Staged, open‐surgical lesioning of the thalamic ventral intermediate nucleus to abate medication‐refractory essential tremor (mrET) has been historically associated with a high frequency of adverse events (AEs), including speech and balance impairments. 1 More recently, the real‐time imaging and thermometry guidance offered by magnetic resonance‐guided focused ultrasound (MRgFUS) have allowed for minimally‐invasive thalamotomies with increased focal accuracy to address upper limb tremor in ET patients. 2 Qualitative and semi‐quantitative trials evaluating the safety of staged, bilateral MRgFUS thalamotomy have reported AEs that were mostly transient with mild to moderate severity based on patients’ and physicians’ perceived deficits. 3 , 4 , 5 However, a comprehensive quantitative analysis of the potential post‐thalamotomy changes in stance and gait balance (ie, equilibrium), and oro‐motor function is lacking. Further, cognitive assessments have been mostly limited to screening instruments. We comprehensively evaluated the incidence and severity of AEs, including changes in equilibrium, speech, and cognition, following staged, bilateral MRgFUS thalamotomy for patients with mrET.
This prospective, open‐label trial (NCT04720469) screened 16 consecutive mrET patients who had undergone MRgFUS thalamotomy at least 1 year prior (MRgFUS1). Three patients were excluded due to mild cognitive impairment. Two others were excluded given abnormal gait, measured using the Scale for Assessment and Rating of Ataxia (SARA). 6 The SARA's speech item revealed no baseline impairment. Eleven patients underwent MRgFUS2 (seven males; median age 71 years, range 52–80 years; eight right‐sided thalamotomy; Table S1). Tremor severity of individual hands was measured pre‐ and 3–6 months post‐MRgFUS (median 3.9 months post‐MRgFUS1; median 4.4 post‐MRgFUS2) using Part A (resting, postural, action/intention) and Part B (Archimedes spiral/line drawing, water pouring) of the Clinical Rating Scales for Tremor (CRST). AEs were systematically captured during MRgFUS2 and up to 6 months post‐MRgFUS2. Pre‐ and post‐MRgFUS2 speech measures from eight patients were assessed using the Frenchay Dysarthria Assessment‐2 (FDA‐2), 7 a 99‐word passage, and diadochokinetic single syllable repetition (eg, /pa/, /ta/, /ka). Vocal tremor was extracted as Frequency (FTrP) and Amplitude (ATrP) Tremor Power from maximum phonation task recordings (ie, “say ‘ah’ for as long as you can”) using the TREMOR.PRAAT algorithm. 8 The neuropsychological battery consisted of the Montreal Cognitive Assessment and tests measuring processing speed, attention/working memory, executive function, language, verbal fluency, and visuospatial perception. Least‐square mean (LS‐mean) with 95% confidence intervals (95%CI) were reported using generalized estimating equations in SAS9.4.
CRST scores for the targeted hand improved after each MRgFUS (P < 0.001), while the untargeted‐hand tremor had no significant change (Fig. 1A). Twenty‐one AEs were identified with 81% rated as mild per the Common Terminology Criteria for AEs v5.0, consistent with other trials. 3 , 4 , 5 No severe AEs were reported. AEs included perioperative, short‐lasting (<24 h) leg/shoulder pain (n = 2), nausea (n = 1) and headache (n = 2), and one event of pin site pain and scalp bruising, lasting 7 days. There was acute‐on‐chronic shoulder pain in a patient with tendinopathy, which resolved in 30 days. Mild thalamotomy‐related peri‐procedural peri‐oral or finger paresthesia with no impact on ADL persisted at follow‐up (n = 4).
Figure 1.
