Abstract
Background
Primary intracerebral hemorrhage (ICH) accounts for 85% of ICH cases and is associated with high morbidity and mortality rates. Fever can cause secondary injury after ICH; however, relevant studies have reported inconsistent results regarding the effects of fever on functional outcomes after ICH. This study examined the effects of early fever on the prognosis of ICH, particularly on long-term functional outcomes.
Methods
This prospective study recruited patients with primary ICH at a tertiary medical center between 2019 and 2021. Early fever was defined as a tympanic body temperature of ≥ 38 °C upon admission. Barthel Index (BI) and modified Rankin scale (mRS) were examined at 1 year after ICH. A BI of ≤ 60 or mRS of ≥ 4 was considered as indicating severe disability.
Results
We included 100 patients, and early fever was significantly associated with less functional independence at 1 year post-ICH, as determined using the mRS (p = 0.048; odds ratio [OR] = 0.23), and with severe functional dependency at 1 year post-ICH, as determined using the BI (p = 0.043; OR = 3) and mRS (p = 0.045; OR = 3). In addition, patients with early fever had a longer length of hospital stay (p = 0.002; 95% confidence interval = 21.80–95.91).
Conclusions
Fever is common among patients with primary ICH. Our data indicate a significant association between early fever and worse functional outcomes in ICH survivors at 1 year after ICH. Additionally, patients with early fever had a significantly longer length of hospital stay after ICH.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-023-03426-w.
Keywords: Intracerebral hemorrhage˙functional outcome˙prognosis˙survivors ˙ fever
Background
Nontraumatic intracerebral hemorrhage (ICH) constitutes10–15% of all stroke cases and is associated with high morbidity and mortality. Primary ICH accounts for 85% of all ICH cases and is associated with high morbidity and mortality rates [1]. ICH can lead to primary or secondary brain injury. Primary brain injury involves initial damage to the parenchyma induced by the blood clot, and secondary brain injury involves damage to blood vessels caused by various mechanisms, including oxidative stress, excitotoxicity, inflammatory response, mitochondrial dysfunction, and cell death [1, 2]. Excessive production of reactive oxygen species (ROS) leads to excessive oxidative stress, which aggravates secondary brain injury [2]. The aforementioned processes may be accelerated by fever. Therefore, fever is considered a potential contributor to secondary brain injury after ICH [3]. Previous study showed that hyperthermia was associated with poor outcomes at 3 months in both ischemic and hemorrhagic stroke patients, though the underlying mechanism may be different [4]. Fever is common in patients with ICH and is not usually associated with a clear infectious etiology. A study showed around 40% spontaneous ICH patients developed fever, but only 9% had an infectious etiology [5]. Studies have reported that patients with ICH with early fever had higher short-term mortality rates than did other patients; nevertheless, such studies have reported conflicting results regarding the effects of fever on functional outcomes after ICH [3, 6, 7]. In addition, most studies have applied a retrospective design and have used only the modified Rankin scale (mRS) score as their outcome measure. Extant studies have also used short follow-up periods; consequently, the long-term effects of fever in ICH survivors remain unclear [3]. To address these limitations, we conducted this prospective study to examine the effects of early fever on the prognosis of primary ICH; in particular, we examined the effects of fever on long-term functional outcomes in patients with primary ICH.
Methods
Study population
This prospective observational study examined 1-year functional outcomes in patients with primary ICH undergoing poststroke rehabilitation between 2019 and 2021 at a tertiary medical center. Patients were included in this study if they [1] were aged ≥ 20 years; [2] were admitted to a neurology, neurosurgery, or rehabilitation ward with a primary diagnosis of primary ICH, which was confirmed using a brain computed tomography or magnetic resonance imaging (MRI) scan; and [3] had provided written informed consent themselves or through their legal representatives. Patients were excluded if they died before discharge or transferred from other hospital for rehabilitation (i.e. not in early stage of ICH). Follow-up assessments were performed at 3, 6, and 12 months after the onset of ICH; the assessments involved phone interviews conducted by a physician.
