Abstract
The hormone ethylene is crucial in the regulation of ripening in climacteric fruit, such as bananas. The transcriptional regulation of ethylene biosynthesis throughout banana fruit ripening has received much study, but the cascaded transcriptional machinery of upstream transcriptional regulators implicated in the ethylene biosynthesis pathway is still poorly understood. Here we report that ethylene biosynthesis genes, including MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8, were upregulated in ripening bananas. NAC (NAM, ATAF, CUC) transcription factor, MaNAC083, a ripening and ethylene-inhibited gene, was discovered as a potential binding protein to the MaACS1 promoter by yeast one-hybrid screening. Further in vitro and in vivo experiments indicated that MaNAC083 bound directly to promoters of the five ethylene biosynthesis genes, thereby transcriptionally repressing their expression, which was further verified by transient overexpression experiments, where ethylene production was inhibited through MaNAC083-modulated transcriptional repression of ethylene biosynthesis genes in banana fruits. Strikingly, MaMADS1, a ripening-induced MADS (MCM1, AGAMOUS, DEFICIENS, SRF4) transcription factor, was found to directly repress the expression of MaNAC083, inhibiting trans-repression of MaNAC083 to ethylene biosynthesis genes, thereby attenuating MaNAC083-repressed ethylene production in bananas. These findings collectively illustrated the mechanistic basis of a MaMADS1–MaNAC083–MaACS1/MaACOs regulatory cascade controlling ethylene biosynthesis during banana fruit ripening. These findings increase our knowledge of the transcriptional regulatory mechanisms of ethylene biosynthesis at the transcriptional level and are expected to help develop molecular approaches to control ripening and improve fruit storability.
Introduction
Ethylene, a versatile phytohormone, modulates numerous physiological processes, spanning seed germination to organ senescence [1]. Due to its critical involvement in regulating agronomically valuable traits in plants, particularly fruit ripening and quality formation, ethylene production has been a topic of intense study [2]. The ethylene biosynthetic pathway includes two enzyme steps. S-Adenosyl-l-methionine (SAM) is converted to 1-aminocyclopropane-1-carboxylic acid (ACC) via catalysis by ACC synthase (ACS) [3]. The second step, converting ACC to ethylene, is catalyzed through ACC oxidase (ACO) [4]. Thus, ethylene production is controlled by ACS and ACO, the rate-limiting enzymes for ethylene biosynthesis. Multigene families that encode the enzymes ACS and ACO are a longstanding area of interest in fruit ripening research [5].
Gene expression regulation requires precise transcriptional programs, involving binding of transcription factors (TFs) to cis-acting regions of gene promoter DNA [6]. With the advent of deep research on tomato non-ripening mutants rin and nor, researchers have steadily been interested in the central TFs' role in regulating fruit ripening. Members of the MADS and NAC families of TFs are encoded by the nor and rin genes, respectively [7]. RIN and NOR were found to regulate fruit ripening by acting upstream of the ethylene pathway, as evaluated by the phenotypic characterization of mutants [8]. Throughout tomato fruit ripening, RIN is the first transcription factor discovered to control ethylene biosynthetic gene expression, including SlACS2 and SlACO4 [9, 10]. NOR was also found to favorably control ethylene biosynthesis through direct induction of SlACS2 and SlACS4 [11]. Regulation of the ACS and ACO genes throughout fruit ripening (apples, plums, bananas, and melons) is also believed to be controlled by other TFs, including ERF, MYB, MYC, and ABF [12–15].
The banana (Musa acuminata) is the most widespread type of fresh fruit in the world, produced mainly in developing countries due to the special climatic conditions needed to grow them, and are a multimillion-dollar export product consumed primarily in wealthier nations [16]. Currently, commercialized bananas are dominated by Cavendish cultivars with an AAA triploid genome [17]. Bananas are typically collected in a green, mature phase and shipped to wholesale markets, where they are artificially ripened to a golden yellow fruit with good edible quality by application of ethylene [18]. Hence, the regulating mechanisms of ethylene production during postharvest banana fruit ripening have received considerable study. During postharvest ripening, ACS and ACO are induced and modulate ethylene biosynthesis in banana fruit [19, 20]. MaACS1 (also MaACS7 [21], Ma04_t35640) and MaACO1 (also MaACO8, [21] Ma07_g19730) are widely recognized as key ethylene biosynthesis genes involved in banana ripening [22]. Various ethylene signaling and biosynthesis-related gene groups have been further investigated in banana fruit utilizing transcriptome and genome profiling [21, 23]. Furthermore, there is a significant emphasis on the transcriptional regulation of the genes responsible for ethylene biosynthesis in bananas. Multiple transcription factors have been identified to have critical functions in banana fruit ripening, including ERF [13], bHLH [18], NAC [24], EIL [25], and MYB [26]. MaNAC029 and MaERF9 function as positive regulators, while MaERF11 is a negative transcriptional regulator of the ethylene biosynthetic pathway [13, 20, 24]. However, the relationship among these upstream transcription factors of the ethylene biosynthesis pathway, especially their cascaded transcription mechanism, remains unclear.
Therefore, this study aims to analyze the cascaded transcriptional machinery of ethylene biosynthesis during banana fruit ripening. The findings of this research will offer novel transcriptional insights into fruit ripening and provide clues for improving banana storage and for breeding high-quality fruits.
Results
Identification of key genes for ethylene biosynthesis related to banana fruit ripening
RNA-seq of banana fruits in different stages was conducted to detect potential ethylene biosynthesis genes associated with fruit ripening. This transcriptome analysis revealed nine ethylene biosynthesis genes, of which one ACS gene and four ACO genes were induced by ripening, and two SAM synthetase (SAMS) genes and one ACS gene were repressed by ripening (Supplementary Data Fig. S1 and Supplementary Data Table S1). These ripening-related genes, including MaSAMS4, MaSAMS11, MaACS1, MaACS12, MaACO1, MaACO4, MaACO5, and MaACO8, were subsequently selected, and their expression pattern during the whole ripening process was measured by qRT–PCR. After the initiation of ripening with exogenous ethylene treatment, a color change was initiated in the banana peel from green to yellow on the third day and the peel was completely yellow on the fifth day (Fig. 1A), which was also indicated by a sharp rise in the color index (CI) from −9.28 at day 0 to −0.37 on the seventh day (Fig. 1B). Pulp firmness reduced progressively after 3 days of storage and ultimately dropped to a minimum level of 6.02 N on the seventh day (Fig. 1C). The endogenous generation of ethylene in banana fruits elevated significantly after 1 storage day, reaching a maximum on day 3 and then declining (Fig. 1D). In parallel, the expression of five ethylene biosynthesis genes, MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8, was initially elevated, peaked on the third or fifth day, and subsequently marginally declined during ripening, consistent with the ethylene production (Fig. 1E). However, the expression of MaSAMS4, MaSAMS11, and MaACS12 showed downward trends during the postharvest ripening process. These results suggested that MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 are good candidates associated with ethylene biosynthesis during banana ripening.
Figure 1.
