Abstract
Outbred ICR mice were immune suppressed either with hydrocortisone or with 5-fluorouracil and were infected intranasally with Aspergillus fumigatus. Beginning 3 days before infection some groups of mice were given recombinant human granulocyte colony-stimulating factor (G-CSF), SCH56592 (an antifungal triazole), or both. Corticosteroid-pretreated mice responded to SCH56592 and had reduced counts in lung tissue and prolonged survival. In these mice, G-CSF strongly antagonized the antifungal activity of SCH56592. Animals treated with both agents developed large lung abscesses with polymorphonuclear leukocytes and large amounts of Aspergillus. In contrast, mice made neutropenic with 5-fluorouracil and then infected with A. fumigatus conidia benefited from either G-CSF or triazoles, and the effect of the combination was additive rather than antagonistic. Host predisposing factors contribute in different ways to the outcome of growth factor therapy in aspergillosis.
Aspergillus species are molds which are ubiquitous and which grow rapidly in most culture media. Humans are infected after they inhale Aspergillus conidia, which rapidly convert to mycelia (3). Aspergillus fumigatus is the most virulent of these species, but it still rarely attacks the immune-competent host (3, 5, 7, 9, 16, 18, 22, 23, 27, 28). Aspergillus conidia are readily killed by alveolar macrophages, and hyphae are readily killed by polymorphonuclear leukocytes (PMNs) (12, 14, 24, 37, 38). The immune-competent host can deal with large numbers of Aspergillus conidia, but invasive aspergillosis is a dreaded complication of immune suppression. Chief among the predisposing factors are neutropenia and corticosteroid use (3, 4, 10, 11, 24, 28–30). Steroids markedly increase the number of circulating neutrophils (PMNs) in the peripheral blood, but they impair their response to chemotaxins and also impair monocyte phagocytic and microbicidal activities against Aspergillus hyphae (13). In addition, cytotoxic agents cause sustained neutropenia, which also allows overgrowth and invasion of Aspergillus (1, 8, 11, 17, 27, 28).
Granulocyte colony-stimulating factor (G-CSF) is a recombinant human hormone which induces the bone marrow to accelerate the production of PMNs and also to increase their microbicidal activity (34, 35, 40, 41). G-CSF may be useful both in neutropenic patients, with whom most clinical studies have been done, and in patients with normal numbers of PMNs by further increasing the numbers and activity of the PMNs. Also, G-CSF may improve the effects of antifungal drugs (41). In murine candidiasis, we found that the benefit of G-CSF is additive when it is given with fluconazole antifungal therapy (21). Corticosteroids in vitro impair the ability of PMN to damage Aspergillus hyphae. G-CSF ameliorates this effect (34). Likewise, G-CSF in vitro corrects a serum-mediated suppressive effect of neutrophil-mediated killing of A. fumigatus in some children with human immunodeficiency virus (HIV) infection (33). When administered in vivo to healthy subjects, G-CSF activates PMNs so that their level of killing of A. fumigatus increases as much as 15-fold (25). In neutropenic mice intravenously infected with A. fumigatus, G-CSF is beneficial in prolonging survival, but it is less helpful in mice pretreated with corticosteroids (32).
Despite the studies described above, however, there is no clear evidence that G-CSF benefits patients with aspergillosis. In some trials, G-CSF was administered with other antifungal therapy in open studies (15). In a recent large review, Geller (19) found no evidence from multiple randomized prospective trials that there were significant reductions in fungal infections in patients with acute myelocytic leukemia given G-CSF. Therefore, in the present studies we sought to clarify whether G-CSF alone or combined with a novel broad-spectrum triazole, SCH56592, offered any benefit in vivo. For these studies we used two different murine models of pulmonary invasive aspergillosis.
MATERIALS AND METHODS
Drug.
SCH56592 is an antifungal triazole with considerable activity in vitro and in vivo against A. fumigatus in mice (20). SCH56592 was obtained from the Schering Plough Research Institute and was prepared in carboxymethyl cellulose.
Pathogen.
A. fumigatus 94-2766, a clinical isolate, was maintained on Sabouraud agar. The MIC of SCH56592 (Schering) for this organism was <0.03 μg/ml at 24 h and at 0.06 μg/ml at 48 h by the method adapted by the National Committee for Clinical Laboratory Standards for molds (31). Conidia were induced by culturing the mold on sporulation agar plates at room temperature. Conidia were harvested by flooding the plate with water and disrupting the culture with a magnetic spinning bar. Conidia and mycelial fragments were filtered through sterile nylon wool to separate the mycelial fragments, and the conidia were washed, counted with a hemacytometer, and suspended in water at the desired inoculum per 0.60 μl. The inoculum size was confirmed by quantitative serial dilution cultures, and the viable inoculum was recorded.
Mice.
Outbred ICR male mice weighing approximately 30 g were obtained from Charles River Laboratories. Mice were housed in groups of five per cage and were given food and water ad libitum. For infection, mice were briefly anesthetized with ketamine. A 6-μl droplet containing A. fumigatus conidia was placed on the nares while the diaphragm was compressed. The diaphragm was released and the mouse inhaled the droplet.
Immune suppression and treatment.