Outcome measures
Early fever was defined as a tympanic body temperature of ≥ 38 °C upon admission. The duration from the symptom onset to admission was recorded. All patients with fever upon admission were treated with acetaminophen as anti-pyretic treatment. mRS scores and Barthel Index (BI) values at 3, 6, and 12 months were the primary outcome measures (Appendix A, B). An mRS score of ≥ 4 was considered as indicating severe disability, and a BI value of ≤ 60 was considered as indicating severe dependency in activities of daily living (ADLs). Moreover, an mRS score of ≤ 2 or a BI value of > 90 was considered as indicating functional independence. The length of hospital stay was recorded by physicians through chart reviews and phone interviews (Appendix C).
Statistical analysis
All statistical analyses were conducted using Stata software (version 14.0; StataCorp LLC, USA). Patient demographics and baseline characteristics were analyzed using descriptive statistics and are presented herein as mean ± standard deviation or percentage. Pearson’s chi-square test was used to examine the association between categorical variables, and a t test was used to examine the association between numerical variables and early fever. Multiple linear and logistic regression analyses were conducted to assess the association between early fever and functional outcomes. Area under the receiver operating characteristic curve (AUC) curve was also analyzed. P < 0.05 was considered statistically significant.
Results
Baseline characteristics
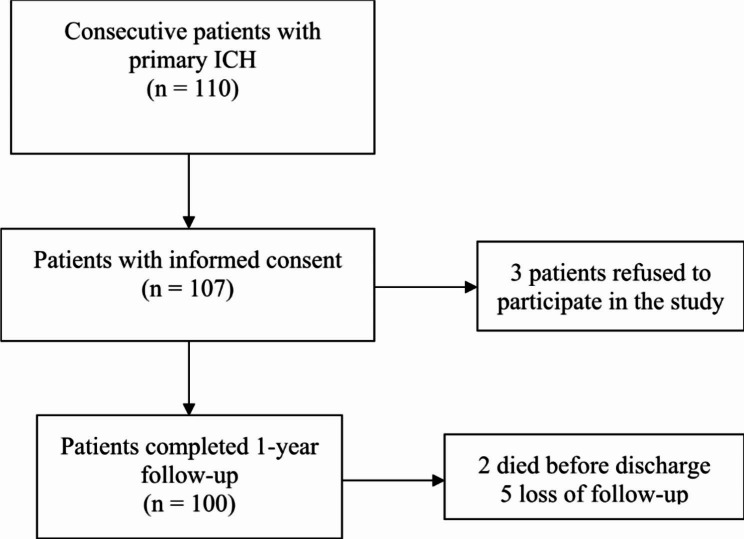
We collected the data of 100 consecutive patients (mean age: 60.69 years) who completed 1-year follow-up (Fig. 1). Of the included patients, 60% were men. The mean ICH volume was 20.34 mL. The mean initial National Institute of Health Stroke Scale (NIHSS) score was 14.16 (median: 14). The mean BI at admission was 15.24, and 16% of the patients had early fever upon admission. 97% patients had admission within 24 h from symptom onset, and the median time from onset to admission was 1 h. Among the patients with early fever, 4 had infectious fever and 12 had non-infectious fever upon admission. 3 patients were diagnosed as having pneumonia, and 1 patient had urinary tract infection. All the 4 patients with infectious fever were treated with antibiotics (3 with Ampicillin-Sulbactam and 1 with Ceftriaxone) (Table 1).
Fig. 1.
The study population
Table 1.
