Abstract
Background
Since 2014, there has been increasing public outreach effort regarding isolated/idiopathic rapid eye movement (REM) sleep behavior disorder (iRBD) in Montreal.
Objective
To assess if, over time, milder iRBD cases are presenting earlier.
Methods
Disease‐free survival was compared in two iRBD recruitment epochs: 2004 to 2013 (“earlier”) versus 2014to 2022 (“later”) and by referral type (“self‐referral” vs. “conventional‐referral”) in three large centers.
Results
In Montreal, among 209 subjects followed prospectively, shorter time to phenoconversion was observed in the earlier epoch (5‐year phenoconversion = 42% earlier vs. 23% later); diagnosis before 2014 had a 1.8‐fold phenoconversion hazard. However, no difference was observed in 248 subjects from Barcelona and 166 from Innsbruck. Analysis of Montreal data found that increased survival in the later epoch was driven by an increasing number of self‐referrals, who phenoconverted at 1/3 the rate of physician‐referred subjects.
Conclusions
Increased patient awareness of iRBD results in earlier presentation to clinical attention, with a longer time to phenoconversion.
Keywords: REM sleep behavior disorder, secular trends, Parkinson's disease, dementia with Lewy bodies, prevalence
The prodromal stages of the synucleinopathies, which include Parkinson's disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA), have become an increasing focus in the development of neuroprotective therapies. 1 Isolated/idiopathic rapid eye movement (REM) sleep behavior disorder (iRBD) in particular is the strongest prodromal condition and provides an unprecedented opportunity to potentially intervene with neuroprotective therapies. 2 A key component of neuroprotective trial design is a clear understanding of how subjects normally progress over time. Recently, two international multicenter studies identified clinical predictors of phenoconversion, tracked their natural evolution, determined optimal trial outcomes, and estimated the overall rate of phenoconversion to overt disease. 3 , 4
One lingering question remains whether clinical progression and phenoconversion rates remain stable or if they are subject to secular changes. As a disease becomes increasingly recognized in the general population, cases may come to medical attention earlier, leading to longer latencies between iRBD diagnosis and phenoconversion. This may be critical in a clinical trial setting that aims to recruit participants via screening of the general population.
Starting in 2014, increasing public outreach about iRBD was started in Montreal, resulting in local news reports, public lectures, and articles in the local press. As a result, we have informally observed an increasing number of subjects referring themselves directly to our center for evaluation rather than through conventional means (ie, referrals from their primary physician or through general neurologists). Additionally, in 2014, we recruited subjects directly from the community using a strategy that began with a screening newspaper advertisement. 5
To evaluate the possibility of secular changes, we compared the clinical progression of two recruitment epochs at a single center (Montreal): those recruited between 2004 and 2013 (“earlier epoch”) and those recruited between 2014 and 2022 (“later epoch”). We compared our findings with two other large sleep centers that recruited patients over the same periods (Barcelona and Innsbruck). Finally, within the Montreal cohort, we determined whether there was a role of increased patient awareness of iRBD, as determined by those referring themselves directly to the clinic (“self‐referrals”) versus those referred by physicians (“conventional referrals”).
Methods
Patients
Patients were recruited between 2004 and 2022 and followed annually until phenoconversion to overt disease at three clinical academic centers (Center for Advanced Research in Sleep Medicine, Hôpital du Sacré‐Coeur de Montréal, Canada; Department of Neurology of Innsbruck Medical University, Austria; Sleep Disorders Center of the Neurology Service at the Hospital Clinic de Barcelona, Spain). Subjects recruited between 2004 and 2013 were grouped into the “earlier” recruitment epoch; subjects recruited between 2014 and 2022 were grouped into the “later” epoch. All subjects had polysomnogram‐confirmed iRBD per standard criteria 6 and were without baseline parkinsonism or dementia. The cohorts have been described in detail previously. 7 , 8 iRBD symptom duration was derived from interviews with the patient and bed‐partner (if available). Phenoconversion was defined according to diagnostic criteria at the time of phenoconversion. 9 , 10 , 11 , 12 , 13
In all three centers, patients may self‐refer, but are more typically referred by general practitioners, neurologists, or other specialists. A subgroup of the post‐2013 Montreal cohort included subjects (n = 28) that self‐referred to the clinic (“self‐referrals”). To assess the effects of referral source, these were randomly matched in a 1:2 ratio by year of recruitment with subjects referred by physicians (“conventional referrals”). Patient recruitment in Barcelona and Innsbruck was similar to Montreal, but without specific targeted awareness campaigns or newspaper advertisements for recruitment.
