Abstract
Background
To investigate the value of fluid-attenuated inversion recovery vascular hyperintensity (FVH) within asymmetrical prominent veins sign (APVS) on susceptibility-weighted imaging predicting collateral circulation and prognosis in patients with acute anterior circulation ischemic stroke.
Method
Patients with severe stenosis or occlusion of ICA or MCA M1, who underwent MRI within 72 h from stroke onset were reviewed. The Alberta Stroke Program Early CT Score was used to evaluate the volume of infarction on DWI, the degree of FVH and APVS. Spearman correlation analysis was used to evaluate the correlation between FVH and APVS. All patients were divided into the good prognosis group and the poor prognosis group according to the score of the modified ranking scale (mRS) 90 days after the stroke. Logistic regression analysis was used to explore the relationship between FVH and APVS and functional prognosis, while receiver operating characteristic (ROC) curves were plotted to assess the value of FVH and APVS in predicting prognosis.
Results
Spearman correlation analysis revealed moderate positive correlations between FVH and APVS (r = 0.586, P < 0.001). The poor prognosis group had a higher rate of a history of atrial fibrillation, a larger cerebral infarction volume, a higher NIHSS score at admission, and a higher FVH and APVS score compared with the good prognosis group (all P < 0.05). A further logistic regression indicated that the NIHSS score, cerebral infarction volume, FVH and APVS were independent risk factors for a poor functional prognosis. In terms of FVH, APVS, alone and their combination for the diagnosis of poor prognosis, the sensitivity, specificity, area under the ROC curve (AUC), and 95% confidence interval (CI) were 86.8%, 83.3%, 0.899 (95% CI 0.830–0.968); 60.5%, 93.7%, 0.818 (95% CI 0.723–0.912); 86.8%, 89.6%, 0.921 (95% CI 0.860–0.981), respectively.
Conclusion
The presence of FVH and APVS can provide a comprehensive assessment of collateral circulation from the perspective of veins and arteries, and the correlation between the two is positively correlated. Both of them were independent risk factors for poor prognosis, their combination is complementary and can improve the predictive value.
Keywords: Acute ischemic stroke, Asymmetrical prominent veins sign, Susceptibility-weighted imaging, FLAIR vascular hyperintensity, Collateral circulation, Prognosis
Introduction
Acute ischemic stroke (AIS) has a large burden of morbidity and mortality worldwide, accounting for 87% of all total strokes [1]. Identification of irreversible infarct core and salvageable penumbra is essential to guide the therapeutic decision. The clinical practice found that there was a mismatch between the volume of infarction and the therapeutic effects, as well as the functional prognosis. This diversity may be partly explained by the different compensatory capacities of collateral circulation [2, 3]. Good collaterals enable timely reperfusion of blood flow to the ischemic penumbra, including reducing the volume of cerebral infarction, prolonging the therapeutic window, decreasing neurological impairments, and improving prognosis [4]. Recently, imaging techniques with contrast media have been introduced for the evaluation of collateral circulation, however, considering the complication of irreversible renal damage caused by contrast injection, and the inability to perform it in patients with renal insufficiency or contrast medium allergy, it is necessary to find a more non-invasive imaging method to estimate collaterals [5].
Fluid-attenuated inversion recovery (FLAIR) is now routinely used to improve the detection of lesions in patients with AIS because of its ability to suppress cerebrospinal fluid hyper-signal. FLAIR vascular hyperintensity (FVH) was probably due to compensatory reflux from the distal to the proximal of a stenotic or occluded vessel, as the slow blood flow would lead to a loss of the flowing void effect and result in hyperintensity, serving as a non-contrast visualization marker of leptomeningeal collaterals [6–8]. When the primary collateral circulation cannot provide enough blood perfusion, the secondary and tertiary collateral vessels will be established. Among them, the opening of leptomeningeal collaterals is an important pathway. FVH could serve as an objective basis for the formation of leptomeningeal collateral circulation after stroke [9]. Many studies have confirmed that FVH was correlated with circulation as well as the severity of hemodynamic impairments, opening up a new idea of exploring noninvasive assessment of the status of intracranial collaterals and prognosis [10–12].
Susceptibility-weighted imaging (SWI) is a novel, high-resolution, three-dimensional gradient-echo MR technique, characterized by being highly sensitive to paramagnetic substances and has been implemented in the assessment of cerebral hemodynamics following AIS [13]. Asymmetrical dilated-vessel-like signal loss seen in the ischemic cerebral hemisphere on SWI, namely asymmetrical prominent veins sign (APVS), can indirectly show an increase of oxygen extraction fraction (OEF) in the penumbral, has been increasingly recognized as an alternative to assess collaterals and an imaging biomarker for predicting prognosis [14–16]. It has been widely believed that the APVS correlated with uncoupling between the oxygen supply and demand in the hypoperfusion tissue, resulting in a relative increase in deoxyhemoglobin [17]. To our knowledge, no studies have been found on exploring the correlation between the combination of FVH and APVS in the evaluation of collateral circulation in AIS, and predicting the functional prognosis. Therefore, this study aimed to investigate the relationship between FVH, APVS and prognosis, to explore the correlation between the status of collaterals and relevant factors affecting prognosis, and to provide an imaging basis for the blood perfusion status in the ischemic area, individualized precise treatment and prognosis evaluation.
Methods
Participants
We performed a retrospective study of patients with AIS hospitalized in the Affiliated Yantai Yuhuangding Hospital of Qingdao University between July 1, 2021 and October 1, 2022. Patients were eligible if they met the following inclusion criteria: (i) age ≥ 18 years; (ii) diagnosis of anterior circulation stroke due to occlusion or server stenosis (> 70%) of the unilateral internal carotid artery (ICA) and/or M1 segment of the middle cerebral artery (MCA); (iii) multimodal MR protocol including diffusion-weighted imaging (DWI), fluid-attenuated inversion recovery (FLAIR), time-of-flight MR angiography (TOF-MRA) and SWI sequence was performed within 72 h after symptom onset; and (iv) pre-stroke modified Rankin scale score ≤ 1; The exclusion criteria were (i) MRI-confirmed of the posterior circulation or bilateral infarction; (ii) absence of complete clinical and imaging data; (iii) intracranial hemorrhage evident on CT imaging; and (iv) patients who underwent intravenous or intra-arterial thrombolytic therapy.