Baseline characteristics of the population (n = 100)
| Variables | N (% or SD) |
|---|---|
| Age (years) | 60.69 (13.11) |
| Gender | |
| Female | 40 (40) |
| Male | 60 (60) |
| ICH volume (mL) | 20.34 (21.83) |
| Hypertension | 85 (85) |
| Diabetes mellitus | 24 (24) |
| Dyslipidemia | 30 (30) |
| Initial NIHSS | 14.16 (7.29) |
| ICH score | |
| 0 | 40 (43.01) |
| 1 | 26 (27.96) |
| 2 | 19 (20.43) |
| 3 | 6 (6.45) |
| 4 | 2 (2.15) |
| Infratentorial ICH origin | 12 (12) |
| Ventriculostomy | 7 (7) |
| Surgical hematoma evacuation | 22 (22) |
| Presence of fever at admission | 16 (16) |
| Infectious fever | 4 (4) |
| Non-infectious fever | 12 (12) |
| History of smoking | 20 (20) |
| Mean BI at admission | 15.24 (18.85) |
N, number; SD, standard deviation; ICH, intracerebral hemorrhage; NIHSS, National Institute of Health Stroke Scale; BI, Barthel Index
Functional outcomes at 1 year
The mean BI improved from 15.24 at admission to 72.22 at 1 year. Furthermore, at the 1-year follow-up, 29 patients (29%) exhibited severe dependency in ADLs. By contrast, at the 1-year follow-up, 41 (41%) patients achieved functional independence, as determined on the basis of a BI value of > 90; additionally, 34 (34%) patients achieved functional independence, as determined on the basis of an mRS score of
Table 2.
Functional outcomes at one year (n = 100)
| Variables | N (% or SD) |
|---|---|
| mRS | |
| 1 | 13 (13) |
| 2 | 21 (21) |
| 3 | 26 (26) |
| 4 | 23 (23) |
| 5 | 9 (9) |
| 6 | 2 (2) |
| Mean BI | 72.22 (29.08) |
| Severe dependency | |
| BI |
29/100 (29) |
| mRS |
40/100 (40) |
| Functional independence | |
| BI > 90 | 41/100 (41) |
| mRS |
34/100 (34) |
N, number; SD, standard deviation; BI, Barthel Index; mRS, modified Rankin Scale
Early fever and functional outcomes
Our results revealed no significant difference in age, sex, initial Glasgow Coma Scale (GCS) score, or initial BI between patients with or without early fever (Table 3). At the 1-year follow-up, early fever was significantly associated with both lower chance of achieving functional independence defined as mRS score
Table 3.
Comparison between ICH survivors with or without early fever (n = 100)
| Variables | Early fever (n = 16) N (% or SD) |
Without early fever (n = 84) N (% or SD) |
P-value |
Odds Ratio
(OR) |
|---|---|---|---|---|
| Age (years) | 56.63.26 (11.67) | 61.46 (13.30) | 0.15 | |
| Gender (female) | 7/16 (43.75) | 33/84 (39.29) | 0.74 | |
| Initial GCS | 12.69 (3.73) | 12.58 (3.21) | 0.91 | |
| Initial BI | 13.75 (27.5) | 15.31 (18.26) | 0.87 | |
| Functional independence at 1-year | ||||
| BI > 90 | 4/16 (25) | 37/84 (44.05) | 0.16 | 0.32† |
| mRS |
2/16 (12.5) | 32/84 (38.1) | 0.048* | 0.23† |
|
Severe dependency at 1-year |
||||
| BI |
8/16 (50) | 21/84 (25) | 0.043* | 3‡ |
| mRS |
10/16 (62.5) | 30/84 (35.72) | 0.045* | 3‡ |
| Surgery | 7/16 (43.75) | 15/84 (17.86) | 0.02* | 3.58 |
| Ventriculostomy | 2/16 (12.5) | 5/84 (5.96) | 0.35 | 1.98 |
| Length of hospital stay (days) | 123.62 (100.48) | 66.24 (52.29) | 0.002* |
*p < 0.05
† Odds ratio for better outcome (BI > 90 or mRS score
‡ Odds ratio for worse outcome (BI ≤ 60 or mRS score ≥ 4)
N, number; SD, standard deviation; GCS, Glasgow coma scale; BI, Barthel Index; mRS, modified Rankin Scale
After adjusted for age and sex, early fever was not significantly associated with functional independence (defined as mRS score
Table 4.
