Abstract
We assessed the efficacy and safety of combining bevacizumab with temsirolimus in patients with advanced extra-pancreatic neuroendocrine tumors. This NCI-sponsored multicenter, open-label, phase II study (NCT01010126) enrolled patients with advanced, recurrent, or metastatic extra-pancreatic neuroendocrine tumors. All patients were treated with temsirolimus and bevacizumab until disease progression or unacceptable toxicity. Temsirolimus 25 mg was administered intravenously on days 1, 8, 15, and 22, and bevacizumab 10 mg/kg intravenously on days 1 and 15 of a 4-week cycle. Discontinuation of temsirolimus or bevacizumab did not require discontinuation of the other agent. The primary endpoints were objective response rate and 6-month progression-free survival rate. Fifty-nine patients were enrolled in this study, and 54 were evaluable for efficacy and adverse events. While median progression-free survival was 7.1 months, median duration of treatment with temsirolimus was 3.9 months, and with bevacizumab was 3.5 months. The objective response rate of combination therapy was 2%, and 6-month progression-free survival was 48%. The most frequently reported grade 3–4 adverse events included fatigue (13%), hypertension (13%), and bleeding (13%). Close to 54% of patients discontinued treatment due to adverse events, refusal of further treatment, or treatment delays. Three deaths occurred on study, with 2 treatment-related fatal bowel perforations. Given the minimal efficacy and increased toxicity seen with the combination of bevacizumab and temsirolimus, we do not recommend the use of this regimen in patients with advanced extra-pancreatic neuroendocrine tumors.
Keywords: bevacizumab, temsirolimus, neuroendocrine, carcinoid, mTOR inhibitor
Introduction
Neuroendocrine tumors (NETs) most frequently originate in the gastroenteropancreatic (GEP) system (55%), followed by the bronchopulmonary system (30%).(Dasari et al., 2017) Low-to-intermediate grade NETs of the aerodigestive tract (also referred to as carcinoid tumors) typically behave in an indolent fashion, while high-grade neuroendocrine tumors are aggressive. In patients with unresectable or metastatic extra-pancreatic NETs, options for treatment are limited. Lanreotide was approved by the US Food and Drug Administration (FDA) in 2014 for the treatment of patients with well or moderately differentiated advanced NETs due to its ability to delay progression as demonstrated by the CLARINET trial.(Caplin et al., 2014) In 2016, the efficacy of everolimus, an inhibitor of the mammalian target of rapamycin (mTOR), in gastrointestinal and lung NETs was confirmed by results from the RADIANT-4 trial leading to FDA approval. (Yao et al., 2016) These results were consistent with those from the RADIANT-3 trial in pancreatic NETs, which also showed a progression-free survival benefit. (Yao et al., 2011) In 2018, Lu-177 dotatate became the first radiopharmaceutical approved for the treatment of GEP-NETs based on the results of the NETTER-1 trial. (Strosberg et al., 2017b)
NETs are highly vascular, and there is evidence that angiogenesis inhibitors have anti-tumor activity in advanced NETs. A randomized phase II study of octreotide with bevacizumab compared to octreotide with pegylated interferon-alpha (IFN) in patients with unresectable or metastatic carcinoid tumors demonstrated a higher response rate (18% vs. 0%) in the bevacizumab arm, and an improved progression-free survival (PFS) rate at 18 weeks of 95% for the bevacizumab arm versus 68% for the interferon arm. (Yao et al., 2008) This led to a phase III trial in 427 patients with NETs of bevacizumab with octreotide versus IFN with octreotide (SWOG 0518) where no significant difference was found in progression-free survival (16.6 months vs. 15.4 months; HR=0.93; p=0.55). (Yao et al., 2017)
There is clinical evidence of the efficacy of simultaneously targeting the mTOR and VEGF pathways in the treatment of pancreatic NETs. CALGB 80701 was a randomized phase II trial of the combination of everolimus and octreotide with or without bevacizumab in 150 patients with advanced pancreatic NETs. There was a statistically significant increase in the response rate in the bevacizumab group (31% vs. 12%; p=0.005) and a trend toward improved median progression-free survival (16.7 months vs. 14 months; HR=0.80; p=0.12).
Temsirolimus, another inhibitor of mTOR, was studied in a single-arm phase II trial of patients with advanced NETs and showed a partial response rate of 6% and a PFS rate at 6 months of 48%. (Duran et al., 2006) Of the 2 patients in this trial who achieved a confirmed partial response, 1 had an extra-pancreatic NET and one had a pancreatic NET. (Kulke et al., 2015)
We hypothesized that the combination of temsirolimus and bevacizumab would demonstrate clinical efficacy in patients with NETs. Based on the known anti-tumor activity and safety of bevacizumab and temsirolimus as single agents, we sought to investigate the clinical efficacy and safety of the combination. We report the results of the extra-pancreatic NET cohort within a multi-tumor phase II trial using bevacizumab and temsirolimus in patients with endometrial cancer, ovarian cancer, hepatocellular carcinoma, extra-pancreatic NETs, or well-differentiated pancreatic NETs (pNETs) (NCI protocol 8233, Mayo Phase 2 Consortium).
Materials and Methods
Patient Eligibility.
Eligible patients had histologically or cytologically confirmed locally advanced, recurrent, or metastatic extra-pancreatic NETs, were 18 years of age or older, and had an ECOG performance status of 0–1. An amendment was made to the initial protocol that required patients to have well- to moderately differentiated NETs; be on a stable dose of octreotide for greater than or equal to 2 months prior to study entry, and have evidence of progressive disease as documented by the revised Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 within 7 months prior to study entry. Tumor grade was ascertained using the WHO grading system (2000). (Hamilton SR, 2000, Capella C)
Patients with high-grade NETs who had enrolled in the study prior to this amendment were permitted to continue in the study. To account for the need to clinically control carcinoid syndrome in a subset of patients and to prevent a confounding bias that could affect the primary endpoint, all patients with extra-pancreatic NETs were required to continue concurrent somatostatin analog therapy while on the study. After an observation of increased bleeding rates, a second amendment to the protocol excluded patients from receiving anticoagulation while on the study. Other eligibility criteria included: absolute neutrophil count (ANC) ≥ 1500/mm3; platelets ≥ 75,000/mm3; hemoglobin ≥ 9.0 g/dL; total bilirubin ≤ 1.5 times the upper limit of normal (ULN); alkaline phosphatase and serum glutamic-oxaloacetic transaminase (SGOT) ≤ 2.5 times ULN (≤ 5 times ULN if liver metastases are present); creatinine < 1.5 times ULN; urinalysis < 2+ protein; international normalized ratio (INR) ≤ 1.5; fasting serum cholesterol ≤ 350mg/dL (≤ 9.0 mmol/L); and triglycerides ≤ 1.5 times ULN. Patients who had prior anthracycline chemotherapy were required to have a normal left ventricular ejection fraction prior to registration.
Prior systemic treatments for metastatic disease were permitted, including ≤ 2 prior cytotoxic chemotherapy regimens, interferons, radiolabeled octreotide therapy, and other investigational therapy. Radiation therapy or regional treatments, including selective internal radiation therapy, transhepatic artery chemoembolization, and radiofrequency ablation were also permitted prior to study entry. All prior treatment had to be completed for 4 or more weeks prior to initiation. Patients with significant cardiovascular disease, untreated central nervous system (CNS) metastases, surgical resection of CNS metastases within 3 months of registration, a major surgical procedure, or evidence of a bleeding event within 6 months of registration were excluded from the study. Patients with known HIV infection or who were pregnant or breastfeeding were excluded. Prior therapy with either VEGF-targeting agents or with mTOR inhibitors was also an exclusion criterion.