Ethics approval was obtained from the local institutional boards of each center with subject consent in accordance with the Declaration of Helsinki.
Statistical Analyses
Statistical analyses were performed using R (v4.2.2). Survival analyses were performed using Kaplan–Meier curves and Cox proportional hazards modeling. 14 , 15 Time until phenoconversion or censoring was the outcome of interest, with t = 0 set to the date of iRBD diagnosis. The onset of phenoconversion was estimated to be the half‐way point between the prior “disease‐free” assessment and the visit in which phenoconversion was diagnosed. Those not phenoconverting were censored at their last visit. Cox proportional hazards modeling was used to evaluate the contributions of year of recruitment, baseline age, referral type, and iRBD symptom duration on the hazard of phenoconversion.
Results
Baseline Descriptive Results (Montreal)
Demographics are shown in the Table 1. In total, 209 subjects (101 recruited between 2004 and 2013, and 108 recruited between 2014 and 2022) were followed over a mean of 4.5 ± 3.4 years. Subjects in the earlier epoch were significantly older (earlier = 67.6 ± 9.4, later = 65.0 ± 7.8 years, P = 0.03) and had longer iRBD symptom duration (earlier = 9.4 ± 10.3, later = 6.3 ± 4.9 years, P = 0.01).
TABLE 1.
Baseline demographic, clinical, and phenoconversion characteristics in Montreal
| A Early epoch (n = 101) | B Late epoch (n = 108) | C Late epoch self‐referral (n = 28) | D Matched late epoch conventional‐referral (n = 56) | A vs. B P‐value | C vs. D P‐value | |
|---|---|---|---|---|---|---|
| Variables | ||||||
| Age at diagnosis, years | 67.6 (9.4) | 65.0 (7.8) | 66.6 (6.4) | 65.7 (7.5) | 0.03 | 0.58 |
| Sex (% female) | 27.7 | 22.2 | 35.7 | 19.6 | 0.45 | 0.18 |
| Education (years) | 12.5 (4.1) | 14.2 (3.7) | 14.8 (2.9) | 13.8 (4.3) | 0.01 | 0.28 |
| Symptom duration (years) | 9.4 (10.3) | 6.3 (4.9) | 7.8 (6.2) | 7.2 (7.8) | 0.01 | 0.69 |
| Follow‐up duration (years) | 5.6 (4.1) | 3.9 (2.4) | 4.7 (2.5) | 5.3 (3.1) | n.a.* | 0.41 |
| UPDRS‐I | 1.7 (1.8) | 1.4 (1.6) | 1.3 (1.7) | 1.4 (1.6) | 0.22 | 0.75 |
| UPDRS‐II | 1.5 (1.9) | 1.7 (2.2) | 1.0 (1.0) | 1.7 (2.1) | 0.38 | 0.18 |
| UPDRS‐III | 4.37 (4.28) | 4.26 (3.8) | 3.8 (3.3) | 4.7 (4.3) | 0.78 | 0.37 |
| MoCA | 25.1 (2.9) | 25.9 (3.0) | 26.5 (2.0) | 25.7 (3.0) | 0.21 | 0.19 |
| UPSIT‐12 (z‐score) | −2.3 (2.3) | −2.2 (2.2) | −1.9 (2.2) | −2.3 (2.2) | 0.59 | 0.48 |
| Postural systolic drop (mm Hg) | 13.2 (14.9) | 10.9 (13.7) | 10.7 (11.5) | 11.1 (14.8) | 0.25 | 0.92 |
| Phenoconversion (%) | 56.4 | 22.2 | 17.9 | 30.4 | <0.001 | 0.33 |
| PD | 40.4 | 58.3 | 10.7 | 19.6 | – | – |
| DLB | 52.6 | 37.5 | 7.1 | 8.9 | – | – |
| MSA | 7.0 | 4.2 | 0.0 | 1.8 | – | – |
Note: Continuous data are expressed as mean (standard deviation). Bold indicates P < 0.05.
No statistical test is performed because, by definition, follow‐up duration is longer in the earlier epoch.
Abbreviations: n.a., not applicable; UPDRS, Unified Parkinson's Disease Rating Scale; MoCA, Montreal Cognitive Assessment; UPSIT, University of Pennsylvania Smell Identification Test; PD, Parkinson's disease; DLB, dementia with Lewy bodies; MSA, multiple system atrophy.