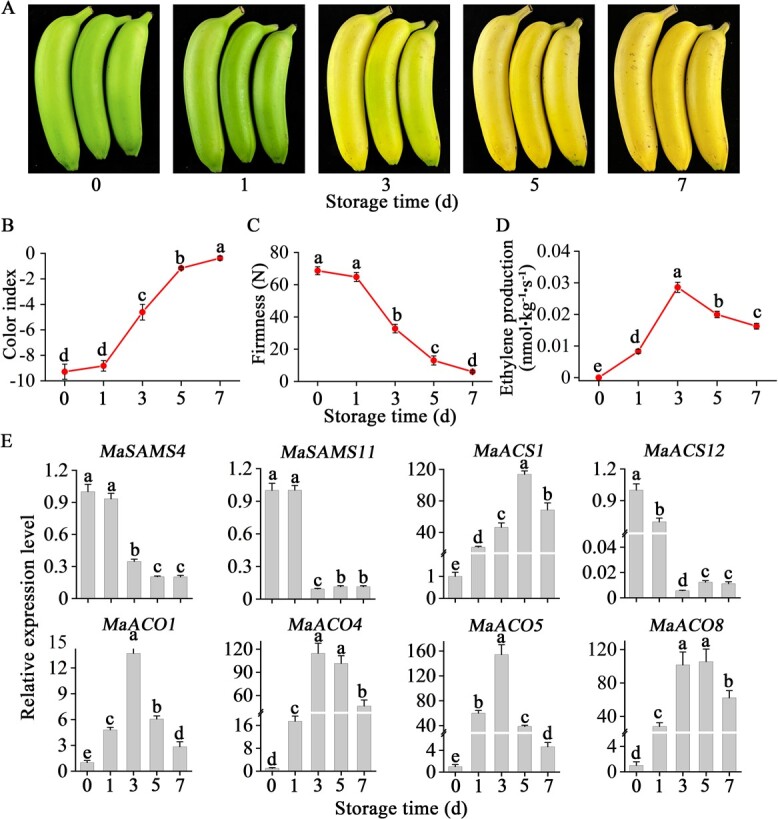
Ethylene production and ethylene biosynthesis genes expression in banana fruit during ethylene-induced ripening. (A) Ethylene-induced banana ripening phenotype alteration. (B–D) CI changes (B), fruit firmness (C), and ethylene generation (D) in fruit during ripening. (E) Banana fruit ripening expression of MaSAMS4, MaSAMS11, MaACS1, MaACS12, MaACO1, MaACO4, MaACO5, and MaACO8. The expression level is represented as a ratio to day 0 (set at 1). Data are represented as means ± standard error from n = 6 (B-–D) and n = 3 (E) replicates, and different letters denote statistical variation (one-way ANOVA, P < .05).
MaNAC083 directly targets ethylene biosynthesis genes
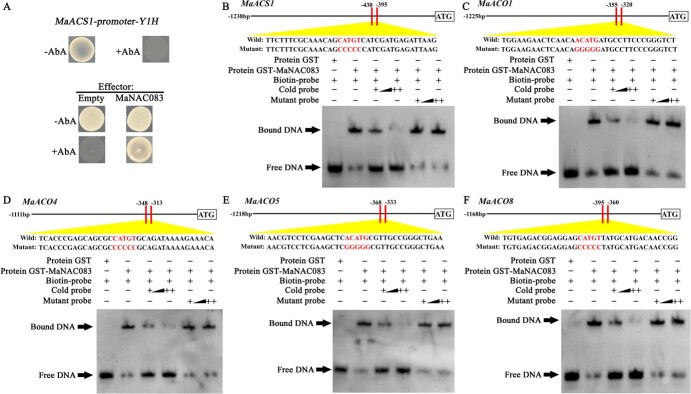
To understand the regulatory techniques that influence the development of ethylene biosynthetic genes, we conducted a screening process utilizing yeast one-hybrid (Y1H) libraries in search of proteins interacting with their respective promoters. Due to the inability of aureobasidin A (AbA) to inhibit promoter activities of MaACO1, MaACO4, MaACO5, and MaACO8, the banana fruit cDNA library was screened using the MaACS1 promoter as bait. After screening, a fragment of NAC gene cDNA (Ma10_g12120) was detected. Previous studies showed that Ma10_g12120 has the closest evolutionary relationship with Arabidopsis ANAC083 [27]; hence, it was designated MaNAC083. The Y1H assay validated the interaction between MaNAC083 and the MaACS1 promoter by employing MaNAC083 full-length coding DNA sequences as prey. The MaACS1 promoter did not exhibit basal activity in yeast with AbA, while yeast cells expressing MaNAC083 triggered the AbA resistance gene expression controlled by the MaACS1 promoter and developed well on media containing AbA, showing that MaNAC083 protein physically interacts with the MaACS1 promoter (Fig. 2A).
Figure 2.
MaNAC083 bound to promoters of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8. (A) Y1H study of MaNAC083's effect on the MaACS1 promoter. (Top) The MaACS1 promoter was not expressed in yeast developed on SD media without Leu and with 500 ng mL−1 AbA. (Bottom) A yeast growth assay was conducted after introducing plasmids containing cassettes that expressed either MaNAC083 effector or empty (pGADT7; negative control) to Y1H reporter strains. The interaction between them was evaluated based on their capacity to grow when transformed yeast was cultivated in SD medium without Leu under the influence of AbA. (B) MaNAC083 binding to MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 promoters via EMSA. The wild type and mutant probe sequences for ethylene biosynthesis gene promoters are shown at the top. Purified GST protein (negative control) or recombinant GST-MaNAC083 protein was subjected to an incubation process along with probes. The resulting DNA–protein complexes were separated on native polyacrylamide gels. Triangles show competing quantities of unlabeled wild-type or mutated probes.
Promoter analysis of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 showed the presence of the NAC recognition sequence (NACRS) in their promoters (Supplementary Data Fig. S2). In vitro, an electrophoretic mobility shift assay (EMSA) was utilized to determine if MaNAC083 can interact with all promoters directly. The purified recombinant MaNAC083 protein (Supplementary Data Fig. S3) bound to NACRS-containing fragments from these five promoters, resulting in mobility changes that were reversed by unlabeled probes with the same sequence but without altered competitors (Fig. 2B–F).
These data illustrate that MaNAC083 directly targets MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 promoters via the NACRS.
Molecular characterization of MaNAC083
RNA-seq data analysis revealed that MaNAC083 mRNA levels were lower in ripe banana fruit than in unripe fruit (Fig. 3A). The expression pattern during the ethylene-induced ripening process was further measured by RT–qPCR to investigate the potential correlation between MaNAC083 and fruit ripening. The MaNAC083 gene transcripts obviously decreased during ripening (Fig. 3B). Furthermore, ethylene decreased the activity of the MaNAC083 promoter, as illustrated by a transient expression assay based on the dual-luciferase reporter (DLR) system (Fig. 3C). To investigate the subcellular localization, GFP tagged with MaNAC083 was transiently produced in tobacco BY2 protoplasts. Then, the nucleus-targeted mCherry (NLS-mCherry) was co-expressed as a control to monitor the nucleus. The result demonstrated that GFP control fluorescence could be seen in the cytoplasm and nucleus. The green and red fluorescent signals of MaNAC083-GFP and NLS-mCherry showed nuclear localization (Fig. 3D). MaNAC083's transcriptional ability was examined utilizing a GAL4-responsive yeast reporter system. Full-length MaNAC083 exhibited self-transcriptional activation activity among yeast cells (Fig. 3E). Utilizing the DLR system, MaNAC083's transcriptional ability was further evaluated in Nicotiana benthamiana leaves by coupling the LUC reporter with a TATA box and five GAL4 DNA-binding components. VP16, a potent transcriptional stimulator, served as the positive control (Fig. 3F). MaNAC083 considerably decreased the LUC/REN ratio compared with an empty BD vector. These findings indicated that MaNAC083 could be a transcriptional repressor that mediates banana ripening.
