and regardless whether the final TICI was included in the regression model (eFigures 2–6, eTable 5).
Multiple Time Point Outcome Measures
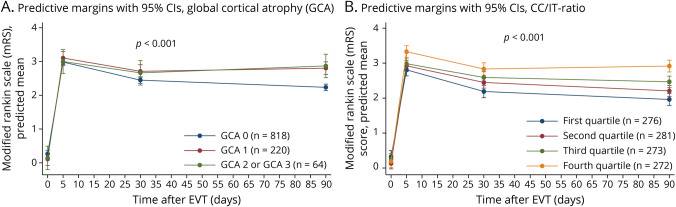
In the repeated-measures analyses, heterogeneity was seen among different degrees of cortical and subcortical atrophy (p < 0.001). This means that the different mRS trajectory plots differed significantly when grouped according to the severity of brain atrophy. Whereas all participants showed worsening in mRS compared with prestroke by the time of discharge, the mRS scores of participants with greater atrophy did not recover as much as those with no cortical atrophy (GCA = 0) by 30 days (adjusted difference for GCA1 = 0.41, [95% CI 0.18–0.65] and for GCA2/3 = 0.33 [95% CI −0.08–0.74]),and remained worse at 90 days (adjusted difference for GCA1 = 0.72; [95% CI 0.49–0.95] and for GCA2/3 = 0.75 [95% CI 0.36–1.14]). The same trends were seen for subcortical atrophy, where, compared with participants in the lowest CC/IT ratio quartile, participants in each higher quartile fared worse at 30 days (e.g., adjusted difference for second quartile = 0.45 [95% CI 0.19–0.71], third quartile = 0.47 [95% CI 0.21–0.73], highest/fourth quartile = 0.78 [95% CI 0.52–1.05]). These differences remained at 90 days (e.g., adjusted difference for highest quartile vs lowest quartile = 1.09 [95% CI 0.84–1.35]) (Figure 3). Sensitivity analyses showed similar results, regardless of the region assessed and of the modality used (e.g., MRI) (eFigures 3 and 6, links.lww.com/WNL/D54).
Figure 3. Mixed-Effects Linear Regression for Predicted Mean mRS at Different Time Points for Cortical Atrophy (A) and Subcortical Atrophy (B), Assessed on CT (N = 1,102).
Adjustments were made for age, sex, final infarct volume, total WMD-burden, the interaction between alteplase and nerinetide, NIHSS at baseline, stroke laterality, and presence of chronic infarctions. p values are derived from simple effect models. FIV = final infarct volume; GCA = global cortical atrophy; mRS = modified Rankin Scale; WMD = white matter disease.
Discussion
In this study, we determined the influence of cortical and subcortical brain atrophy on 90-day outcomes and recovery trajectories as visually assessed on acute NCCT in 1,102 AIS patients, undergoing EVT. We found that both cortical and subcortical brain atrophy were associated with worse 90-day mRS (acOR [GCA ≥ 1 vs GCA 0] = 1.62, p = 0.001; acOR [per 0.01 increase in CC/IT ratio] = 1.07, p < 0.001), and lower rates of functional independence (aOR [GCA ≥ 1 vs GCA 0] = 0.65, p = 0.022; aOR [per 0.01 increase in CC/IT ratio] = 0.92, p = 0.001). Cortical atrophy was associated with 90-day mortality (aOR [GCA ≥ 1 vs GCA 0] = 2.12, p = 0.003). Furthermore, functional recovery, represented by repeated mRS assessments up to 90 days, differed by the degree of cortical (p < 0.001) and subcortical atrophy (p < 0.001). We show similar results when assessing different regions or when performing the examinations with MRI.
Several studies have previously explored the role of brain atrophy in AIS patients undergoing EVT. A recent retrospective analysis23 investigated the influence of brain atrophy on outcome after EVT in 295 AIS patients using visual ratings and the Evan index and found a higher risk of futile reperfusion (i.e., mRS >2 despite complete reperfusion) with increasing atrophy. However, as opposed to our study, the Evan index does not account for potential AIS-associated edema or hemorrhage. The Evan index is a marker for ventriculomegaly and is defined by the ratio of the maximum width of the frontal horns of the lateral ventricles and the maximal diameter of the skull, measured at the same level. Another retrospective study24,25 that assessed brain atrophy and functional outcomes in 360 patients from an EVT registry by using automated CSF volume measures found that atrophy was associated with lower odds of 90-day functional independence. By contrast, an analysis from the MR CLEAN trial (n = 410),14 using automated brain volumetry, revealed greater shifts toward better functional outcome with more pronounced atrophy. This may be explained by the markedly poor natural history of stroke in patients with more pronounced atrophy who do not undergo EVT, elevating the treatment effect in these patients compared with patients with less atrophy.