Survival Analyses (Montreal)
Kaplan–Meier analysis (Fig. 1A) revealed a significant difference in survival to phenoconversion between epochs (log rank test, P = 0.015). No difference was observed between those phenoconverting to a parkinsonism‐first versus a dementia‐first phenotype (log rank test, P = 0.51). Five‐year survival for the earlier epoch was (57.8% [95% CI = 48.4, 69.0%]) compared with (77.3% [68.2, 87.5%]) in the later epoch. Univariate Cox proportional hazards modeling (Table S1) showed that an iRBD diagnosis before 2014 was associated with an 80% greater hazard of phenoconversion (HR = 1.8 [1.1, 3.0], P = 0.017). Multivariate Cox proportional hazards modeling using age at diagnosis as a covariate showed hazard of phenoconversion was reduced with each successive year of enrolment after 2004 (HR = 0.95 [0.90, 0.99], P = 0.03). The proportion of those phenoconverting to either PD, DLB, or MSA in each epoch was not statistically different (Kruskal‐Wallis rank sum test, P = 0.143).
FIG. 1.
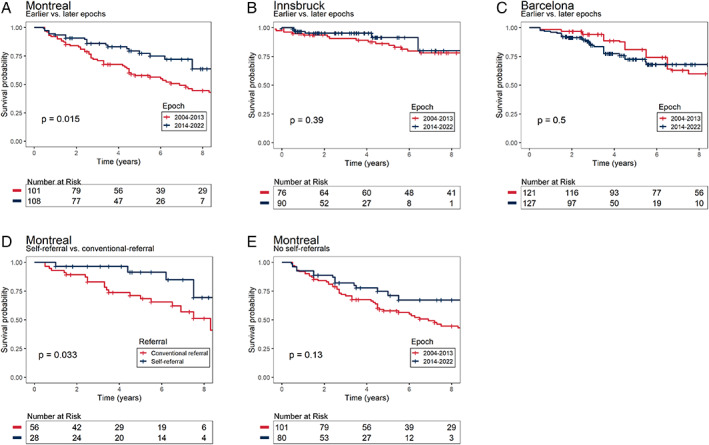
Kaplan–Meier plots of disease‐free survival of patients with idiopathic rapid eye movement sleep behavior disorder (iRBD). (A–C) Comparing disease‐free survival in iRBD subjects at earlier versus later recruitment epochs in three different centers. (D) Comparing disease‐free survival of conventional versus self‐referrals. (E) Comparing rates of disease‐free survival between recruitment epochs after removing self‐referrals. Log‐rank test P‐values are shown.
Survival Analyses in Other Centers (Innsbruck, Barcelona)
To determine if secular changes were unique to a single center (Montreal), we examined data from two other large centers: Innsbruck (n = 166) and Barcelona (n = 248). Age at iRBD diagnosis was not significantly different between epochs at either center. In both centers, no significant differences in survival were observed between subjects diagnosed earlier or later than 2014 (Fig. 1B,C).
Does Referral Type Affect Survival Rate?
With the failure to see changes in other centers, we speculated that increased public awareness of iRBD related to targeted public awareness campaigns in Montreal could explain the longer latency to phenoconversion in the later‐recruited Montreal participants. To test this, we compared a sub‐group in the Montreal cohort that self‐referred for iRBD evaluation (between 2014 and 2019) relative to subjects referred by conventional means during the same period. Baseline demographics were similar between the groups (Table 1). A significant difference was observed in survival between self‐referred versus conventionally referred subjects (log rank test, P = 0.039, Fig. 1D). Moreover, removing self‐referred subjects eliminated the slower progression previously observed (Fig. 1E). Univariate Cox proportional hazards modeling (Table S2) showed that self‐referral was associated with a 64% decreased risk of phenoconversion (HR = 0.36, 95% CI = [0.14, 0.97], P = 0.043). Multivariate Cox proportional hazards modeling using age at diagnosis as a covariate showed significantly improved survival in self‐referred compared with conventionally referred subjects (HR = 0.35 [0.13, 0.94], P = 0.037).
Discussion
This study demonstrates secular changes in a large single‐center iRBD population, in which subjects more recently recruited presented at earlier ages with longer latencies to phenoconversion than those recruited more distantly. This effect, however, was present in only one center and appeared to be driven by site‐specific changes in recruitment, with self‐referred patients having lower risk of phenoconversion.
