ABSTRACT
Background
In Parkinson's disease (PD), impulsivity as a personality trait may be linked to the risk of developing impulse control disorders (ICDs) during dopaminergic therapy. However, studies evaluating differences in trait impulsivity between patients with PD and healthy controls or between patients with PD with and without ICDs reported partly inconsistent findings.
Objectives
We conducted a systematic review and meta‐analysis (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) of studies comparing Barratt Impulsiveness Scale (BIS‐11) scores between patients with PD and healthy controls and between patients with PD with and without ICDs.
Methods
Eligible studies were identified through a systematic search in 3 databases. Mean differences with 95% confidence intervals (CIs) for BIS‐11 total and subscale scores were separately calculated for studies comparing patients with PD and healthy controls and patients with PD with and without ICDs. Meta‐regressions were performed to explore sources of heterogeneity (percentage of men, age, disease duration, and levodopa equivalent daily dose).
Results
A total of 40 studies were included in the quantitative analyses. BIS‐11 total scores were significantly higher in patients with PD compared with healthy controls (mean difference 2.43; 95% CI, 1.03, 3.83), and in patients with PD with active ICDs compared with patients without ICDs (6.62; 95% CI, 5.01, 8.23). No significant moderators emerged by meta‐regression analyses.
Conclusions
The present meta‐analysis supports that impulsivity, as a personality trait, may characterize patients with PD, even in the absence of ICDs. Moreover, these data corroborate findings of clinical studies reporting higher levels of trait impulsivity in PD patients with ICDs compared with patients without ICDs.
Keywords: Parkinson's disease, impulse control disorders, impulsivity, Barratt Impulsiveness Scale
Impulsivity is a multidimensional concept relevant either in the description of normal individual differences in personality or as a maladaptive factor characterizing a variety of pathological conditions with a lack of behavioral control as a common feature. 1 , 2 , 3 An impulsive personality trait may be defined as a tendency toward rapid and unplanned reactions to internal or external stimuli without regard to the negative consequences. 4 This definition incorporates the following 3 core elements of impulsivity: (1) decreased sensitivity to immediate negative feedbacks; (2) rapid, unplanned reactions to stimuli before complete processing of information; and (3) lack of regard for long‐term consequences of behavior. 4 These aspects of impulsivity traits are configured as peculiar features of impulse control disorders (ICDs). 3 , 4
ICDs, which include pathological gambling, hypersexuality, and compulsive eating and shopping and related behaviors such as punding, hoarding, hobbyism, and compulsive medication overuse are commonly reported in patients with Parkinson's disease (PD) during dopaminergic therapy. 5 , 6 ICDs are associated with greater functional impairment, decreased quality of life, and increased caregiver burden and represent a critical issue for the clinical management of patients with PD. ICDs may result from the interaction between predisposing factors (ie, demographic, psychological, clinical, and genetics factors) and dopaminergic medication. 7 , 8
Therefore, it has been suggested that PD patients with high levels of impulsivity, along with other personality characteristics such as novelty seeking, can be at higher risk for developing ICDs. 9 , 10 , 11 In keeping with this hypothesis, higher impulsivity, as assessed by self‐report questionnaire, was reported in PD patients with ICDs compared with patients without ICDs. 9 , 12 , 13 , 14 Moreover, evidence of higher levels of trait impulsivity have been reported in patients with PD when compared with healthy controls even in the absence of ICDs. 14 , 15 However, some studies did not report differences either from the comparison between patients with PD and healthy controls 16 , 17 or between patients with and without ICDs. 18 , 19 Therefore, whether impulsivity may represent a main vulnerability factor for the development of ICDs during dopaminergic treatment still remains an open question.
The overall goal of the present study was to investigate impulsivity as a personality trait in patients with PD using a meta‐analytic approach to the current literature. To this end, we performed 2 separate meta‐analyses aimed to verify: (1) whether impulsivity traits are higher in patients with PD compared with age‐matched healthy individuals and (2) whether and to what extent impulsivity traits differ between PD patients with and without ICDs. Moreover, we explored the possible influence of demographic and clinical factors through meta‐regression analyses. We used the total score of the Barratt Impulsiveness Scale (BIS‐11) as a primary measure. BIS‐11 is the most administered self‐report questionnaire to assess impulsive personality traits. 20 Recently, a relationship between BIS‐11 score and awareness of motor intention has been found both in healthy subjects 21 , 22 and patients with PD. 23
Methods
Search Strategy and Selection Criteria
We performed a systematic and comprehensive literature search up to May 2022 using the databases PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science (https://webofknowledge.com), and PsychINFO (https://search.ebscohost.com/). The selected keywords were combined using the Boolean operator AND and OR. The search input was the following: (“personality trait*” OR “impulsivity trait*” OR “Barratt Impulsiveness Scale” OR “Barratt Impulsivity Scale” OR “BIS‐11”) AND (“Parkinson's disease” OR “Parkinson disease” OR “impulse control disorder*”). Additional studies were searched from the references of all identified publications. No language restrictions were applied. Eligibility was determined by a 2‐step procedure performed by 3 of the authors (F.G., C.N., and G.G.). First, the titles and abstracts of all identified articles were screened. In the second step, the full texts of studies, according to predefined eligibility criteria, were independently examined, and agreement was reached after discussion. Our study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. 24
We included controlled studies published in peer‐reviewed journals reporting impulsivity traits assessed by the BIS‐11 in patients with a diagnosis of idiopathic PD and in age‐matched healthy controls or comparing patients with PD with and without ICDs. Included studies had to provide mean and standard deviation (SD) values of the BIS‐11 scores or data to calculate them. Case reports, conference proceedings, and publications available only in abstract form not reporting detailed data were excluded. Studies reporting impulsivity traits assessed by self‐report questionnaires (eg, Eysenck Personality Questionnaire; Eysenck Impulsiveness Questionnaire–I‐7; Dickman Impulsivity Inventory; Lifetime History of Impulsive Behaviors; the Impulsive/Premeditated Aggression Scale; or the more recent Urgency, Premeditation, Perseverance, Sensation Seeking impulsive behavior scale [UPPS]) other than the BIS‐11 were also excluded. We opted for this conservative approach as different self‐report questionnaires developed to assess impulsive traits are based on different theories and models of impulsivity, emphasizing different aspects of this multifaceted psychological construct.