Our results add to previous literature by providing functional outcome assessments at multiple time points after EVT, in a large RCT-derived population. Our demonstration of persistent differences in functional outcome among patients with greater atrophy from post-EVT until 90 days underscores the longitudinal association of brain atrophy with stroke recovery. Such trajectory data may inform recovery expectations, thereby facilitating rehabilitation planning, disposition, and other facets of EVT follow-up care with patients and their relatives. In addition, we used multiple global and regional atrophy measures and assessed subcortical atrophy. The latter was specifically adapted to the acute stroke setting, minimizing contamination by ipsilateral AIS-induced edema or hemorrhage.21 Such pragmatic use of simple, readily available measures on routinely acquired imaging without the need for postprocessing, as opposed to advanced imaging features involving volumetrics14 or radiomics,26 further corroborates the clinical relevance of our study.
Visual assessments of brain atrophy may potentially find its way as an additional determinant available at baseline, helping to refine outcome prediction models. This may improve prognostication and inform patient counseling and treatment decisions in the acute phase of stroke. This applies especially to the older patients, in whom risk-benefit assessments remain challenging, particularly when relying on chronologic age alone. Although octogenarians and nonagenarians are underrepresented in the large EVT trials, some studies have reported a persistent (albeit lower) benefit of EVT in the older patients compared with younger patients.27,28 By contrast, older age has been associated with futile recanalization, higher risk of symptomatic intracranial hemorrhage, and increased mortality.29-31 The ambiguity of available evidence may partly relate to the unaccounted role of biologic age involving pivotal determinants of functional outcome in addition to the chronologic age.11 In this respect, our findings are compatible with the concept of diminished brain reserve in individuals with brain atrophy, leading to less favorable recovery after EVT compared with patients without atrophy. In addition, future studies should examine the impact of brain atrophy on stroke outcome in non-EVT patients as well to help further generalize the concept of brain reserve to the overall stroke population.
This study also has some limitations. First, the applied atrophy scales were originally developed for imaging evaluation of neurodegenerative disorders on MRI in a nonemergency clinical setting. However, such scales have been used for CT-based sensitivity analyses previously.11 Besides, our separate analysis involving assessments on post-EVT MRI revealed similar results. Second, in time sensitive stroke settings, image acquisitions usually do not include specific postprocessing reconstructions (e.g., for MTA assessments) and may exhibit angulation or tilt that could interfere with the readers' interpretations. However, we mitigated this by using 3D multiplanar reconstructions. Third, visual assessments of atrophy could be prone to potential inter-rater variability. That being said, we used consensus readings from trained experts, and important discrepancies were resolved by an additional independent reader. Finally, all participants enrolled in ESCAPE-NA1 were selected to have favorable baseline imaging and independent premorbid functional status, potentially limiting the generalizability of our results to the broader EVT population. In addition, all included participants underwent EVT, rendering any conclusions on treatment decisions or effect sizes of EVT impossible. Subsequently, we cannot comment on any potential risks that are associated with EVT in patients with increasing burden of atrophy, and we cannot give any estimates such as numbers needed to treat (NNT) and to reflect the effect of EVT (e.g., utility or futility of EVT) in this subgroup of patients. That being said, we also show that patients with high atrophy burden can still achieve good post-EVT outcomes; over 40% of patients with moderate to severe cortical atrophy (GCA scale of 2 or 3) had a 90-day mRS0-2, as did 45% of patients in the highest quartile of subcortical atrophy.
In this large RCT-derived population, participants with cortical and subcortical atrophy, determined by pragmatic visual assessments on acute NCCT imaging, showed less favorable stroke recovery after EVT and worse 90-day functional outcomes compared with participants with no atrophy.
Acknowledgment
All authors contributed to conceptualization, drafting and critical revision. A. Ganesh, F. Benali, J. Fladt, F. Bala, N. Singh, and T. Jaroenngarmsamer contributed to data collection. M. Tymianski, M.D. Hill, and M. Goyal contributed to critical revisions.