The institutional review boards of each participating medical center and the National Cancer Institute approved the protocol, and patients provided written informed consent prior to entering the study. The trial is registered at ClinicalTrials.gov (NCT01010126).
Study Design and Treatment.
This was a multicenter, single-arm, open-label, phase II trial. Patients were treated with temsirolimus 25 mg intravenously (IV) on days 1, 8, 15, and 22 of a cycle and bevacizumab 10 mg/kg IV on days 1 and 15 of a cycle. One cycle was defined as 28 days. The continuation of concurrent long-acting somatostatin analog therapy was required. Patients were not permitted to receive any concomitant chemotherapy or radiotherapy; however, they could receive supportive care. Treatment was continued until disease progression, unacceptable toxicity, intercurrent illness that prevented further treatment with study drug, treatment delay of greater than 4 weeks, need for radiation therapy for symptomatic disease, or patient decision to discontinue study drug(s). Dose interruption of bevacizumab was permitted for commonly known adverse events, including bleeding or vascular events, hypertension, nephrotic syndrome, bowel perforation or obstruction, and specific grade 4 hematologic or clinical events. No dose reductions of bevacizumab were permitted; rather, the dose could be omitted until resolution of the adverse event or discontinued entirely. Temsirolimus dose modifications were planned for expected adverse events, including neutropenia, thrombocytopenia, hypercholesterolemia, hypertriglyceridemia, pneumonitis, and vascular events. Dose reductions from 25 mg of temsirolimus to 20, 15, or 10 mg were allowed, and once reduced, the dose could not be re-escalated with subsequent treatments. If either bevacizumab or temsirolimus was omitted or discontinued, the patient could continue on the study and receive the other agent.
Evaluation During Study.
A complete history; physical examination; blood tests, including a complete blood count (CBC) with differential; renal and liver function tests; cholesterol; triglycerides; coagulation studies; endocrine tests as applicable to each patient (serum chromogranin A and 24-hour urinary 5-HIAA); urinalysis; left ventricular ejection fraction assessment if prior therapy with anthracycline; chest X-ray; and tumor measurement by radiographic imaging (computed tomography or magnetic resonance imaging) were obtained in all patients at baseline. History and physical examination, CBC with differential, renal and liver function tests, cholesterol, and triglycerides were repeated every 4 weeks, prior to administration of subsequent cycles and at study end (either at time of progression or withdrawal from study). Coagulation tests were measured as clinically indicated. Radiographic imaging, using the same technique as at baseline, was performed every 8 weeks during the course of study treatment. Change in tumor burden was assessed according to the revised RECIST criteria, version 1.1. Tumor response was categorized as complete response (CR), partial response (PR), progressive disease (PD), or stable disease. In addition, certain tumor markers produced by neuroendocrine tumors were measured in patients’ serum at baseline and every 8 weeks during treatment. Adverse events were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v3.0.
Statistical Considerations.
The primary co-endpoints of this study were objective response rate and 6-month progression-free survival (PFS) rate. A confirmed tumor response was defined to be either a complete response (CR) or partial response (PR) noted as the objective status on two consecutive evaluations at least 8 weeks apart. All patients meeting the eligibility criteria who signed a consent form and received at least 1 dose of study treatment were evaluable for response. Patients who died without documentation of progression were considered to have progressed on the date of their death. Some patients ended their study treatment before 6 months due to adverse events, and they were neither progressed nor dead at the 6-month follow-up. Because these patients may have received alternative treatment after discontinuing study treatment, we adopted both a conservative approach and standard approach for defining progression-free status at 6 months. In the standard approach, if patients received at least 1 dose of study treatment and had not progressed at 6 months, they were considered progression-free at 6 months (regardless of whether they were on study treatment or alternative treatment at 6 months). In the conservative approach, patients were defined to be progression-free at 6 months if they were both on study treatment at 6 months and had not progressed at 6 months. In our results, we will focus on data calculated by the conservative approach because we believe this model accounts for patients who were able to receive study treatment up until the 6-month evaluation, and that outcomes may be attributed to the effects of the study treatment in this model. The conservative approach to reporting PFS was adopted post-hoc. If 5 or more of the first 25 patients experienced grade 4/5 non-hematologic adverse events that were probably, possibly, or definitely related to study treatment, accrual to the study had to be suspended to allow for investigation.
The study was conducted using a modified two-stage Simon’s design with a maximum sample size of 55 patients. This included an a priori allowance for an extra 10% of patients to replace those deemed not evaluable due to ineligibility, major treatment violation, or cancellation. If more than 5 of the first 25 evaluable patients enrolled achieved a confirmed tumor response during the first 6 cycles of treatment, or more than 12 of the first 25 evaluable patients enrolled were progression-free at 6 months by the standard criteria, then enrollment would continue to the second stage of another 25 evaluable patients. If not, patient accrual was to be terminated and the regimen would be considered inactive in this patient population. The sample size was derived by assuming an alternative hypothesis of 35% (versus a historical control rate of 15%) for response rate, and an alternative hypothesis of 65% (versus a historical control rate of 45%) for 6-month progression-free survival rate. Descriptive statistics such as mean (SD), median (range), frequency (percentage), and statistical graphs are used to summarize patient characteristics, toxicity, and tumor response. Kaplan-Meier methodology was used to summarize progression-free survival and overall survival.
Results
Patient Characteristics.
From October 2009 to January 2012, 59 patients were enrolled in this study from multiple sites in the United States and Canada, including Moffitt Cancer Center, Mayo Clinic Cancer Center, The Ohio State University Comprehensive Cancer Center, University of California-Davis Medical Center, Memorial Sloan-Kettering Cancer Center, University of Chicago, Princess Margaret Hospital, Montefiore Einstein Center for Cancer Care, Washington University, and Maplewood Cancer Center. Two patients were deemed to have pancreatic NETs and excluded from this analysis, 2 patients were ineligible for the study, and 1 did not proceed with study treatment. Fifty-four patients received at least 1 dose of study treatment with bevacizumab and temsirolimus and were evaluable for assessment of efficacy (Figure 1). All patients had advanced or metastatic disease, with liver and lymph nodes as the most common metastatic disease sites (Table 1). Six (11%) patients had high-grade (grade III/IV) neuroendocrine tumors and had been enrolled prior to the amendment that clarified that patients must have low- or intermediate-grade (I/II) NETs (Figure 1). More than half the patients had a history of hypertension (n=30, 56%), while hyperlipidemia (n=10, 19%) was less common; diabetes mellitus (n=7, 13%) and thrombosis (n=1, 2%) were uncommon. A total of 37% (20/54) of the cohort had documented progression by RECIST criteria in the previous 7 months.
Figure 1.