Figure 3.
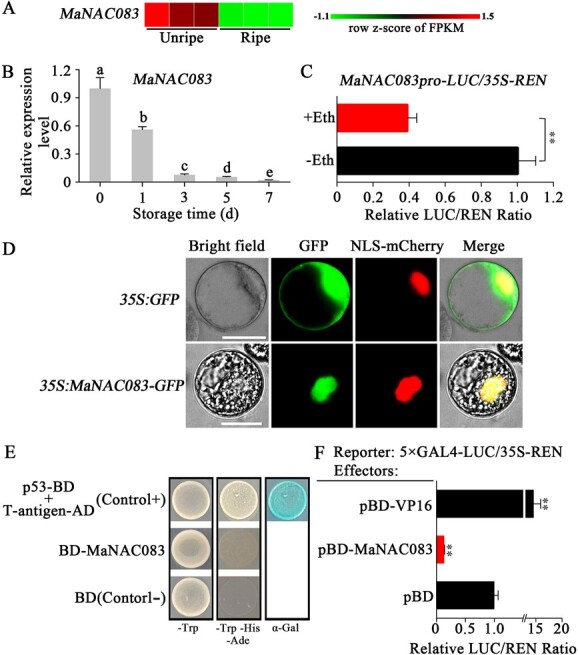
MaNAC083 molecular characteristics. (A) Heat map of gene expression of MaNAC083 in transcriptome analysis of unripe and ripe fruit. (B) MaNAC083 expression in ripening banana fruit. Data are means ± standard error from three replicates and different letters denote statistical variation (one-way ANOVA, P < .05). (C) MaNAC083 promoter activity responding to ethylene. (D) MaNAC083 localization in tobacco BY-2 protoplasts. NLS-mCherry was utilized as a nuclear marker. Scale bars, 25 μm. (E) MaNAC083 transcriptional stimulation in yeast cells. The full MaNAC083 length was merged with the GAL4 DNA-binding domain. Two controls were employed: the pGBKT7 vector (BD) served as a negative control, while p53-BD + T-antigen-AD functioned as a positive one. (F) MaNAC083 transcriptional activity in tobacco leaves. Using VP16 (an extremely strong transcriptional activator) as a positive control, we standardized the LUC/REN ratio of the empty pBD vector (negative control) to 1. Error bars in (C) and (F) denote the standard error from six replicates. Student's t-test, **P < .01.
MaNAC083 represses the expression of ethylene biosynthesis genes and inhibits ethylene production
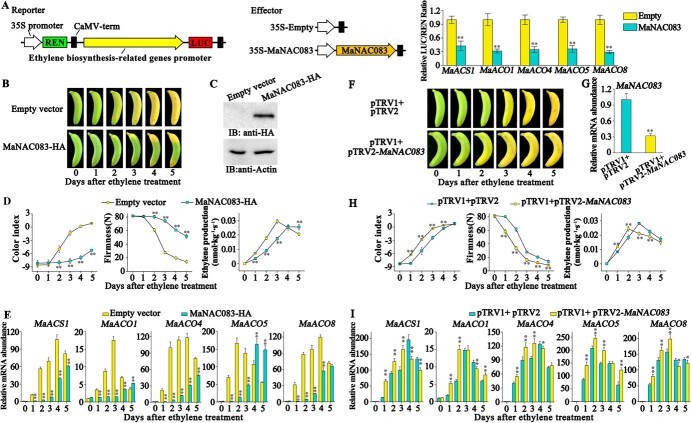
Since MaNAC083 revealed transcriptional repression activity and was directly bound to ethylene biosynthesis gene promoters (Figs 2 and3E–F), a transient DLR assay was conducted to assess if MaNAC083 represses ethylene biosynthesis gene transcription. The MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 promoter activities were inhibited after MaNAC083 co-transfection when compared with co-transfection with an empty vector. A lower LUC/REN ratio was reported, showing that MaNAC083 could repress the ethylene biosynthesis gene promoter (Fig. 4A).
Figure 4.
MaNAC083 inhibits ethylene biosynthesis gene expression and ethylene production. (A) MaNAC083 represses the activity of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 promoters in a DLR assay in tobacco leaves. Schematic representation of reporter and effector constructs is illustrated in the left panel. LUC/REN ratio of an empty vector with ethylene biosynthetic gene promoters was utilized as a calibrator (set to 1). (B–E) Transient overexpression of MaNAC083 in banana fruit. (B) During ripening, MaNAC083 and empty vector were transiently overexpressed in banana fruit. (C) Western blot showing MaNAC083 protein level transiently MaNAC083-overexpressing and empty vector in bananas. Protein detection employed fruit pulp tissues from the injection region . Actin was detected as the loading control. (D) CI, firmness, and ethylene production of MaNAC083-overexpressing and control banana fruit throughout the ripening process. (E) Relative expression of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 in MaNAC083-overexpressing and control banana fruit. (F–I) Transient silencing of MaNAC083 in banana fruit. (F) Appearance of banana fruit infiltrated with empty vector (control) and pTRV2-MaNAC083. (G) RT–qPCR showing MaNAC083 mRNA level in infiltrated banana fruit. (H) CI changes, firmness, and ethylene production in banana fruit as shown in (F). (I) Relative expression of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 in banana fruit as shown in (F). Error bars in (A, D, H), and (E, G, I) denote standard error from six replicates and triplets, respectively. Student's t-test, **P < .01.
To verify the MaNAC083-mediated transcriptional regulation of ethylene biosynthesis genes in bananas, we performed transient overexpression and silencing of MaNAC083 in banana fruit. Banana fruit with transiently overexpressed MaNAC083 showed slowed fruit ripening, and MaNAC083-overexpressing fruit revealed yellowing reduction compared with controls (Fig. 4B and C). Also, MaNAC083-overexpressing fruit exhibited reduced CI and a higher firmness than control fruit (Fig. 4D). Furthermore, MaNAC083 overexpression significantly inhibited ethylene generation compared with the control (Fig. 4D). In parallel, the expressions of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 were downregulated in the overexpressing fruits during ripening (Fig. 4E). In contrast, suppression of endogenous MaNAC083 expression through virus-induced gene silencing (VIGS) hastened fruit ripening and resulted in a faster yellowing phenotype (Figs. 4F–G). Concomitantly, higher CI and lower firmness of the fruit were found in MaNAC083-silenced fruit (Fig. 4H). More importantly, suppression of MaNAC083 expression significantly accelerated the rise in ethylene production relative to control fruit (Fig. 4H). In parallel, MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 in MaNAC083-silenced fruits were significantly upregulated compared with the control fruit during the ripening progress (Fig. 4I).
