Abstract
Background
Brain metastases are the most common intracranial tumours. Variation exists in the use of stereotactic radiosurgery for patients with 10 or more brain metastases. Concerns include an increasing number of brain metastases being associated with poor survival, the lack of prospective, randomised data and an increased risk of toxicity.
Methods
We performed a systematic review and meta-analysis to assess overall survival of patients with ten or more brain metastases treated with stereotactic radiosurgery as primary therapy. The search strings were applied to MEDLINE, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL). Log hazard ratios and standard errors were estimated from each included study. A random-effects meta-analysis using the DerSimonian and Laird method was applied using the derived log hazard ratios and standard errors on studies which included a control group.
Results
15 studies were included for systematic review. 12 studies were used for pooled analysis for overall survival at set time points, with a predicted 12 month survival of 20–40%. The random-effects meta-analysis in five studies of overall survival comparing ten or greater metastases against control showed statistically worse overall survival in the 10 + metastases group (1.10, 95% confidence interval 1.03–1.18, p-value = < 0.01, I2 = 6%). A funnel plot showed no evidence of bias. There was insufficient information for a meta-analysis of toxicity.
Discussion
Overall survival outcomes of patients with ten or more brain metastases treated with SRS is acceptable and should not be a deterrent for its use. There is a lack of prospective data and insufficient real-world data to draw conclusions on toxicity.
PROSPERO ID
CRD42021246115
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-11452-7.
Keywords: Stereotactic radiosurgery, Radiotherapy, Multiple metastases
Key points
Patients with 10 or more brain metastases treated with stereotactic radiosurgery can have prolonged survival outcomes.
There is a lack of prospective trials to inform on treatment decisions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-11452-7.
Importance of the study
This systematic review and meta-analysis is the first of its kind in the literature and provides information on overall survival outcomes and toxicities encountered in patients with ten or more brain metastases treated with stereotactic radiosurgery. Centres treating patients with ten or more brain metastases are doing so based only on retrospective real-world data analyses, the vast majority of which are from single centres and single radiotherapy platforms. This review provides an additional evidence resource for practitioners of stereotactic radiosurgery to aide in the management of this difficult patient group. The methods used to predict survival outcomes through the calculation of log hazard ratios and standard errors allowed analysis of small, retrospective case series. To our knowledge, this is the first meta-analysis of this patient group gives evidence for acceptable overall survival outcomes post-treatment, and provides further evidence for the use of stereotactic radiosurgery for these patients.
Overall survival following stereotactic radiosurgery for ten or more brain metastases: a systematic review and meta-analysis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-11452-7.
Introduction
Brain metastases (BMs) are the most common intracranial tumours in adults. One population-based study over 28 years found primary malignant sites with the highest incidence proportions of BMs were lung (19.9%), melanoma (6.9%), renal (6.5%) and breast cancers (5.1%) [1]. However, BMs may arise from any malignancy and although their exact incidence is not known, they are thought to account for more than half of all intracranial tumours, with an incidence up to 40,000 per annum in the United States [2]. Management of brain metastases is dependent on a multitude of factors. Surgery is considered most appropriate for patients with severe mass effect or impending herniation and is also considered in patients with oligometastatic disease as a potentially curative approach. Radiotherapy is considered a standard approach for patients without evidence of severe mass effect or where multiple metastases are found. While historically whole brain radiotherapy (WBRT) was the preferred radiation method of choice, it is being supplanted by stereotactic radiosurgery (SRS) for many cases where there are a limited number of brain metastases. SRS is the delivery of a set number of doses of radiation delivered to a small target. This is achieved using many, non-coplanar radiation beans which converge to a single point. Healthy tissue is protected from the high doses of radiation by the steep drop-off in dose which occurs away from the intended target volume. While novel systemic anti-cancer agents with good brain penetrance may change this treatment algorithm, further studies are required to determine if they can replace local therapies for the management of BMs. SRS is now considered a standard option without adjuvant WBRT for “limited” metastases, although the definition of “limited” varies across the literature.
Prospective, randomised data has provided evidence for the use of SRS alone for cases of 1–4 BMs [3–5]. Based on data such as the prospective, observational JLGK0901 study [6] which demonstrated non-inferiority in treating 5–10 BMs as compared to 1–4 BMs, a number of guidelines have eliminated the set BM number criterion for determining eligible patients for SRS [7, 8]. However, significant discrepancies exist in the use of SRS in patients with 10 or greater metastases. While the Congress of Neurological Surgeons guidelines recommend more than 4 metastases can be treated with SRS up to a volume of 7 cc, this is only given a level 3 recommendation [9]. The American Radium Society’s guidelines in the management of 11–20 BMs resulted in no recommendation that SRS could be used alone [10], and the latest American Society for Radiation Oncology (ASTRO) guidelines also does not recommend SRS for more than 10 BMs [11]. Concerns may be due to an increasing number of BMs being associated with poor survival [12], the lack of prospective, randomised data and the increased risk of toxicities such as radiation necrosis when treating multiple targets with SRS. The aim of this systematic review and meta-analysis was to collate the available literature in the use of SRS as primary therapy in patients with 10 or more BMs and evaluate resulting overall survival. We also aimed to collect information on rates of radiation-induced complications, in particular rates of radiation necrosis.