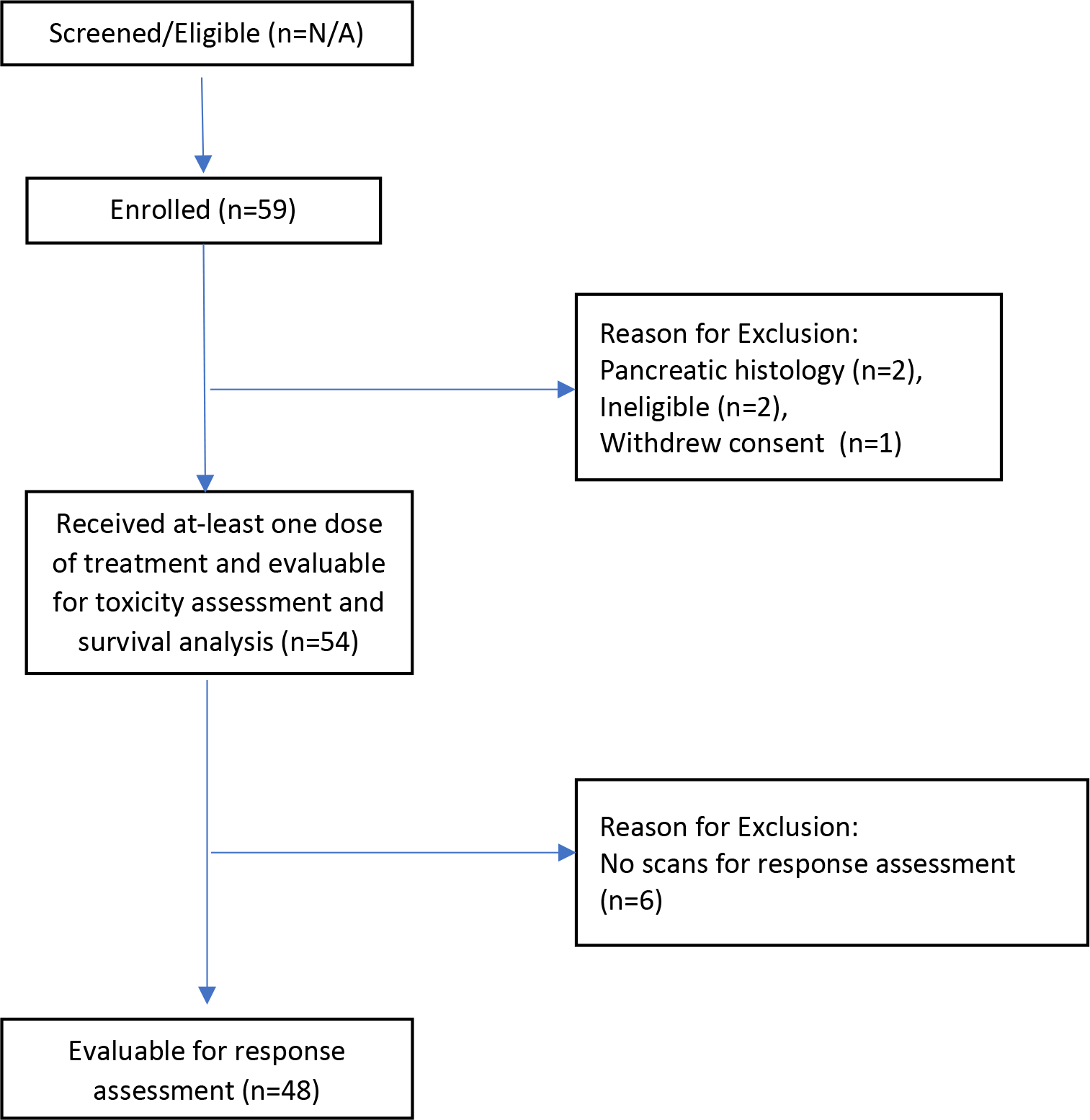
CONSORT Diagram
Table 1.
Patient Characteristics at Baseline
| Characteristics | Total # Patients, N=54 |
|---|---|
| Age (y) | |
| Median | 62 |
| Range | 35–89 |
| Sex, n (%) | |
| Female | 29 (54) |
| Male | 25 (46) |
| ECOG performance status, n (%) | |
| 0 | 31 (57) |
| 1 | 23 (43) |
| Status of primary tumor, n (%) | |
| Resected, without residual disease | 12 (22) |
| Resected, with residual disease | 18 (33) |
| Unresected | 15 (28) |
| Recurrent | 5 (9) |
| Unknown | 4 (7) |
| Tumor grade, n (%) | |
| Grade I | 39 (72) |
| Grade II | 7 (13) |
| Grade III | 3 (6) |
| Grade IV | 3 (6) |
| Unknown | 2 (4) |
| Metastatic sites, n (%) | |
| Liver | 48 (89) |
| Lymph nodes | 34 (63) |
| Lung | 13 (24) |
| Bone | 12 (22) |
| Subcutaneous | 1 (2) |
| Brain | 1 (2) |
| Sum of target lesions in cm, median (range) | 12.5 (2.6–32.0) |
| Prior therapy, n (%) | |
| Systemic or regional therapy | 46 (85) |
| Surgery | 43 (80) |
| Radiation | 13 (24) |
| Progressive disease in preceding 7 months, n (%) | |
| Yes | 20 (37) |
| No | 2 (4) |
| Unknown | 32 (59) |
| Tumor Marker not detected | 21 (39%) |
| Tumor Marker detected | |
| 5-HIAA & Chromogranin A | 3 (6%) |
| 5-HIAA alone | 1 (2%) |
| Chromogranin A | 14 (26%) |
| Gastrin | 1 (2%) |
| Gastrin & Pancreastatin | 1 (2%) |
| Pancreastatin | 5 (9%) |
| Serotonin | 4 (7%) |
| Unknown/Missing | 4 (7%) |
Treatment Administered.
The median duration of treatment with temsirolimus was 3.9 months (range, 0.1–12.6 months), and the median duration of treatment with bevacizumab was 3.4 months (range, 0.1–10.6 months). Dose adjustments of temsirolimus were required in 34 patients (63%) and of bevacizumab in 14 patients (26%). The median duration of follow-up for all patients was 17.1 months (range, 0.6–30.1 months). Treatment discontinuation occurred in 46 patients and was related to disease progression (n=13, 28%). Among patients with discontinuation not due to progression (n=33, 72%), the reasons were: adverse events (n=18, 39%); refusal of further treatment (n=9, 20%); death (n=2, 4%); treatment delays (n=2, 4%); other/personal reasons, including non-study-related traumatic events and travel issues (n=2, 4%).
The adverse events leading to treatment discontinuation included bleeding (n=4), poor overall quality of life (n=3), lab value abnormalities (n=2), fatigue (n=1), weight loss (n=1), dehydration (n=1), ischemic stroke (n=1), bowel perforation (n=1), bowel ischemia (n=1), altered mental status (n=1), skin rash (n=1), and diarrhea (n=1).
Efficacy.
At the interim analysis, 19 out of the first 25 patients were progression-free at 6 months including one patient with an unconfirmed partial response (unconfirmed interim objective response rate of 4%) and the enrollment of a further 25 patients was permitted. Forty-eight of 54 patients had a response assessment after baseline assessment, and best response to treatment is summarized in the form of a waterfall plot (Figure 2). One of 48 evaluable patients experienced a confirmed partial response, and no patients had a complete response. The objective response rate was 2% (1/48). Five out of 48 patients (10%) had an unconfirmed partial response as their best response, and 42 out of 48 patients (88%) achieved stable disease as their best response. The maximum percentage change in target lesion size is shown in Figure 2. Of note, 19 of 48 patients (40%) had a greater than 20% reduction in sum of index tumor lesions. Among the total enrolled cohort (n=54), the median PFS was 7.1 months (95% CI, range 4.6–8.7 months) (Figure 3), and median OS was not reached (Figure 4).
Figure 2.