These results demonstrated that MaNAC083 inhibits ethylene biosynthesis in banana ripening by blocking MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 gene activities.
MaMADS1 directly binds to the MaNAC083 promoter
To assess MaNAC083 upstream regulators, a Y1H screen was done utilizing the MaNAC083 promoter as bait. Therefore, a cDNA fragment encoding the MADS transcription factor MaMADS1 peptide was obtained [28]. Utilizing full-length MaMADS1 as prey in one-to-one Y1H assays, the relationship between the MaMADS1 and MaNAC083 promoters was further verified. Yeast cells developed well on AbA-containing media when co-expressing MaMADS1, which promoted the AbA resistance gene expression regulated by the MaNAC083 promoter (Fig. 5A).
Figure 5.
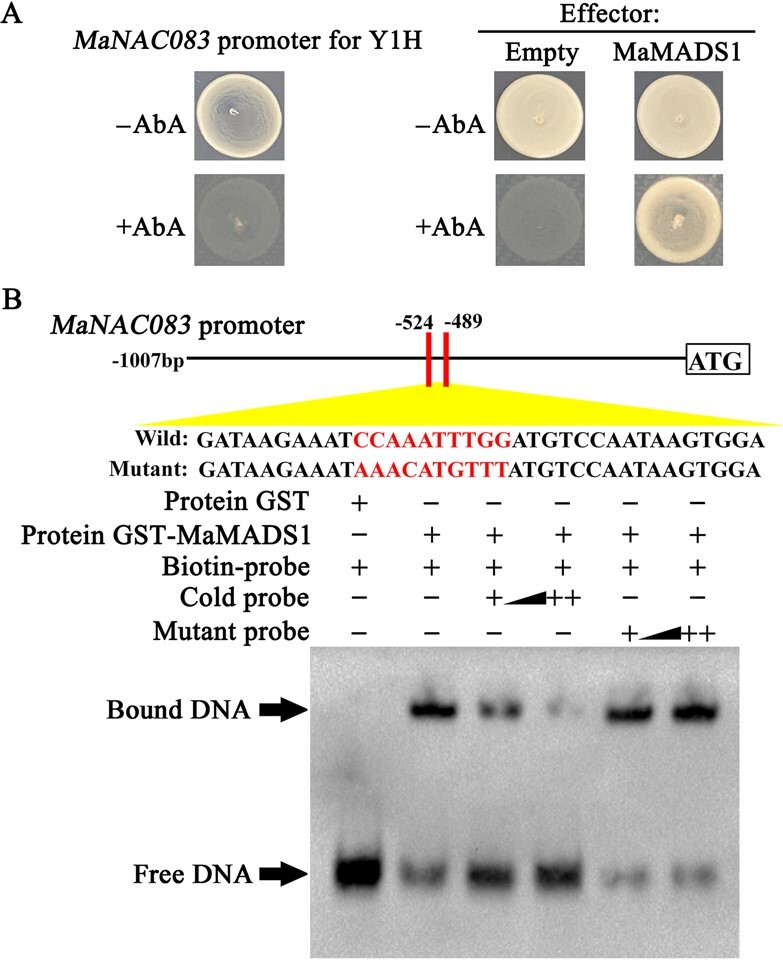
MaMADS1 directly binds to the MaNAC083 promoter. (A) Y1H study of MaMADS1's interaction with the MaNAC083 promoter. (Left) The MaNAC083 promoter showed no basal expression in yeast grown on Leu-deficient SD media supplemented with 500 ng mL−1 AbA. (Right) After transforming plasmids containing cassettes that constitutively express the MaMADS1 effector or negative control (pGADT7), a yeast growth assay was performed on Y1H reporter strains. The interaction was evaluated by yeast growth after transformation on SD media without Leu in AbA's presence. (B) MaMADS1 binding to MaNAC083 promoter in an EMSA assay. The probe sequences for ethylene biosynthetic gene promoters are displayed at the top. DNA–protein complexes from probes exposed to pure GST (negative control) or recombinant GST-MaMADS1 protein were resolved on native polyacrylamide gels. Triangles show competing quantities of unlabeled wild-type or mutated probes.
It is generally accepted that MADS TFs tend to bind to their target promoters' CArG boxes [29]. Sequence analyses have considered the CArG box in the MaNAC083 promoter (Supplementary Data Fig. S4). Subsequently, we performed an EMSA, which showed that the purified MaMADS1 (Supplementary Data Fig. S5) directly bound to the CArG box with a fragment derived from the MaNAC083 promoter and resulted in obvious mobility shifts (Fig. 5B). Furthermore, the introduction of unlabeled probes possessing identical sequences resulted in the disappearance of displaced bands. In contrast, mutated competitors had no similar effect.
Together, these studies demonstrate that MaMADS1 specifically targets the MaNAC083 promoter.
MaMADS1 promotes ethylene biosynthesis through transcriptional repression of MaNAC083
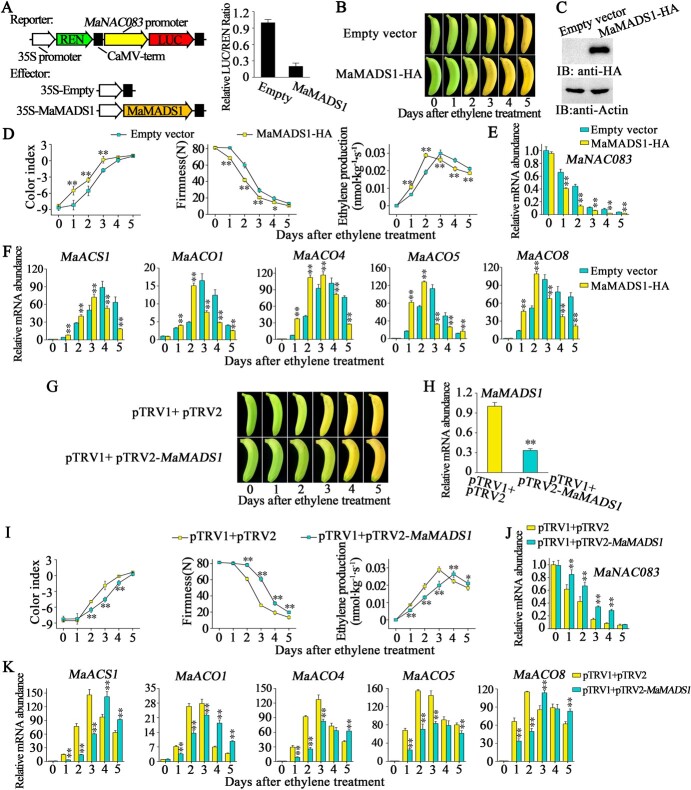
The expression of the MaMADS1 gene was ripening-induced, and its protein possessed transcriptional repression activity (Supplementary Data Fig. S6), so we postulated that MaMADS1 could transcriptionally repress the expression of MaNAC083. To verify this speculation, a transient DLR-based trans-activation was conducted. The promoter activity of MaNAC083 was greatly decreased in the presence of MaMADS1, with a considerably lower LUC/REN ratio than the control (Fig. 6A).