Cytotoxic chemotherapy was used to predispose one set of mice, and corticosteroid pretreatment was used to predispose the other. For the model of neutropenia, cyclophosphamide was administered at 200 mg/kg of body weight intraperitoneally on the day of infection. 5-Fluorouracil was given to the mice intravenously at 150 mg/kg, also on the day of infection. These mice were considered predisposed by neutropenia. In prior studies we found that the peripheral blood PMN count was <100/μl for 10 or more days after treatment. G-CSF was administered intraperitoneally at 125, 250, 500, or 600 μg/kg/dose daily beginning on day −3 and continuing through day 5 after infection. With this regimen, we found that peripheral blood PMN counts could be raised to approximately 30,000/μl in a week of daily treatment with 300 or 600 μg of G-CSF per kg. For corticosteroid pretreatment, hydrocortisone was administered to mice at 100 mg/kg subcutaneously beginning on day −2 before infection and continuing for 3 days (39). These mice were considered predisposed by steroids. In control studies with groups of three to five mice per group, we found that administration of G-CSF beginning 3 days before infection raised the mean peripheral blood neutrophil (PMN) count from 2.8 × 105 to 23 × 105/ml by 7 days of treatment. Recipients of hydrocortisone alone had reduced mean peripheral blood PMN counts of 0.9 × 105/μl. Recipients of G-CSF and hydrocortisone combined had a mean count of 86 × 105 PMNs/μl. SCH56592 was prepared in carboxymethyl cellulose at 1, 5, 10, or 25 mg/kg and was administered once daily in 0.2 ml by oral gavage from day 1 through day 9 after infection. In earlier studies we had found that 5 mg/kg was the minimal protective dose and that 25 mg/kg was consistently protective (20). Peak concentrations of SCH56592 in serum, determined by high-performance liquid chromatography at Schering Plough, were 5 or 12 μg/ml after the administration of single doses of 20 or 80 mg of SCH56592 per kg (data from Schering Plough Research Institute). Bioassay values were slightly higher. The terminal half-life of SCH56592 is approximately 20 h in mice.
Statistics.
For studies of survival, data were analyzed by the log-rank and Wilcoxon tests in two phases. In the first phase, tests that included data for all treatment groups in a study were performed. If the results of these tests were significant, then the relevant pairwise tests were carried out. For this second phase of analysis, the error rate (alpha) was adjusted so that the overall error rate for the entire study would be less than or equal to 0.05. Counts in tissue were analyzed by both parametric and nonparametric methods. The parametric analysis consisted of a one-way analysis of variance of the natural logarithms of the counts in tissue, followed by a multiple comparison test. The nonparametric method consisted of a Kruskall-Wallis test followed by pairwise Wilcoxon rank sum tests. As in the analysis of the survival data, for these pairwise comparisons the error rate (alpha) was adjusted so that the overall error rate for the entire study would be less than or equal to 0.05. Differences described as significant thus met the criteria for pairwise comparisons, which are set to reflect a P value of <0.05 for the entire study with multiple comparisons.
RESULTS
Neutropenic mice.
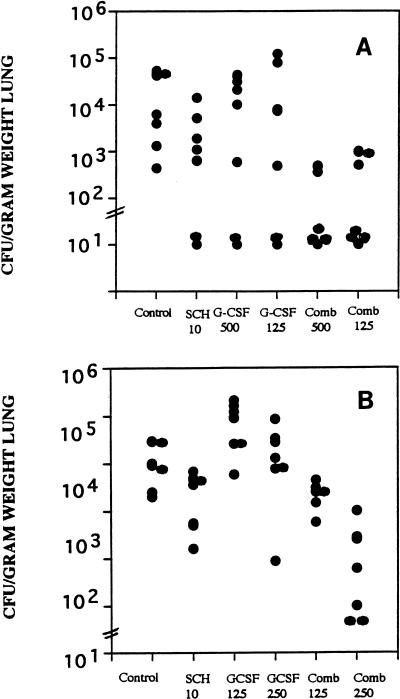
In the neutropenic model our cytotoxic regimen is sublethal, but it lowers the peripheral blood PMN count to <100/μl for 10 or more days after administration of the dose. The parameter for efficacy was a reduction of the counts in lung tissue. Four studies were done. In the first study the inoculum was very high (>108 CFU) (Fig. 1A). In this study the only regimens which significantly reduced the counts in lung tissue were G-CSF at 125 and 500 μg/kg combined with SCH56592 at 10 mg/kg. In a second study, with a lower infecting dose of 1.3 × 107 CFU, SCH56592 at 10 mg/kg/day slightly but significantly reduced the counts in lung tissue (Fig. 1B). G-CSF at 125 μg/kg was ineffective when it was used alone, and G-CSF did not benefit SCH56592 when G-CSF was combined with SCH56592. G-CSF had no benefit when it was given alone at 250 μg/kg. The combination of G-CSF and SCH56592 was more effective than no treatment or treatment with either agent alone. A third study was done with SCH56592 at a dose of 5 mg/kg and G-CSF at a dose of 125 or 500 μg/kg. SCH56592 and both combinations, but not G-CSF alone, reduced the counts significantly compared with those for the controls (data not shown). Combined therapy was not superior to G-CSF given alone.