Studies conducted in patients with PD undergoing deep brain stimulation (DBS) were excluded unless the assessments were clearly carried out before the implantation.
Data Extraction
Data were collected independently by 3 authors (F.G., C.N., and G.G.) using a standardized data extraction form. For each study, the mean and SD of the BIS‐11 total score were extracted or calculated. If available also subscale scores (ie, attentional, motor, and nonplanning) were extracted. BIS‐11 score values were retrieved from text, tables, or estimated by graphs (details are given in Supplementary Material S1). In case of discrepancies, data from tables were chosen. Moreover, authors were contacted to retrieve missing or incomplete data. Other details on data extraction are given in Supplementary Material S1.
In addition, the following data were also extracted for each study: number of participants for each group, mean age, percentage of men, disease duration, Hoehn and Yahr Scale, mean score of the Unified Parkinson Disease Rating Scale (UPDRS) Part III on and/or off medication, mean levodopa equivalent daily dose (LEDD), and mean dopamine agonist LEDD. Moreover, data on the presence of ICDs in the groups were also extracted. Data were independently extracted and cross‐checked by 3 review authors (F.G., C.N., and G.G.), who also independently assessed the methodological quality of each study.
Data are available from the corresponding author on request.
Primary Measure
The BIS‐11 is a 30‐item self‐report questionnaire widely used to measure impulsive personality traits. 25 Each item is measured on a 4‐point Likert scale, with higher values indicating higher impulsivity level. The BIS‐11 includes the following 3 subscales: (1) inability to focus attention or concentrate on the task at hand (attentional impulsivity), (2) tendency to act on the spur of the moment without thinking (motor impulsivity), and (3) lack of planning and forethought (nonplanning impulsivity). Translations of the BIS‐11 are available in several languages. 20 The internal consistencies (Cronbach's α) reported for the BIS‐11 total score from different translations all fall within an acceptable range (0.71–0.83). 20 Similarly, the test–retest reliability was acceptable. Therefore, the BIS‐11 is considered a valid tool to assess the construct of impulsiveness in both clinical and nonclinical samples. 20 A short version of the scale (15 items instead of 30) has been recently validated, and normative data have been provided. 26 This short version can be used as a quick screening tool to assess impulsivity in a clinical setting. However, to the best of our knowledge, the BIS short version has not been used yet in patients with PD.
Data Analysis
The meta‐analysis has been conducted using the software RevMan version 5.4.1 (Review Manager, The Cochrane Collaboration, 2020). Mean differences (continuous data) with 95% confidence intervals (CIs) for BIS‐11 total score were separately calculated for studies comparing (1) patients with PD and age‐matched healthy controls and (2) patients with PD with and without ICDs. Included studies reporting BIS‐11 subscale scores (attentional, motor, and nonplanning) were used for a secondary analysis comparing patients with PD with and without ICDs.
Heterogeneity between studies has been assessed by I 2 and Cochran's Q test. Given the heterogeneity among studies (see the Results), data were analyzed using a random‐effects model.
A weighted least squares linear meta‐regression was performed to explore sources of heterogeneity in the BIS‐11 total score mean difference between patients with PD and healthy controls. The following factors were used as independent variables: percentage of men in the sample, age, disease duration, and LEDD in patients with PD. Because of the rates of missingness across studies, each potential moderator was evaluated in a separate meta‐regression model. To evaluate the influence of LEDD in the mean difference of BIS‐11 total score between patients with PD with and without ICDs, the mean LEDD difference was used as an independent variable in a meta‐regression. The meta‐regression model was weighted by the inverse of variance of each study. The meta‐regression analysis was performed using the software IBM (Armonk, NY) SPSS 20.0; significance was set at P < 0.05.