Methods
Search strategies and selection criteria
We performed a systematic review and meta-analysis to assess overall survival of patients with ten or more brain metastases treated with SRS as primary therapy. The study was prospectively registered with PROSPERO (CRD42021246115) and performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist [13]. Search terms were devised by HR in conjunction with a medical librarian from Imperial College London. Patients with ten or more brain metastases treated with stereotactic radiotherapy alone were included. The full inclusion and exclusion criteria, and the search strings used, are available in the appendix in Sect. 1.1 and 1.2 respectively.
The search strings were applied to MEDLINE, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL). Searches were restricted to human studies available in English, and relevant grey literature. Trial registries were also searched. Studies published from 1st January 1960 to 1st April 2021 were included and transferred to Covidence (www.covidence.org) for analysis. Abstract screening and full-text eligibility were performed independently by HR and JC. Disputes were resolved by the senior author (MW).
Data analysis
Data extraction was performed by HR. If different studies involving the same patient cohort was found, meta-analysis was performed only with the study with the largest cohort to reduce bias. All studies were included for narrative synthesis.
Total number of patients in the study, the number of patients with 10 or greater metastases, the proportion of primary malignancies, the median number of metastases in the multi-metastases group, the median survival, the median follow up time, the median cumulative volume of metastases treated and the number of patients alive at follow up were collated. The main summary measure was proportion of patients alive at set time points, with a primary outcome of overall survival. Survival outcomes were extracted where documented in the study manuscript or were otherwise derived from survival curves. Information on rates of radiation necrosis were also collected where available.
Log hazard ratios and standard errors were estimated from each included study using the methods and spreadsheet as developed by Tierney, et al. [14]. All studies with sufficient information available underwent a pooled proportion analysis via a generalised linear mixed-effects model to create an estimated pooled survival curve of all included studies at set time points (3, 6, 9, 12, 15, 18 and 24 months). Heterogenicity was assessed using the I2 test. P values < 0.05 were considered statistically significant. As significant levels of heterogeneity were anticipated between the studies analysed, a random-effects meta-analysis using the DerSimonian and Laird method was applied using the derived log hazard ratios and standard errors on studies which included a control group. A funnel plot was generated as a measure of reporting bias.
Statistical analysis was performed using R version 4.1.0, and packages tidyverse, meta and dmetar were utilised. A bias analysis was conducted using the Risk Of Bias In Non-randomised Studies - of Interventions (ROBINS-I) tool [15]. A summary of the evidence was rated using the GRADE system [16]. The certainty of evidence for overall survival was assessed by considering the risk of bias in the studies, inconsistency, imprecision, indirectness, and the possibility for publication bias.
Results
Our database search yielded 822 studies, from which 4 duplicate studies were removed. Abstract and full text screening was performed as shown in Fig. 1, culminating in 15 studies which were included in the systematic review.
Fig. 1.
Study selection performed on the Covidence online platform as per the PRISMA guidelines. Studies were searched for in Embase, MEDLINE and CENTRAL databases. Screening performed by two authors (HR and JC) with disputes resolved by senior author (MW)
A summary of the included studies for narrative synthesis is shown in Table 1. The total number of treated patients with ten or more BMs was 2360, with one of the included studies not documenting the number of 10 + metastases patients. Six studies included centres based in the United States, six in Japan, two in the Republic of Korea, one in Italy and one in Australia. Most included patients had a primary malignancy of lung cancer (1424), breast cancer (343), and melanoma (133). Median overall survival from all studies was 8.15 months (interquartile range 2.5-9.875). Studies included patients treated from 1994 to 2018. All except one study was conducted on the Gamma Knife, with one study (Minniti, et al. 2020) using linear accelerator technology. Median cumulative tumour volume treated was not reported in every study but of those presented ranged from 0.38 to 9.98 cc.
Table 1.
Summary of studies included in systematic review following screening process
| Centre, country | Follow up (months) | No. of patients included | Period patients undergoing treatment | Primary cancer sites include (%) | No. of patients metastases in 10 + group | Median number of mets in multi-mets group | Control group included? | Median survival in multi-metastases group (months) | Median survival in limited metastases group (months) | Cumulative volume (cm3) |
SRS platform used | No. of patients alive at the end of follow up | Rates of AREs documented? | Included in meta-analysis? | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ali 2017 [17] |
University of California, USA Gamma Knife House, Japan Tokyo Gamma Knife Centre, Japan |
6.4 (median) | 5750 | 1994–2014 |
Breast (12.3) GI (12.0) Lung (65.0) Melanom (4.9) RCC (5.5) |
981 | Not specified | Yes (2–9 metastases) | 5.5 | 6.4 |
Breast 6.90 GI 9.17 Lung 3.75 Melanoma 1.99 RCC 5.35 |