Early fever and functional outcomes at one year after ICH (n = 100)
| Model | p-value | 95% CI | AUC |
|---|---|---|---|
| Functional independence | |||
| BI > 90 † | 0.085 | (-2.37, 0.15) | 0.6714 |
| mRS |
0.031* | (-3.39, -0.17) | 0.7143 |
| Functional dependence | |||
| BI |
0.003* | (0.34, 1.73) | 0.7088 |
| mRS |
0.025* | (0.17, 2.52) | 0.6848 |
| Hospital Stay ‡ | 0.002* | (21.80, 95.91) | NA |
BI, Barthel Index; mRS, modified Rankin Scale; ICH, intracerebral hemorrhage; CI, confidence interval; AUC, area under the receiver operating characteristic curve; NA, not applicable
*p < 0.05
† Logistic regression, adjusted for age and sex
‡ Multiple linear regression, adjusted for age and sex
In addition, early fever was associated with a longer length of hospital stay after ICH (p = 0.002; 95% CI = 21.80–95.91 days) and was associated with potential surgery during the treatment course (p = 0.02; OR = 3.58; Table 3).
Different fever definitions and functional outcomes
The authors performed further analysis examining functional outcomes at 1 year by different definitions of fever. If fever was defined as body temperature
If fever was defined as body temperature
Discussion
Early fever and functional outcomes after ICH
The present study is the first to examine the association between early fever and long-term functional outcomes in ICH survivors. Our findings reveal that early fever at admission was associated with worse functional outcomes at 1 year after ICH in patients undergoing rehabilitation and was associated with a longer length of hospital stay after ICH. By contrast, a 2022 review of 19 clinical studies concluded that fever was associated with an increased risk of short-term mortality but was not associated with poor outcomes among ICH survivors [3]. This discrepancy may stem from differences in sample characteristics, fever definitions, and temperature measurements. In addition, most studies were retrospective, and some included patients with a diagnosis other than primary ICH. The largest study involved post hoc analyses of the INTERACT2 trial, which randomly assigned 2839 patients to intensive (< 140 mmHg) or guideline-recommended (< 180 mmHg) blood pressure (BP) management after acute cerebral hemorrhage [8]. The post hoc analyses showed that early pyrexia (≥ 37.5 °C) upon admission is associated with a larger perihematomal edema volume in 24 h and a higher mortality risk but not with major disability (mRS ≥ 3) at 3 months [9]. However, the mentioned study included only patients with mild to moderate ICH with elevated BP (> 150 mmHg), and the follow-up duration was relatively short. Another prospective study with a longer follow-up duration demonstrated that fever is associated with worse health-related quality of life at 1 year; nevertheless, the mentioned study did not assess other measures of functional outcomes [10]. As for the etiology of early fever in ICH patient, it could be dichotomized as infectious or non-infectious (central) fever [7]. One-fourth of the patients with early fever in the present study were diagnosed as having infectious fever, and the ratio of infectious to non-infectious fever was similar to previous studies [6, 7]. Studies for primary ICH patients showed that both infectious and non-infectious fever were associated with worse outcomes in short term (at discharge or at 3 months) [6, 7]. However, a subgroup analysis in the present study was not performed due to the small sample size.