The publication bias has been evaluated by funnel plot inspection. A symmetric funnel plot suggests no publication bias. The presence of asymmetry in the funnel plot was statistically evaluated by Egger's regression asymmetry test using the open‐source software Jeffreys's Amazing Statistics Program ‐ JASP (version 0.16.2; JASP Team 2022, University of Amsterdam, Amsterdam, The Netherlands).
Results
Results of the Study Search
The flowchart of the article selection is illustrated in Figure 1. Our search yielded 124 potentially eligible studies. After full‐text assessment of these articles, 40 studies (reference list is provided in Supplementary Material S1) from 2007 to 2022 were included in our quantitative analyses (28 studies for the comparison between patients with PD and age‐matched controls and 18 studies for the comparison between patients with PD with and without ICDs). The main characteristics of the studies included in the analysis are reported in Tables 1 and 2. A total of 4 studies were conducted or reported data on newly diagnosed drug‐naïve patients. 19 , 27 , 28 , 29
FIG. 1.
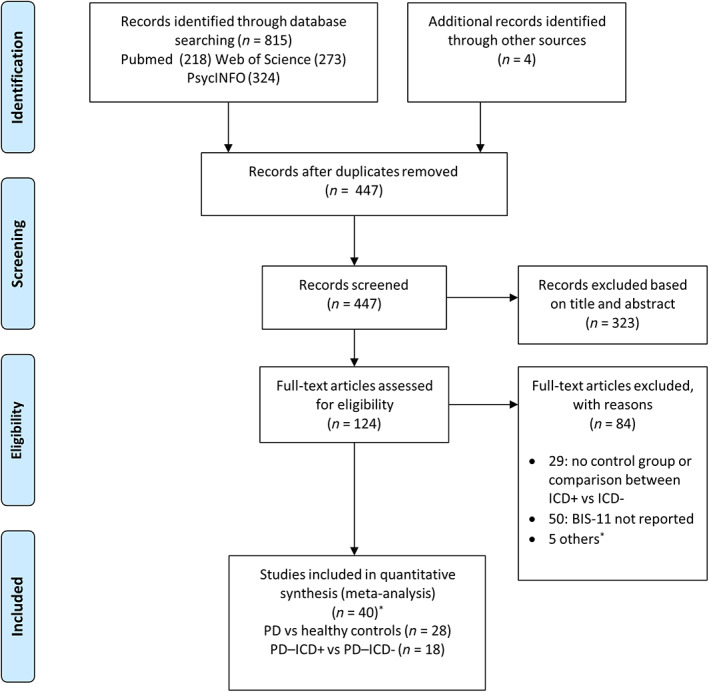
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flowchart (*see Supplementary Material S1 for details). BIS‐11, Barratt Impulsiveness Scale; ICDs, impulse control disorders; PD, Parkinson's disease.
TABLE 1.
Characteristics of studies included in the analysis (patients with PD vs. healthy controls)
| Study | Patients with PD | Healthy Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Sex, % Male | Age, y, Mean ± SD or Range | Disease Duration, y | H&Y, Mean or Range | UPDRS III, On Medication | UPDRS III, Off Medication | LEDD, mg, Mean ± SD or Range | DA‐LEDD, mg, Mean ± SD or Median [Interquartile Range] | N | Age, y, Mean ± SD or Range | Sex, % Male | |
| Chen et al, 2022 15 | 50 | 62 | 60.4 ± 7.5 | NR | NR | NR | 27.9 ± 12.5 | 638 ± 414.5 | NR | 90 | 56.2 ± 13.2 | 51.1 |
| de Chazeron et al, 2021, 32 , a | 200 | 60 | 67.5 ± 9.9 | 5.8 | NR | 16.7 ± 9.8 | NR | 500 (257–850) | 35 [0; 210] | 200 | 67.5 ± 9.9 | 60 |
| Aumann et al, 2020 14 | 68 | 77.9 | 65 ± 8.2 | 5 | NR | 27.6 ± 12.3 | NR | 741 ± 411 | NR | 93 | 58.0 ± 8.0 | 53.7 |
| Hlavatá et al, 2020 38 | 22 | 45.5 | 69.2 ± 5.5 | 6.95 | 2.5 ± 0.7 | NR | NR | 1025.5 ± 567.2 | NR | 36 | 58.0 ± 8.1 | 52.8 |
| Izzo et al, 2020, 33 , a | 47 | 54.9 | 70.3 ± 7.1 | NR | NR | NR | NR | NR | NR | 42 | 69.17 ± 7.5 | 71.4 |
| Koh et al, 2020 44 | 45 | 40 | 65 ± 9.2 | 6.3 | NR | 23.1 ± 12.8 | NR | 469 (319–663) | 150 (0–285) | 21 | 64.3 ± 10.3 | 61.9 |
| Pickering et al, 2020 45 | 25 | 68 | 63.8 ± 5.3 | 8.1 | 2 ± 0.6 | 26.6 ± 12.6 | NR | NR | NR | 25 | 68.9 ± 5.6 | 48 |