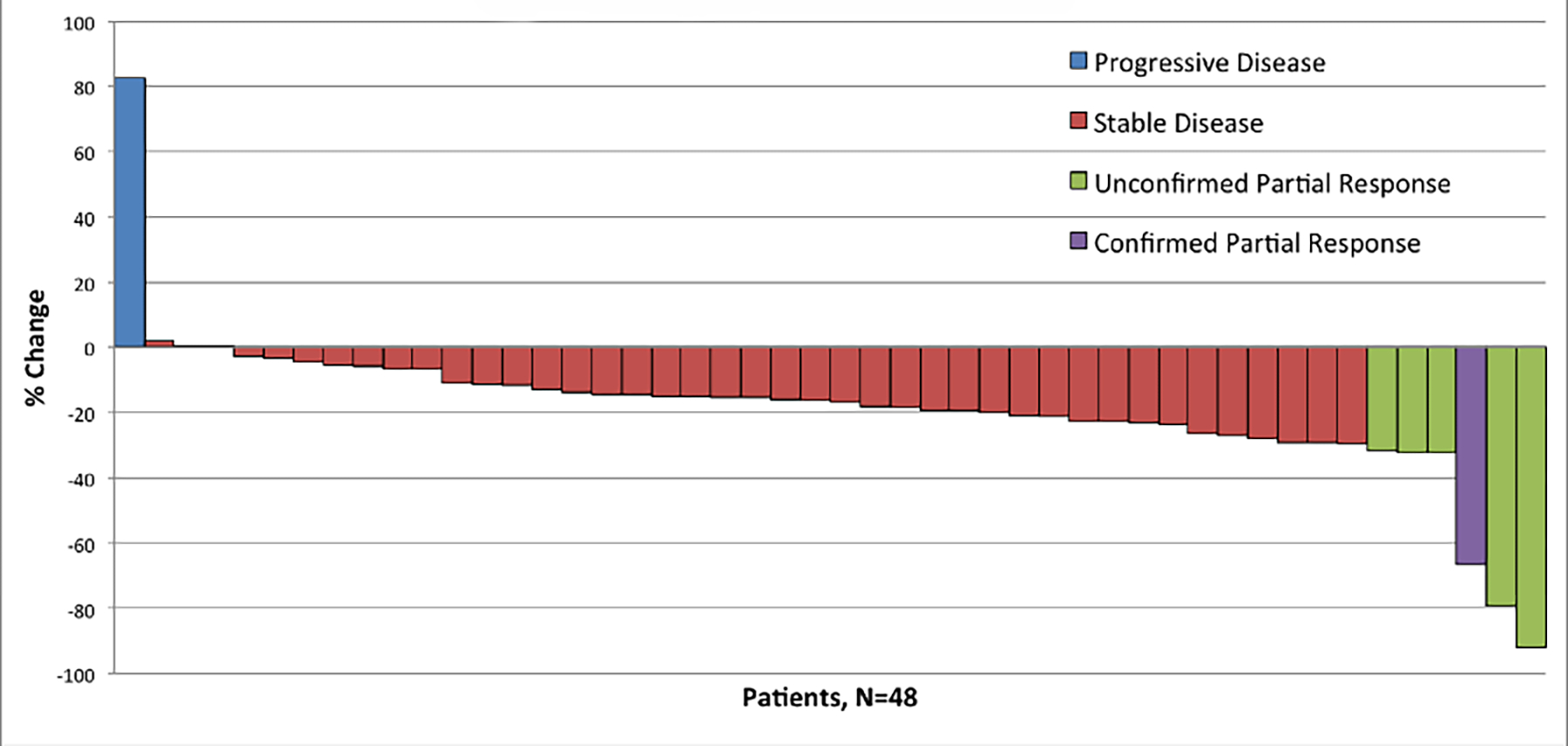
Waterfall Plot of Best Response
Figure 3.
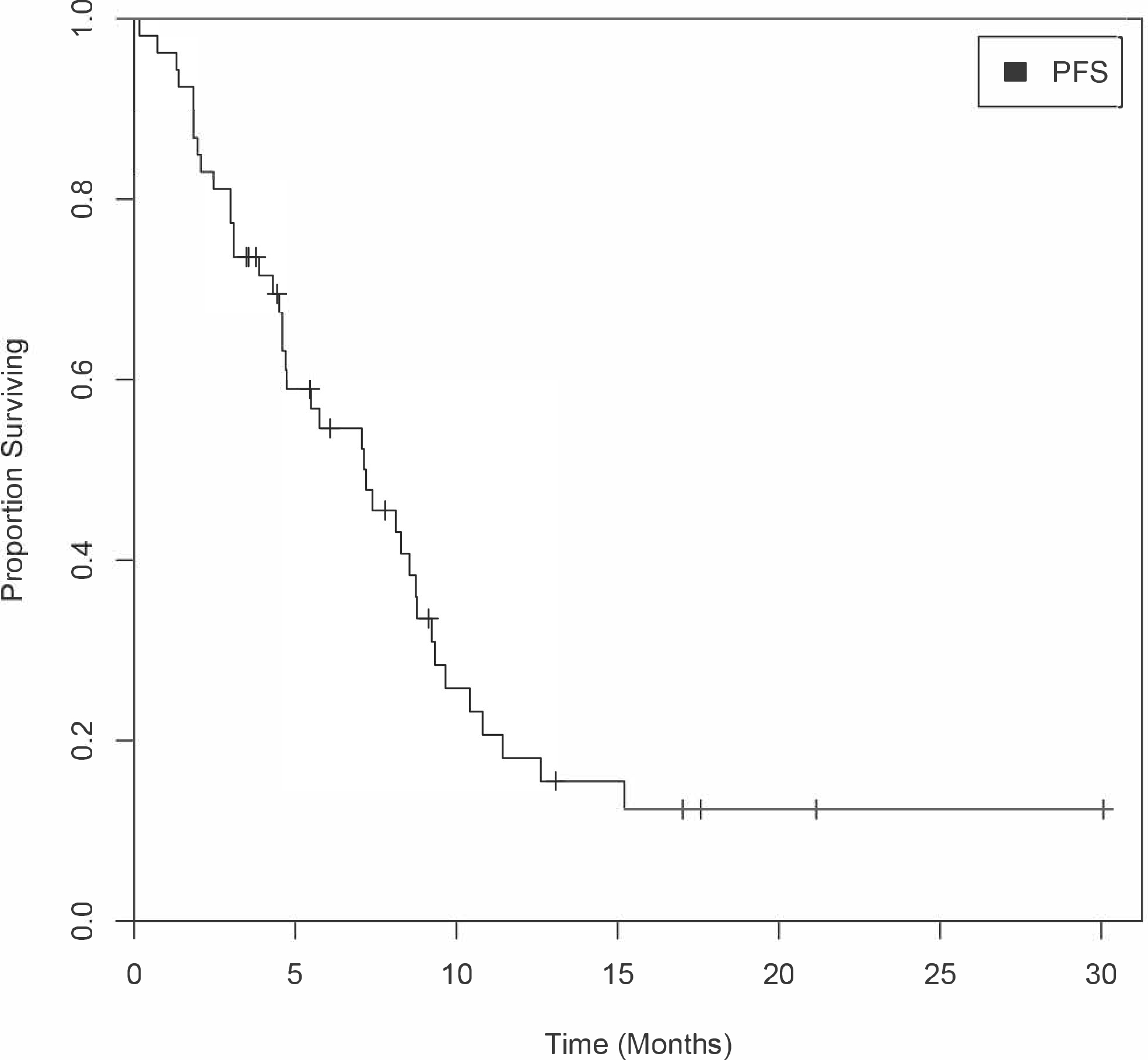
Kaplan-Meier Curve Estimating PFS
Figure 4:
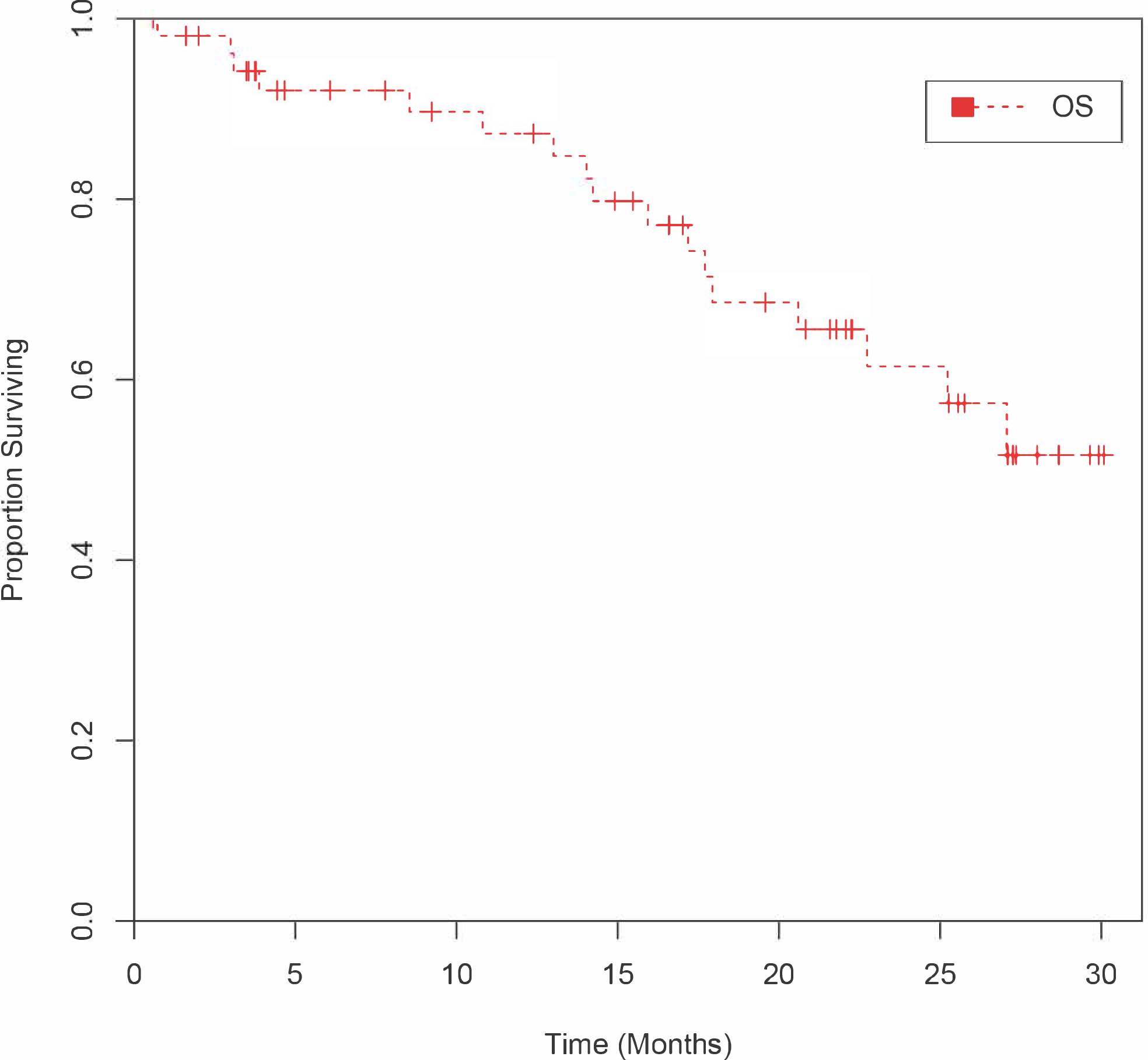
Kaplan-Meier Curve Estimating OS
As NETs can be slow-growing in nature and many patients stopped the study drug early due to toxicities, a standard definition of progression-free survival would overestimate the clinical benefit of this combination. Therefore, a conservative approach excluding patients who discontinued treatment was employed to assess progression-free survival. By this conservative approach of statistical analysis, 26 patients were progression-free at 6 months, with a conservative 6-month PFS rate of 48%. An additional 13 patients were progression-free at 6 months, but were no longer on study treatment, for a 6-month PFS rate of 72% by standard criteria. These 13 patients discontinued treatment due to adverse events or patient preference, and many received alternative treatments following study discontinuation. Three patients were continued on octreotide therapy, no subsequent treatment was reported in 4 patients, and off-study treatment or procedures were reported in 6 patients: one received paclitaxel, one received off-study bevacizumab, one received off-label sunitinib and later pasireotide on clinical trial, one underwent palliative radiation, one underwent radiofrequency ablation, and one underwent trans-arterial chemoembolization to the right and left liver lobes.
Serum Tumor Markers.
Baseline chromogranin A measurements, along with at least one subsequent chromogranin A measurement, were available in 9 patients. No consistent correlation was found between the change in chromogranin A levels from baseline, and the change in tumor size from baseline.
Adverse Events.
Fifty-four patients were evaluable for toxicity. The most frequently reported treatment-related laboratory adverse events included thrombocytopenia, anemia, leukopenia, hypercholesterolemia, hypocalcemia, and proteinuria (Table 2). The most frequently reported (>30%) treatment-related clinical adverse events of any grade were fatigue, hypertension, diarrhea, mucositis, anorexia, nausea, and