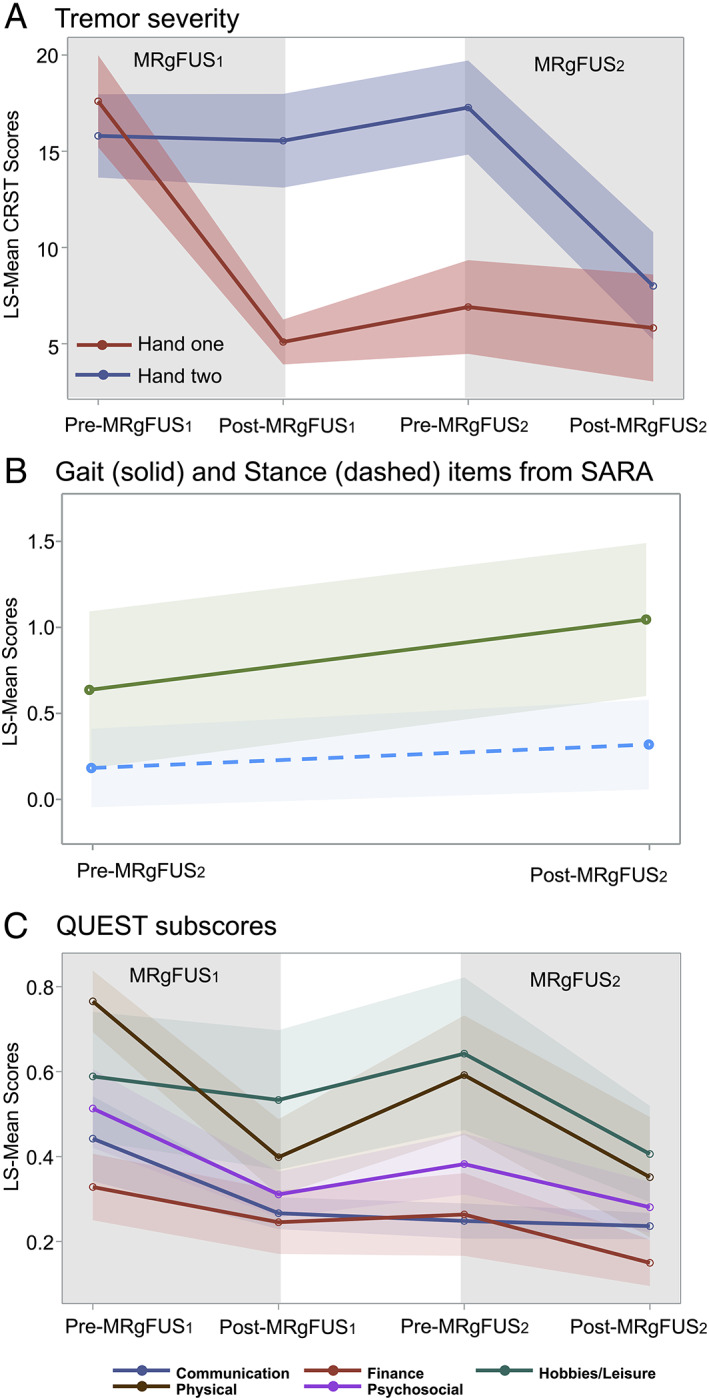
(A) Tremor severity. Group scores from Part A (resting, postural, action/intention) and Part B (Archimedes spiral/line drawing, water pouring) of the CRST for the hand treated in the first procedure (Hand one; MRgFUS1) and the second procedure (Hand two, MRgFUS2) plotted as least square means (CRST LS‐MEAN). Shaded areas CI‐95%. Baseline measures were collected <2 weeks prior to each MRgFUS procedure. Follow‐up measures were collected median 3.9 months after MRgFUS1 and median 4.4 months after MRgFUS2. Higher scores suggest worse tremor. (B) Equilibrium assessment for MRgFUS2. Gait and stance items of the SARA plotted as least square means (SARA LS‐MEAN). Higher scores suggest worse equilibrium. Shaded areas CI‐95%. (C) Quality of life. QUEST domain scores were reported as a percentage of the highest possible total for that given domain. Higher scores suggest worse quality of life. Shaded areas CI‐95%.