As would be expected, Montreal iRBD subjects in the later epoch were of younger age and had shorter RBD symptom duration, which are associated with longer latencies to phenoconversion. 16 However, an effect of age or symptom duration was not observed when comparing self‐referred versus conventionally referred subjects, which suggests that additional factors that are indicative of earlier iRBD were present. Notably, the targeted newspaper advertisements were placed in a seniors’ newspaper, and therefore, were directed to an older population, whereas symptom duration is necessarily subjective, based on the recall of the subject or bed‐partner. We speculate that the main reason for our findings is that self‐referrers, being made aware that iRBD is a strong marker of prodromal synucleinopathy, recognized their symptoms and presented directly for sleep evaluation at milder/earlier stages of their disease course. Increasing awareness of iRBD among non‐specialists might also eventually lead to a similar earlier referral to specialized sleep centers, although we saw no evidence of any change in physician‐referred patients over time. Other baseline clinical variables, although nominally less severe in self‐referrals versus conventional referrals, were not significantly different between earlier and later epochs. This may be explained by the fact that many clinical variables are slowly progressive with a long latency (eg, olfaction, autonomic dysfunction) or tend to dramatically worsen close to the time of phenoconversion (eg, motor, cognitive). 4 , 7
This study may have implications for clinical trials in iRBD. Studies examining the natural progression of iRBD will be used to inform the design of clinical trials, 3 , 4 and an underlying assumption is that the two study populations will resemble one another. Whereas the absence of any clear secular change is reassuring, we demonstrate here that an adjustment for referral source, in addition to study center, may be critical in the planning of trials. Data from other centers using different referral strategies are needed to determine if this effect is potentially widespread. If patients are actively recruited with outreach methods in the general population, progression may be slower than what has been observed in sleep center cohorts, therefore, requiring longer clinical trials if the main outcome is phenoconversion. Indeed, a recent study observed that the onset of iRBD at a younger age was associated with longer latency to phenoconversion. 17
Some limitations should be noted. First, for ease of presentation, we dichotomized the two epochs into “earlier” and “later” using 2014 as a somewhat arbitrary cutoff. However, note that our findings were also present when using recruitment year as a continuous variable. Second, our study population was restricted to centers that followed patients for 20 years with a similar protocol; over time, we will be able to expand the analysis to diverse centers that started recruiting later. Third, given the lengthy study period, different diagnostic criteria were applied according to the time of phenoconversion, which may have affected diagnostic accuracy over time, although the primary diagnostic criteria for parkinsonism and dementia, per se, have had only relatively minor changes.
To conclude, increased patient awareness of iRBD, particularly if related to outreach initiatives, may result in earlier presentation to clinical attention and subsequent slower progression. Therefore, adjustment for referral source may be necessary in the planning of neuroprotective trials.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
S.J.: 1B, 1C, 2A, 2B, 3A
A.Iranzo: 1B, 1C, 3B
A.S.: 1B, 1C, 3B
A.P.: 1B, 1C, 3B
M.S.: 1C, 3B
A.M.L.: 1C, 3B
A.Ibrahim: 1C, 3B
E.H.: 1C, 3B
J.Y.M.: 1B, 1C, 3B
G.M.: 1C, 3B
J.S.: 1C, 3B
C.G.: 1C, 3B
M.B.: 1C, 3B
E.B.: 1C, 3B
B.H.: 1B, 1C, 3B
J.F.G.: 1B, 1C, 3B
R.B.P.: 1A, 1B, 1C, 2C, 3B
Disclosures
Ethical Compliance Statement: All participants gave written informed consent. An ethical standards committee on human experimentation reviewed and approved the study at each participating site. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: S.J. was supported by an Edmond J. Safra Fellowship in Movement Disorders from The Michael J. Fox Foundation. J.F.G. was funded by a grant from the Canadian Institutes of Health Research (CIHR) and he holds a Canada Research Chair in Cognitive Decline in Pathological Aging. R.B.P. was funded by a grant from CIHR. The authors report no relevant conflicts of interest.