Figure 6.
MaMADS1 inhibits MaNAC083 expression and promotes ethylene biosynthesis. (A) MaMADS1 represses MaNAC083 promoter activity in a DLR assay in tobacco leaves. The left panel shows reporter and effector constructs schematically. For the empty vector with the ethylene biosynthesis gene promoter reporter, the LUC/REN ratio was set as 1. (B–F) Transient overexpression of MaMADS1 in banana fruit. (B) Banana fruit ripening transiently overexpresses MaMADS1 and empty vector. (C) Western blot showing MaMADS1 protein level in bananas transiently overexpressing empty vector and MaMADS1. Protein recognition used fruit pulp tissues from the injection area, with actin as a loading control. (D) CI, firmness, and ethylene production during the ripening process in MaMADS1-overexpressing and control banana fruit. (E) Relative expression of MaNAC083 in MaMADS1-overexpressing and control banana fruit. (F) MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 gene expression in MaMADS1-overexpressing and control bananas. (G–K) Transient silencing of MaMADS1 in banana fruit. (G) Appearance of banana fruit infiltrated with empty vector (control) and pTRV2-MaMADS1. (H) RT–qPCR showing MaMADS1 mRNA level in infiltrated banana fruit. (I) Changes in CI, firmness, and ethylene production in banana fruit as shown in (G). (J) Relative expression of MaNAC083 in banana fruit as shown in (G). (K) Expression of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 in banana fruit as shown in (G). Error bars in (A, D, G, I) and (E, F, J, K) denote the standard error from six replicates and triplets, respectively. Student's t-test, **P < .01.
To assess the role of MaMADS1 in regulating MaNAC083 and its downstream targets, banana fruit exhibited transient overexpression and silencing. Rapid ripening of banana fruit was induced by transient overexpression of MaMADS1, as MaMADS1-overexpressing fruits showed accelerated yellowing compared with the control fruits (Fig. 6B and C). Furthermore, MaMADS1 overexpression significantly accelerated the increase in CI and ethylene production and the decline in fruit firmness compared with the control (Fig. 6D). Noticeably, the MaNAC083 transcript level was obviously inhibited in MaMADS1-overexpressing fruits compared with the control (Fig. 6E). In contrast, the expression of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 was dramatically upregulated in MaMADS1-overexpressing fruit during ripening (Fig. 6F). However, suppression of MaMADS1 expression delayed fruit ripening, as the MaMADS1-silencing fruit showed a less yellowing peel compared with control fruit (Fig. 6G–H). In addition, silencing of MaMADS1 in banana fruit significantly retarded the rise in CI and ethylene production and the decline in fruit firmness during ripening (Fig. 6I). Furthermore, MaNAC083 was upregulated but MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 were downregulated in MaMADS1-silenced fruit (Fig. 6J–K).
These findings confirmed that MaMADS1 inhibits the expression of MaNAC083, which then attenuates MaNAC083-mediated repression of ethylene biosynthesis genes and thus promotes ethylene biosynthesis in banana fruit.
Discussion
Ethylene is a vital hormone for modulating climacteric fruit ripening, including bananas [25, 30]. On the one hand, ethylene triggers banana ripening to promote fruit quality formation, while on the other hand, it accelerates fruit ripening and senescence to decrease shelf life [31, 32]. Hence, investigation of the regulatory mechanism of ethylene production throughout banana ripening is crucial for fruit quality improvement and shelf life extension. Fruit ripening is a finely controlled process that includes several TFs [33]. Various research has identified the role of multiple TFs in banana ripening-associated transcriptional mechanisms [34, 35], but the upstream transcriptional regulator cascade involved in the ethylene biosynthesis pathway remains unclear. Here we discuss and identify the molecular mechanism for the regulation of ethylene biosynthesis mediated by the MaMADS1–MaNAC083 transcription factor cascade through banana ripening.
The induction of ACS and ACO genes is crucial in ethylene biosynthesis regulation during climacteric fruit ripening [3, 5]. Earlier studies found that the expression of MaACS1 (also MaACS7 [21], Ma04_t35640) and MaACO1 (also MaACO8 [21], Ma07_g19730) increases significantly at banana ripening onset, consistent with ethylene production [22, 23]. Genome-wide transcript profiling further showed that, apart from MaACS1 and MaACO1, other ACO genes, such as MaACO5 (also MaACO2, Ma05_g09360) and MaACO8 (also MaACO11, Ma10_g16100) exhibit high expression levels during postharvest banana ripening [21]. Consistently, our present study demonstrated that the expression patterns of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 generally align with ethylene production during banana ripening (Fig. 1). Together, these findings confirm the pattern of ethylene biosynthesis gene expression during banana ripening and serve as a foundation for future investigations into the regulatory network governing banana ripening.
NAC transcription factors, an extensive group of plant-specific transcription factors, serve crucial functions in plant growth, development, and stress responses [36, 37]. The non-ripening tomato mutant nor was identified and NAC TFs' role in fruit ripening was investigated [7, 38]. The nor mutation significantly inhibits ethylene production and fruit quality formation [39, 40]. Additional molecular investigation reveals that NAC-NOR binds directly to the promoter and enhances the expression of the ethylene biosynthesis gene SlACS2 [11]. Furthermore, SlNAC1 and SlNOR-like1 have been found to directly regulate both ACS and ACO gene expressions in tomato ripening [41, 42]. Several additional members of the NAC family in other fruit, such as kiwifruit AdNAC2/3 and banana MaNAC029, have also been implicated in fruit ripening by controlling ethylene production genes [43]. However, these NAC TFs are all positive transcriptional regulators that function upstream of the ethylene biosynthesis pathway. NAC-mediated negative transcriptional regulation of ethylene biosynthesis is unclear. The banana genome encodes 167 NACs [22], among which MaNAC083 is a putative ortholog of Arabidopsis negative regulator of senescence ANAC083 (VNI2) [44]. However, to date no biological function has been identified for MaNAC083. This study found that MaNAC083 is a transcriptional repressor that directly binds to the promoters of the MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 genes and represses their expression, which in turn inhibits ethylene biosynthesis and banana ripening (Figs 2–4). These findings illustrate the mechanism of the negative regulatory function of MaNAC083 in ethylene biosynthesis, enhancing our information on how NAC TFs regulate fruit ripening.