| Hammes et al, 2019, 17 , a | 62 | 67.7 | 68 ± 9.9 | 4.7 | 2 | NR | 25.7 ± 9.9 | 465 ± 285 | 142 ± 117 | 18 | 67 ± 8.4 | 44.4 |
| Kubera et al, 2019 46 | 22 | 36.4 | 64.6 ± 2.2 | 5.6 | 1.8 ± 0.6 | 24.7 ± 10.9 | NR | 557.2 ± 363.7 | NR | 18 | 62.7 ± 2.3 | 50 |
| Girard et al, 2019 47 | 14 | 100 | 57 ± 9 | 6.8 | NR | 12.6 ± 6.0 | 28.4 ± 9.1 | 1068.7 ± 398.8 | 295.1 ± 161.3 | 14 | 54.4 ± 5 | 100 |
| Picazio et al, 2018 48 | 28 | 50.0 | 69.0 ± 7.3 | 9 | NR | 14.1 ± 3.2 | 27.4 ± 3.2 | 591.2 ± 183.1 | NR | 10 | 68.3 ± 8.4 | 50 |
| Aiello et al, 2017 16 | 18 | 66.7 | 60.2 ± 6.9 | 9.8 | NR | 16.5 ± 9 | 42.7 ± 11.8 | 1113.9 ± 436.3 | NR | 18 | 61.6 ± 8.9 | 61.1 |
| Duprez et al, 2017 49 | 32 | 56.3 | 58.7 ± 9.8 | 9.5 | 2.7 ± 1.3 | 11.2 ± 8.9 | NR | 995.4 ± 316.4 | NR | 32 | 55.5 ± 8.9 | 31.3 |
| Sharp et al, 2016 50 | 22 | 59.1 | 61.1 ± 6.5 | 6.8 | NR | 13.3 ± 6.0 | 18.6 ± 6.0 | 715 ± 273 | NR | 21 | 62.8 ± 6.8 | 52.4 |
| Fonoff et al, 2015, 31 , a | 28 | 57.1 | 59.3 ± 10.3 | 13.3 | 2.8 ± 0.6 | 16.2 ± 7.3 | 45.5 ± 10.7 | 1125.6 ± 511.8 | NR | 28 | 59.3 ± 11.7 | 39.3 |
| Grogan et al, 2015 51 | 15 | NR | 71.5 ± 2.4 | 5.2 | NR | 19.9 ± 3.2 | 24.9 ± 3.9 | 603 ± 71.6 | NR | 15 | 71.5 ± 2.6 | |
| Herz et al, 2014 52 | 26 | 57.7 | 68.2 ± 8.5 | 6.8 | NR | 20.6 ± 6.3 | 32.7 ± 8.7 | 823.2 ± 371.9 | NR | 13 | 68.4 ± 4.9 | 69.2 |
| Nombela et al, 2014 53 | 30 | 46.7 | 66.4 ± 10.5 | NR | 2.2 ± 0.6 | 23.3 ± 11.1 | NR | NR | NR | 30 | 62.4 ± 7.5 | 46.7 |
| Piray et al, 2014 54 | 40 | 77.5 | 63.7 ± 3.9 | 9.4 | NR | 20.1 ± 5.8 | NR | NR | NR | 20 | 66.4 ± 4.7 | 65 |
| Schomaker et al, 2014 55 | 21 | 71.4 | 61.8 (51–69) | NR | (2, 3) | 21.8 | 29.1 | 851.1 ± 581.1 | NR | 21 | 60 (49–69) | 52.4 |
| Florin et al, 2013 56 | 29 | 100 | 57.4 ± 9.2 | 5.3 | NR | 25.8 ± 7.8 | NR | 661 ± 528.6 | NR | 19 | 56.5 ± 7.2 | 100 |
| Leroi et al, 2013 57 | 55 | 70.9 | 62.5 ± 9.2 | 8.1 | 2.4 ± 0.7 | 28.9 ± 13.4 | NR | 732.2 ± 589.9 | 156.54 ± 161.11 | 20 | 57.9 ± 13.6 | 55 |
| Rustamov et al, 2013 58 | 20 | 55 | 58.9 ± 8.3 | 5.4 | 2.0 ± 0.9 | 15.1 ± 6.8 | NR | 544.4 ± 359.5 | NR | 20 | 54.9 ± 4.9 | 40 |
| van der Vegt et al, 2013 29 | 13 | 61.5 | 58 ± 10 | 3 | NR | 25.6 ± 8.7 | NR | Drug‐naïve | 12 | 60 ± 7 | 41.7 | |
| Canesi et al, 2012, 30 , a | 36 | NR | 61 ± 7.5 | 9.7 | 2.1 ± 0.4 | 19.4 ± 8 | NR | 650 ± 222.6 | NR | 36 | 60.2 ± 9.7 | NR |
| Poletti et al, 2012 28 | 42 | 66.7 | 64.9 ± 7.9 | NR | NR | 18.2 ± 12.6 | NR | Drug‐naïve | 30 | 66.1 ± 7.6 | 60.0 | |
| Cools et al, 2010 59 | 15 | 46.7 | 64.5 ± 8.5 | 8.1 | NR | 13.2 ± 9.8 | 20.1 ± 11.7 | NR | NR | 14 | 66.5 ± 6.2 | 35.7 |
| Isaias et al, 2008 9 | 36 | 66.7 | 65 ± 9 | 8 | NR | 18.8 ± 6.4 | NR | 622.0 ± 294.0 | NR | 80 | 63 ± 9 | 50 |
Studies including some patients with impulse and compulsive behaviors in the PD group.