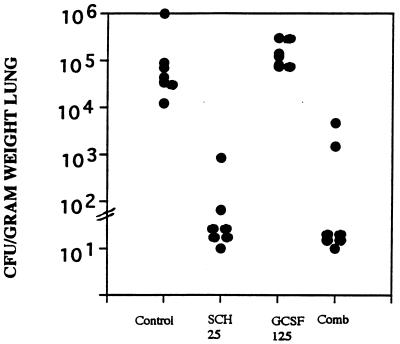
FIG. 1.
Burden of A. fumigatus in lung tissue. Groups of seven mice each were treated with G-CSF beginning on day −3 before infection, with cyclophosphamide at 200 mg/kg and 5-fluorouracil at 150 mg/kg on the day of infection, and with SCH56592 beginning 1 day after infection. Treatment was stopped on day 5 after infection, and mice were killed on day 6 after infection. (A) Mice were infected with >108 conidia, and only combined (Comb) treatment with SCH56592 at 10 mg/kg/dose and GCSF at 125 or 500 μg/kg significantly reduced tissue burden below that for the untreated controls. (B) Mice were infected with 3.7 × 107 CFU, and the effects of G-CSF at 125 or 250 μg/kg alone, SCH56592 alone, and the combined (Comb) treatment regimen of SCH56592 and G-CSF were evaluated; only the combined treatment regimen with G-CSF at 250 μg/kg/day significantly lowered the lung tissue counts below that for the untreated controls.
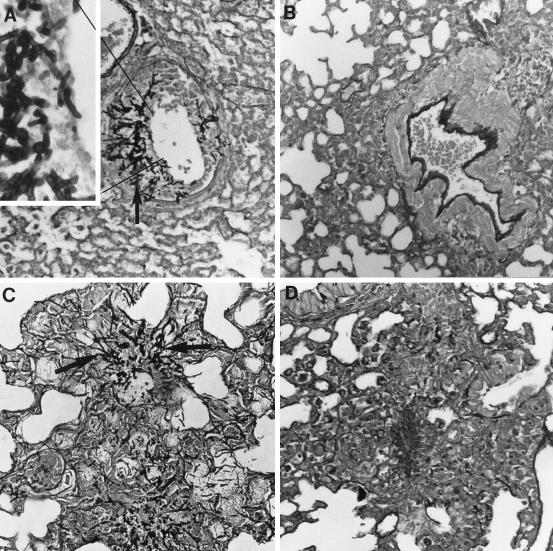
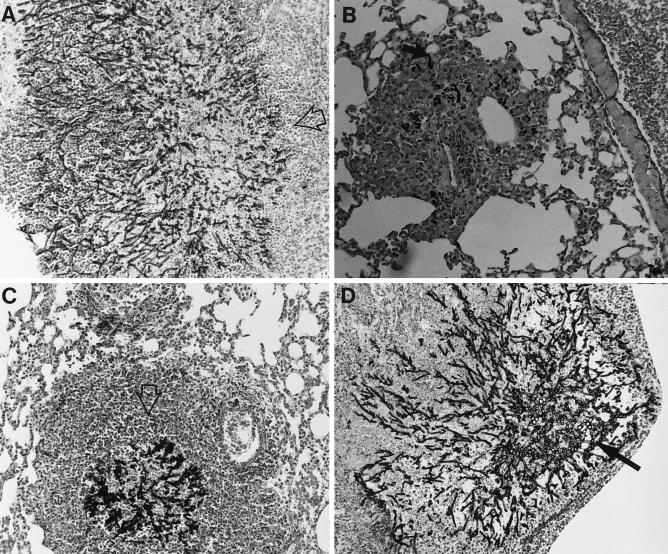
The fourth study (Fig. 2) was a histopathologic examination of the lungs of groups of three mice infected with 107 CFU and treated with G-CSF at 600 μg/kg, SCH56592 at 25 mg/kg, both, or neither. Mice were sacrificed after 6 days of treatment. In this study the controls showed mononuclear infiltrates with abundant hyphae (Fig. 2A). SCH56592 reduced the infiltrates to a minimal amount (Fig. 2B). No hyphae were seen. Mice treated with G-CSF had mycotic bronchitis and pneumonia, with some PMNs and mononuclear cell infiltrates, and hyphe were seen in the lungs of all three mice (Fig. 2C). Mice receiving combination therapy with G-CSF and SCH56592 showed predominantly mononuclear cell infiltrates, with no hyphae seen in any mice (Fig. 2D).
FIG. 2.
Histopathology of lungs of neutropenic mice infected with 107 CFU conidia, treated from days 1 through 6, and killed. There were three mice per group. Representative sections are shown at × 440 magnification, with the inset at × 1,000 magnification. The stain was hematoxylin and eosin combined with Gomori’s methenamine silver. (A) Untreated control mice. Two mice had extensive mycotic bronchitis and one had minimal infiltrates, which were monocytic. Large numbers of hyphae were seen invading the bronchioles in two mice (the inset shows hyphae invading a bronchiole; the solid arrow indicates hyphae). (B) Mice treated with SCH56592 at 10 mg/kg/day. Minimal monocyte infiltrates were seen in two mice. No hyphae were seen. A lesion from one mouse is shown. (C) G-CSF at 600 μg/kg/day. Many large collections of PMNs were seen in one mouse, and mycotic bronchitis and pneumonia were observed in the others. Abundant hyphae were seen in lesions from all three mice (solid arrow). Some bacterial overgrowth is also apparent in this section. (D) G-CSF and SCH56592. Minimal mononuclear infiltrates were seen in all three mice, with some PMNs seen in the lesions of one mouse. No hyphae were seen. A lesion is shown.