Explanation of worse outcomes associated with fever after ICH
In ischemic stroke, fever aggravates secondary brain injury through increases in inflammatory response, blood–brain barrier permeability, leukocyte recruitment and activation, and free-radical production [11, 12]. By contrast, the mechanism through which fever contributes to secondary brain injury in ICH remains poorly understood. Two preclinical studies have reported that hematoma extension and functional outcomes after ICH in young adult male rats were not influenced by hyperthermia in the short term [13, 14]. However, in animal models, hyperthermia is induced through physical heating methods, such as an infrared lamp; these methods may differ from the means through which fever and the subsequent cascade are induced in patients with ICH. The post hoc analyses of the INTERACT2 trial revealed that early hyperthermia is associated with worse cerebral edema in patients with ICH, [9] implying that the mechanism underlying poor outcomes after ICH may be comparable to that in ischemic stroke. However, additional studies are warranted to more comprehensively investigate the mechanism through which hyperthermia worsens outcomes after ICH. In the present study, the difference in functional outcomes between patients with or without early fever turned significant gradually with time (as shown in Results Sect. 3.3), which implied that early fever could potentially impact the outcomes of ICH by delaying recovery rather than exacerbating the initial injury. If this hypothesis is true, studies examining early fever and ICH outcomes may not be able to detect the difference in prognosis between two groups without a long-term follow-up. This may help explain why previous studies on ICH showed inconsistent results.
The investigators also examined the association between early fever and functional outcomes by various fever definitions as sensitivity analyses (Results Sect. 3.4), and the theory that hyperthermia worsens outcomes after ICH was further consolidated by the results.
Therapeutic hypothermia is an emerging intervention for ICH. Two systematic reviews and meta-analyses including ≥ 18 preclinical and clinical studies have demonstrated that therapeutic hypothermia yields significant benefits in behavioral outcomes and cerebral edema. However, heterogeneity between studies in terms of cooling protocols and reported clinical outcomes precluded a definitive conclusion [15, 16]. Although the exact mechanism underlying the effects of therapeutic hypothermia remains unclear, it may reduce free radicals and endothelial cell swelling and prevent inflammatory cells from entering the injured brain [17].
Early fever and length of hospital stay
Few studies have evaluated the effect of fever on the length of hospital stay in patients with ICH. We observed that ICH survivors with early fever had a prolonged length of hospital stay (p = 0.002; 95% CI = 21.80–95.91). By contrast, a 2017 retrospective study including 351 patients with ICH observed that ICH survivors with fever had a shorter length of hospital stay than did patients without fever [7]. Taiwan’s National Health Insurance system covers in-patient rehabilitation after stroke for up to 6 months and may thus alleviate any bias in the results due to heterogeneity in health-care access.
Limitations
This study has several limitations. First, this was a single-center study with a relatively small sample size. Second, because this was not an interventional study, no standard protocol was followed for fever management, possibly leading to considerable interindividual variations in terms of treatment effects. Third, the follow-up was conducted through phone interviews rather than on-site interviews, which may have resulted in bias. Fourth, the present study excluded patients who died before discharge, which impair the generalizability to all ICH patients. Finally, body temperature was measured using the tympanic method. Therefore, our findings may differ from those of other studies using other methods of measuring body temperature, such as axillary or rectal temperature measurement methods.
Conclusions
This prospective study revealed a significant association between early fever and worse functional outcomes at 1 year after ICH. In addition, patients with ICH presenting with fever at admission (early fever) had a significantly prolonged length of hospital stay.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- ICH
Intracerebral hemorrhage
- ROS
Reactive oxygen species
- mRS
Modified Rankin Scale
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- BI
Barthel Index
- ADLs
Activities of daily living
- GCS
Glasgow Coma Scale
- IVH
Intraventricular hemorrhage
- AUC
Area under the receiver operating characteristic curve
- SD
Standard deviation
- OR
Odds ratio
- CI
Confidence interval
Authors’ contributions
WC Tseng and MY Hsiao contributed to the study design, YH Chiu contributed to the data collection, WC Tseng was responsible for the data analysis and manuscript draft. YC Chen and HS Chen revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This research was supported by grants from National Taiwan University Hospital (grant #109-N4458).
Data Availability
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of National Taiwan University Hospital (No. 201905045RINB). All procedures and analyses in this study were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. All participants consent to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rajashekar D, Liang JW. Intracerebral hemorrhage. StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright. © 2022, StatPearls Publishing LLC.; 2022.