This study protocol was approved by the institutional review board of our hospital. All subjects provided informed consent in accordance with the Declaration of Helsinki.
Collection of clinical data
Demographic characteristics (age and sex) and clinical characteristics, including vascular risk factors (hypertension, diabetes, dyslipidemia, ischemic heart disease, atrial fibrillation, prior stroke/transient ischemic attack (TIA), active smoking and drinking history), the admission National Institute of Health Stroke Scale (NIHSS) score was collected from electronic medical records. All patients received antiplatelet or anticoagulation therapy as the guideline recommended [18]. Clinical outcomes were assessed with the modified Rankin Scale (mRS) at 90 days after stroke onset. Good and poor functional outcomes were defined by the mRS scores of 0–2 and 3–5, respectively [19].
MRI protocol
Multimodal MRI was performed using a 3.0-T system (GE Discovery 750, USA). The imaging protocol included T1 (time repetition (TR) = 1750 ms, time echo (TE) = 24 ms, field of view (FOV) = 220 mm, slice thickness = 5 mm, matrix = 320 × 320, duration = 1 min 51 s), T2 (TR = 5392 ms, TE = 120 ms, Matrix = 240 × 240, FOV = 240 mm, section thickness = 5 mm, duration = 1 min 21 s), DWI(TR = 5237 ms, TE = 101 ms, b-value = 1000 s/mm2, FOV = 240 mm, section thickness = 5 mm, section gap = 1.5 mm, matrix = 240 × 240, acquisition duration = 42 s), FLAIR(TR = 9000 ms; TE = 100 ms; flip angle = 16°; matrix = 300 × 240; section thickness = 5 mm; acquisition duration = 1 min 57 s;), three-dimensional time-of-flight magnetic resonance angiography (TOF-MRA) (TR = 20 ms; TE = 2.5 ms; flip angle = 20°; matrix = 272 × 240; section thickness = 1.4 mm; acquisition duration = 5 min 10 s) and SWI (TR = 86.1 ms, TE = 45 ms, Matrix = 267 × 240, flip angle = 15°, FOV = 240 mm, section thickness = 2.0 mm, duration = 5 min 40 s).
Image analysis
One experienced neuroradiologist and one trained neurologist, blinded to the clinical parameters of the patients, assessed all MR imaging independently. Any discrepancies were resolved and consensus. The volume of infarction on DWI images was assessed by the standard Alberta Stroke Program Early CT score (DWI-ASPECTS). Fresh infarcts were visible in each region with a score of 1. The total score ranges from 10 (complete ischemic involving the whole MCA territory) to 0 (without early ischemic change) [20].
The FHV was defined as linear or serpentine high signal intensity relative to gray matter along the cortical sulci or brain surface in the cerebral hemisphere, reflecting the status of leptomeningeal collaterals [21]. The FVH-ASPECTS [22], a scoring system ranging following insular and M1-M6 regions was used to quantify the FVH (Fig. 1). The presence of FVH in each region was scored as 1.0 indicating the absence of FVH and 7 suggesting prominent FVH.
Fig. 1.
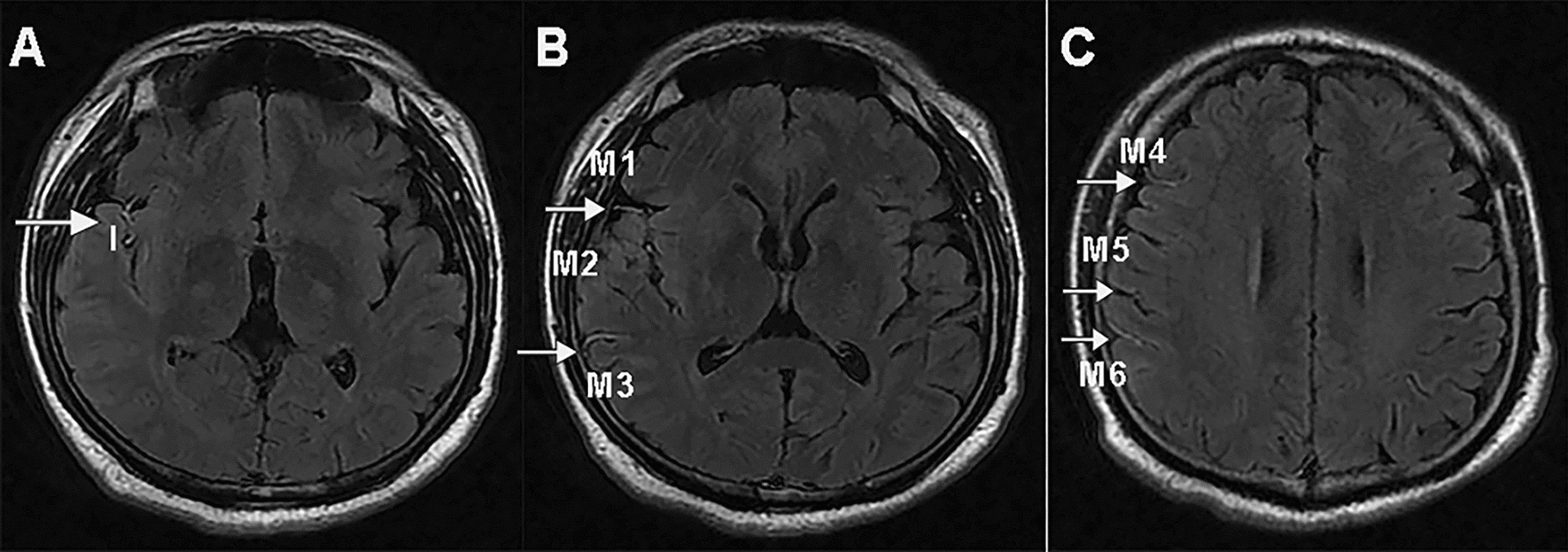
Images illustrating FVH: blood supply in area of the left MCA (M1, M2, M3, Insular, M4, M5, M6) showing FVH and FVH-ASPECTSTS score was 7; I, insular cortex; M1, the anterior MCA cortex, corresponded with the frontal operculum; M2, the MCA cortex lateral to the insular ribbon, corresponded with the anterior temporal lobe; M3, the posterior MCA cortex, corresponded with the posterior temporal lobe. M4, M5, and M6, the anterior, lateral, and posterior MCA territories immediately superior to M1, M2, and M3, respectively
APVS was defined as a prominence of hypointense vessels with either more numerous or larger veins and greater signal loss in the ischemic target compared with the contralateral hemisphere [23, 24]. The ASPECTS score was used to assess the extent of APVS [25, 26]. The MCA territory was divided into ten areas (Caudate nucleus, Lentiform nucleus, Internal capsule, Insular, M1–M6) (Fig. 2). A score on a scale of 0–10 was given, with 0 being none APVS and 10 being APVS was presented in all areas of the MCA.