Corticosteroid-pretreated mice.
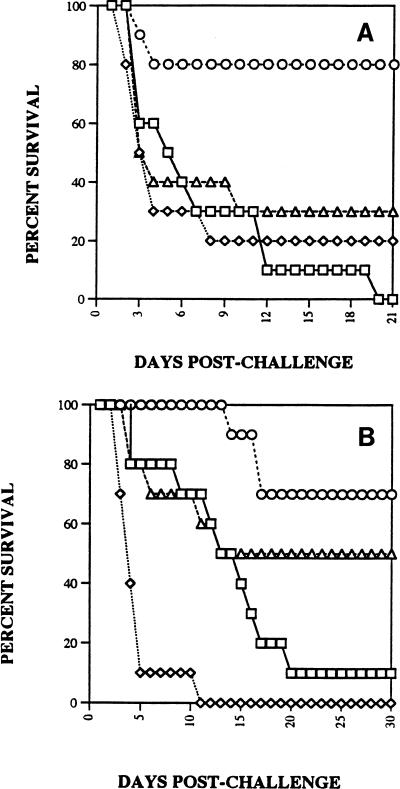
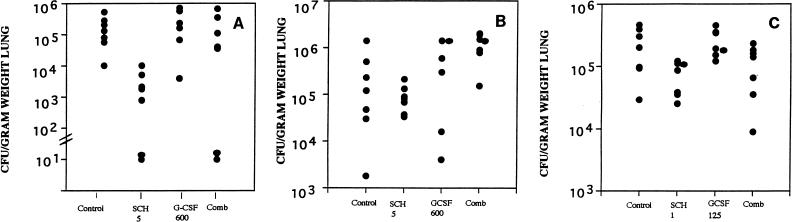
We had found in earlier studies that SCH56592 both prolonged survival and reduced the lung tissue burden for corticosteroid-pretreated mice infected with A. fumigatus (20 [two additional studies are reported therein]). SCH56592 at either 5 mg/kg (Fig. 3A) or 25 mg/kg (Fig. 3B) significantly increased the survival rate for infected mice. In contrast, G-CSF at 600 μg/kg/dose significantly shortened survival compared with that for the controls and abrogated the benefit of SCH56592. The effect was similar with the higher inoculum of 1.3 × 108/mouse (Fig. 3A) or a 2-log lower dose (Fig. 3B). A study of tissue burden was done in parallel with the study whose results are presented in Fig. 3A (Fig. 4A). This showed a reduction in the counts in lung tissue with SCH56592 at 5 mg/kg/dose and abrogation of that effect by combined therapy with G-CSF. A second study (data not shown) confirmed this. Follow-up studies of the counts in lung tissue were done to explore the effects of lower infecting doses of Aspergillus and lower doses of G-CSF (Fig. 4B and C). When given at 600 μg/kg/day, G-CSF added no benefit to SCH56592 when SCH56592 was used at the marginally effective dosage of 5 mg/kg/day (Fig. 4B). A lower G-CSF dose of 125 μg/kg combined with SCH56592 at 1 mg/kg also showed that G-CSF gave no benefit and had no interaction with SCH56592 (Fig. 4C). Finally, because none of the preceding studies with inocula of between 107 and 108 CFU showed protection, we conducted a study of the counts in lung tissue using an inoculum of only 4.5 × 104 CFU, a sublethal challenge, and treatment with SCH56592 at a high dosage of 25 mg/kg/day or G-CSF at a dosage of 125 μg/kg/day, or both (Fig. 5). At 25 mg/kg, SCH56592 markedly reduced the counts in tissue, and the effect of G-CSF appeared to be worse than that of no treatment, with higher counts found in the lung tissue of mice treated with G-CSF. At the high dose of SCH56592 (25 mg/kg), combined therapy again offered no benefit, and the effect was similar to that of SCH56592 alone.
FIG. 3.
Survival after intranasal infection of groups of 10 mice with conidia of A. fumigatus. Mice were treated with G-CSF at 600 μg/kg beginning on day −3 before infection and continuing through day 5 after infection. Hydrocortisone at 100 mg/kg was begun subcutaneously on day −2 before infection and was continued through day 1 after infection. SCH56592 was begun on day 1 and was continued through day 5 after infection. □, control; ◊, G-CSF at 600 μg/kg; ○, SCH56592; ▵, G-CSF at 600 μg/kg and SCH56592. (A) Mice were infected with 1.3 × 108 CFU. Only SCH56592 at 5 mg/kg/day significantly prolonged survival beyond that for untreated controls. (B) Mice were infected with 2.2 × 106 CFU. SCH56592 at 25 mg/kg/day alone prolonged survival. The response to combined therapy was significantly shorter than that to therapy with SCH56592 alone.