Abbreviations: PD, Parkinson's disease; SD, standard deviation; H&Y, Hoehn and Yahr Scale; UPDRS III, Unified Parkinson's Disease Rating Scale–Motor subscale; LEDD, levodopa equivalent daily dose; DA‐LEDD, dopamine agonist levodopa equivalent daily dose; NR, not reported.
TABLE 2.
Characteristics of studies included in the analysis (ICD positive vs. ICD negative)
| Study | PD patients with ICDs | Patients with PD without ICDs | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Sex, % Male | Age, y, Mean ± SD or Range | Disease Duration, y | H&Y, Mean ± SD or Range | UPDRS III, On Medication | LEDD, mg, Mean ± SD | DA‐LEDD, mg, Mean ± SD | N | Sex, % Male | Age, y, Mean ± SD or Range | Disease Duration, y | H&Y, Mean ± SD or Range | UPDRS III, On Medication | LEDD, mg, Mean ± SD | DA‐LEDD, mg, Mean ± SD | |
| Ricciardi et al, 2021 42 | 6 | NR | 54.6 ± 6.0 | 8.8 | NR | 17.0 ± 12.0 | 852.8 ± 391.3 | 190.0 ± 119.8 | 17 | NR | 60.6 ± 5.4 | 10.0 | NR | 19.5 ± 9.4 | 1112.5 ± 410.8 | 214.7 ± 166.0 |
| Aumann et al, 2020 14 | 43 | 58.1 | 60.9 ± 6.9 | 4.07 | NR | 25.9 ± 13.1 | 642.6 ± 397.0 | NR | 68 | 77.9 | 64.9 ± 8.2 | 5.0 | NR | 27.6 ± 12.3 | 740.9 ± 410.6 | NR |
| Hlavatá et al, 2020 38 | 15 | 73.3 | 59.3 ± 8.9 | 8.87 | 2.5 ± 0.6 | NR | 1289.7 ± 543.9 | NR | 22 | 45.5 | 69.2 ± 5.5 | 7.0 | 2.5 ± 0.7 | NR | 1025.5 ± 567.2 | NR |
| Lee et al, 2019 19 | 50 | 80.0 | 59.6 ± 9.2 | 6.55 | 2.0 ± 0.6 | 18.5 ± 7.9 | 545.0 ± 456.3 | 197.1 ± 118.6 | 60 | 43.3 | 63.9 ± 7.4 | 7.7 | 1.9 ± 0.5 | 17.0 ± 8.5 | 562.6 ± 264.5 | 160.4 ± 87.4 |
| Girard et al, 2019 47 | 13 | 100 | 58.5 ± 8.3 | 7.5 | NR | 11.1 ± 5.1 | 973.1 ± 422.6 | 282.1 ± 185.1 | 14 | 100 | 57.0 ± 9.0 | 6.8 | NR | 12.6 ± 6.0 | 1068.7 ± 398.8 | 295.1 ± 161.3 |
| Balconi et al, 2018 60 | 15 | 86.7 | 65.2 ± 6.3 | 9.8 | 2.1 ± 0.6 | 16.5 ± 8.9 | 783.1 ± 320.1 | NR | 17 | 76.5 | 60.7 ± 9.1 | 8.5 | 1.8 ± 0.8 | 13.1 ± 7.6 | 761.9 ± 323.9 | NR |
| Balconi et al, 2018 61 | 17 | 82.4 | 60.7 ± 6.1 | NR | 2.0 ± 0.7 | 17.0 ± 7.8 | 771.2 ± 320.1 | NR | 20 | 85.0 | 63.9 ± 7.1 | NR | 1.7 ± 0.5 | 13.8 ± 7.3 | 755.7 ± 320.2 | NR |
| Marín‐Lahoz et al, 2018 18 | 31 | 54.8 | 63.5 ± 9.8 | 6.48 | 2 (2–2) | 19.9 ± 9.6 | 702.2 ± 416.9 | NR | 69 | 53.6 | 63.5 ± 9.8 | 5.5 | 2 (2–2.5) | 23.4 ± 9.9 | 533.1 ± 451.6 | NR |
| Ruitenberg et al, 2018 62 | 21 | 66.7 | 60.0 ± 15.0 | 4.77 | (1–3) | 25.9 ± 9.9 | 561.0 ± 322.0 | NR | 30 | 63.3 | 62.0 ± 8.0 | 3.7 | (1–3) | 25.3 ± 9.5 | 486.0 ± 332.0 | NR |