epistaxis (Table 3). The majority of these events were grade 1–2. The most common clinical grade 3–4 treatment-related adverse events were fatigue (n=8), hypertension (n=7), and bleeding (n=7). One patient, a 67-year-old female, was on concurrent warfarin when she experienced a treatment-related grade 3 duodenal hemorrhage and subsequently, a grade 3 rectal hemorrhage. Most sites of hemorrhage were distant from the primary tumors including bronchopulmonary (n=2), lower GI (n=1), epistaxis (n=1), and hemorrhoids (n=1). Three patients died while on the study, and two of these deaths were treatment related. One death occurred in a 67-year-old male with a history of NETs of the mesentery and prior bowel surgeries, who experienced a grade 5 treatment-related perforated viscus. One patient, an 89-year-old female, died because of sepsis related to skin ulceration and infection, deemed unrelated to study treatment by the treating physician. One additional patient, a 68-year-old female, experienced a grade 4 treatment-related colonic perforation and required an emergent right hemi-colectomy. She was removed from the study on the date of surgery and died 13 days later.
Table 2.
Laboratory Adverse Events
| Laboratory Adverse Eventsa | Number of patients, N = 54, n (%) | |
|---|---|---|
| Grade 1–2b | Grade 3–4c | |
| Hematologic | ||
| Thrombocytopenia | 34 (63) | 4 (7) |
| Anemia | 32 (59) | 4 (7) |
| Leukopenia | 28 (52) | 1 (2) |
| Neutropenia | 15 (28) | 1 (2) |
| Lymphopenia | 7 (13) | 4 (7) |
| Hepatic | ||
| AST elevation | 16 (30) | 0 |
| ALT elevation | 13 (24) | 1 (2) |
| Alkaline phosphatase elevation | 13 (24) | 1 (2) |
| Hypoalbuminemia | 9 (17) | 0 |
| Hyperbilirubinemia | 4 (4) | 1 (2) |
| Lipids | ||
| Hypercholesterolemia | 29 (53) | 0 |
| Hypertriglyceridemia | 22 (41) | 1 (2) |
| Metabolic | ||
| Proteinuria | 27 (50) | 1 (2) |
| Hyperglycemia | 19 (35) | 2 (4) |
| Elevated Creatinine | 14 (26) | 0 |
| Hypokalemia | 11 (20) | 5 (9) |
| Hypocalcemia | 6 (11) | 0 |
| Hypophosphatemia | 3 (6) | 3 (6) |
| Coagulation | ||
| Prolonged aPTT | 3 (6) | 0 |
National Cancer Institute Common Terminology Criteria for Adverse Events, criteria 3.0.