Fruit ripening is a coordinated transcriptional regulatory process that is mediated by a large count of transcription factors [33]. Until now, several TFs belonging to different families have been implicated in banana ripening through direct regulation of ripening-related structural genes. For example, MaERF9, MaMYB4, and MabZIP21 have been shown to implicate fruit softening and ethylene synthesis by directly controlling the transcription of ethylene biosynthesis and cell wall modifying genes [13, 32, 45]. MabHLH6 and MaMYB3 directly target starch degradation-related genes to positively and inversely modulate starch degradation throughout the ripening process, respectively [18, 34]. Furthermore, MaMYB60 and MaEIL9 function as upstream regulators of carotenoid biosynthesis and the chlorophyll degradation pathway in banana ripening, respectively [19, 20]. However, the upstream transcriptional regulators of these TFs, and the connections among them, remain unclear. MADS and NAC are well recognized as two important classes of TFs contributing to fruit ripening [46, 47]. Genome ENCODE analyses reveal that NACs and MADSs form the dual-loop molecular circuit controlling banana ripening [48], but direct evidence of their interconnections is still lacking. Previous studies showed that MaMADS1, which is homologous to the tomato ripening regulator RIN, is necessary for banana fruit ripening [28], but its precise regulatory mechanisms, especially its target genes, remain elusive. The present study showed that MaMADS1 directly targeted the promoter of MaNAC083 to inhibit its expression, which in turn attenuated MaNAC083's repression of the MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 genes, and eventually promoted ethylene biosynthesis and fruit ripening (Figs 5 and6). These findings indicate that MaMADS1 and MaNAC083 constitute a transcriptional cascade to regulate ethylene biosynthesis genes. Moreover, ethylene induced the expression and promoter activity of MaMADS1 (Supplementary Data Fig. S6A and B). These data also establish that ethylene inhibits MaNAC083-repressed ethylene biosynthesis by activating MaMADS1-mediated transcriptional repression of MaNAC083, which is a feedback regulation.
Based on the present findings, a working model is proposed to reveal the MaMADS1–MaNAC083 module that regulates ethylene biosynthesis during banana fruit ripening (Fig. 7). During postharvest ripening, MaMADS1 gene expression is activated. MaMADS1 binds directly to the MaNAC083 promoter and inhibits its expression. This negative regulation antagonizes MaNAC083's transcriptional repression of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8, and after this it induces ethylene biosynthesis. Our findings represent a transcriptional regulatory cascade, MaMADS1–MaNAC083–MaACS1/MaACOs, regulating ethylene biosynthesis during ripening, offering novel transcriptional insights into fruit ripening development. These findings may also contribute to different molecular approaches to improve the storability of fleshy fruits.
Figure 7.
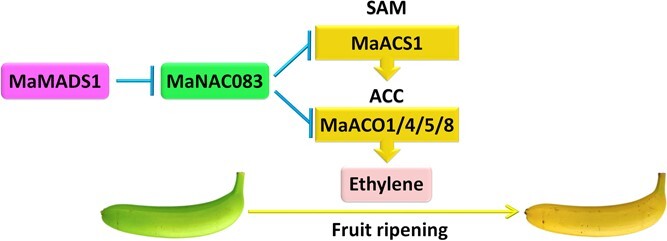
A proposed model for the transcriptional cascade function of MaMADS1–MaNAC083 in ethylene biosynthesis throughout banana ripening. MaMADS1 binds the MaNAC083 promoter and represses its expression, which attenuates the MaNAC083-mediated repression of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8, and eventually promotes ethylene biosynthesis.
Materials and methods
Plant materials and samples
Pre-climacteric bananas (M. acuminata, AAA group, cv. ‘Cavendish’) at 75–80% maturity were gathered from a plantation near Guangzhou, China. The fruits were then subjected to ripening by incubation with 100 μL L−1 ethylene for 18 h. As Zhu et al. [25] described, the fruits were ripened at 20°C for 7 days in closed polyethylene bags (0.01 mm thickness). CI, firmness and ethylene generation were monitored at all sampling points, adopting a previously published method [49]. The samples were snap-frozen in liquid nitrogen and placed in cold storage at −80°C until further use.
Gene expression analysis
Total banana fruit RNA was isolated utilizing the hot borate technique [44]. Using the Hieff® qPCR SYBR Green Master Mix Kit (Yeasen), qRT–PCR reactions were carried out via CFX96 real-time PCR (Bio-Rad, USA). As Chen et al. [50] indicated, primer premier (version 5.0) was used to build primer sequences, and MaRPS4 was employed as an internal reference gene.
Yeast one-hybrid assay
Y1H screening was done utilizing the Matchmaker™ Gold Yeast One-Hybrid System (Clontech, Takara, Japan). The short fragment of the MaACS1 promoter (−499 bp upstream of ATG) or MaNAC083 promoter (−676 bp upstream of ATG) was cloned into the pAbAi vector to generate the bait plasmid, followed by linearization and transformation into the Y1H Gold strain to produce the bait-specific reporter strain, which was preceded by screening a cDNA library of banana fruits. MaNAC083 or MaMADS1 coding sequences were cloned into the pGADT7 prey vector and introduced into bait-reporter yeast strains for re-transformation. According to the manufacturer's guidelines, DNA–protein interactions were assessed based on the co-transformants' ability to grow on SD/−Leu media with AbA.
Electrophoretic mobility shift assay
The MaNAC083 coding sequence was inserted in-frame into pGEX-4 T-1 vector with a GST tag. Using glutathione–Sepharose 4B beads, GST-MaNAC083 fusion protein in Escherichia coli BM Rosetta (DE3) was purified. Fragments containing NACRS in the promoter of MaACS1, MaACO1, MaACO4, MaACO5, and MaACO8 were manufactured (Sangon Biotech, Shanghai, China), and labeled with biotin at the 5′ end. As stated in our previously published work [30], EMSA was performed using a LightShift Chemiluminescent EMSA kit (Thermo Scientific). After incubating biotin-labeled probes with GST-MaNAC083 recombinant protein, the bound and free probes were determined on a native acrylamide gel. Unlabeled probes were used as competitors, whereas GST protein acted as a negative regulator.
Promoter activity and subcellular localization assay
Using a previously described technique [30], promoter activity and subcellular localization assays were performed on tobacco BY-2 suspension culture cell protoplasts. Briefly, MaNAC083 or MaMADS1 promoter was extracted and placed in the pGreenII 0800-LUC reporter vector to induce the firefly luciferase reporter (LUC) gene. CaMV35S-REN/MaNAC083pro-LUC or CaMV35S-REN/MaMADS1pro-LUC constructs were transferred into tobacco protoplasts using a PEG-mediated method. Using a dual LUC assay kit (Promega), LUC and Renilla luciferase (REN) activities of transformed protoplasts supplemented with 0 (–ETH) or 10 μL L−1 ethylene (+ETH) were measured.
MaNAC083 and MaMADS1 coding sections were transferred into the pBI221-GFP vector to bind with the GFP reporter gene utilizing NLS-mCherry [30] as a nuclear marker for subcellular localization. Co-transfection of MaNAC083-GFP or MaMADS1-GFP and NLS-mCherry into BY-2 protoplasts was performed as previously described. A Zeiss Axioskop 2 Plus fluorescence microscope was utilized to record GFP and mCherry fluorescence signals.
Dual-luciferase transient expression assay
Transcriptional capability was accessed by inserting full-length MaNAC083 or MaMADS1 coding sequence into the pGreenII 62SK-BD vector as an effector. The double-reporter vector featured an LUC gene controlled by five GAL4-binding element copies. The effector and recombinant reporter constructs were co-transfected into tobacco leaves, as in our prior research [34]. As reported in the promoter activity experiment, LUC and REN activities were assessed 60 h after injection.