FIG. 4.
Lung tissue burden of A. fumigatus as CFU per gram of lung weight. (A) Mice were concurrently infected with those in the survival study (Fig. 3A) and were infected with 1.3 × 108 CFU. Only SCH56592 at 5 mg/kg/day (SCH 5) significantly reduced the tissue burden. Slashed bars across the y axis indicate the minimum detectable counts, usually 20 to 30 CFU/g of lung tissue. (B) Mice were infected with 4 × 107 CFU and were given G-CSF at 600 mg/kg/day beginning on day −3. The G-CSF and combined (Comb) treatment groups had only seven mice because of cannibalism of several mice that succumbed early. No significant differences were seen, although the result for SCH56592 alone was close to significance compared with the result for the controls. (C) Mice were infected with 107 CFU and were given either SCH56592 at a low dose of 1 mg/kg/day (SCH 1), GCSF at 125 μg/kg/day, or both. No significant reduction in counts in tissue compared with the counts for untreated controls was noted for any group.
FIG. 5.
Lung tissue burden of A. fumigatus. Mice were infected with a sublethal inoculum of 4.5 × 104 CFU and treated with SCH56592 at 25 mg/kg/dose, GCSF at 125 μg/kg/dose, or both (Comb). With this regimen SCH56592 alone and combined therapy reduced the counts in lung tissue significantly more than no treatment did. Combined therapy was not superior to SCH56592 alone.
Finally, we examined the histopathology of lungs obtained from groups of three mice each after 6 days of treatment with the following: no treatment (controls), G-CSF at 600 μg/kg, SCH56592 at 10 mg/kg, and combined therapy. Representative tissue sections are shown in Fig. 6. All control mice had large numbers of Aspergillus hyphae, with modest local, predominantly monocytic infiltrates, although some PMNs were present. Mice given SCH56592 had similar cell infiltrates, but with almost no fungi were seen. All mice given either G-CSF alone or G-CSF combined with SCH56592 had dense abscesses composed of PMNs and large numbers of fungal mycelia within the necrotic abscesses.
FIG. 6.
Histopathology of the lungs of mice 6 days after infection with 107 A. fumigatus. Hematoxylin and eosin stain combined with Gomori’s methenamine silver was used. Three mice were examined for each treatment group. Magnifications, × 400. (A) Untreated control mice. Large numbers of hyphae are seen invading the bronchioles. Foci of hyphae are surrounded by a modest infiltrate of predominantly monocytic cells with a few PMNs (open arrow). (B) Mice treated with SCH56592 at 10 mg/kg/day. Few hyphae are seen (solid arrow), with modest surrounding infiltrates of mononuclear cells. (C) G-CSF at 600 μg/kg/day. Many large collections of PMNs and necrotic debris (open arrow) surround foci with large numbers of hyphae present. (D) G-CSF and SCH56592. Large abscesses with PMNs surrounding foci of A. fumigatus hyphae. Hyphae (solid arrow) are more numerous than in mice treated with SCH56592 alone.
DISCUSSION
G-CSF is one of the first recombinant growth factors to become available for clinical use. Prospective, randomized, placebo-controlled trials with patients with acute myelocytic leukemia have shown that G-CSF treatment shortens the time that the PMN counts are <500/μl, shortens the period of hospitalization for chemotherapy, and reduces the number of neutropenia-associated infections. However, the benefits appear to be rather modest at best and are not consistent for all studies, at least in patients with acute myelocytic leukemia (19). G-CSF in vitro causes increased microbicidal activation of PMNs against Staphylococcus aureus but not Candida albicans (35). In studies with PMNs taken from volunteers treated with G-CSF, the G-CSF caused an increase in the level of damage to Aspergillus hyphae caused by PMNs compared with that for untreated controls (25). However, it has been difficult to demonstrate clinically that G-CSF plays a protective role in candidiasis or aspergillosis.
A potential answer may be sought in reviewing the predisposing factors for aspergillosis. Neutropenia absolutely reduces the number of circulating phagocytic cells, so there are fewer cells to attack Aspergillus hyphae. The recovery of numbers of neutrophils and the activation of neutrophils, which is accomplished by G-CSF, returns the host’s immune response to a normal state and should restore (to some degree) host defenses. In this context, we found an additive effect of G-CSF and antifungal therapy with SCH56592 in all three studies. Unlike amphotericin B, which activates macrophages, in addition to its direct antifungal effects, triazoles have relatively little effect on the host’s immune response. Therefore, the additive reduction of lung tissue burden in our studies reflects increased PMN numbers and the activity of G-CSF and also the antifungal activity of the triazole. Histopathologic studies confirmed that mice given SCH56592 and combined treatment with both SCH56592 and G-CSF had no demonstrable hyphae. These studies support and extend those of Polak-Wyss (32), who found that G-CSF and genaconazole (another broad-spectrum triazole) prolonged survival over that for the controls and suggested that G-CSF combined with a low dose of genaconazole were superior to genaconazole alone.