Gamma Knife | None | Not specified | Yes |
| Bowden 2019 [18] | Pittsburgh, USA | Not specified | 93 | Dec 2008 – Dec 2017 |
Breast (34.8) Lung (42.4) Melanoma (22.8) |
93 (15 + mets) |
Breast: 23 Lung: 21 Melanoma: 21 |
No |
Breast 18.0 Lung 9.4 Melanoma 6.3 |
n/a |
Breast 8.75 Lung 6.89 Melanoma 9.98 |
Gamma Knife | Not specified | Not specified | No – no control group |
| Chang 2010 [19] | Yonsei University, Republic of Korea |
6 (mean) 0–44 (range) |
323 | Oct 2005 – Oct 2008 |
NSCLC (39) Breast (12.4) RCC (8.7) CRC (8.4) SCLC (4.6) Gynae (3.4) HCC (2.5) Thyroid (1.5) H&N (1.5) Prostate (1.2) |
50 total (17 (11–15 mets group) 33 (> 15 mets group)) |
Not specified | Yes (1–5 metastases) |
13.0 (11–15 mets group) 8.0 33 (> 15 mets group) |
10.0 (1–5 metastases group) | Not specified | Gamma Knife | Not specified |
ARE in 23 pts (7.12%). Only 1 pts (3.0%) in > 15 mets group |
Yes |
| Ehrlich 2019 [20] |
Zucker School of Medicine at Hofstra/Northwel, USA |
Not specified | 55 | Mar 2014 – Apr 2018 |
NSCLC (47%) Breast (22%) Melanoma (16%) Other (15%) |
40 (synchronously treated) |
10–15 mets: 32 pts 16–20 mets: 9 pts > 20 mets: 14 pts |
No | 9.5 | n/a | 0.3167–54.86 | Gamma Knife | Not specified | Not specified | No – no contorl group |
| Izard 2019 [21] | Macquarie University Hospital, Australia | 11 (0–62.4 months) | 180 | Aug 2010 – Jul 2017 |
NSCLC (27%) Breast (27) Melanoma (24) Other (22) |
47 |
11–20 mets: 30 pts > 20 mets: 17 patients |
Yes (4–9 mets) | 8.5 months | 6 months | Median: 0.57 (range < 0.005–5.44) | Gamma Knife | 39 | Long-term radionecrosis in 22 pts (12.2%) | Yes |
| Kim 2008 [22] | Sungkyunkwan University School of Medicine, Seoul, Korea | Not specified | 26 | Aug 2002 – Dec 2007 |
NSCLC (80) Breast (12) Unknown (8) |
26 | 16.6 | No | 7.82 | n/a | 10.9 cc (1.0-42.2) | Gamma Knife | 8 | Not specified | No – no control group |
| Minniti 2020 [23] | University of Siena, Italy | 10.8 (median) | 40 | Jan 2017 – Dec 2018 |
Lung (42.5%) Melanoma (25%) Breast (17.5%) Kidney (15) |
40 | 13 | No | 14.1 | n/a | 0.38 Range 0.17–6.8 | LINAC | 21 | Imaging suggestive of necrosis in 14 pts (35%). Grade 2 or 3 in 7 pts (14.5%) | No – no control group |
| Mizuno 2019 [24] | Komaki City Hospital, Japan |
6.7 (SRS group) 6.4 (WBRT group) |
44 | Jan 2009 – Dec 2016 | NSCLC (100%) | 24 | 20 | Yes | 7.3 | 7.2 | Not specified | Gamma Knife | Not specified | Not specified | Yes |
| Nakazaki 2013 [25] | Ota Memorial Hospital; Chiba University; Tsukiji Neurologic Clinic, Japan |
4.0 (median) 0.5–21.2 (range) |
47 | Jan 1999 – Mar 2011 | SCLC (100%) | 17 | 30 | Yes (1–10 mets) | 5 | 9 | Not specified | Gamma Knife | 5 | No AREs reported in any pts included in study | Yes |
| Raldow 2013 [26] | Yale School of Medicine, USA | Not specified | 103 | 2000–2010 |
Melanoma (33%) NSCLC (32%) Breast (17) RCC (6) SCLC (5) Others (7) |
18 | 84 | Yes (5–10 mets) | 8.3 | 7.6 | 0.33–34.6 | Gamma Knife | Not specified | Not specified | Yes |
| Rava 2013 [27] | Tufts Medical Center Boston, USA | 7.5 (median) | 53 | 2004–2010 |
NSCLC (38) Breast (34) Others (28) |
53 | 10.9 | No | 6.5 | n/a | Not specified | Gamma Knife | Not specified | Not specified | No – no control group |
| Serizawa 2006 [28] | Chiba University, Japan | Not specified | 1127 | Not specified |
Lung (69.8%) GI (12.3) Breast (6.6) Urinary (5) Others (6.5) |
Not specified | Not specified | Yes (1–4 metastases) | 10 | 15 | 0.8 range 0.1–24 | Gamma Knife | Not specified | AREs not noted in any pts | No – missing no of 10 + mets patients. Data overlaps with Nakazawa 2013 |
| Susko 2020 [29] | University of California, USA | 7.4 (2.7–15.9) | 143 | Mar 1999 – Dec 2016 |
Breast (36.4) NSCLC (34.3) Melanoma (21.0) Other (8.3) |
143 | 13 | No | 11.7 | n/a | 4.1 (IQR, 2.0-9.9) | Gamma Knife | 6 |
16 pts (11%) with ARE on imaging. 3 pts (2%) symptomatic |
No – no control group, overlap with Ali 2017 |
| Suzuki 2000 [30] | Shin-Koga Hospital Gamma Knife Cente, Japan | 3.0 (0 -8.7) | 24 | Jul 1998 – Jan 2000 |
Lung (83) Breast (12.5) CRC (4.5) |
24 | 20 | No | 2.5 | n/a | 0.66 (range 0.003–18.4 ) | Gamma Knife | 12 | Not specified | No – no control group |
| Yamamoto 2021 [31] |
Katsuta Hospital Mito Gamma House; Ibaraki Tokyo Gamma Unit Center, Japan |
15.5 (IQR 1.3–22.8) | 1515 | 1998–2018 |
NSCLC (59) Breast (13.1) SCLC (11.2) GI (7.9) Kidney (2.2) Other (6.6) |
804 | 14 (12–17) | Yes (5–10 mets) | 6.5 | 7.7 |
54% < 7 46% ≥ 7 |
Gamma Knife | 174 | Not specified | No -overlap with Ali 2017 |
ARE: adverse radiation effect; IQR: interquartile range; LINAC: linear accelerator; mets: metastases; NSCLC: non-small cell lung cancer; pts: patients; RCC: renal cell carcinoma. Twelve studies (80% of all studies) were used for pooled analysis for overall survival at set time points. The summary table and resulting confidence band chart is shown in Table 2; Fig. 2 respectively. There was significant study heterogenicity at each of the calculated time points (I2 range 73.9–84.4%) which is why a confidence band chart is used to visual the data, highlighting the uncertainty in the results. Raw data for each time point is available in the appendix (Sect. 1.3)
All included studies were retrospective case series. Six of the fifteen studies compared patients with ten or more metastases against a control group; one control group was WBRT in ten or more metastases (Mizuno 2019) and so was not included in the meta-analysis, the remaining five studies were patients with fewer than ten metastases and so were included. 12 of 15 studies were included in the pooled analysis of survival outcomes. Of the three excluded studies, Susko, et al. 2020 and Yamamoto, et al. 2021 had overlapping data with Ali et al., 2017, and Serizawa, et al. 2006 did not specify the number of patients with more than ten metastases.