Financial Disclosures for the Previous 12 Months: R.B.P. holds grants and personal fees from Fonds de la Recherche en Sante, grants from CIHR, grants from The Michael J. Fox Foundation, grants from Webster Foundation, personal fees from Takeda, grants and personal fees from Roche, personal fees from Biogen, personal fees from AbbVie, personal fees from Curasen, personal fees from Lilly, personal fees from Novartis, personal fees from Eisai, other from Parkinson Canada, personal fees from Paladin, grants from National Institute of Health (NIH), personal fees from International Parkinson and Movement Disorders Society, personal fees from Merck, personal fees from Vaxxinity. J.F.G. reports funding from the CIHR, Fonds de Recherche du Québec – Santé (FRQ‐S), Canada Research Chair, and NIH/National Institute on Aging (NAI). J.Y.M. holds a Canada Research Chair in Cognitive Decline in Pathological Aging, and reports personal fees from Takeda, Novartis, and Merck Pharmaceuticals outside the submitted work. A.S. holds royalties from Elsevier, grants from Max‐Kade Postdoctoral Fellowship, Tyrolean Science Funding 2021, Dr. Johannes and Hertha Tuba Research Funding 2022. B.H. reports personal fees from Jazz, travel support from Habel Medizintechnik, and grants from the Austrian Science Fund I4891 and Austrian Science Fund 5894. S.J., A. Iranzo, A.P., M.S., A.M.L, A. Ibrahim, E.H., J.S., C.G., M.B., E.B., and B.H. report no further financial disclosures.
Supporting information
Table S1. Univariate Cox proportional hazards model evaluating the hazard of phenoconversion (by baseline year of recruitment) in Montreal.
Table S2. Univariate Cox proportional hazards model evaluating the hazard of phenoconversion (by referral type) in Montreal.
Relevant disclosures and conflict of interest are listed at the end of this article.
References
- 1. Postuma RB, Berg D. Prodromal Parkinson's disease: the decade past, the decade to come. Mov Disord 2019;34:665–675. 10.1002/MDS.27670. [DOI] [PubMed] [Google Scholar]
- 2. Postuma RB. Neuroprotective trials in REM sleep behavior disorder. Neurology 2022;99:19–25. 10.1212/WNL.0000000000200235. [DOI] [PubMed] [Google Scholar]
- 3. Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 2019;142:744–759. 10.1093/BRAIN/AWZ030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joza S, Hu MT, Jung K‐Y, et al. Progression of clinical markers in prodromal Parkinson's disease and dementia with Lewy bodies: a multicentre study. Brain 2023;139:16–17. 10.1093/brain/awad072. [DOI] [PubMed] [Google Scholar]
- 5. Postuma RB, Pelletier A, Berg D, Gagnon J‐F, Escudier F, Montplaisir J. Screening for prodromal Parkinson's disease in the general community: a sleep‐based approach. Sleep Med 2016;21:101–105. 10.1016/j.sleep.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 6. Sateia MJ. International classification of sleep disorders‐third edition. Chest 2014;146:1387–1394. 10.1378/CHEST.14-0970. [DOI] [PubMed] [Google Scholar]
- 7. Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB. Evolution of prodromal Parkinson's disease and dementia with Lewy bodies: a prospective study. Brain 2019;142:2051–2067. 10.1093/BRAIN/AWZ111. [DOI] [PubMed] [Google Scholar]
- 8. Iranzo A, Stefani A, Serradell M, et al. Characterization of patients with longstanding idiopathic REM sleep behavior disorder. Neurology 2017;89:242–248. 10.1212/WNL.0000000000004121. [DOI] [PubMed] [Google Scholar]
- 9. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–1601. 10.1002/MDS.26424. [DOI] [PubMed] [Google Scholar]
- 11. McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 2005;65:1863–1872. 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 12. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J‐P, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies. Neurology 2017;89:88–100. 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Therneau T. A Package for Survival Analysis in R. v3.4–0; https://CRAN.R-project.org/package=survival Accessed March 14, 2023.
- 15. Alboukadel K, Kosinski M, Biecek P. survminer: Drawing Survival Curves using “ggplot2.”; https://CRAN.R-project.org/package=survminer. https://CRAN.R-project.org/package=survminer Accessed March 20, 2023.
- 16. Iranzo A, Molinuevo JL, Santamaría J, Serradell M, Martí MJ, Valldeoriola F, Tolosa E. Rapid‐eye‐movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 2006;5:572–577. 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 17. Zhou L, Huang B, Wang J, et al. Early‐ and late‐onset of isolated rapid eye movement sleep behavior disorder: a retrospective cohort study. Sleep Med 2023;105:1–8. 10.1016/j.sleep.2023.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate Cox proportional hazards model evaluating the hazard of phenoconversion (by baseline year of recruitment) in Montreal.
Table S2. Univariate Cox proportional hazards model evaluating the hazard of phenoconversion (by referral type) in Montreal.