Glossary
- AIS
acute ischemic stroke
- CTA
computed tomography angiography
- EVT
endovascular thrombectomy
- FIV
final infarct volumes
- GCA
global cortical atrophy
- ICD
intercaudate distance
- IQR
interquartile ranges
- ITW
inner table width
- LVO
large vessel occlusion
- mRS
modified Rankin Scale
- NCCT
noncontrast computed tomography
Appendix. Authors
| Name | Location | Contribution |
| Faysal Benali, MD | Maastricht University Medical Center+ (MUMC+); Calgary Stroke Program, Department of Clinical Neurosciences, University of Calgary Cumming School of Medicine, Canada | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Joachim Fladt, MD | Calgary Stroke Program, Department of Clinical Neurosciences, University of Calgary Cumming School of Medicine, Canada | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| lTanaporn Jaroenngarmsamer, MD | Calgary Stroke Program, Department of Clinical Neurosciences, University of Calgary Cumming School of Medicine, Canada | Major role in the acquisition of data; analysis or interpretation of data |
| Fouzi Bala, MD | Calgary Stroke Program, Department of Clinical Neurosciences, University of Calgary Cumming School of Medicine, Canada | Major role in the acquisition of data |
| Nishita Singh, MD | Calgary Stroke Program, Department of Clinical Neurosciences, University of Calgary Cumming School of Medicine, Canada | Major role in the acquisition of data |
| Johanna Maria Ospel, MD | Calgary Stroke Program, Department of Clinical Neurosciences, University of Calgary Cumming School of Medicine, Canada | Drafting/revision of the manuscript for content, including medical writing for content |
| Michael Tymianski, MD, PhD, FRCSC | NoNO, Toronto, ON, Canada | Study concept or design |
| Michael D. Hill, MD, MSc, FRCPC | Calgary Stroke Program, Department of Clinical Neurosciences, University of Calgary Cumming School of Medicine, Canada | Study concept or design; analysis or interpretation of data |
| Mayank Goyal, MD, PhD | Calgary Stroke Program, Department of Clinical Neurosciences, University of Calgary Cumming School of Medicine, Canada | Study concept or design |
| Aravind Ganesh | Calgary Stroke Program, Department of Clinical Neurosciences, University of Calgary Cumming School of Medicine, Canada | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Footnotes
CME Course: NPub.org/cmelist
Study Funding
The authors report no targeted funding.
Disclosure
Dr. J. Ospel reported being supported by the Julia Bangerter Rhyner Foundation, the University of Basel Research Foundation, and Freiwillige Akademische Gesellschaft Basel. Dr M. Tymianski reported being chief executive officer of NoNO Inc, a company developing neuroprotectants for the treatment of acute ischemic stroke. Dr. M. H. Hill reported receiving personal fees from Sun Pharma and Merck; receiving nonfinancial support from Hoffmann-La Roche Canada; holding a patent for US 10,916,346 licensed to Circle NVI and a patent for US 62/086,077 licensed to Circle NVI; owning stock in PureWeb Inc; serving as a director of the Canadian Federation of Neurologic Sciences, the Canadian Stroke Consortium, and Circle NeuroVascular; and receiving grants from Alberta Innovates Health Solutions, the Canadian Institutes of Health Research, Heart & Stroke Foundation of Canada, Covidien, Boehringer-Ingleheim, Biogen, Stryker, Medtronic, and the National Institutes of Neurologic Disorders and Stroke. Dr. M. Goyal reported receiving personal fees from Medtronic, Stryker, Microvention, and Mentice during the conduct of the study; receiving unrestricted research grants to the University of Calgary from NoNO, Stryker, and Medtronic; holding a patent for systems of acute stroke diagnosis, licensed to GE Healthcare; and having an ownership interest in Circle Neurovascular. Dr. A. Ganesh reported receiving grants from the Wellcome Trust, the Canadian Institutes of Health Research, Canadian Cardiovascular Society, Alberta Innovates, Campus Alberta Neuroscience, and Sunnybrook Research Institute INOVAIT; receiving personal fees