SARA's gait LS‐mean scores worsened post‐MRgFUS2 by a small magnitude (P = 0.03, Fig. 1B), while stance LS‐mean scores were stable (P = 0.39). Mild to moderate disequilibrium was reported by four patients, and included one fall. No limb ataxia was detected as LS‐mean scores were unchanged post‐MRgFUS2 for finger chase, fast alternating movements, and heel‐shin‐slide. Nose‐finger task improved significantly in agreement with post‐MRgFUS2 CRST measures (P < 0.05).
There were no post‐MRgFUS2 group changes in dysarthria or speech intelligibility FDA‐2 LS‐mean scores (Table S2). Similarly, there were no post‐MRgFUS2 changes in total speech duration, pause events or duration during the passage read, or changes in oro‐motor control during single‐syllable repetition. There was no significant change in vocal tremor FTrP and ATrP measures. On an individual level, one patient experienced mild, transient dysphagia and subjective word‐finding difficulty. Mild to moderate dysgeusia resolved near the post‐MRgFUS2 visit in one patient but persisted for another three with interference in oral intake. Similar to previous reports, 5 no significant changes in cognitive function were detected (Table S3).
Despite mild to moderate AEs, post‐MRgFUS2 function and quality of life improved further as measured by CRST Part C disability LS‐mean scores (P < 0.05) and the finance (P = 0.043), hobbies/leisure (0.007), physical (P = 0.001) and psychosocial (P = 0.002) domains of the Quality of Life in ET 9 (QUEST; Fig. 1C). Patients’ sense‐of‐self may have increased with the ability to perform tasks that require two hands.
Herein, staged, bilateral MRgFUS thalamotomy was safe in a small sample of patients with mrET. As with unilateral MRgFUS thalamotomy, there was increased risk of disequilibrium. 2 In contrast to early, open‐surgical thalamotomies 1 speech dysfunction was not detected. Consistent with an increased risk of dysgeusia in bilateral relative to unilateral deep brain stimulation, 10 we noted a 25% increase in dysgeusia incidence relative to unilateral MRgFUS thalamotomies performed at our institution. Future larger studies may consider a topography analysis of the thalamic lesion and gustatory processing fibers.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
N.S.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
C.R.R.: 1B, 1C, 2C, 3A, 3B
Y.Y.: 1A, 1B, 1C, 2B, 3B
J.S.R: 1A, 1B, 1C, 2A, 2C, 3B
Y.H.: 1A, 1B, 1C, 3B
R.M.J.: 1A, 1B, 1C, 3B
Y.M.: 1A, 2C, 3B
C.H.: 1A, 1C, 3B
G.G.: 1C, 2A, 2B, 2C, 3B
S.M.: 1A, 1C, 2A, 2B, 3A, 3B
I.J.S.: 1C, 2A, 2B, 2C, 3B
M.M.: 1C, 3B
R.M.: 1C, 3B
B.L.: 1C, 3B
K.H.: 1A, 3B
M.L.S.: 1A, 1C, 3B
N.L.: 1A, 1B, 1C, 3B
A.A.: 1A, 1B, 1C, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors acknowledge support from the Harquail Centre for Neuromodulation. The authors have no conflict of interest to declare.
Financial Disclosures for Previous 12 Months: Grants: The authors acknowledge support from the Harquail Centre for Neuromodulation, Sunnybrook Research Institute and the Hurvitz Brain Sciences Program. J.S.R. also receives support from the Dr. Sandra Black Centre for Brain Resilience & Recovery. Y.Y. receives support from the National Institute on Deafness and Other Communication Disorders, the Natural Sciences and Engineering Research Council, and the Canada Brain Research Fund. N.L. receives support from InSightec, Veteran's Affairs Canada, Power Corporation, and the Alternate Planning Fund. K.H. receives support from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health, and the Temerty Chair in Focused Ultrasound Research at Sunnybrook Health Sciences Centre. Y.Y., C.H., K.H. and N.L. are supported by the Canadian Institutes of Health Research. C.H. and N.L. are supported by the New Frontiers in Research Fund. A.A. and N.L. received support from the Multiple Sclerosis Society of Canada, and the Focused Ultrasound Foundation. Y.Y. and A.A. receive support from the ALS Society of Canada. N.L. and K.H. received support from the Weston Brain Institute. Honoraria: A.A. has received an honorarium from Mitsubishi Tanabe Pharma and Amylyx.