Fig. 2.
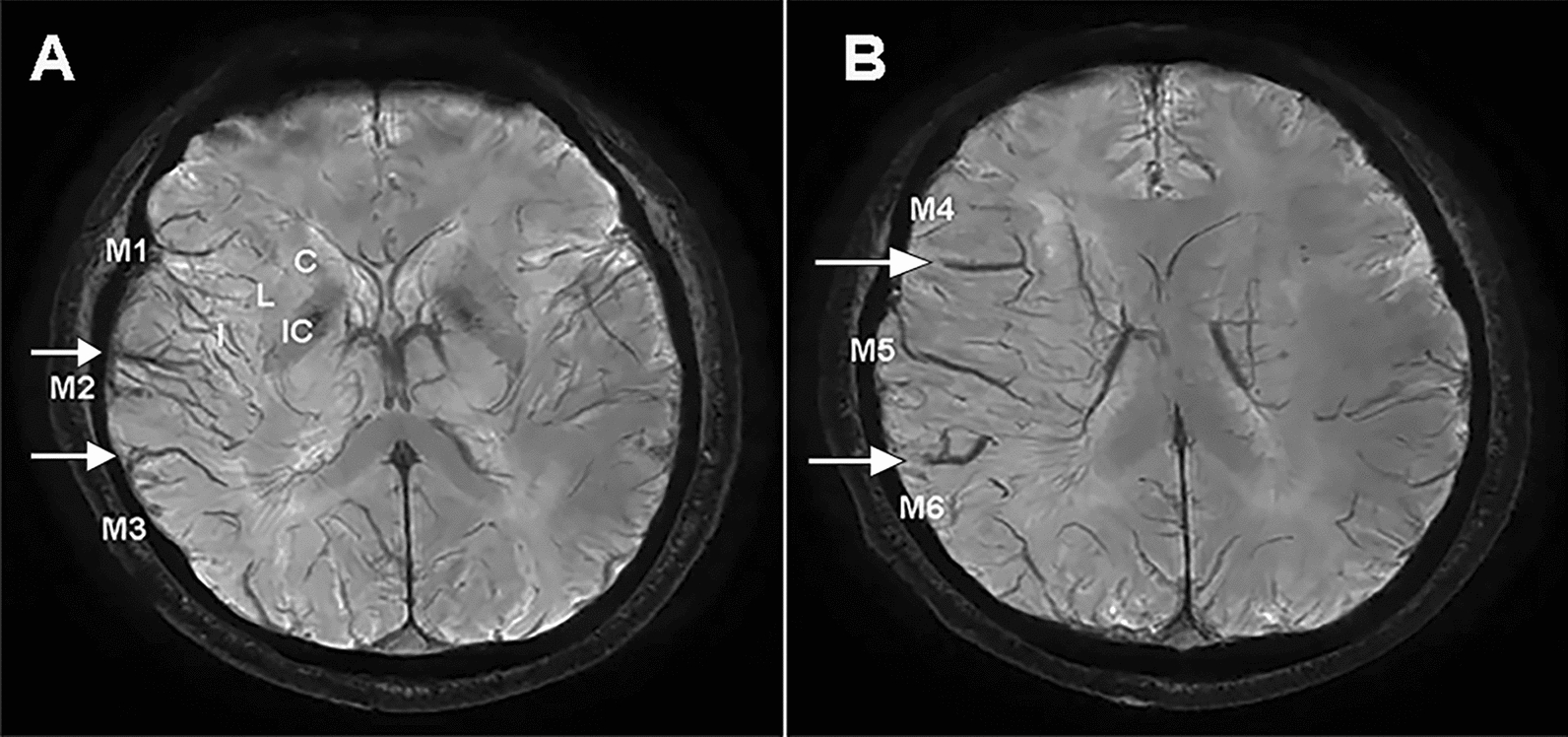
Example of APVS-ASPECTS score. A typical extensive APVS image which are visible on seven areas of the middle cerebral artery (MCA) territory (M1, M2, M3, Insula, M4, M5, M6) and the APVS-ASPECTS score was 7
Statistical analysis
The data were analyzed using SPSS v.24.0. All metric and normally distributed variables were reported as means and standard deviations (SD), and non-parametric distributed variables were described as medians and interquartile ranges (IQR). Categorical variables were presented as frequencies and percentages. The student t-test was used to compare normally distributed continuous variables; the Mann–Whitney U test was used when continuous variables failed tests for normality; the chi-square test or Fisher’s exact test for categorical variables as appropriate. Inter-observer agreement was assessed using the intraclass correlation coefficient (ICC). Spearman’s correlation was used to assess the correlation between the FVH score and the APVS score. The good and poor prognosis were analyzed by univariate analysis. Then all variables with P < 0.1 were included in the multivariable analysis. Multivariable logistic regression analysis was performed to identify independent factors associated with poor prognosis. The receiver operating characteristic (ROC) curve was used to analyze the predictive value of FVH, APVS and their combination in assessing the poor prognosis. P-values < 0.05 were considered statistically significant.
Results
Patient demographics and variables’ correlation analysis
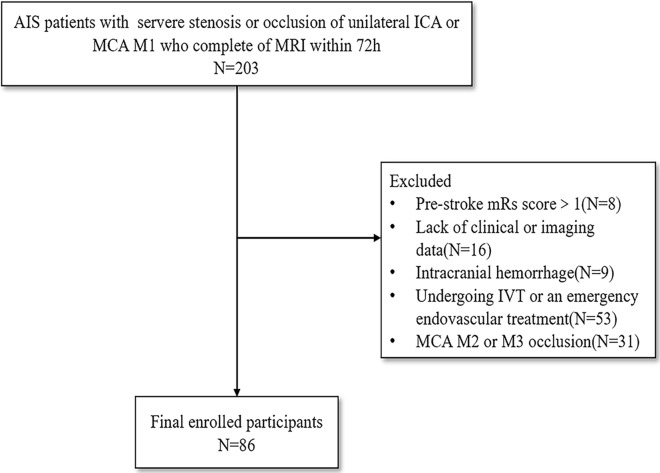
A total of 203 patients were assessed for study eligibility and 86 patients were enrolled in this study (Fig. 3). The mean age was 66.14 ± 11.39 years. The overall rate of FVH presence was 86.0%, and the rate of APVS presence was 70.9%. The media FVH-ASPECTS was 3.0 (interquartile range [IQR], 2.0–5.0), the media APVS-ASPECTS was 4.0 (IQR, 1.0–6.0). The media of DWI-ASPECTS score was 4.0 (IQR, 3.0–6.0), the median admission NIHSS score was 8.5 (IQR, 5.0–12.0), and the median time from onset to MRI imaging was 42.0 h (IQR, 31.0–53.3).