| Pettorruso et al, 2014 63 | 34 | 76.5 | 62.9 ± 9.6 | 8.6 | NR | 19.0 ± 9.7 | 672.8 ± 373.1 | 171.4 ± 113.1 | 120 | 50.0 | 67.7 ± 9.4 | 7.0 | NR | 20.4 ± 8.4 | 575.0 ± 420.0 | 130.0 ± 112.0 |
| Piray et al, 2014 54 | 16 | 87.5 | 64.4 ± 3.3 | 9.63 | 2.47 ± 0.5 | 19.0 ± 5.3 | NR | NR | 15 | 77.5 | 63.7 ± 3.9 | 9.4 | 2.5 ± 0.6 | 20.1 ± 5.8 | NR | NR |
| Leroi et al, 2013 57 | 35 | 77.1 | 58.9 ± 9.0 | 8.04 | 2.2 ± 0.7 | 26.8 ± 9.9 | 994.7 ± 585.4 | 166.74 ± 175.23 | 55 | 70.9 | 62.5 ± 9.1 | 8.1 | 2.4 ± 0.6 | 28.9 ± 13.4 | 732.2 ± 589.9 | 156.54 ± 161.11 |
| Bentivoglio et al, 2013 64 | 17 | 82.4 | 62 ± 10.1 | 6.9 | 2.0 ± 0.8 | 23.8 ± 11.0 | 606.1 ± 319.2 | 172.9 ± 112.2 | 17 | 64.7 | 63.9 ± 9.2 | 7.3 | 2.3 ± 0.5 | 22.5 ± 6.9 | 616.2 ± 367.8 | 192.5 ± 88.5 |
| Ray et al, 2012 65 | 7 | 59.7 ± 10.9 | 10.43 | NR | 21.0 ± 8.0 | 888.3 ± 479.9 | NR | 7 | NR | 60.6 ± 9.7 | 8.1 | NR | 17.1 ± 6.4 | 644.4 ± 337.7 | NR | |
| Antonini et al, 2011 27 | 18 | 83.3 | 58.3 ± 9.7 | 1.6 | 1.4 ± 0.5 | 16.5 ± 8.1 | drug–naïve | 85 | 61.2 | 60.9 ± 9.1 | 1.2 | 1.6 ± 0.5 | 16.4 ± 8.9 | drug–naïve | ||
| Voon et al, 2011 6 , 13 | 282 | 67.7 | 60.8 ± 8.4 | 7.4 | NR | 19.2 ± 12.4 | 946.1 ± 609.6 | 266.3 ± 228.4 | 282 | 67.7 | 61.3 ± 8.4 | 7.4 | NR | 19.6 ± 12.4 | 809.2 ± 609.6 | 265.2 ± 228.4 |
| Isaias et al, 2008 9 | 14 | 50.0 | 60.0 ± 9.0 | 8.5 | NR | 16.7 ± 6.0 | 656.0 ± 252.0 | NR | 36 | 66.7 | 65.0 ± 9.0 | 8.0 | NR | 18.8 ± 6.4 | 622.0 ± 294.0 | NR |
| Voon et al, 2007 12 | 21 | 71.4 | 60.2 ± 8.9 | 9.2 | 2.0 ± 0.5 | 15.2 ± 6.9 | 874.2 ± 495.6 | 268.3 ± 194.3 | 42 | 50.0 | 65.7 ± 9.9 | 6.9 | 2.2 ± 0.8 | 22.1 ± 13.9 | 746.9 ± 322.5 | 192.1 ± 105.3 |
Abbreviations: ICD, impulse control disorder; PD, Parkinson's disease; H&Y, Hoehn and Yahr Scale; UPDRS III, Unified Parkinson's Disease Rating Scale–Motor subscale; LEDD, levodopa equivalent daily dose; DA‐LEDD, dopamine agonist‐LEDD; NR, not reported.
Quantitative Analysis: PD Versus Healthy Controls
The 28 selected studies included 1061 patients and 1000 healthy age‐matched control subjects. The meta‐analysis revealed a statistically significant mean difference (2.43; 95% CI, 1.03, 3.83), with higher BIS‐11 total scores in patients with PD compared with control subjects (Fig. 2). The heterogeneity was high (I 2 = 77%, Cochran's Q test P < 0.001).
FIG. 2.
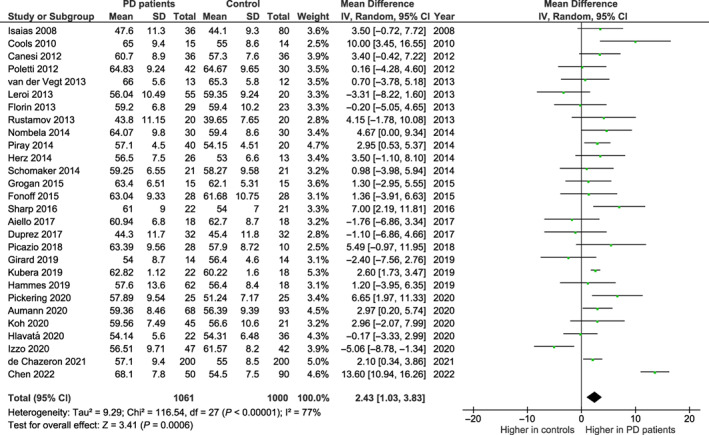
Barratt Impulsiveness Scale total scores in the 28 studies selected for the meta‐analysis comparing patients with Parkinson's disease (PD) to age‐matched healthy controls. CI, confidence interval; df, degree of freedom; SD, standard deviation.