Table 2.
Summary of pooled survival outcomes at set time points formed by a linear mixed-effects model
| Month | Survival | L-CI | U-CI | I 2 |
|---|---|---|---|---|
| 3 | 0.7699 | 0.6478 | 0.8589 | 76.0 |
| 6 | 0.5326 | 0.4031 | 0.6579 | 73.9 |
| 9 | 0.3682 | 0.2496 | 0.5053 | 81.1 |
| 12 | 0.3051 | 0.2054 | 0.4273 | 83.9 |
| 15 | 0.2442 | 0.1640 | 0.3473 | 84.4 |
| 18 | 0.1786 | 0.1162 | 0.2644 | 79.3 |
| 24 | 0.1283 | 0.0804 | 0.1984 | 81.2 |
L-CI: lower bound of 95% confidence interval; U-CI: upper bound of 95% confidence interval
Fig. 2.
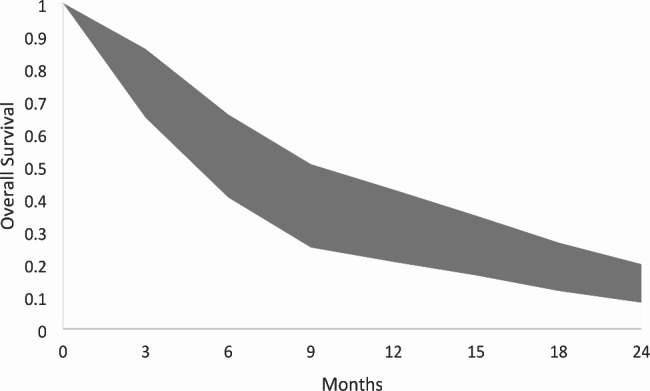
Confidence band chart of pooled overall survival outcomes created through the lower and upper confidence intervals presented in Table 2
The random-effects meta-analysis of ten or greater metastases against control groups is shown in Fig. 3. Five studies including a control group were used. The pooled hazard ratio of survival favoured the control group, and reached statistical significance with high levels of homogeneity between studies (1.10, 95% confidence interval 1.03–1.18, p-value = < 0.01, I2 = 6%). The study by Ali, et al. contributed a significant weight to the meta-analysis, although their analysis found no statistically significant difference in survival between the 2–9 and 10 + BM groups [17]. The corresponding funnel plot did not demonstrate evidence of bias and is shown in the appendix (Sect. 1.4).
Fig. 3.
Random-effects meta-analysis of ten or greater metastases against control, showing a statistically significant trend for improved overall survival outcomes in the fewer than 10 brain metastases group
There was only limited data available on rates of radiation necrosis and so was deemed unsuitable for meta-analysis. Rates of radiation necrosis found on imaging in patients treated with Gamma Knife ranged from 0 to 12.2%. The study by Chang, et al. 2010 specified necrosis in 3% of treated patients with 15 or greater metastases, and no treated patients with 11–14 metastases. The remaining studies did not distinguish rates of necrosis between those patients with 10 or greater metastases and those with fewer. No studies used histological determination for confirmation of radiation necrosis, and instead relied on radiologically-suspicious features. The single linear accelerator-based study (Minniti, et al. 2020) reported necrosis in 35% of patients treated, but only half of these were symptomatic and required treatment (57% required medical therapy only, 43% required surgery).
All studies were assessed for bias, and a summary is presented in Table 3. Three of the included studies demonstrated serious risks of bias as per the ROBINS-I criteria.
Table 3.
ROBINS-I analysis to assess the risk of bias in each included study
| Bias due to confounding | Bias in selection of participants into the study | Bias in classification of intervention | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias | |
|---|---|---|---|---|---|---|---|---|
| Ali 2017 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | Moderate |
| Bowden 2019 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | Moderate |
| Chang 2010 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | Moderate |
| Erlich 2019 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | Moderate |
| Izard 2019 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | Moderate |
| Kim 2008 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | Moderate |
| Minniti 2020 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | Moderate |
| Mizuno 2019 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | Serious |
| Nakazaki 2013 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | Serious |
| Raldow 2013 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | Moderate |
| Rava 2013 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | Moderate |
| Serizawa 2006 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | Moderate |
| Susko 2020 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | Moderate |
| Suzuki 2000 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | Serious |
| Yamamoto 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Moderate |
0 = no information/unclear, 1=“Low risk”, 2=“Moderate risk”, 3=“Serious risk”, 4=“Critical risk” of bias. The quality of evidence for assessing overall survival in patients with 10 or more BMs is shown in Table 4. The quality of evidence was downgraded to very low as per the GRADE criteria due to limitations in study design and the risk of bias, particularly in the largest studies included for analysis
Table 4.