Fig. 3.
Flow diagram of enrollment of study patients
The quality of MRI images of enrolled patients met the diagnostic standards and allowed to identify FVH and APVS. The agreement between readers was excellent for FVH on FLAIR images (ICC = 0.982) and for APVS on SWI (ICC = 0.951). Scatter plots showed a moderate positive correlation between FVH-ASPECTS and APVS-ASPECTS (r = 0.586, P < 0.001). Figure 4 shows typical serial images.
Fig. 4.
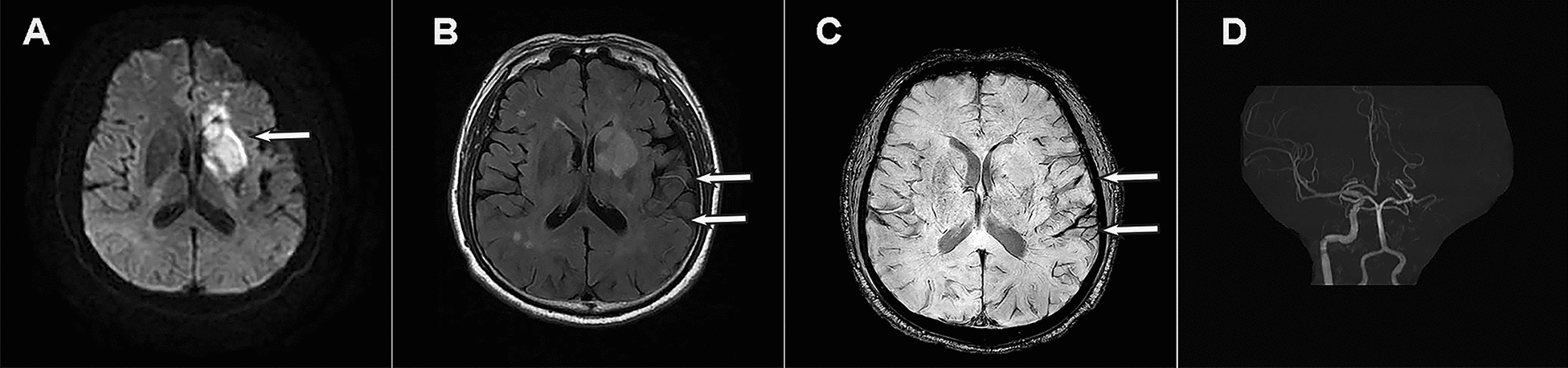
Presentation of a typical patient. A 66-year-old man with left limb weakness was admitted to the hospital, with a 30-h between onset to MR examination. A DWI showed acute infarction; B FLIAR image demonstrated vascular hyperintensity (FVH); C SWI indicated asymmetrical prominent veins sign (APVS); D MRA demonstrated disappearance of left ICA and MCA signals
Factors associated with prognosis
Patients with poor prognosis had a higher rate of atrial fibrillation history, a significantly higher admission NIHSS score, larger cerebral infarction volume, higher APVS-ASPECTS and FVH-ASPECTS score than patients with good prognosis (all P < 0.05). There were no significant differences between the groups in age, sex, stroke risk factors (hypertension, diabetes mellitus, hyperlipidemia, ischemic heart disease, previous stroke or TIA, active smoking and drinking), MRI time from stroke onset and the vascular site and degree of steno-occlusion. The details are presented in Table 1.
Table 1.
Comparisons of clinical and imaging characteristics in patients with good and poor outcome
| Characteristics | Good outcome (n = 48) |
Poor outcome (n = 38) |
P-value |
|---|---|---|---|
| Age at onset, mean ± SD | 65.60 ± 10.82 | 66.82 ± 12.19 | 0.627 |
| Male, n (%) | 24(50.0) | 26(68.4) | 0.085 |
| Medical history, n (%) | |||
| Hypertension | 33(68.8) | 23(60.5) | 0.427 |
| Diabetes mellitus | 13(27.1) | 9(23.7) | 0.720 |
| Hyperlipidemia | 14(29.2) | 10(26.3) | 0.770 |
| Ischemic heart disease | 7(14.6) | 2(5.3) | 0.161 |
| Atrial fibrillation | 6(12.5) | 13(34.2) | 0.016 |
| Previous stroke or TIA | 12(25.0) | 5(13.2) | 0.171 |
| Drinking | 8(16.7) | 11(28.9) | 0.173 |
| Smoking | 15(31.3) | 17(44.7) | 0.199 |
| Time to MRI examination, median (IQR) | 40.5(31.0–53.0) | 43.5(30.0–54.0) | 0.913 |
| NIHSS score at admission, median (IQR) | 6.0(4.0–8.0) | 12.0(9.0–16.0) | < 0.001 |
| DWI-ASPECTS, median (IQR) | 3.0(2.0–4.0) | 6.0(4.8–7.3) | < 0.001 |
| Site of steno-occlusion, n (%) | 0.224 | ||
| ICA or M1 severe stenosis | 13(27.1) | 8(21.1) | |
| ICA occlusion | 12(25.0) | 4(10.5) | |
| M1 occlusion | 14(29.2) | 17(44.7) | |
| ICA and M1 occlusion | 9(18.8) | 9(23.7) | |
| APVS-ASPECTS | 6.0(4.0–7.0) | 2.0(0–4.0) | < 0.001 |
| FVH-ASPECTS | 5.0(4.0–6.0) | 2.0(0.3–3.0) | < 0.001 |
NIHSS, National Institute of Health Stroke Scale; DWI, Diffusion weighted imaging; ASPECTS, Alberta Stroke Program Early CT Score; ICA, internal carotid artery; M1, M1 segment of middle cerebral artery; APVS, asymmetrical prominent veins sign; FVH, Fluid-attenuated inversion recovery vascular hyperintensity
Multivariable logistic regression analysis showed that FVH-ASPECTS (Odd ratio (OR) 2.485, 95% confidence interval (CI) 1.145–5.390, P = 0.021), APVS-ASPECTS [OR 1.801; 95% CI 1.094–2.965; P = 0.021], admission NIHSS score (OR 1.869, 95% CI 1.107–3.155, P = 0.019) and DWI-ASPECTS score (OR 3.104, 95% CI 1.104–8.730, P = 0.032) were independently associated with a poor prognosis (Table 2).