In contrast, corticosteroids, whose use is another major predisposing factor for aspergillosis, do not deplete the numbers of PMNs in the peripheral blood but actually may increase them. However, the PMNs and monocytes are somewhat dysfunctional at ingesting and killing microbes, including Aspergillus conidia (13). PMNs and monocytes exposed to corticosteroids do not efficiently release tumor necrosis factor alpha and do not move well toward chemotactic stimuli. Thus, patients with AIDS, in whom progressive invasive aspergillosis is a dreaded complication of end-stage disease, have dysfunctional phagocytic cells and may also be receiving corticosteroid therapy but often are not neutropenic (22).
In our studies, G-CSF was ineffective at low doses, and the effect of concurrent therapy with G-CSF at higher doses along with a triazole was either indifferent or antagonistic. The causes for this are not entirely clear. However, the rise in leukocyte counts and the development of large pulmonary abscesses suggest a destructive process that is at least somewhat analogous to a period of vulnerability which occurs in humans after recovery from neutropenia. At this point in the infection the pneumonia caused by Aspergillus may actually increase in size as large numbers of PMNs migrate to the site of infection. If the foci of infection are near the pulmonary artery, there is a heightened risk of lethal hemorrhage (2, 6). Furthermore, in patients with Aspergillus pneumonic processes located near the pulmonary arteries, either during neutropenia or after recovery, there is sufficient risk of hemorrhage that very aggressive resectional surgery has been recommended (11, 26, 30). We suspect that the large necrotic foci in our corticosteroid-treated mice reflect a similar migration into areas of Aspergillus infection and also that the PMNs, under the influence of steroids, are unable to kill the hyphae in these large abscesses. Of interest, Polak-Wyss (32) found that cortisone-pretreated mice infected intranasally had an increase in survival from a mean of 3.8 days to one of 10.7 days when a low dose of G-CSF (1 μg/mouse) was used. However, as the G-CSF dose was raised, the survival shortened progressively to 2.5 days when G-CSF was used at 5 μg/dose/mouse. This suggests a narrow range for the therapeutic benefit of G-CSF, with a strong adverse effect of higher doses of G-CSF. Polak-Wyss (32) also found that genaconazole was protective in both neutropenic and steroid-pretreated mice infected intranasally. In our steroid-pretreated mice, the antagonistic effect of G-CSF on triazole therapy was thus unexpected. This may be caused by the failure of the drug to penetrate the dense abscesses in which the fungal hyphae were seen. This effect was evident at a low dose of 5 mg/kg (Fig. 4C). At a higher dose of SCH56592 (25 mg/kg) (Fig. 5), the triazole was highly effective alone, and the addition of G-CSF did not antagonize this effect. This may have occurred because higher drug concentrations penetrated to the center of the abscesses or because the infecting dose was lower and caused a less destructive pulmonary process. It is not due to an inadequate dose of SCH56592 administered orally, because in the absence of G-CSF the counts in tissue were reduced on quantitative cultures and very few hyphae were seen in histopathologic examination of mice.
Another growth factor, granulocyte-macrophage colony-stimulating factor (GM-CSF) has been shown to provide protection against fungal infections, including aspergillosis, in at least one clinical trial with patients with acute myelocytic leukemia (36). Unlike G-CSF, GM-CSF does not cause such dramatic increases in leukocyte counts as G-CSF. GM-CSF does activate PMNs, monocytes, and macrophages so that they have increased microbicidal activity. It is possible that GM-CSF may add to host defenses more by activating cells than by inducing absolute rises in cell numbers, with the resulting lesser damage from an acute inflammatory process.
Finally, many patients with acute invasive aspergillosis are receiving not only cytotoxic therapy but also are receiving corticosteroids at the same time (3, 28). It may be that G-CSF, by increasing the numbers of dysfunctional PMNs in corticosteroid-treated mice, contributes to abscess formation and does not have much of an antifungal effect. If a similar process occurs in patients treated with corticosteroids, this may in part explain why there has thus far been no clinical demonstration of an in vivo benefit of G-CSF treatment in aspergillosis. Such an adverse interaction would be a significant adverse consequence of using concurrent G-CSF and corticosteroid therapy. This also calls into question our sometimes casual tendency to group fungi, such as Candida and Aspergillus, together in considering them a single target for growth factor therapy. This concept may be naive.
ACKNOWLEDGMENT
This study was supported by Schering-Plough Research Institute, Kenilworth, N.J.