Summary of findings for overall survival outcomes
| Certainty of evidence (GRADE) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Study population and study design | Summary effect | Methodological limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality |
| Overall survival | 15 studies (2360 patients) | Overall survival of patients treated with SRT for 10 + BMs is comparable to patients with 1–9 BMs | Critical | Not serious | Not serious | Critical | Not serious | Very low a |
adowngraded due to the small, non-randomised retrospective nature of studies without incorporating confounding factors for survival. The relatively high confidence intervals within studies also strongly suggests imprecision
Discussion
This systematic review and meta-analysis collated the available data on overall survival of patients with ten or more BMs treated with SRS. Our results demonstrate that overall survival in patients with 10 or more BMs treated with SRS is slightly worse than patients with 2–9 BMs [12]. However, a median survival of 8.15 months for patients with ten or more BMs is more impressive when considered that many of the patients were treated before 2010, and therefore before the widespread use of intracranially active systemic anti-cancer treatments. Some patients were treated as early as 1994, although unfortunately no breakdown of when individual patients were treated is available.
Our pooled survival analysis allowed the construction of a survival curve for the reader to gauge survival outcomes in patients with ten or more BMs. For example, the 24-month survival 95% confidence interval of 0.08–0.20 months is likely greater than most treating physicians may predict and may inform treatment decisions for this patient group. The visual representation of long-term survival in this patient cohort and the review dedicated to patients with ten or more BMs has not been previously published.
Our review highlights the lack of prospective, randomised data to inform treatment decisions for patients with ten or greater BMs. Our pooled random effects meta-analysis comparing ten or more BMs to a control group showed statistically significantly worse overall survival in the 10 + group compared to the < 10 BMs group, and the corresponding I2 test showed low heterogeneity between studies. The meta-analysis was dominated by the largest study by Ali, et al. [17] but whereas their study did not demonstrate a statistically significant difference in overall survival between high and low BM groups, the meta-analysis presented here does show a statistically significant difference. However we do not believe this should deter the use of SRS in patients with 10 or more BMs. The lower limit of the 95% confidence interval is close to unity (1.03), and confounding factors such as total volume of intracranial disease and primary malignancy have not been corrected for. The corresponding funnel plot demonstrates a low risk of publication bias. In light of the lack of prospective, randomised data, our review suggests non-inferiority for overall survival in patients with ten or more BMs.
A review of this nature will not provide a definitive answer to predict survival outcomes in this patient group. We believe the two most significant omissions in the current literature are firstly the lack of prospective, randomised data, and secondly the lack of detail in patient factors which impact on survival as well as SRS, including but not limited to: detailed cancer histology including mutation status; preceding, concurrent, and proceeding systemic anti-cancer therapy; and preceding and proceeding local intracranial therapy besides SRS, namely surgery and WBRT. This data is lacking in the studies included in this review.
Although RCTs would represent an ideal evidence base, and there are several randomised trials currently recruiting patients with multiple BMs and randomising between SRS and WBRT (NCT02953717, NCT03075072 and NCT03550391), there have been previous issues with trials of this nature. A Dutch randomised control trial of 4–10 BMs closed early due to a failure to accrue sufficient patients [32] and the North American Gamma Knife Consortium trial (NCT01731704) which had planned to randomise patients with 4–10 BMs treated with SRS against WBRT has also closed early. A further randomised controlled trial of patients with non-melanoma primaries and 4–15 BMs has been reported in abstract form, but has only 36 patients in each treatment arm, thus limiting interpretation of their results [33]. Other studies have reported outcomes from treating patients with multiple metastases, but in a staged form [34], and planning studies have confirmed that patients treated with SRS receive a low hippocampal radiotherapy dose [35].
A reason for the failure for randomised trials to accrue sufficient patients may be due to the lack of equipoise which exists in treating radiation oncologists and neurosurgeons. A patterns of care survey performed amongst the German Society for Radiation Oncology (DEGRO) found that WBRT is the preferred treatment modality in most multiple BMs cases over SRS. In an example case of a patient with NSCLC, 15 BMs and a prognosis over 6 months found that 89.1% of 165 respondents favoured WBRT over SRS [36]. Similarly, a patterns of care survey in the United States of 116 radiation oncologists found 82.5% of respondents report using WBRT for ten or more BMs over SRS [37]. A survey of practitioners allied to a radiosurgical society were however more likely to utilise SRS for patients with 7 or more metastases [38].This suggests that there may be a discrepancy between physicians with easy and regular access to dedicated SRS equipment. There may be an ingrained approach some physicians have towards treating patients with multiple BMs, and this may limit the numbers of patients referred for randomised trials.
The treatment paradigm for patients with multiple BMs is changing in light of the increasing number of novel systemic anti-cancer agents with good brain penetrance. Patients with oncogenic driver mutations such as epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements may derive significant benefit in treating their intracranial disease from oral systemic therapy. This may avoid or delay the need for local intracranial treatment. There are a number of benefits in trying to avoid local therapies in this patient group. Intracranial tumours have shown significant response rates when targeted agents against these driver mutations are used. Patients with these driver mutation are living longer, and this increased survival will allow more time for side effects to develop from local therapies [39, 40]. Their use in lieu of local therapies, particularly in asymptomatic patients, is likely to increase. In the absence of prospective, randomised controlled data, studies need to include detailed information regarding the tumour mutation status and systemic anti-cancer therapy history of their patient cohort to adjust for their impact on overall survival.