The symmetry of the plots as well as Egger's test suggested no evidence of publication bias for BIS‐11 score mean difference (z = −0.371; P = 0.711) (Supplementary Material S1).
As a sensitivity analysis, the meta‐analysis was repeated after excluding 5 studies 17 , 30 , 31 , 32 , 33 that included in the PD groups some patients with ICDs. Similarly, a statistically significant mean difference (2.85; 95% CI, 1.23, 4.46), with higher BIS‐11 total scores in patients with PD compared with control subjects emerged. The heterogeneity was high (I 2 = 77%, Cochran's Q test P < 0.001).
Meta‐regressions did not reveal statistically significant effects of percentage of men in the sample, age, disease duration, and LEDD as moderators (β = −0.011, F1,24 = 0.077, P = 0.783; β = −0.287, F1,26 = 2.000, P = 0.169; β = 0.004, F1,21 < 0.001, P = 0.985; and β = −0.252, F1,19 = 1.290, P = 0.270, respectively).
Of 28 studies, 9 reported BIS‐11 subscale scores (Fig. S1). Higher levels of attentional (mean difference: 1.80; 95% CI, 1.54, 2.05) and nonplanning (1.69; 95% CI, 1.31, 2.07) impulsivity emerged for PD patients with ICDs compared with patients without ICDs. No significant difference was observed for the motor impulsivity subscale score (−0.08; 95% CI, −0.42, 0.25).
Quantitative Analysis: Patients with PD with Versus without ICDs
The 18 selected studies included 655 and 976 patients with and without ICDs, respectively. The meta‐analysis revealed a statistically significant mean difference (6.62; 95% CI, 5.01, 8.23), with higher BIS‐11 total scores in PD patients with ICDs compared with patients without ICDs (Fig. 3). The heterogeneity was moderate (I 2 = 52%, Cochran's Q test P < 0.001).
FIG. 3.
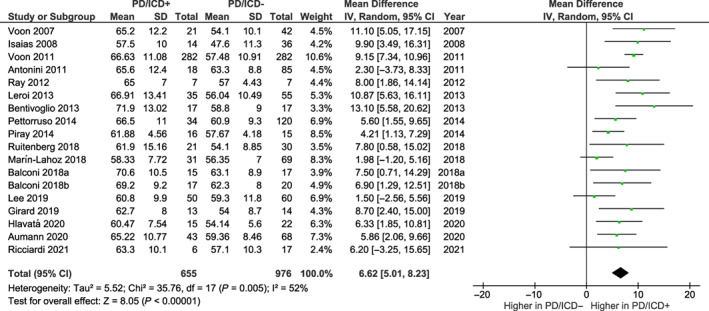
Barratt Impulsiveness Scale total scores in the 18 studies selected for the meta‐analysis comparing patients with Parkinson's disease (PD) with and without impulse control disorders (ICDs). CI, confidence interval; df, degree of freedom; SD, standard deviation.
The symmetry of the plots as well as Egger's test suggested no evidence of publication bias for BIS‐11 score mean difference (z = 1.222; P = 0.222) (Supplementary Material S1).
When mean LEDD difference between patients with and without ICDs was evaluated as moderator in the meta‐regression analysis, no statistically significant effect was observed (β = 0.163, F1,15 = 0.381, P = 0.547).
In 8 of 18 studies reporting BIS‐11 subscale scores, higher levels of attentional (mean difference: 1.79; 95% CI, 1.35, 2.22), motor (2.14; 95% CI, 0.96, 3.32), and nonplanning (3.05; 95% CI, 2.36, 3.73) impulsivity emerged for PD patients with ICDs compared with patients without ICDs (Fig. S2).
Discussion
The 2‐fold aim of the present meta‐analysis was (1) verifying whether impulsivity trait, as assessed by the BIS‐11, is higher in patients with PD with respect to age‐matched healthy individuals and (2) evaluating whether and to what extent the impulsivity traits differ between patients with PD with and without ICDs. The results showed a significantly higher level of impulsivity in patients with PD compared with healthy controls. In addition, BIS‐11 total scores are significantly higher in patients with PD with active ICDs compared with patients without ICDs. Overall, the results of the present meta‐analysis corroborate evidence showing elevated levels of impulsivity in PD, particularly in patients with ICDs. 9 , 13 , 14 , 15 , 34
Although the BIS‐11 mean difference between patients with PD and controls was quite small, such a difference remained significant even after excluding studies in which a proportion of patients with PD reported ICDs. It is noteworthy here that in most of the reviewed studies, ICDs are not systematically assessed in the healthy group and, as a consequence, the difference between patients with PD and healthy controls could be underestimated; even more so if we assume that also healthy subjects may have ICDs and related behaviors. 9
Differences between patients with PD and controls were not significantly influenced by any demographic or clinical factor (ie, percentage of men in the sample, age, disease duration, and LEDD) within the patient groups as revealed by meta‐regression analyses. Particularly noteworthy is that the mean LEDD did not emerge as a significant moderator of the relationship between impulsivity traits and ICDs.