Ethical Compliance Statement: All activities were performed under approval of the Sunnybrook Research Ethics Board (Project ID 3341). Written informed consent was obtained from each patient prior to participation. We confirm that we read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Supporting information
Table S1. Demographics and tremor severity at MRgFUS2.
Table S2. Per cent change in speech. Speech duration, pause duration and number of pause events were measured from a 99‐word passage read by patients at baseline and follow‐up of MRgFUS2.
Table S3. Neuropsychological test results.
Acknowledgments
We wish to thank the research personnel who made this study possible, particularly Ruby Endre, Sangkyu Moon, Zuzana Fless, Madhura Kulkarni and Garry Detzler.
Nir Lipsman and Agessandro Abrahao are Co‐senior authors.
References
- 1. Alshaikh J, Fishman PS. Revisiting bilateral thalamotomy for tremor. Clin Neurol Neurosurg 2017;158:103–107. [DOI] [PubMed] [Google Scholar]
- 2. Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2016;375:730–739. [DOI] [PubMed] [Google Scholar]
- 3. Fukutome K, Hirabayashi H, Osakada Y, Kuga Y, Ohnishi H. Bilateral magnetic resonance imaging‐guided focused ultrasound thalamotomy for essential tremor. Stereotact Funct Neurosurg 2022;100:44–52. [DOI] [PubMed] [Google Scholar]
- 4. Iorio‐Morin C, Yamamoto K, Sarica C, et al. Bilateral focused ultrasound thalamotomy for essential tremor (best‐fus phase 2 trial). Mov Disord 2021;36:2653–2662. [DOI] [PubMed] [Google Scholar]
- 5. Martínez‐Fernández R, Mahendran S, Pineda‐Pardo JA, et al. Bilateral staged magnetic resonance‐guided focused ultrasound thalamotomy for the treatment of essential tremor: a case series study. J Neurol Neurosurg Psychiatry 2021;92:927–931. [DOI] [PubMed] [Google Scholar]
- 6. Weyer A, Abele M, Schmitz‐Hübsch T, Schoch B, Frings M, Timmann D, Klockgether T. Reliability and validity of the scale for the assessment and rating of ataxia: a study in 64 ataxia patients. Mov Disord 2007;22:1633–1637. [DOI] [PubMed] [Google Scholar]
- 7. Enderby PM. Frenchay Dysarthria Assessment. San Diego, Califonia: College‐Hill Press; 1983. [Google Scholar]
- 8. Bruckl M, Viallet F, Ghio A. Measurement of tremor in the voices of speakers with Parkinson's disease. Procedia Comput Sci 2018;128:47–54. [Google Scholar]
- 9. Tröster AI, Pahwa R, Fields JA, Tanner CM, Lyons KE. Quality of life in essential tremor questionnaire (QUEST): development and initial validation. Parkinsonism Relat Disord 2005;11:367–373. [DOI] [PubMed] [Google Scholar]
- 10. Carlson JD, McLeod KE, Mark JB, McLeod PS, Bremer BA. Dysgeusia in deep brain stimulation for essential tremor. J Clin Neurosci 2018;50:242–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographics and tremor severity at MRgFUS2.
Table S2. Per cent change in speech. Speech duration, pause duration and number of pause events were measured from a 99‐word passage read by patients at baseline and follow‐up of MRgFUS2.
Table S3. Neuropsychological test results.