To evaluate MaNAC083 or MaMADS1's influence on promoters of target genes, cloning of MaNAC083 or MaMADS1 was performed into pGreenII 62-SK vector effector, and cloning of target gene promoters was performed into pGreenII 0800-LUC reporter vector [51]. Tobacco was co-transformed with an effector and a reporter plasmid utilizing Agrobacterium tumefaciens strain EHA105 and pSoup vector. The LUC/REN ratio reflected target promoter transcriptional repression. Each pair had six transient assay replicates.
Transient overexpression and silencing analysis in banana fruit
As previously mentioned, transient overexpression investigation and silencing were done in banana fruit [26, 30]. The open reading frames of MaNAC083 or MaMADS1 were cloned into the pCXUN-HA vector, respectively. For vector construction in VIGS, MaNAC083 or MaMADS1 was cloned into the pTRV2 vector. Cells of the A. tumefaciens strain EHA105 carrying recombinant pTRV2 and pTRV1 were combined at a 1:1 ratio. The pulp was injected with A. tumefaciens harboring the constructed plasmids. On day 1 following inoculation, the transformed fruits were exposed to 100 μL L−1 ethylene and kept at 20°C for 5 days. The samples were collected to evaluate ethylene generation, gene expression, and protein accumulation.
Statistical analysis
SPSS (v.19.0, SPSS Inc.) was used for execute statistical analysis. Data were represented as mean ± standard error of three or six independent biological replicates. Analysis of variance (ANOVA) or Student's t-test was utilized, as appropriate, to identify statistically significant differences between samples.
Primers
Supplementary Data Table S2 lists the primers employed for this study.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (grant no. 2022YFD2100103), the National Natural Science Foundation of China (grant no. 32072279), and the China Agriculture Research System of MOF and MARA (grant no. CARS-31).
Author contributions
W.S. conceived and designed the study, while W.W. performed experiments alongside W.S. Data analysis was carried out by a team consisting of W.W., Y.Y.Y, C.J.W, J.F.K., W.J.L., and W.S. The manuscript writing and revision were done by J.Y.C., W.S., and W.W.; all authors participated in manuscript discussions.
Data availability
All study data are incorporated in the submitted article.
Conflict of interest
None declared.
Supplementary data
Supplementary data is available at Horticulture Research online.
Supplementary Material
Contributor Information
Wei Wei, Guangdong Provincial Key Laboratory of Postharvest Science of Fruits and Vegetables/Engineering Research Center of Southern Horticultural Products Preservation, Ministry of Education, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Ying-ying Yang, Guangdong Provincial Key Laboratory of Postharvest Science of Fruits and Vegetables/Engineering Research Center of Southern Horticultural Products Preservation, Ministry of Education, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Chao-jie Wu, Guangdong Provincial Key Laboratory of Postharvest Science of Fruits and Vegetables/Engineering Research Center of Southern Horticultural Products Preservation, Ministry of Education, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Jian-fei Kuang, Guangdong Provincial Key Laboratory of Postharvest Science of Fruits and Vegetables/Engineering Research Center of Southern Horticultural Products Preservation, Ministry of Education, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Jian-ye Chen, Guangdong Provincial Key Laboratory of Postharvest Science of Fruits and Vegetables/Engineering Research Center of Southern Horticultural Products Preservation, Ministry of Education, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Wang-jin Lu, Guangdong Provincial Key Laboratory of Postharvest Science of Fruits and Vegetables/Engineering Research Center of Southern Horticultural Products Preservation, Ministry of Education, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
Wei Shan, Guangdong Provincial Key Laboratory of Postharvest Science of Fruits and Vegetables/Engineering Research Center of Southern Horticultural Products Preservation, Ministry of Education, College of Horticulture, South China Agricultural University, Guangzhou 510642, China.
References
- 1. Binder BM. Ethylene signaling in plants. J Biol Chem. 2020;295:7710–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kou X, Zhou J, Wu CEet al. The interplay between ABA/ethylene and NAC TFs in tomato fruit ripening: a review. Plant Mol Biol. 2021;106:223–38 [DOI] [PubMed] [Google Scholar]
- 3. Boller T, Herner RC, Kende H. Assay for and enzymatic formation of an ethylene precursor, 1-aminocyclopropane-1-carboxylic acid. Planta. 1979;145:293–303 [DOI] [PubMed] [Google Scholar]
- 4. Adams DO, Yang SF. Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA. 1979;76:170–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pattyn J, Vaughan-Hirsch J, Poel B. The regulation of ethylene biosynthesis: a complex multilevel control circuitry. New Phytol. 2021;229:770–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strader L, Weijers D, Wagner D. Plant transcription factors – being in the right place with the right company. Curr Opin Plant Biol. 2022;65:102136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giovannoni JJ. Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol. 2007;10:283–9 [DOI] [PubMed] [Google Scholar]
- 8. Seymour GB, Granell A. Fruit development and ripening preface. J Exp Bot. 2014;65:4489–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ito Y, Kitagawa M, Ihashi Net al. DNA-binding specificity, transcriptional activation potential, and the rin mutation effect for the tomato fruit-ripening regulator RIN. Plant J. 2008;55:212–23 [DOI] [PubMed] [Google Scholar]
- 10. Li L, Wang X, Zhang Xet al. Unraveling the target genes of RIN transcription factor during tomato fruit ripening and softening. J Sci Food Agric. 2017;97:991–1000 [DOI] [PubMed] [Google Scholar]
- 11. Gao Y, Wei W, Fan ZQet al. Re-evaluation of the nor mutation and the role of the NAC-NOR transcription factor in tomato fruit ripening. J Exp Bot. 2020;71:3560–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang S, Sawaki T, Takahashi Aet al. Melon EIN3-like transcription factors (CmEIL1 and CmEIL2) are positive regulators of an ethylene- and ripening-induced 1-aminocyclopropane-1-carboxylic acid oxidase gene (CM-ACO1). Plant Sci. 2010;178:251–7 [Google Scholar]
- 13. Han YC, Kuang JF, Chen JYet al. Banana transcription factor MaERF11 recruits histone deacetylase MaHDA1 and represses the expression of MaACO1 and expansins during fruit ripening. Plant Physiol. 2016;171:1070–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Espley RV, Leif D, Plunkett Bet al. Red to brown: an elevated anthocyanic response in apple drives ethylene to advance maturity and fruit flesh browning. Front. Plant Sci. 2019;10:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadka A, Qin Q, Feng Jet al. Ethylene response of plum ACC synthase 1 (ACS1) promoter is mediated through the binding site of abscisic acid insensitive 5 (ABI5). Plants (Basel). 2019;8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aurore G, Parfait B, Fahrasmane L. Bananas, raw materials for making processed food products. Trends Food Sci Technol. 2009;20:78–91 [Google Scholar]