Table 2.
Multivariate logistic regression of risk factors for predicting poor outcome
| Variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Atrial fibrillation | 3.611 | 0.291–44.726 | 0.317 |
| Admission NIHSS | 1.869 | 1.107–3.155 | 0.019 |
| DWI-ASPECTS | 3.104 | 1.104–8.730 | 0.032 |
| APVS-ASPECTS | 1.801 | 1.094–2.965 | 0.021 |
| FVH-ASPECTS | 2.485 | 1.145–5.390 | 0.021 |
ASPECTS, Alberta Stroke Program Early CT Score; APVS, asymmetrical prominent veins sign; FVH, Fluid-attenuated inversion recovery vascular hyperintensity; CI, confidence interval
The ROC curves for FVH and APVS individually and their combination for predicting poor prognosis are presented in Fig. 5. Interestingly, the area under the curve (AUC) of FVH was 0.899 (95% CI 0.830, 0.968), the sensitivity was 0.868, the specificity was 0.833. While the AUC of APVS was 0.818 (95% CI 0.723, 0.912), with a sensitivity was 0.605, and a specificity was 0.937. Moreover, the AUC of their combination was 0.921(95% CI 0.860, 0.981, P < 0.001), and the sensitivity and specificity to predict poor prognosis were 86.8% and 89.6% respectively. The FVH, APVS and their combination with an optimal cut-off 0.459, 0.665, and 0.452, respectively (Table 3).
Fig. 5.
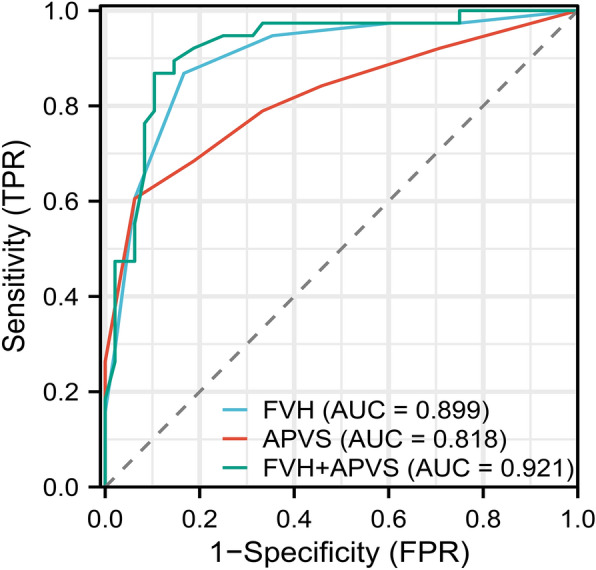
The ROC curve of FVH and APVS alone and their combination to predict a poor outcome. ROC, receiver operating characteristic; FVH, FLAIR vascular hyperintensity; APVS, asymmetrical prominent veins sign
Table 3.
Areas under the ROC curves for each parameter of FVH and APVS individually and their combination for predicting poor prognosis
| Parameters | Cut-off | Sensitivity | Specificity | AUC | 95% CI | P-value |
|---|---|---|---|---|---|---|
| FVH | 0.459 | 0.868 | 0.833 | 0.899 | 0.830–0.968 | < 0.01 |
| APVS | 0.665 | 0.605 | 0.937 | 0.818 | 0.723–0.912 | < 0.01 |
| FVH + APVS | 0.452 | 0.868 | 0.896 | 0.921 | 0.860–0.981 | < 0.01 |
AUC, area under the curve; APVS, asymmetrical prominent veins sign; FVH, Fluid-attenuated inversion recovery vascular hyperintensity; CI, confidence interval
Discussion
Our results showed a positive correlation between the FVH-ASPECTS and APVS-ASPECTS, suggesting that the presence of extensive FVH and APVS in the acute phase of stroke can both reflect the status of collaterals. We also found that the significant predictors of a poor prognosis in AIS treated without recanalization due to occlusion or stenosis of the unilateral ICA and/or M1 segment of the MCA within 72 h of symptom onset were higher baseline NIHSS scores, larger baseline DWI lesion volume, more extensive FVH and APVS grading. In addition, the combination of the FVH and APVS was more predictive of poor prognosis than either alone.
Good collateral flow is increasingly recognized as a crucial element in therapeutic and prognostic assessment, as it can markedly reduce penumbra loss and prolong the salvaged time [27]. The new concept of collateral histology reveals that collateral circulation includes the entire cerebral circulatory system including the arterial, microvascular and venous systems. Consequently, collateral vessels can be judged indirectly via capillary horizontal perfusion, metabolic brain tissue status and changes in intracranial drainage venous pressure besides direct visual imaging technology [28]. FVH and APVS can reflect the hypoperfusion state of damaged brain tissue in patients with AIS and provide information on blood perfusion for assessing the status of the collateral circulation. This study used the ASPECTS score system to quantify the extent of FVH and APVS, and analyzed the correlation between them on this basis. Regarding FVH and APVS, previous studies reached a consensus about their mechanism that patients with severe stenosis or occlusion of large vessels were more likely to have extensive FVH and APVS [15, 29]. When the infarction caused by severe stenosis or occlusion of large vessels occurred, brain tissue in the infarct core area experienced a sequence of pathophysiological mechanisms, including ischemia, hypoxia, cell edema and so on. Under the stimulation of ischemia and hypoxia, the establishment and opening of the leptomeningeal collaterals occurred, along with changes in brain oxygen metabolism and vein expansion, resulting in an increase in oxygen uptake fraction to maintain the basic metabolic level of brain cells. Our study also demonstrated a positive correlation between FVH score and APVS score, which further confirmed the hemodynamic mechanisms of FVH and APVS from both arterial and venous perspectives. Despite the distinct physiological mechanisms of FVH and APVS, they both can indicate inadequate perfusion of brain tissue and reflect the status of collateral circulation in the acute phase.