REFERENCES
- 1.Adelman B A, Bentman A, Rosenthal P, Smith B R, Bridges K R, Holmes W. Treatment of aspergillosis in leukemia. Ann Intern Med. 1979;91:323–324. doi: 10.7326/0003-4819-91-2-323_2. [DOI] [PubMed] [Google Scholar]
- 2.Albelda S M, Talbot G H, Gerson S L, Miller W T, Cassileth P A. Pulmonary cavitation and massive hemoptysis in invasive pulmonary aspergillosis: influence of bone marrow recovery in patients with acute leukemia. Am Rev Respir Dis. 1985;131:115–120. doi: 10.1164/arrd.1985.131.1.115. [DOI] [PubMed] [Google Scholar]
- 3.Andriole, V. T. 1993. Infections with Aspergillus species. Clin. Infect. Dis. 17(Suppl. 2):S481–S486. [DOI] [PubMed]
- 4.Berenguer J, Allende M C, Lee J W, Garret K, Lyman C, Ali N M, Bacher J, Pizzo P A, Walsh T J. Pathogenesis of pulmonary aspergillosis—granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am J Respir Crit Care Med. 1995;152:1079–1086. doi: 10.1164/ajrccm.152.3.7663787. [DOI] [PubMed] [Google Scholar]
- 5.Binder R E, Faling L J, Pugatch R D, Mahasaen C, Snider G L. Chronic necrotizing pulmonary aspergillosis: a discrete clinical entity. Medicine (Baltimore) 1982;61:109–124. doi: 10.1097/00005792-198203000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Borkin M H, Arena F P, Brown A E, Armstrong D. Invasive aspergillosis with massive fatal hemoptysis in patients with neoplastic disease. Chest. 1980;78:835–839. doi: 10.1378/chest.78.6.835. [DOI] [PubMed] [Google Scholar]
- 7.Brown E, Freedman S, Arbeit R, Come S. Invasive pulmonary aspergillosis in an apparently nonimmunocompromised host. Am J Med. 1980;69:624–627. doi: 10.1016/0002-9343(80)90478-7. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J, Denning D W, Viviani M A EORTC Invasive Fungal Infection Cooperative Group. Epidemiology of invasive aspergillosis in European cancer centres. Eur J Clin Microbiol Infect Dis. 1993;12:392–393. doi: 10.1007/BF01964440. [DOI] [PubMed] [Google Scholar]
- 9.Cook D J, Achong M R, King D E L. Disseminated aspergillosis in an apparently healthy patient. Am J Med. 1990;88:74–76. doi: 10.1016/0002-9343(90)90132-w. [DOI] [PubMed] [Google Scholar]
- 10.Denning D W, Follansbee S F, Scolaro M, Norris S, Edelstein H, Stevens D A. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:654–662. doi: 10.1056/NEJM199103073241003. [DOI] [PubMed] [Google Scholar]
- 11.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 12.deRepentigny L, Petitbois S, Boushira M, Michaliszyn E, Senechal S, Gendron N, Montplaisir S. Acquired immunity in experimental murine aspergillosis is mediated by macrophages. Infect Immun. 1993;61:3791–3802. doi: 10.1128/iai.61.9.3791-3802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond R D. Inhibition of monocyte-mediated damage to fungal hyphae by steroid hormones. J Infect Dis. 1983;147:160. doi: 10.1093/infdis/147.1.160. [DOI] [PubMed] [Google Scholar]
- 14.Diamond R D, Krezicki R, Epstein B, Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. Am J Pathol. 1978;91:313–327. [PMC free article] [PubMed] [Google Scholar]
- 15.Dornbusch H J, Urban C E, Pinter H, Ginter G, Fotter R, Becker H, Miorini T, Berghold C. Treatment of invasive pulmonary aspergillosis in severely neutropenic children with malignant disorders using liposomal amphotericin B (AmBisome), granulocyte colony-stimulating factor, and surgery: report of five cases. Pediatr Hematol Oncol. 1995;12:577–586. doi: 10.3109/08880019509030772. [DOI] [PubMed] [Google Scholar]
- 16.Edge J R, Stansfield D, Fletcher D E. Pulmonary aspergillosis in an unselected hospital population. Chest. 1971;59:407–413. doi: 10.1378/chest.59.4.407. [DOI] [PubMed] [Google Scholar]
- 17.Finegold S M, Will D, Murray J F. Seminar on mycotic infections: aspergillosis. Am J Med. 1959;27:463–482. [Google Scholar]
- 18.Gefter W B, Weingrad T R, Epstein D M, Ochs R H, Miller W T. “Semi-invasive” pulmonary aspergillosis. Radiology. 1981;140:313–321. doi: 10.1148/radiology.140.2.7255704. [DOI] [PubMed] [Google Scholar]
- 19.Geller R B. Use of cytokines in the treatment of acute myelocytic leukemia: a critical review. J Clin Oncol. 1996;14:1371–1382. doi: 10.1200/JCO.1996.14.4.1371. [DOI] [PubMed] [Google Scholar]
- 20.Graybill J, Najvar L, Bocanegra R, Fothergill A, Luther M. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Treatment of murine pulmonary aspergillosis with SCH56592 (SCH), abstr. F99; p. 116. [Google Scholar]
- 21.Graybill J R, Bocanegra R, Luther M. Antifungal combination therapy with granulocyte colony-stimulating factor and fluconazole in experimental disseminated candidiasis. Eur J Clin Microbiol Infect Dis. 1995;14:700–703. doi: 10.1007/BF01690878. [DOI] [PubMed] [Google Scholar]
- 22.Khoo, S. H., and D. W. Denning. 1994. Invasive aspergillosis in patients with AIDS. Clin. Infect. Dis. 19(Suppl. 1):S41–S48. [DOI] [PubMed]