Grade 1–2 adverse events that were possibly, probably, or definitely related to study treatment, and occurred greater than or equal to 5% of the time.
Grade 3–4 adverse events that were possibly, probably, or definitely related to study treatment, regardless of frequency.
Table 3.
Clinical Adverse Events
| Clinical Adverse Eventsa,b | Lower | |
|---|---|---|
| Grade 1–2c | Grade 3–4d | |
| Cardiovascular | ||
| Hypertension | 24 (44) | 6 (11) |
| Thrombosis | 1 (2) | 1 (2) |
| Visceral arterial ischemia | 0 | 1 (2) |
| Constitutional | ||
| Fatigue | 27 (50) | 7 (13) |
| Weight loss | 14 (26) | 2 (4) |
| Fever | 4 (7) | 0 |
| Chills | 4 (7) | 0 |
| Dermatology | ||
| Rash, acneiform | 19 (35) | 0 |
| Rash, desquamating | 12 (22) | 1 (2) |
| Pruritis | 8 (15) | 0 |
| Alopecia | 6 (11) | 0 |
| Dry skin | 6 (11) | 0 |
| Nail disorder | 4 (7) | 0 |
| Skin disorder | 4 (7) | 0 |
| Skin ulceration | 3 (6) | 0 |
| Gastrointestinal | ||
| Anorexia | 21 (39) | 1 (2) |
| Diarrhea | 22 (41) | 2 (4) |
| Mucositis, oral | 20 (37) | 2 (4) |
| Nausea | 18 (33) | 1 (2) |
| Vomiting | 10 (19) | 0 |
| Taste alteration | 10 (19) | 0 |
| Gastrointestinal disorder | 6 (11) | 0 |
| Constipation | 5 (9) | 1 (2) |
| Hemorrhoids | 3 (6) | 1 (2) |
| Dry mouth | 3 (6) | 0 |
| Colonic perforation | 0 | 1 (2) |
| Hemorrhage | ||
| Epistaxis | 18 (33) | 1 (2) |
| Petechaie | 7 (13) | 0 |
| Pulmonary hemorrhage | 6 (11) | 2 (4) |
| Lower GI hemorrhage | 3 (6) | 3 (6) |
| Upper GI hemorrhage | 4 (8) | 1 (2) |
| Lymphatics | ||
| Edema of extremities | 9 (17) | 2 (4) |
| Localized edema | 4 (7) | 0 |
| Musculoskeletal | ||
| Muscle weakness | 3 (6) | 0 |
| Neurology | ||
| Insomnia | 6 (11) | 0 |
| Neurologic disorder NOS | 3 (6) | 0 |
| Peripheral sensory neuropathy | 3 (6) | 0 |
| Pain | ||
| Headache | 9 (17) | 3 (6) |
| Oral/pharyngolaryngeal pain | 7 (13) | 0 |
| Abdominal pain | 5 (9) | 2 (4) |
| Anal/rectal pain | 3 (6) | 1 (2) |
| Buttock pain | 1 (2) | 1 (2) |
| Pulmonary | ||
| Dyspnea | 6 (11) | 0 |
| Cough | 3 (6) | 0 |
| Nasal congestion | 3 (6) | 0 |
| Respiratory disorder | 3 (6) | 0 |
| Change in voice | 3 (6) | 0 |
National Cancer Institute Common Terminology Criteria for Adverse Events, criteria 3.0.
Three Grade 5 adverse events that occurred on trial (n=3) are described in the text.
Grade 1–2 adverse events that were possibly, probably, or definitely related to study treatment and occurred greater than or equal to 5% of the time. Not included in the table is one patient (1.8%) who experienced a Grade 2 intracranial hemorrhage without neurologic deficits, concurrent with a hypertensive emergency (Grade 4 HTN and Grade 3 headache).
Grade 3–4 adverse events that were possibly, probably, or definitely related to study treatment, regardless of frequency.
Discussion
This trial of temsirolimus and bevacizumab was initiated in 2009, prior to the approval of everolimus in NETs, and sought to study the efficacy and safety of the combination of VEGF and mTOR inhibition in a group of patients with few approved treatment options at the time. The trial results show that bevacizumab and temsirolimus in combination achieve a confirmed objective response rate of 2%, a median PFS of 7.1 months, and a 6–month PFS of 48% by conservative criteria. The median duration of treatment with temsirolimus and bevacizumab were 3.9 months and 3.4 months, respectively, indicating that the majority of patients stopped treatment before progression due to toxicities. This is lower than the partial response rate of 12% observed in the bevacizumab arm in the SWOG-0518 trial. (Yao et al., 2017) Unlike SWOG-0518, an amendment was made to the inclusion criteria to enroll an aggressive subset of extra-pancreatic neuroendocrine patients with RECIST progression in the 7 months prior to study entry. Our results are also in contrast to the pancreatic NET cohort of this trial, where the response rate in the first 56 patients was 41% (23/56 patients). (Hobday et al., 2015) We hypothesize that this limited antitumor activity seen in the extra-pancreatic NET cohort may be due to underlying differences in tumor biology. Recent tumor genome sequencing confirms profound differences in the genomic landscape of gastrointestinal NETs compared to pNETs; mechanistic studies need to be undertaken to better understand differences in susceptibility to anti-VEGF approaches in these tumors. (Jiao et al., 2011, Banck et al., 2013, Scarpa et al., 2017)
A second possible explanation is that responses in extra-pancreatic NETs may not be well captured by traditional RECIST criteria. In patients achieving stable disease, 13 patients (24%) did achieve a tumor shrinkage of >20% but less than 30%, arguably a clinically meaningful benefit in a disease with a longer natural history. By including these patients with a minor response, the rate of patients achieving tumor reduction is 40%. Finally, the toxicity of this regimen in this population limited the duration of therapy in many patients, likely compromising durable disease control, as well as perhaps not allowing the slowly evolving deepening responses over several months’ time that are typical in this disease.
The toxicity of the combination of bevacizumab and temsirolimus was greater than anticipated, with 29 patients (54%) discontinuing treatment due to adverse events, refusal of further treatment, or treatment delays, and 3 patient deaths (7%). Close to 72% of patients discontinued therapy due to adverse events or personal preference. Specifically, grade 3–4 bleeding (n=7, 13%) was increased in our patients receiving the combination regimen of bevacizumab and temsirolimus, as compared to historical standards for bevacizumab. In the pancreatic NET cohort from the same trial, only 30% (17/58) of patients stopped treatment due to adverse events or refusal and around 5% experienced grade 3 or 4 hemorrhage. (Hobday et al., 2015)
In large, randomized phase III clinical trials that evaluated bevacizumab alone in metastatic colorectal cancer patients, grade 3–4 hemorrhage was reported at frequencies of 2–3%. (Hurwitz et al., 2004, Giantonio et al., 2007, Saltz et al., 2008) One reason for the increased bleeding might be the higher dose (10 mg/kg as opposed to the 5mg/kg dose used for colorectal cancer). A second reason may be related to the concurrent use of anticoagulant drugs in some of the patients in our study, which was allowed prior to an amendment of the study protocol. There was also a relatively high (7%) rate of grade 3–4 thrombocytopenia in our study, as compared to the reported rate of 1% in renal cell carcinoma patients treated with temsirolimus. (Hudes et al., 2007) While severe thrombocytopenia may have increased the likelihood of bleeding in our study, the numbers of patients who experienced grade 3–4 thrombocytopenia (n=4) are too few to draw meaningful conclusions.