In clinical practice, research has shown a higher incidence of disability in individuals with anterior cerebral circulation stroke. Subsequently, the early identification of useful prognostic markers plays a critical role in treatment selection and timely intervention. Our study discovered that patients with poor outcomes exhibited increased occurrences of atrial fibrillation in their medical history, higher NHISS scores at baseline, larger baseline infarct volumes, and more extensive FVH and APVS. We suggested that a history of atrial fibrillation is associated with a poor prognosis, possibly due to the abrupt onset of stroke in patients with cardiogenic embolism, the lack of time for collateral branches to form around the ischemic tissue and insufficient compensation of collaterals can result in more severe hypoperfusion [30]. This also highlights the significance of distinguishing stroke subtypes caused by different pathological mechanisms for predicting functional prognosis. The NIHHS score is a widely-used scale for assessing the severity of neurological deficits and plays a crucial role in predicting their functional prognosis. Generally, higher NIHHS scores indicate greater neurological impairment and poorer functional outcomes, and vice versa. A further logistic regression analysis showed that the NIHSS score was an independent risk factor for a poor prognosis. The volume of infarction strongly predicted clinical prognosis has been reported earlier [31]. Our study similarly identified an independent correlation between infarct volume and poor prognosis in AIS patients. The larger the irreversible damage volume of brain tissue, the more severe the clinical dysfunction and unfavorable recovery of the ischemic semidark zone, and the worse the prognosis.
Currently, research on the relationship between FVH and functional outcomes varies with time. The duration of time from symptom onset to imaging has been reported as an essential factor for evaluating the prognostic value of FVH [32]. In most studies that suggested a correlation between FVH and favorable outcomes, the time from symptom onset to imaging was less than 6 h [33–35]. In comparison, in the majority of studies that demonstrated an association between FVH and unfavorable outcomes, the symptom-to-imaging time was 12–24 h or longer [36–38]. Our findings confirmed that FVH was independently associated with poor prognosis, which we believe is related to the fact that the patients we enrolled who did not receive timely vessel recanalization and underwent MR scanning within 72 h. Previous studies believed that FVH may serve as an imaging marker of leptomeningeal collaterals within 6 h of symptom onset or within the time window of reperfusion treatment [39]. The extent of FVH may indicate the amount of brain tissue vulnerable to ischemia, which can be reversed with reperfusion therapy to reduce the size of the eventual lesion and improve functional outcomes. This clarifies why FVH-positive patients tend to have superior clinical outcomes compared to those FVH-negative patients during this specific time interval. However, when FVH appears beyond the time window for reperfusion therapy, it may indicate persistent occlusion of the vessel and hypoperfusion. Thus, patients with FVH may be more susceptible to hemodynamic instability than patients with and without FVH. This variability might be related to the severity of clinical impairment and unfavorable outcomes in patients with FVH during this period. We recommended further investigation into the potential mechanisms underlying this difference.
APVS was found to be another independent factor associated with a 90-day poor prognosis in our study. To some extent, extensive APVS represents an acute ischemic penumbra with poor perfusion. As the hypoperfusion time increases, the size of the ischemic penumbra diminishes, which is predictive of early neurological deterioration (END) and stroke progression [16]. Several studies have investigated the correlation between APVS and prognosis. Some argued that APVS was not associated with prognosis [40, 41], while others believed that patients with APVS had a worse prognosis than those patients without APVS [26, 42, 43]. Chen et al. [40] considered that extensive APVS was associated with poor prognosis, whereas patients without APVS tended to have better collaterals compensation and no obvious hypoperfusion area, thus making it easier to obtain a good prognosis. Sun et al. [44] demonstrated that APVS was a predictor of poor prognosis and was independently associated with END. In our study, patients with extensive APVS had a worse functional prognosis, possibly due to the absence of thrombolytic treatment. Conservative therapy was primarily given to individuals with contraindications to thrombolytic or over a time-window, with more underlying conditions. Although collateral circulation existed at this time, it was more susceptible to stroke progression due to the inability to receive timely and effectively. As a result, it carries a higher overall probability of resulting in a poor prognosis [45]. This outcome may provide clinicians with novel insights into the precise management of stroke, as timely endovascular therapy within the time window is crucial for improving prognosis. We further clarified the value and efficacy of FVH and APVS alone and their combination in predicting poor outcome by ROC curves. The combination AUC was higher than that of each individual index and can compensate for the limitations of the evaluation of each individual index. These findings also offered a degree of reference for the clinical treatment assessment and functional prognosis prediction.
There are several limitations in this study. It is a retrospective analysis and has a potential risk of selection bias, without analysis of patients who underwent thrombolysis or endovascular therapy. Infarct volume determined by ASPECTS is influenced by the quality of the images and this may lead to bias; Finally, FVH and APVS were not observed dynamically in this study, a longitudinal study investigating the association with the change of FVH and APVS and the progression of collateral as well as prognosis is needed.
Conclusions
When stroke occurred, apart from the rapid establishment and opening of the leptomeningeal collaterals in the ischemia and hypoxia area, there is also an increase in the oxygen uptake fraction of brain tissue to co-compensate for the ischemic and hypoxic state. The presence of FVH and APVS can comprehensively assess collateral circulation from both the vein and artery perspective, and there is a positive correlation between the two. Besides, the two indicators were independently related to a poor prognosis at 90 days, and their combination can enhance the predictive accuracy. Using the FLAIR and SWI sequence together is useful for an early non-invasive assessment of collateral status, the selection of individualized treatment and improves the prediction of clinical outcome. Further studies with a larger sample size are needed to confirm our results.
Acknowledgements
The authors are grateful to all participants and physicians for their contribution to this study and acknowledge the efforts of research staff, who worked on the clinical and neuroimaging data collection.
Author contributions
WX and ZGL conceived and designed the study. MMZ and ZWS involved in the data collection, YDL and CZZ involved in data analysis and the interpretation of data. WX and HCW were responsible for initial manuscript. ZGL and HGZ revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the Ministry of Science and Technology of Yantai (No. 2018SFGY092, 2021YD033).