- 2.Shao L, Chen S, Ma L. Secondary Brain Injury by oxidative stress after cerebral hemorrhage: recent advances. Front Cell Neurosci. 2022;16:853589. doi: 10.3389/fncel.2022.853589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liddle LJ, Dirks CA, Almekhlafi M, Colbourne F. An ambiguous role for fever in worsening Outcome after Intracerebral Hemorrhage. Transl Stroke Res. 2022. [DOI] [PMC free article] [PubMed]
- 4.Campos F, Sobrino T, Vieites-Prado A, Pérez-Mato M, Rodríguez-Yáñez M, Blanco M, et al. Hyperthermia in human ischemic and hemorrhagic stroke: similar outcome, different mechanisms. PLoS ONE. 2013;8(11):e78429. doi: 10.1371/journal.pone.0078429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iglesias-Rey R, Rodríguez-Yáñez M, Arias S, Santamaría M, Rodríguez-Castro E, López-Dequidt I, et al. Inflammation, edema and poor outcome are associated with hyperthermia in hypertensive intracerebral hemorrhages. Eur J Neurol. 2018;25(9):1161–8. doi: 10.1111/ene.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honig A, Michael S, Eliahou R, Leker RR. Central fever in patients with spontaneous intracerebral hemorrhage: predicting factors and impact on outcome. BMC Neurol. 2015;15:6. doi: 10.1186/s12883-015-0258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillow SJ, Ouyang B, Lee VH, John S. Factors Associated with Fever in Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis. 2017;26(6):1204–8. doi: 10.1016/j.jstrokecerebrovasdis.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355–65. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 9.Malavera A, You S, Zheng D, Delcourt C, Anderson CS. Prognostic significance of early pyrexia in acute intracerebral haemorrhage: the INTERACT2 study. J Neurol Sci. 2021;423:117364. doi: 10.1016/j.jns.2021.117364. [DOI] [PubMed] [Google Scholar]
- 10.Bush RA, Beaumont JL, Liotta EM, Maas MB, Naidech AM. Fever burden and health-related quality of Life after Intracerebral Hemorrhage. Neurocrit Care. 2018;29(2):189–94. doi: 10.1007/s12028-018-0523-y. [DOI] [PubMed] [Google Scholar]
- 11.Castillo J, Dávalos A, Noya M. Aggravation of acute ischemic stroke by hyperthermia is related to an excitotoxic mechanism. Cerebrovasc Dis. 1999;9(1):22–7. doi: 10.1159/000015891. [DOI] [PubMed] [Google Scholar]
- 12.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371(9628):1955–69. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 13.Williamson MR, Colbourne F. Evidence for decreased brain parenchymal volume after large intracerebral hemorrhages: a potential mechanism limiting intracranial pressure rises. Transl Stroke Res. 2017;8(4):386–96. doi: 10.1007/s12975-017-0530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkinson CM, Kalisvaart ACJ, Kung TFC, Maisey DR, Klahr AC, Dickson CT, et al. The collagenase model of intracerebral hemorrhage in awake, freely moving animals: the effects of isoflurane. Brain Res. 2020;1728:146593. doi: 10.1016/j.brainres.2019.146593. [DOI] [PubMed] [Google Scholar]
- 15.Baker TS, Durbin J, Troiani Z, Ascanio-Cortez L, Baron R, Costa A, et al. Therapeutic hypothermia for intracerebral hemorrhage: systematic review and meta-analysis of the experimental and clinical literature. Int J Stroke. 2022;17(5):506–16. doi: 10.1177/17474930211044870. [DOI] [PubMed] [Google Scholar]
- 16.Melmed KR, Lyden PD. Meta-analysis of pre-clinical trials of therapeutic hypothermia for Intracerebral Hemorrhage. Ther Hypothermia Temp Manag. 2017;7(3):141–6. doi: 10.1089/ther.2016.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu TC, Grotta JC. Hypothermia for acute ischaemic stroke. Lancet Neurol. 2013;12(3):275–84. doi: 10.1016/S1474-4422(13)70013-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.