We were unable to perform the planned meta-analysis on rates of radiation necrosis due to information on their incidence only being included in five studies. The disparate nature they were reported meant they were included for narrative synthesis only. Nonetheless, rates of necrosis detected on imaging were low, particularly in patients treated with Gamma Knife. Brain necrosis is also notorious difficult to diagnose and differentiate from disease progression using imaging alone. It is not specified whether the patients included in these studies would have undergone a biopsy to confirm necrosis, although this is not common practice in many centres. Rates of true necrosis may therefore not correlate well to those documented. The relatively low rates of necrosis across the studies presented provide assurance to treating physicians managing patients with ten or more metastases. Adverse radiation effects are difficult to accurately report in trials as they can occur more than ten years following radiation exposure [41]. This may be mitigated by studies demonstrating superior normal brain dosimetry for SRS over hippocampal-sparing WBRT in patients with 10 to 30 BMs [42]. Based on planning studies, SRS appears to deliver little hippocampal dose even in patients with multiple metastases [35]. Other studies have looked at multiple cumulative BMs which included toxicity data [34], but this may not correlate closely with single session SRS.
To reduce the impact of bias in this review we assessed each included study with design-specific criteria as set out by Viswanathan, et al. [43]. We also only included the largest cohort when data overlapped with more than one study. Nonetheless, the studies included are at significant risk of participation and attrition bias. The studies also showed high levels of heterogeneity in pooled survival analysis, likely due in part to the variations in primary malignancies, systemic therapies cancer genetics and comorbidities. Our GRADE assessment showed high levels of discrepancies in patient populations, size of effects and publication bias, and this is consistent with many systematic reviews of retrospective case series. Our statistical analysis is also limited by not having access to individual patient data, and instead basing analysis on aggregates derived from variable outcome measures reporting in each individual study. Interpretation of the results presented should be considered in this context. A further issue is the likelihood of overlap between patient cohorts from different studies. The cohorts as presented by Nakazaki [25] may have overlapping patients with the cohorts published by Serizawa and Yamamoto [28, 31]. However as only the Nakazaki cohort was included in the meta-analysis, and they only contributed 47 patients, we believe this is unlikely to significantly affect the results of our analysis.
We have not made any corrections for different treatment platforms, and have considered the most commonly used platforms of LINACs, Cyberknife and Gamma Knife as equivalent. While dosimetric data exists suggesting a difference in coverage and dose spill, no randomised prospective trials have shown a different in outcomes, whether tumour response or complication rates [44]. Advancements in the ability to deliver SRT with LINACs, coupled with their comparative availability, will likely increase its use for SRT in the future. Given much of the current data is based on the Gamma Knife platform, future studies will need to assess whether a change in platform leads to different outcomes in survival and tumour response.
In conclusion, this systematic review and meta-analysis provides insight into the survival outcomes of patients treated with SRS for ten or more BMs. Outcomes are similar to those published for unselected patients treated with SRS for more than one BM, downplaying the importance of number of BMs in patient survival. A meta-analysis of five studies demonstrated a significant survival difference in patients with ten or more BMs treated with SRS versus control, a finding not seen when reviewing the largest single analysis alone. However the lower limit of the 95% confidence interval was close to unity and data is likely to be effected by confounding factors for survival such as volume of disease. Our data suggests SRS is a suitable option for selected patients with ten or more BMs. Future work would ideally be in the form of a randomised, controlled clinical trial. Observational data requires more extensive detail on the primary malignancy, on additional intracranial and systemic treatments received, and on rates of radiation necrosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not Applicable.
Authors’ contributions
HR, JC and MW made substantial contributions to the conception and design of the study. HR, JC and MW made substantial contributions to the writing of the manuscript. HR, JC and MW approved the version to be published.
Funding
JC is supported by the Guangdong International Young Research Talents Training Programme for Postdoctoral Researchers and receives funding from the China Postdoctoral Science Foundation Grant (2019M653210), the Science and Technology Project of Jiangmen (2019030102480013011), and the Medical Science Foundation of Jiangmen Central Hospital (J201901). MW receives funding from the Imperial/NIHR Biomedical Research Centre and Imperial College Healthcare NHS Trust.
Data Availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Local institution approval, and UK health research authority research ethics committee approval (ID ID 19/LO/1763).
Consent for publication
Not applicable.
Competing interests
No authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–72. 10.1200/JCO.2004.12.149. [DOI] [PubMed]
- 2.Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am. 2011;22(1):1–6, v. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21109143. [DOI] [PubMed]
- 3.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16757720. [DOI] [PubMed]
- 4.Kondziolka D, Patel A, Lunsford LDD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol. 1999;45(2):427–34. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0360301699001984. [DOI] [PubMed]
- 5.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–72. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0360301699001984. [DOI] [PubMed]
- 6.Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–95. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1470204514700610. [DOI] [PubMed]
- 7.Royal College of Radiologists. Radiotherapy dose fractionation Third edition. 2019 [cited 2021 Jul 30]. p. 24–31. Available from: https://www.rcr.ac.uk/system/files/publication/field_publication_files/brfo193_radiotherapy_dose_fractionation_third-edition.pdf.