Availability of data and materials
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Declarations
Ethics approval and consent to participate
This study involves human participants. The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The institutional review board approved the study (Approval number 2021-175). All methods were performed in accordance with the relevant guidelines and regulations. We confirm that the informed consent was obtained from all subjects and/or their legal guardian(s).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Xiang and Hongchun Wei contributed equally to this work and share the first authorship.
References
- 1.Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, Abbasifard M, Abbasi-Kangevari M, Abd-Allah F, Abedi V, Abualhasan A. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer OC, Berkefeld J, Nolte CH, Bohner G, Reich A, Wiesmann M, et al. Collateral vessels in proximal middle cerebral artery occlusion: the ENDOSTROKE study. Radiology. 2015;274(3):851–858. doi: 10.1148/radiol.14140951. [DOI] [PubMed] [Google Scholar]
- 3.Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke. 2003;34(11):2750–2762. doi: 10.1161/01.STR.0000095791.85737.65. [DOI] [PubMed] [Google Scholar]
- 4.Zhou H, Sun J, Ji X, Lin J, Tang S, Zeng J, et al. Correlation between the integrity of the circle of willis and the severity of initial noncardiac cerebral infarction and clinical prognosis. Medicine. 2016;95(10):e2892. doi: 10.1097/MD.0000000000002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghoshal S, Freedman BI. Mechanisms of stroke in patients with chronic kidney disease. Am J Nephrol. 2019;50(4):229–239. doi: 10.1159/000502446. [DOI] [PubMed] [Google Scholar]
- 6.Cosnard G, Duprez T, Grandin C, Smith AM, Munier T, Peeters A. Fast FLAIR sequence for detecting major vascular abnormalities during the hyperacute phase of stroke: a comparison with MR angiography. Neuroradiology. 1999;41(5):342–346. doi: 10.1007/s002340050761. [DOI] [PubMed] [Google Scholar]
- 7.Sanossian N, Saver JL, Alger JR, Kim D, Duckwiler GR, Jahan R, et al. Angiography reveals that fluid-attenuated inversion recovery vascular hyperintensities are due to slow flow, not thrombus. AJNR Am J Neuroradiol. 2009;30(3):564–568. doi: 10.3174/ajnr.A1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamran S, Bates V, Bakshi R, Wright P, Kinkel W, Miletich R. Significance of hyperintense vessels on FLAIR MRI in acute stroke. Neurology. 2000;55(2):265–269. doi: 10.1212/wnl.55.2.265. [DOI] [PubMed] [Google Scholar]
- 9.Liebeskind DS. Collateral circulation. Stroke. 2003;34(9):2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 10.Legrand L, Tisserand M, Turc G, Naggara O, Edjlali M, Mellerio C, et al. Do FLAIR vascular hyperintensities beyond the DWI lesion represent the ischemic penumbra? AJNR Am J Neuroradiol. 2015;36(2):269–274. doi: 10.3174/ajnr.A4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Xu G, Yue X, Wang X, Ma M, Zhang R, et al. Hyperintense vessels on FLAIR: a useful non-invasive method for assessing intracerebral collaterals. Eur J Radiol. 2011;80(3):786–791. doi: 10.1016/j.ejrad.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 12.Lyu J, Hu J, Wang X, Bian X, Wei M, Wang L, et al. Association of fluid-attenuated inversion recovery vascular hyperintensity with ischaemic events in internal carotid artery or middle cerebral artery occlusion. Stroke Vasc Neurol. 2022 doi: 10.1136/svn-2022-001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santhosh K, Kesavadas C, Thomas B, Gupta AK, Thamburaj K, Kapilamoorthy TR. Susceptibility weighted imaging: a new tool in magnetic resonance imaging of stroke. Clin Radiol. 2009;64(1):74–83. doi: 10.1016/j.crad.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Roh HG, Lee SB, Jeon YS, Park JJ, Lee TJ, et al. Collateral estimation by susceptibility-weighted imaging and prediction of functional outcomes after acute anterior circulation ischemic stroke. Sci Rep. 2021;11(1):21370. doi: 10.1038/s41598-021-00775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang HF, Zhang YQ, Pang JX, Shao PN, Qiu HC, Liu AF, et al. Factors associated with prominent vessel sign on susceptibility-weighted imaging in acute ischemic stroke. Sci Rep. 2021;11(1):5641. doi: 10.1038/s41598-021-84269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kao HW, Tsai FY, Hasso AN. Predicting stroke evolution: comparison of susceptibility-weighted MR imaging with MR perfusion. Eur Radiol. 2012;22(7):1397–1403. doi: 10.1007/s00330-012-2387-4. [DOI] [PubMed] [Google Scholar]
- 17.Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009;30(2):232–252. doi: 10.3174/ajnr.A1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 19.Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603–612. doi: 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- 20.Verma RK, Gralla J, Klinger-Gratz PP, Schankath A, Jung S, Mordasini P, et al. Infarction distribution pattern in acute stroke may predict the extent of leptomeningeal collaterals. PLoS ONE. 2015;10(9):e0137292. doi: 10.1371/journal.pone.0137292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KY, Latour LL, Luby M, Hsia AW, Merino JG, Warach S. Distal hyperintense vessels on FLAIR: an MRI marker for collateral circulation in acute stroke? Neurology. 2009;72(13):1134–1139. doi: 10.1212/01.wnl.0000345360.80382.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn SJ, Suh SH, Lee KY, Kim JH, Seo KD, Lee S. Hyperintense vessels on T2-PROPELLER-FLAIR in patients with acute MCA stroke: prediction of arterial stenosis and perfusion abnormality. AJNR Am J Neuroradiol. 2015;36(11):2042–2047. doi: 10.3174/ajnr.A4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen-Kondering U, Böhm R. Asymmetrically hypointense veins on T2*w imaging and susceptibility-weighted imaging in ischemic stroke. World J Radiol. 2013;5(4):156–165. doi: 10.4329/wjr.v5.i4.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park MG, Yang TI, Oh SJ, Baik SK, Kang YH, Park KP. Multiple hypointense vessels on susceptibility-weighted imaging in acute ischemic stroke: surrogate marker of oxygen extraction fraction in penumbra? Cerebrovasc Dis (Basel, Switzerland) 2014;38(4):254–261. doi: 10.1159/000367709. [DOI] [PubMed] [Google Scholar]