A phase II trial evaluating the combination of bevacizumab and temsirolimus in patients with untreated metastatic renal cell carcinoma also reported a higher-than-anticipated rate of toxicity, with 42% of patients discontinuing treatment due to adverse events, and greater than 50% of patients unable to tolerate the combination over several months. (Négrier et al., 2011) The frequency of grade 3–4 thrombocytopenia was 11% in the renal cell cancer patients studied, higher than observed in our study. However, unlike our study, hemorrhage was not increased as an adverse event. This may reflect the increased likelihood of observing hemorrhage in the gastrointestinal tract, the primary site of the majority of extra-pancreatic NETs.
To compensate for heterogeneity in disease aggressiveness, we amended the inclusion criteria to restrict to patients who had progressive disease within the previous 7 months. Therefore 37% (20/54) of the total cohort had documented progression in the previous 7 months. However, significant heterogeneity of aggressiveness, primary site, grade, hormone production status, etc. remain, which may impact the primary outcome and generalizability of our findings.
This trial highlights the need to continue efforts that explore new targets in the treatment of NETs. For GEP-NETs with somatostatin receptor (SSTR) expression, peptide receptor radiotherapy with lutetium-177 (177Lu-dotatate), a β–particle-emitting radiopharmaceutical, has emerged as a standard line of treatment based on the NETTER-1 study. (Strosberg et al., 2017a) There is strong pre-clinical evidence of radiosensitization with temsirolimus against various neuroendocrine cell lines. (Exner et al., 2021). A pilot study of the combination of everolimus and radiation in neuroendocrine liver metastasis also reported 1- and 2-year local control rates of 97% and 71%. (Myrehaug et al., 2021) Therefore, a possible future avenue of research is the combination of mTOR inhibition with 177Lu-dotatate to try to improve the efficacy of 177Lu-dotatate alone.
Conclusion
Despite compelling preclinical data, the combination of bevacizumab and temsirolimus did not achieve predefined levels of activity in patients with advanced extra-pancreatic neuroendocrine tumors. Around 54% of patients discontinued treatment due to adverse events, refusal of further treatment, or treatment delays. Given the increased toxicity observed with the combination, particularly with regard to severe gastrointestinal bleeding and bowel perforation, we do not recommend the use of bevacizumab and temsirolimus concurrently in patients with advanced extra-pancreatic neuroendocrine tumors.
Table 4:
Clinical Efficacy
| Parameter | Outcome |
|---|---|
|
Median Duration of Therapy with: Temsirolimus Bevacizumab |
3.9 months 3.4 months |
| Median Progression-Free Survival | 7.1 months (95% CI 4.6 – 8.7 months) |
| Objective Response Rate | 2% (1 out of 48) |
| Stable Disease Rate | 88% (42 out of 48) |
Grant Support
This project has been funded in whole or in part with federal funds from the National Cancer Institute, the National Institutes of Health, and the US Department of Health and Human Services, under the following contract numbers: HHSN261201100070C (OSU), HHSN261201100099C (Mayo Clinic), HHSN261201100100C (Moffitt Cancer Center), HHSN261201100038C (University of California-Davis), HHSN261201100071C (University of Chicago), and HHSN261201100032C (Princess Margaret Hospital).
Funding:
Supported by NCI N01 contracts: 00099, 00070, 00100, 00038, 00071, 62206, 00032
Footnotes
Declaration of Interest
Jonathan Strosberg: Honoraria from Speaker’s Bureau from Genentech; Jennifer J. Knox: Commercial research support from Pfizer for an investigator-initiated study; Manisha Shah: Commercial research grant from Novartis and consult/advisory board for Novartis; Vineeth Sukrithan: Research funding (to institution) from Eli Lilly & Company, consulting fees from GE Health and Lantheus. The remaining authors have no conflicts to disclose.
References
- BANCK MS, KANWAR R, KULKARNI AA, BOORA GK, METGE F, KIPP BR, ZHANG L, THORLAND EC, MINN KT, TENTU R, ECKLOFF BW, WIEBEN ED, WU Y, CUNNINGHAM JM, NAGORNEY DM, GILBERT JA, AMES MM & BEUTLER AS 2013. The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest, 123, 2502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPELLA C SE, SOBIN LH et al. ENDOCRINE TUMOURS OF THE SMALL INTESTINE. IN: HAMILTON SR, AALTONEN LA, EDS. PATHOLOGY AND GENETICS OF TUMOURS OF THE DIGESTIVE SYSTEM. LYON, FRANCE: IARC PRESS; 2000:77–82. [Google Scholar]
- CAPLIN ME, PAVEL M, ĆWIKŁA JB, PHAN AT, RADERER M, SEDLÁČKOVÁ E, CADIOT G, WOLIN EM, CAPDEVILA J, WALL L, RINDI G, LANGLEY A, MARTINEZ S, BLUMBERG J & RUSZNIEWSKI P 2014. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. New England Journal of Medicine, 371, 224–233. [DOI] [PubMed] [Google Scholar]
- DASARI A, SHEN C, HALPERIN D, ZHAO B, ZHOU S, XU Y, SHIH T & YAO JC 2017. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol, 3, 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURAN I, KORTMANSKY J, SINGH D, HIRTE H, KOCHA W, GOSS G, LE L, OZA A, NICKLEE T, HO J, BIRLE D, POND GR, ARBOINE D, DANCEY J, AVIEL-RONEN S, TSAO MS, HEDLEY D & SIU LL 2006. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer, 95, 1148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EXNER S, ARREY G, PRASAD V & GRÖTZINGER C 2021. mTOR Inhibitors as Radiosensitizers in Neuroendocrine Neoplasms. Frontiers in Oncology, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANTONIO BJ, CATALANO PJ, MEROPOL NJ, O’DWYER PJ, MITCHELL EP, ALBERTS SR, SCHWARTZ MA & BENSON AB 3RD 2007. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol, 25, 1539–44. [DOI] [PubMed] [Google Scholar]
- HAMILTON SR AL 2000. Pathology and Genetics of Tumours of the Digestive System, IARC Press. [Google Scholar]