fromMDAnalytics, MyMedicalPanel, Creative Research Designs, Atheneum, DeepBench, Research on Mind, Figure 1, Alexion; receiving travel awards from American Academy of Neurology, American Heart Association, and University of Calgary; receiving cash awards from the Association of Indian Neurologists in America; holding stock options from SnapDx, Advanced Health Analytics, and TheRounds.com outside the submitted work; holding a patent for US 63/024,239 pending for a system for patient monitoring and delivery of remote ischemic conditioning or other cuff-based therapies; and serving on the editorial boards of Neurology, Neurology: Clinical Practice, and Stroke. Dr. F. Benali, Dr. J. Fladt, Dr. T. Jaroenngarmsamer, Dr. F. Bala and Dr. N. Singh report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/s0140-6736(16)00163-x [DOI] [PubMed] [Google Scholar]
- 2.Jansen IGH, Mulder MJHL, Goldhoorn RB; MR CLEAN Registry investigators. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ. 2018;360:k949. doi: 10.1136/bmj.k949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganesh A, Ospel JM, Kromm J, Goyal M. Ignorance is not bliss: managing uncertainty in acute stroke treatment in the COVID-19 era. Neuroradiology. 2021;63(1):3-6. doi: 10.1007/s00234-020-02592-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ospel JM, Ganesh A, Kappelhof M, et al. Evaluating outcome prediction models in endovascular stroke treatment using baseline, treatment, and posttreatment variables. Stroke Vasc Interv Neurol. 2021;1(1):e000167. doi: 10.1161/svin.121.000167 [DOI] [Google Scholar]
- 5.Rabinstein AA, Albers GW, Brinjikji W, Koch S. Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. Int J Stroke. 2018;14(1):23-31. doi: 10.1177/1747493018799979 [DOI] [PubMed] [Google Scholar]
- 6.Dąbrowski J, Czajka A, Zielińska-Turek J, et al. Brain functional reserve in the context of neuroplasticity after stroke. Neural Plast. 2019;2019:9708905. doi: 10.1155/2019/9708905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pino A, Fumagalli G, Bifari F, Decimo I. New neurons in adult brain: distribution, molecular mechanisms and therapies. Biochem Pharmacol. 2017;141:4-22. doi: 10.1016/j.bcp.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 8.Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597-608. doi: 10.1038/nrneurol.2014.162 [DOI] [PubMed] [Google Scholar]
- 9.Harrison TM, Maass A, Baker SL, Jagust WJ. Brain morphology, cognition, and beta-amyloid in older adults with superior memory performance. Neurobiol Aging. 2018;67:162-170. doi: 10.1016/j.neurobiolaging.2018.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Z, Li S, Matuskey D, Nabulsi N, Huang Y. PET imaging of synaptic density: a new tool for investigation of neuropsychiatric diseases. Neurosci Lett. 2019;691:44-50. doi: 10.1016/j.neulet.2018.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedraza MI, de Lera M, Bos D, et al. Brain atrophy and the risk of futile endovascular reperfusion in acute ischemic stroke. Stroke. 2020;51(5):1514-1521. doi: 10.1161/strokeaha.119.028511 [DOI] [PubMed] [Google Scholar]
- 12.Cole JH, Ritchie SJ, Bastin ME, et al. Brain age predicts mortality. Mol Psychiatry. 2018;23(5):1385-1392. doi: 10.1038/mp.2017.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauksio I, Lindstrom I, Khan N, et al. Brain atrophy predicts mortality after mechanical thrombectomy of proximal anterior circulation occlusion. J Neurointerv Surg. 2021;13(5):415-420. doi: 10.1136/neurintsurg-2020-016168 [DOI] [PubMed] [Google Scholar]
- 14.Luijten SP, Compagne KC, van Es AC, et al. Brain atrophy and endovascular treatment effect in acute ischemic stroke: a secondary analysis of the MR CLEAN trial. Int J Stroke. 2021;17(8):881-888. doi: 10.1177/17474930211054964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill MD, Goyal M, Menon BK, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395(10227):878-887. doi: 10.1016/s0140-6736(20)30258-0 [DOI] [PubMed] [Google Scholar]