- 23.Lake K B, Browne P M, Van Dyke J J, Ayers L. Fatal disseminated aspergillosis in an asthmatic patient treated with corticosteroids. Chest. 1983;83:138–139. doi: 10.1378/chest.83.1.138. [DOI] [PubMed] [Google Scholar]
- 24.Levitz S M, Diamond R D. Mechanisms of resistance of Aspergillus fumigatus conidia to killing by neutrophils in vitro. J Infect Dis. 1985;152:33–42. doi: 10.1093/infdis/152.1.33. [DOI] [PubMed] [Google Scholar]
- 25.Liles W C, Huang J E, Van Burik J A, Bowden R A. Granulocyte colony-stimulating factor administered in vivo augments neutrophil-mediated activity against opportunistic fungal pathogens. J Infect Dis. 1997;175:1012–1015. doi: 10.1086/513961. [DOI] [PubMed] [Google Scholar]
- 26.Massard G, Lioure B, Wihlm J-M, Morand G. Resection of mycotic lung sequestra after invasive aspergillosis. Ann Thorac Surg. 1993;55:563–564. doi: 10.1016/0003-4975(93)91056-s. [DOI] [PubMed] [Google Scholar]
- 27.McCormick W F, Schochet S S, Weaver P R, McCrary J A. Disseminated aspergillosis. Arch Pathol. 1975;99:353–359. [PubMed] [Google Scholar]
- 28.McWhinney P H M, Kibbler C C, Hamon M D, Smith O P, Gandhi L, Berger L A, Walesby R K, Hoffbrand A V, Prentice H G. Progress in the diagnosis and management of aspergillosis in bone marrow transplantation: 13 years’ experience. Clin Infect Dis. 1993;17:397–404. doi: 10.1093/clinids/17.3.397. [DOI] [PubMed] [Google Scholar]
- 29.Minamoto G Y, Barlam T F, Vander Els N J. Invasive aspergillosis in patients with AIDS. Clin Infect Dis. 1992;14:66–74. doi: 10.1093/clinids/14.1.66. [DOI] [PubMed] [Google Scholar]
- 30.Moreau P, Zahar J R, Milpied N, Baron O, Mahé B, Wu D, Germaud P, Despins P, Delajartre A Y, Harousseau J L. Localized invasive pulmonary aspergillosis in patients with neutropenia: effectiveness of surgical resection. Cancer. 1993;72:3223–3226. doi: 10.1002/1097-0142(19931201)72:11<3223::aid-cncr2820721115>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 31.Pfaller M A, Bale M, Buschelman B, Lancaster M, Espinel-Ingroff A, Rex J H, Rinaldi M G, Cooper C R, McGinnis M R. Quality control guidelines for National Committee for Clinical Laboratory Standards-recommended broth macrodilution testing of amphotericin B, fluconazole, and flucytosine. J Clin Microbiol. 1995;33:1104–1107. doi: 10.1128/jcm.33.5.1104-1107.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polak-Wyss A. Protective effect of human granulocyte colony-stimulating factor (hG-CSF) on Cryptococcus and Aspergillus infections in normal and immunosuppressed mice. Mycoses. 1991;34:205–215. doi: 10.1111/j.1439-0507.1991.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 33.Roilides E, Holmes A, Blake C, Pizzo P A, Walsh T J. Impairment of neutrophil antifungal activity against hyphae of Aspergillus fumigatus in children infected with human immunodeficiency virus. J Infect Dis. 1993;167:905–911. doi: 10.1093/infdis/167.4.905. [DOI] [PubMed] [Google Scholar]
- 34.Roilides E, Uhlig K, Venzon D, Pizzo P A, Walsh T J. Prevention of corticosteroid-induced suppression of human polymorphonuclear leukocyte-induced damage of Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993;61:4870–4877. doi: 10.1128/iai.61.11.4870-4877.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roilides E, Walsh T J, Pizzo P, Rubin M. Granulocyte colony stimulating factor enhances the phagocytic and bactericidal activity of normal and defective human neutrophils. J Infect Dis. 1991;163:579–583. doi: 10.1093/infdis/163.3.579. [DOI] [PubMed] [Google Scholar]
- 36.Rowe J M, et al. A randomized, placebo-controlled phase III study of granulocyte-macrophage colony-stimulating factor in adult patients (>55 to 70 years of age) with acute myelogenous leukemia; a study of the Eastern Cooperative Oncology Group (E1490) Blood. 1995;86:457–462. [PubMed] [Google Scholar]
- 37.Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982;69:617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaffner A, Douglas H, Braude A I, Davis C E. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect Immun. 1983;42:1109–1115. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidransky H, Friedman L. The effect of cortisone and antibiotic agents on experimental pulmonary aspergillosis. Am J Pathol. 1959;35:169–184. [PMC free article] [PubMed] [Google Scholar]
- 40.Uchida K, Yamamoto Y, Klein T W, Friedman H, Yamaguchi H. Granulocyte-colony stimulating factor facilitates the restoration of resistance to opportunistic fungi in leukopenic mice. J Med Vet Mycol. 1992;30:293–300. doi: 10.1080/02681219280000381. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto Y, Uchida K, Klein T W, Friedman H, Yamaguchi H. Immunomodulators and fungal infections: use of antifungal drugs in combination with GCSF. In: Freidman H, editor. Microbial infections. New York, N.Y: Plenum Press; 1992. pp. 231–241. [DOI] [PubMed] [Google Scholar]