- 8.Nabors LB, Portnow J, Ahluwalia M, Baehring J, Brem H, Brem S et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18(11):1537–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33152694. [DOI] [PubMed]
- 9.Graber JJ, Cobbs CS, Olson JJ. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Use of Stereotactic Radiosurgery in the Treatment of Adults With Metastatic Brain Tumors. Neurosurgery. 2019;84(3):E168–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30629225. [DOI] [PubMed]
- 10.Milano MT, Chiang VLS, Soltys SG, Wang TJC, Lo SS, Brackett A et al. Executive summary from American Radium Society’s appropriate use criteria on neurocognition after stereotactic radiosurgery for multiple brain metastases. Neuro Oncol. 2020;22(12):1728–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32780818. [DOI] [PMC free article] [PubMed]
- 11.Gondi V, Bauman G, Bradfield L, Burri SH, Cabrera AR, Cunningham DA et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2022;12(4):265–82. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1879850022000546. [DOI] [PubMed]
- 12.Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU et al. Survival in Patients With Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J Clin Oncol. 2020;38(32):3773–84. 10.1200/JCO.20.01255. [DOI] [PMC free article] [PubMed]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700–b2700. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed]
- 14.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17555582. [DOI] [PMC free article] [PubMed]
- 15.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;i4919. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed]
- 16.Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed]
- 17.Ali MA, Hirshman BR, Wilson B, Carroll KT, Proudfoot JA, Goetsch SJ et al. Survival Patterns of 5750 Stereotactic Radiosurgery–Treated Patients with Brain Metastasis as a Function of the Number of Lesions. World Neurosurg. 2017;107:944–951.e1. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1878875017311622. [DOI] [PMC free article] [PubMed]
- 18.Bowden G, Faramand A, Niranjan A, Lunsford LD, Monaco E 3. rd. Gamma Knife Radiosurgery for the Management of More Than 15 Cerebral Metastases. World Neurosurg. 2019;126:e989–97. Available from: https://linkinghub.elsevier.com/retrieve/pii/S187887501930645X. [DOI] [PubMed]
- 19.Chang WS, Kim HY, Chang JHJW, Park YG, Chang JHJW. Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: is stereotactic radiosurgery effective for multiple brain metastases? J Neurosurg. 2010;113(Special_Supplement):73–8. Available from: https://thejns.org/view/journals/j-neurosurg/113/Special_Supplement/article-p73.xml. [DOI] [PubMed]
- 20.Ehrlich MI, Schiff E, Knisely JPS, Chang J, Qian X, Goenka A et al. Tumor control and survival in patients with ten or more brain metastases treated with stereotactic radiosurgery: a retrospective analysis. J Neurooncol. 2019;143(1):167–74. Available from: http://link.springer.com/10.1007/s11060-019-03153-8. [DOI] [PubMed]
- 21.Izard MA, Moutrie V, Rogers JM, Beath K, Grace M, Karle B, et al. Volume not number of metastases: Gamma Knife radiosurgery management of intracranial lesions from an australian perspective. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2019;133:43–9. doi: 10.1016/j.radonc.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Kim C-H, Im Y-S, Nam D-H, Park K, Kim J-H, Lee J-I. Gamma Knife Radiosurgery for Ten or More Brain Metastases. J Korean Neurosurg Soc. 2008;44(6):358. Available from: http://jkns.or.kr/journal/view.php?doi=10.3340/jkns.2008.44.6.358. [DOI] [PMC free article] [PubMed]
- 23.Minniti G, Capone L, Nardiello B, El Gawhary R, Raza G, Scaringi C et al. Neurological outcome and memory performance in patients with 10 or more brain metastases treated with frameless linear accelerator (LINAC)-based stereotactic radiosurgery. J Neurooncol. 2020;148(1):47–55. Available from: http://link.springer.com/10.1007/s11060-020-03442-7. [DOI] [PubMed]
- 24.Mizuno T, Takada K, Hasegawa T, Yoshida T, Murotani K, Kobayashi H, et al. Radiosurgery for patients with more than ten brain metastases. J Neurosurg. 2019;84(1):390–6. [Google Scholar]
- 25.Nakazaki K, Higuchi Y, Nagano O, Serizawa T. Efficacy and limitations of salvage gamma knife radiosurgery for brain metastases of small-cell lung cancer after whole-brain radiotherapy. Acta Neurochir (Wien). 2013;155(1):104–7. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med10&NEWS=N&AN=23065044. [DOI] [PubMed]
- 26.Raldow AC, Chiang VL, Knisely JP, Yu JB. Survival and Intracranial Control of Patients With 5 or More Brain Metastases Treated With Gamma Knife Stereotactic Radiosurgery. Am J Clin Oncol. 2013;36(5):486–90. Available from: https://journals.lww.com/00000421-201310000-00012. [DOI] [PubMed]
- 27.Rava P, Leonard K, Sioshansi S, Curran B, Wazer DE, Cosgrove GR et al. Survival among patients with 10 or more brain metastases treated with stereotactic radiosurgery. J Neurosurg. 2013. [DOI] [PubMed]
- 28.Serizawa T, Higuchi Y, Ono J, Matsuda S, Nagano O, Iwadate Y et al. Gamma Knife surgery for metastatic brain tumors without prophylactic whole-brain radiotherapy: results in 1000 consecutive cases. J Neurosurg. 2006;105 Suppl:86–90. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med6&NEWS=N&AN=18503337. [DOI] [PubMed]