- 16.Pasquier F, Leys D, Weerts JG, Mounier-Vehier F, Barkhof F, Scheltens P. Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol. 1996;36(5):268-272. doi: 10.1159/000117270 [DOI] [PubMed] [Google Scholar]
- 17.Torisson G, van Westen D, Stavenow L, Minthon L, Londos E. Medial temporal lobe atrophy is underreported and may have important clinical correlates in medical inpatients. BMC Geriatr. 2015;15(1):65. doi: 10.1186/s12877-015-0066-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claus JJ, Staekenborg SS, Holl DC, et al. Practical use of visual medial temporal lobe atrophy cut-off scores in Alzheimer's disease: validation in a large memory clinic population. Eur Radiol. 2017;27(8):3147-3155. doi: 10.1007/s00330-016-4726-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koedam EL, Lehmann M, van der Flier WM, et al. Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol. 2011;21(12):2618-2625. doi: 10.1007/s00330-011-2205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho VB, Chuang HS, Rovira MJ, Koo B. Juvenile huntington disease: CT and MR features. AJNR Am J Neuroradiol. 1995;16(7):1405-1412. [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Oh CW, Han JH, et al. The effect of brain atrophy on outcome after a large cerebral infarction. J Neurol Neurosurg Psychiatry. 2010;81(12):1316-1321. doi: 10.1136/jnnp.2009.197335 [DOI] [PubMed] [Google Scholar]
- 22.Butzkueven H, Kolbe SC, Jolley DJ, et al. Validation of linear cerebral atrophy markers in multiple sclerosis. J Clin Neurosci. 2008;15(2):130-137. doi: 10.1016/j.jocn.2007.02.089 [DOI] [PubMed] [Google Scholar]
- 23.Pedraza MI, de Lera M, Bos D, et al. Brain atrophy and the risk of futile endovascular reperfusion in acute ischemic stroke. Stroke. 2020;51(5):1514-1521. doi: 10.1161/strokeaha.119.028511 [DOI] [PubMed] [Google Scholar]
- 24.Diprose WK, Diprose JP, Wang MTM, Barber PA. Intracranial reserve in ischemic stroke: is the skull half-full or half-empty? Neurocrit Care. 2020;33(3):858. doi: 10.1007/s12028-020-01102-2 [DOI] [PubMed] [Google Scholar]
- 25.Diprose WK, Diprose JP, Wang MTM, Tarr GP, McFetridge A, Barber PA. Automated measurement of cerebral atrophy and outcome in endovascular thrombectomy. Stroke. 2019;50(12):3636-3638. doi: 10.1161/strokeaha.119.027120 [DOI] [PubMed] [Google Scholar]
- 26.Bretzner M, Bonkhoff AK, Schirmer MD, et al. Radiomics derived brain age predicts functional outcome after acute ischemic stroke. Neurology. 2023;100(8):e822-e833. doi: 10.1212/WNL.0000000000201596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharobeam A, Corato DJ, Manning N, Cheung A, Wenderoth J, Cappelen-Smith C. Functional outcomes at 90 days in octogenarians undergoing thrombectomy for acute ischemic stroke: a prospective cohort study and meta-analysis. Front Neurol. 2019;10:254. doi: 10.3389/fneur.2019.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majidi S, Lee J, Balushi AA, Fifi JT, Singh IP. Endovascular thrombectomy in octogenarians and nonagenarians with large vessel occlusion: technical aspects and clinical outcome. J Stroke Cerebrovasc Dis. 2020;29(10):105120. doi: 10.1016/j.jstrokecerebrovasdis.2020.105120 [DOI] [PubMed] [Google Scholar]
- 29.Alawieh A, Starke RM, Chatterjee AR, et al. Outcomes of endovascular thrombectomy in the elderly: a 'real-world' multicenter study. J Neurointerv Surg. 2019;11(6):545-553. doi: 10.1136/neurintsurg-2018-014289 [DOI] [PubMed] [Google Scholar]
- 30.van Horn N, Kniep H, Leischner H, et al. Predictors of poor clinical outcome despite complete reperfusion in acute ischemic stroke patients. J Neurointerv Surg. 2021;13(1):14-18. doi: 10.1136/neurintsurg-2020-015889 [DOI] [PubMed] [Google Scholar]
- 31.Sussman ES, Martin B, Mlynash M, et al. Thrombectomy for acute ischemic stroke in nonagenarians compared with octogenarians. J Neurointerv Surg. 2020;12(3):266-270. doi: 10.1136/neurintsurg-2019-015147 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The ESCAPE-NA1 data are not currently publicly available for distribution, but a future public data set may be made available. Researchers interested in the data are asked to contact the corresponding author with a proposal.