- HOBDAY TJ, QIN R, REIDY-LAGUNES D, MOORE MJ, STROSBERG J, KAUBISCH A, SHAH M, KINDLER HL, LENZ HJ, CHEN H & ERLICHMAN C 2015. Multicenter Phase II Trial of Temsirolimus and Bevacizumab in Pancreatic Neuroendocrine Tumors. J Clin Oncol, 33, 1551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUDES G, CARDUCCI M, TOMCZAK P, DUTCHER J, FIGLIN R, KAPOOR A, STAROSLAWSKA E, SOSMAN J, MCDERMOTT D, BODROGI I, KOVACEVIC Z, LESOVOY V, SCHMIDT-WOLF IG, BARBARASH O, GOKMEN E, O’TOOLE T, LUSTGARTEN S, MOORE L & MOTZER RJ 2007. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med, 356, 2271–81. [DOI] [PubMed] [Google Scholar]
- HURWITZ H, FEHRENBACHER L, NOVOTNY W, CARTWRIGHT T, HAINSWORTH J, HEIM W, BERLIN J, BARON A, GRIFFING S, HOLMGREN E, FERRARA N, FYFE G, ROGERS B, ROSS R & KABBINAVAR F 2004. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med, 350, 2335–42. [DOI] [PubMed] [Google Scholar]
- JIAO Y, SHI C, EDIL BH, DE WILDE RF, KLIMSTRA DS, MAITRA A, SCHULICK RD, TANG LH, WOLFGANG CL, CHOTI MA, VELCULESCU VE, DIAZ LA JR., VOGELSTEIN B, KINZLER KW, HRUBAN RH & PAPADOPOULOS N 2011. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science, 331, 1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULKE MH, NIEDZWIECKI D, FOSTER NR, FRUTH B, KUNZ PL, KENNECKE HF, WOLIN EM & VENOOK AP 2015. Randomized phase II study of everolimus (E) versus everolimus plus bevacizumab (E+B) in patients (Pts) with locally advanced or metastatic pancreatic neuroendocrine tumors (pNET), CALGB 80701 (Alliance). Journal of Clinical Oncology, 33, 4005–4005. [Google Scholar]
- MYREHAUG S, CHAN DL, RODRIGUEZ-FREIXINOS V, CHUNG H, HALLET J, LAW C, PATEL C, MILOT L, HUDSON J, CHEN H & SINGH S 2021. A pilot study of everolimus and radiation for neuroendocrine liver metastases. Endocrine-Related Cancer, 28, 541–548. [DOI] [PubMed] [Google Scholar]
- NÉGRIER S, GRAVIS G, PÉROL D, CHEVREAU C, DELVA R, BAY JO, BLANC E, FERLAY C, GEOFFROIS L, ROLLAND F, LEGOUFFE E, SEVIN E, LAGUERRE B & ESCUDIER B 2011. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol, 12, 673–80. [DOI] [PubMed] [Google Scholar]
- SALTZ LB, CLARKE S, DÍAZ-RUBIO E, SCHEITHAUER W, FIGER A, WONG R, KOSKI S, LICHINITSER M, YANG TS, RIVERA F, COUTURE F, SIRZÉN F & CASSIDY J 2008. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol, 26, 2013–9. [DOI] [PubMed] [Google Scholar]
- SCARPA A, CHANG DK, NONES K, CORBO V, PATCH AM, BAILEY P, LAWLOR RT, JOHNS AL, MILLER DK, MAFFICINI A, RUSEV B, SCARDONI M, ANTONELLO D, BARBI S, SIKORA KO, CINGARLINI S, VICENTINI C, MCKAY S, QUINN MC, BRUXNER TJ, CHRIST AN, HARLIWONG I, IDRISOGLU S, MCLEAN S, NOURSE C, NOURBAKHSH E, WILSON PJ, ANDERSON MJ, FINK JL, NEWELL F, WADDELL N, HOLMES O, KAZAKOFF SH, LEONARD C, WOOD S, XU Q, NAGARAJ SH, AMATO E, DALAI I, BERSANI S, CATALDO I, DEI TOS AP, CAPELLI P, DAVI MV, LANDONI L, MALPAGA A, MIOTTO M, WHITEHALL VL, LEGGETT BA, HARRIS JL, HARRIS J, JONES MD, HUMPHRIS J, CHANTRILL LA, CHIN V, NAGRIAL AM, PAJIC M, SCARLETT CJ, PINHO A, ROOMAN I, TOON C, WU J, PINESE M, COWLEY M, BARBOUR A, MAWSON A, HUMPHREY ES, COLVIN EK, CHOU A, LOVELL JA, JAMIESON NB, DUTHIE F, GINGRAS MC, FISHER WE, DAGG RA, LAU LM, LEE M, PICKETT HA, REDDEL RR, SAMRA JS, KENCH JG, MERRETT ND, EPARI K, NGUYEN NQ, ZEPS N, FALCONI M, SIMBOLO M, BUTTURINI G, VAN BUREN G, PARTELLI S, FASSAN M, AUSTRALIAN PANCREATIC CANCER GENOME I, KHANNA KK, GILL AJ, WHEELER DA, GIBBS RA, MUSGROVE EA, BASSI C, TORTORA G, PEDERZOLI P, et al. 2017. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature, 543, 65–71. [DOI] [PubMed] [Google Scholar]
- STROSBERG J, EL-HADDAD G, WOLIN E, HENDIFAR A, YAO J, CHASEN B, MITTRA E, KUNZ PL, KULKE MH, JACENE H, BUSHNELL D, O’DORISIO TM, BAUM RP, KULKARNI HR, CAPLIN M, LEBTAHI R, HOBDAY T, DELPASSAND E, VAN CUTSEM E, BENSON A, SRIRAJASKANTHAN R, PAVEL M, MORA J, BERLIN J, GRANDE E, REED N, SEREGNI E, OBERG K, LOPERA SIERRA M, SANTORO P, THEVENET T, ERION JL, RUSZNIEWSKI P, KWEKKEBOOM D, KRENNING E & INVESTIGATORS N-T 2017a. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med, 376, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROSBERG J, EL-HADDAD G, WOLIN E, HENDIFAR A, YAO J, CHASEN B, MITTRA E, KUNZ PL, KULKE MH, JACENE H, BUSHNELL D, O’DORISIO TM, BAUM RP, KULKARNI HR, CAPLIN M, LEBTAHI R, HOBDAY T, DELPASSAND E, VAN CUTSEM E, BENSON A, SRIRAJASKANTHAN R, PAVEL M, MORA J, BERLIN J, GRANDE E, REED N, SEREGNI E, ÖBERG K, LOPERA SIERRA M, SANTORO P, THEVENET T, ERION JL, RUSZNIEWSKI P, KWEKKEBOOM D & KRENNING E 2017b. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. New England Journal of Medicine, 376, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAO JC, FAZIO N, SINGH S, BUZZONI R, CARNAGHI C, WOLIN E, TOMASEK J, RADERER M, LAHNER H, VOI M, PACAUD LB, ROUYRRE N, SACHS C, VALLE JW, FAVE GD, VAN CUTSEM E, TESSELAAR M, SHIMADA Y, OH DY, STROSBERG J, KULKE MH & PAVEL ME 2016. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet, 387, 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAO JC, GUTHRIE KA, MORAN C, STROSBERG JR, KULKE MH, CHAN JA, LOCONTE N, MCWILLIAMS RR, WOLIN EM, MATTAR B, MCDONOUGH S, CHEN H, BLANKE CD & HOCHSTER HS 2017. Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients With Advanced Carcinoid Tumors: SWOG S0518. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 35, 1695–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAO JC, PHAN A, HOFF PM, CHEN HX, CHARNSANGAVEJ C, YEUNG SC, HESS K, NG C, ABBRUZZESE JL & AJANI JA 2008. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol, 26, 1316–23. [DOI] [PubMed] [Google Scholar]
- YAO JC, SHAH MH, ITO T, BOHAS CL, WOLIN EM, VAN CUTSEM E, HOBDAY TJ, OKUSAKA T, CAPDEVILA J, DE VRIES EGE, TOMASSETTI P, PAVEL ME, HOOSEN S, HAAS T, LINCY J, LEBWOHL D & ÖBERG K 2011. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. New England Journal of Medicine, 364, 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]