- 25.Barnes SR, Haacke EM. Susceptibility-weighted imaging: clinical angiographic applications. Magn Reson Imaging Clin N Am. 2009;17(1):47–61. doi: 10.1016/j.mric.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing L, Sui B, Shen M, Qin H, Gao P. Are prominent medullary veins better than prominent cortical veins as predictors of early clinical outcome in patients with acute ischemic stroke? Diagn Interv Radiol (Ankara, Turkey) 2021;27(2):285–292. doi: 10.5152/dir.2021.19644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung S, Gilgen M, Slotboom J, El-Koussy M, Zubler C, Kiefer C, et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain. 2013;136(Pt 12):3554–3560. doi: 10.1093/brain/awt246. [DOI] [PubMed] [Google Scholar]
- 28.Liebeskind DS. Art of expertise in stroke telemedicine: imaging and the collaterome. Stroke. 2015;46(3):610–611. doi: 10.1161/STROKEAHA.114.008444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang L, Chen YC, Zhang H, Peng M, Chen H, Geng W, et al. FLAIR vascular hyperintensity in acute stroke is associated with collateralization and functional outcome. Eur Radiol. 2019;29(9):4879–4888. doi: 10.1007/s00330-019-06022-0. [DOI] [PubMed] [Google Scholar]
- 30.Tu HT, Campbell BC, Christensen S, Collins M, De Silva DA, Butcher KS, et al. Pathophysiological determinants of worse stroke outcome in atrial fibrillation. Cerebrovasc Dis (Basel, Switzerland) 2010;30(4):389–395. doi: 10.1159/000316886. [DOI] [PubMed] [Google Scholar]
- 31.Ospel JM, Hill MD, Menon BK, Demchuk A, McTaggart R, Nogueira R, et al. Strength of association between infarct volume and clinical outcome depends on the magnitude of infarct size: results from the ESCAPE-NA1 trial. AJNR Am J Neuroradiol. 2021;42(8):1375–1379. doi: 10.3174/ajnr.A7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang WJ, Chen HB, Shu LM, Liao HQ, Huang XY, Xiao S, et al. The association between FLAIR vascular hyperintensity and stroke outcome varies with time from onset. AJNR Am J Neuroradiol. 2019;40(8):1317–1322. doi: 10.3174/ajnr.A6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoki J, Suzuki K, Suda S, Okubo S, Mishina M, Kimura K. Negative-FLAIR vascular hyperintensities serve as a marker of no recanalization during hospitalization in acute stroke. J Clin Neurosci. 2020;72:233–237. doi: 10.1016/j.jocn.2019.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Jiang L, Peng M, Geng W, Chen H, Su H, Zhao B, et al. FLAIR hyperintensities-DWI mismatch in acute stroke: associations with DWI volume and functional outcome. Brain Imaging Behav. 2020;14(4):1230–1237. doi: 10.1007/s11682-019-00156-x. [DOI] [PubMed] [Google Scholar]
- 35.Derraz I, Ahmed R, Benali A, Corti L, Cagnazzo F, Dargazanli C, et al. FLAIR vascular hyperintensities and functional outcome in nonagenarians with anterior circulation large-vessel ischemic stroke treated with endovascular thrombectomy. Eur Radiol. 2021;31(10):7406–7416. doi: 10.1007/s00330-021-07866-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhu L, Gong S, Zhu X, Zhang R, Ren K, Zhu Z, et al. FLAIR vascular hyperintensity: an unfavorable marker of early neurological deterioration and short-term prognosis in acute ischemic stroke patients. Ann Palliat Med. 2020;9(5):3144–3151. doi: 10.21037/apm-20-1175. [DOI] [PubMed] [Google Scholar]
- 37.Dong X, Bai C, Nao J. Influential factors and clinical significance of fluid-attenuated inversion recovery vascular hyperintensities in transient ischemic attacks of carotid arterial system. Neuroradiology. 2017;59(11):1093–1099. doi: 10.1007/s00234-017-1906-z. [DOI] [PubMed] [Google Scholar]
- 38.Kim SE, Lee BI, Kim SE, Shin KJ, Park J, Park KM, et al. Clinical significance of fluid-attenuated inversion recovery vascular hyperintensities in borderzone infarcts. Stroke. 2016;47(6):1548–1554. doi: 10.1161/STROKEAHA.115.012285. [DOI] [PubMed] [Google Scholar]
- 39.Zeng L, Chen J, Liao H, Wang Q, Xie M, Wu W. Fluid-attenuated inversion recovery vascular hyperintensity in cerebrovascular disease: a review for radiologists and clinicians. Front Aging Neurosci. 2021;13:790626. doi: 10.3389/fnagi.2021.790626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen CY, Chen CI, Tsai FY, Tsai PH, Chan WP. Prominent vessel sign on susceptibility-weighted imaging in acute stroke: prediction of infarct growth and clinical outcome. PLoS ONE. 2015;10(6):e0131118. doi: 10.1371/journal.pone.0131118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang P, Chen CH, Lin WC, Lin RT, Khor GT, Liu CK. Clinical applications of susceptibility weighted imaging in patients with major stroke. J Neurol. 2012;259(7):1426–1432. doi: 10.1007/s00415-011-6369-2. [DOI] [PubMed] [Google Scholar]
- 42.Li W, Xiao WM, Luo GP, Liu YL, Qu JF, Fang XW, et al. Asymmetrical cortical vein sign predicts early neurological deterioration in acute ischemic stroke patients with severe intracranial arterial stenosis or occlusion. BMC Neurol. 2020;20(1):331. doi: 10.1186/s12883-020-01907-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu YL, Xiao WM, Lu JK, Wang YZ, Lu ZH, Zhong HH, et al. Asymmetrical cortical vessel sign predicts prognosis after acute ischemic stroke. Brain Behav. 2020;10(7):e01657. doi: 10.1002/brb3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun W, Liu W, Zhang Z, Xiao L, Duan Z, Liu D, et al. Asymmetrical cortical vessel sign on susceptibility-weighted imaging: a novel imaging marker for early neurological deterioration and unfavorable prognosis. Eur J Neurol. 2014;21(11):1411–1418. doi: 10.1111/ene.12510. [DOI] [PubMed] [Google Scholar]
- 45.Yu J, Wang L, Li Z, Wang S, Wang G. Related factors of asymmetrical vein sign in acute middle cerebral artery stroke and correlation with clinical outcome. J Stroke Cerebrovasc Dis. 2017;26(10):2346–2353. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author, upon reasonable request.