- 17. Lescot T. Banana genetic diversity: estimated world production by type of banana. FruiTrop. 2020;269:98–102 [Google Scholar]
- 18. Xiao YY, Kuang JF, Qi XNet al. A comprehensive investigation of starch degradation process and identification of a transcriptional activator MabHLH6 during banana fruit ripening. Plant Biotechnol J. 2018;16:151–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inaba A, Liu X, Yokotani Net al. Differential feedback regulation of ethylene biosynthesis in pulp and peel tissues of banana fruit. J Exp Bot. 2007;58:1047–57 [DOI] [PubMed] [Google Scholar]
- 20. Xiao YY, Chen JY, Kuang JFet al. Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J Exp Bot. 2013;64:2499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Z, Miao HX, Liu JHet al. Musa balbisiana genome reveals subgenome evolution and functional divergence. Nat Plants. 2019;5:810–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X, Shiomi S, Nakatsuka Aet al. Characterization of ethylene biosynthesis associated with ripening in banana fruit. Plant Physiol. 1999;121:1257–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jourda C, Cardi C, Mbéguié-A-Mbéguié Det al. Expansion of banana (Musa acuminata) gene families involved in ethylene biosynthesis and signalling after lineage-specific whole-genome duplications. New Phytol. 2014;202:986–1000 [DOI] [PubMed] [Google Scholar]
- 24. Wei W, Yang YY, Chen JYet al. MaNAC029 modulates ethylene biosynthesis and fruit quality and undergoes MaXB3-mediated proteasomal degradation during banana ripening. J Adv Res. 2022 [DOI] [PubMed] [Google Scholar]
- 25. Zhu LS, Chen L, Wu CJet al. Methionine oxidation and reduction of the ethylene signaling component MaEIL9 are involved in banana fruit ripening. J Integr Plant Biol. 2023;65:150–66 [DOI] [PubMed] [Google Scholar]
- 26. Wei W, Yang YY, Lakshmanan Pet al. Proteasomal degradation of MaMYB60 mediated by the E3 ligase MaBAH1 causes high temperature-induced repression of chlorophyll catabolism and green ripening in banana. Plant Cell. 2023;35:1408–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cenci A, Guignon V, Roux Net al. Genomic analysis of NAC transcription factors in banana (Musa acuminata) and definition of NAC orthologous groups for monocots and dicots. Plant Mol Biol. 2014;85:63–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elitzur T, Yakir E, Quansah Let al. Banana MaMADS transcription factors are necessary for fruit ripening and molecular tools to promote shelf-life and food security. Plant Physiol. 2016;171:380–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tilly JJ, Allen DW, Jack T. The CArG boxes in the promoter of the Arabidopsis floral organ identity gene APETALA3 mediate diverse regulatory effects. Development. 1998;125:1647–57 [DOI] [PubMed] [Google Scholar]
- 30. Shan W, Kuang JF, Wei Wet al. MaXB3 modulates MaNAC2, MaACS1, and MaACO1 stability to repress ethylene biosynthesis during banana fruit ripening. Plant Physiol. 2020;184:1153–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ebrahimi A, Zabihzadeh Khajavi M, Ahmadi Set al. Novel strategies to control ethylene in fruit and vegetables for extending their shelf life: a review. Int J Environ Sci Technol. 2022;19:4599–610 [Google Scholar]
- 32. Yang YY, Shan W, Yang TWet al. MaMYB4 is a negative regulator and a substrate of RING-type E3 ligases MaBRG2/3 in controlling banana fruit ripening. Plant J. 2022;110:1651–69 [DOI] [PubMed] [Google Scholar]
- 33. Li XM, Wang XM, Zhang Yet al. Regulation of fleshy fruit ripening: from transcription factors to epigenetic modifications. Hortic Res. 2022;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fan ZQ, Ba LJ, Shan Wet al. A banana R2R3-MYB transcription factor MaMYB3 is involved in fruit ripening through modulation of starch degradation by repressing starch degradation-related genes and MabHLH6. Plant J. 2018;96:1191–205 [DOI] [PubMed] [Google Scholar]
- 35. Kuang JF, Wu CJ, Guo YFet al. Deciphering transcriptional regulators of banana fruit ripening by regulatory network analysis. Plant Biotechnol J. 2021;19:477–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim HJ, Nam HG, Lim PO. Regulatory network of NAC transcription factors in leaf senescence. Curr Opin Plant Biol. 2016;33:48–56 [DOI] [PubMed] [Google Scholar]
- 37. Song LL, Wang JP, Yu YTet al. 1-Methylcyclopropene retards pak choi (Brassica rapa subsp. chinensis) yellowing via BcNAC055-, BcMYB44-, and BcOBF1-mediated regulation of the key chlorophyll degrading gene BcNYC1 during storage at 20 °C. Food Qual Saf. 2022;7:fyac075 [Google Scholar]
- 38. Jiang GX, Zeng J, Li ZWet al. Redox regulation of the NOR transcription factor is involved in the regulation of fruit ripening in tomato. Plant Physiol. 2020;183:671–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gao Y, Zhu N, Zhu XFet al. Diversity and redundancy of the ripening regulatory networks revealed by the fruit ENCODE and the new CRISPR/Cas9 CNR and NOR mutants. Hortic Res. 2019;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kumar R, Tamboli V, Sharma Ret al. NAC-NOR mutations in tomato Penjar accessions attenuate multiple metabolic processes and prolong the fruit shelf life. Food Chem. 2018;259:234–44 [DOI] [PubMed] [Google Scholar]
- 41. Ma NN, Feng HL, Meng Xet al. Overexpression of tomato SlNAC1 transcription factor alters fruit pigmentation and softening. BMC Plant Biol. 2014;14:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gao Y, Wei W, Zhao Xet al. A NAC transcription factor, NOR-like1, is a new positive regulator of tomato fruit ripening. Hortic Res. 2018;5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu YY, Liu XF, Fu BLet al. Methyl jasmonate enhances ethylene synthesis in kiwifruit by inducing NAC genes that activate ACS1. J Agric Food Chem. 2020;68:3267–76 [DOI] [PubMed] [Google Scholar]
- 44. Yang SD, Seo PJ, Yoon HKet al. The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell. 2011;23:2155–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu CJ, Shan W, Liu XCet al. Phosphorylation of transcription factor bZIP21 by MAP kinase MPK6-3 enhances banana fruit ripening. Plant Physiol. 2022;188:1665–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li S, Chen K, Grierson D. A critical evaluation of the role of ethylene and MADS transcription factors in the network controlling fleshy fruit ripening. New Phytol. 2019;221:1724–41 [DOI] [PubMed] [Google Scholar]
- 47. Liu GS, Li HL, Grierson Det al. NAC transcription factor family regulation of fruit ripening and quality: a review. Cell. 2022;11:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lv P, Yu S, Zhu Net al. Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat Plants. 2018;4:784–91 [DOI] [PubMed] [Google Scholar]
- 49. Wei W, Chen JY, Zeng ZXet al. The ubiquitin e3 ligase MaLUL2 is involved in high temperature-induced green ripening in banana fruit. Int J Mol Sci. 2020;21:9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen L, Zhong XY, Kuang JFet al. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta. 2011;234:377–90 [DOI] [PubMed] [Google Scholar]
- 51. Hellens RP, Allan AC, Friel ENet al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods. 2005;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are incorporated in the submitted article.