- 29.Susko MS, Garcia MA, Ma L, Nakamura JL, Raleigh DR, Fogh S et al. Stereotactic Radiosurgery to More Than 10 Brain Metastases: Evidence to Support the Role of Radiosurgery for Ideal Hippocampal Sparing in the Treatment of Multiple Brain Metastases. World Neurosurg. 2020;135:e174–80. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1878875019329298. [DOI] [PMC free article] [PubMed]
- 30.Suzuki S, Omagari J, Nishio S, Nishiye E, Fukui M. Gamma knife radiosurgery for simultaneous multiple metastatic brain tumors. J Neurosurg. 2000;93 Suppl 3:30–1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11143258. [DOI] [PubMed]
- 31.Yamamoto M, Serizawa T, Sato Y, Higuchi Y, Kasuya H. Stereotactic Radiosurgery Results for Patients with 5–10 versus 11–20 Brain Metastases: A Retrospective Cohort Study Combining 2 Databases Totaling 2319 Patients. World Neurosurg. 2021;146:e479–91. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1878875020323263. [DOI] [PubMed]
- 32.Hartgerink D, Bruynzeel A, Eekers D, Swinnen A, Hurkmans C, Wiggenraad R et al. A Dutch phase III randomized multicenter trial: whole brain radiotherapy versus stereotactic radiotherapy for 4–10 brain metastases. Neuro-Oncology Adv. 2021;3(1). Available from: https://academic.oup.com/noa/article/doi/10.1093/noajnl/vdab021/6125292. [DOI] [PMC free article] [PubMed]
- 33.Li J, Ludmir EB, Wang Y, Guha-Thakurta N, McAleer MF, Settle SH, et al. Stereotactic radiosurgery versus whole-brain Radiation Therapy for patients with 4–15 brain metastases: a phase III Randomized Controlled Trial. Int J Radiat Oncol Biol Phys. 2020;108(3):21–2. doi: 10.1016/j.ijrobp.2020.07.2108. [DOI] [Google Scholar]
- 34.Benjamin CG, Gurewitz J, Kavi A, Bernstein K, Silverman J, Mureb M et al. Survival and outcomes in patients with ≥ 25 cumulative brain metastases treated with stereotactic radiosurgery. J Neurosurg. 2022;137(2):571–81. Available from: https://thejns.org/view/journals/j-neurosurg/137/2/article-p571.xml. [DOI] [PubMed]
- 35.Kavi A, Gurewitz J, Benjamin CG, Silverman JS, Bernstein K, Mureb M et al. Hippocampal sparing in patients receiving radiosurgery for ≥ 25 brain metastases. Radiother Oncol. 2021;161:65–71. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167814021062575. [DOI] [PubMed]
- 36.Kraft J, Mayinger M, Willmann J, Brown M, Tanadini-Lang S, Wilke L et al. Management of multiple brain metastases: a patterns of care survey within the German Society for Radiation Oncology. J Neurooncol. 2021;152(2):395–404. Available from: http://ovidsp.ovid.com/ovidwebcgi?T=JS&PAGE=reference&D=prem&NEWS=N&AN=33620657. [DOI] [PMC free article] [PubMed]
- 37.Blomain ES, Kim H, Garg S, Bhamidipati D, Guo J, Kalchman I, et al. Stereotactic radiosurgery practice patterns for brain metastases in the United States: a national survey. J Radiat Oncol. 2018;7(3):241–6. doi: 10.1007/s13566-018-0353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutta SW, Sheehan JP, Niranjan A, Lunsford LD, Trifiletti DM. Evolution in the role of stereotactic radiosurgery in patients with multiple brain metastases: an international survey. J Clin Neurosci off J Neurosurg Soc Australas. 2018;57:6–12. doi: 10.1016/j.jocn.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 39.Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR -Mutated Advanced Non–Small-Cell Lung Cancer. J Clin Oncol. 2018;36(33):3290–7. 10.1200/JCO.2018.78.3118. [DOI] [PubMed]
- 40.Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T et al. Alectinib versus crizotinib in patients with ALK -positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673617305652. [DOI] [PubMed]
- 41.Strenger V, Lackner H, Mayer R, Sminia P, Sovinz P, Mokry M et al. Incidence and clinical course of radionecrosis in children with brain tumors. A 20-year longitudinal observational study. Strahlenther Onkol. 2013;189(9):759–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23963155. [DOI] [PubMed]
- 42.Nguyen TK, Sahgal A, Detsky J, Soliman H, Myrehaug S, Tseng C-L et al. Single-Fraction Stereotactic Radiosurgery Versus Hippocampal-Avoidance Whole Brain Radiation Therapy for Patients With 10 to 30 Brain Metastases: A Dosimetric Analysis. Int J Radiat Oncol Biol Phys. 2019;105(2):394–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31283978. [DOI] [PubMed]
- 43.Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M et al. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. 2008. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22479713. [PubMed]
- 44.Scorsetti M, Navarria P, Cozzi L, Clerici E, Bellu L, Franceschini D, Marzo AM, Franzese C, Torri V, Reggiori G, Lobefalo F, Raspagliesi L, Attuati L, Pessina F, Franzini A, Picozzi P, Tomatis S. Radiosurgery of limited brain metastases from primary solid tumor: results of the randomized phase III trial ( NCT02355613) comparing treatments executed with a specialized or a C-arm linac-based platform. Radiat Oncol. 2023;18(1):28. doi: 10.1186/s13014-023-02216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].