All BIS‐11 domains were higher in patients with PD who were ICD positive compared with ICD negative. In keeping with Aumann et al, 14 the larger difference between the 2 groups emerged for the nonplanning impulsivity domain. In a recent study, 15 patients with PD showed elevated scores in all domains of the BIS‐11 compared with healthy controls, whereas patients with cerebellar ataxia exhibited differences in specific domains. Conversely, in the present meta‐analysis, significant differences emerged in the attentional and nonplanning domains, but not in the motor subscale. It must be said, however, that few studies reported the subscale (attentional, motor, and nonplanning) scores. Hence, no robust conclusion can be drawn from the present meta‐analysis on the impulsivity profile characterizing patients with PD.
Among the reviewed articles, 4 studies reported BIS‐11 scores in newly diagnosed drug‐naïve patients, allowing some considerations on the relationship between dopamine replacement therapy and impulsivity traits. 19 , 27 , 28 , 29 In the study by Antonini et al, 27 a large sample of drug‐naïve patients with PD were screened for the presence of ICDs and assessed for levels of impulsivity and obsessive‐compulsive symptoms. The proportion of patients who reported at least 1 ICD was 17.5%, a frequency similar to that reported in age‐matched healthy controls. 9 In patients with PD, the mean BIS‐11 total scores (63.7 ± 9.5; range, 45–91) was below the normative mean values in the age‐matched healthy population. 27 Patients with PD who were ICD positive showed higher scores in the attentional impulsiveness subscale of the BIS‐11 compared with patients with PD who were ICD negative, with no differences in the total score. Similarly, no differences between patients with de novo PD and healthy controls were reported by Poletti et al 28 in the BIS‐11 total score (64.8 ± 9.2 vs. 64.7 ± 9.6, respectively). van der Vegt et al 29 evaluated 13 drug‐naïve patients with PD and 12 healthy age‐matched control subjects who underwent functional magnetic resonance imaging recording during a 2‐choice gambling task. The BIS‐11 total score did not differ between the groups (66.0 ± 5.6 vs. 65.3 ± 5.8 in patients with PD and in healthy controls, respectively). Recently, Lee et al 19 conducted a multicenter, open‐label trial in which the baseline characteristics of 50 patients with PD with ICD were compared with those of 60 medicated and 40 drug‐naïve PD control groups. The BIS‐11 total score did not differ between the 3 groups of patients. Hence, available data on drug‐naïve patients seem to downsize the role of impulsive personality trait in predicting the risk of developing ICDs. Interestingly, a recent study conducted in patients with de novo PD identified 3 phenotypes based on personality traits and their relationships with motor and neuropsychiatric symptoms. 35 Impulsivity was observed in the “neuropsychiatric phenotype” characterized by high harm avoidance, low novelty seeking, hypodopaminergic neuropsychiatric symptoms, and higher impulsivity trait. Given the heterogeneity of PD in the early stages, it is conceivable that specific phenotypes may be more associated with the risk of developing ICDs. Moreover, it has been hypothesized that the level of impulsivity may be involved in boosting the severity of ICDs rather than increasing their risk of occurrence. 18 Reasoning on the results of the present meta‐analysis study, it is evident that a clear definition of the role of impulsivity traits as predisposing factors for the development of ICDs can only be drawn from longitudinal studies. Such studies should aim to assess impulsivity personality profile in patients with de novo PD before starting dopaminergic treatment and to verify longitudinally the incidence of ICDs in individuals with baseline levels of impulsivity exceeding normative values. To the best of our knowledge, no such longitudinal studies have been conducted yet.
A limitation of the present meta‐analysis is that only studies assessing impulsivity traits by the BIS‐11 were selected. This choice may limit the generalizability of the results. However, it should be noted that the literature based on different self‐report tools assessing impulsivity in patients with PD is quite limited for some questionnaires and absent for others.
In a relatively small number of studies, the UPPS was used to assess impulsivity traits in patients with PD instead of or in addition to the BIS‐11. In the study by Bayard et al,34 patients with PD without ICDs had greater levels of urgency, lack of premeditation, and lack of perseverance with respect to healthy controls, whereas levels of sensation seeking were higher in patients with ICDs compared with patients without ICDs. Similarly, in some dimensions of the UPPS, higher scores were also reported by Dawson et al 36 and Olley et al 37 in patients with ICDs. In contrast, some studies did not observe significant differences between patients with PD and healthy controls in the UPPS scores. 38 , 39 Interestingly, Hlavatá et al 38 reported significant group differences in the BIS‐11 scores but not in the UPPS subscale scores, confirming that different questionnaires evaluate different dimensions of impulsivity.
There is broad consensus that impulsivity is a multidimensional and heterogeneous concept that should not be considered as a unitary construct, instead consisting of a series of independent subtypes reflecting a variety of behaviors and processes. 40 Accordingly, using voxel‐based morphometry analyses, Marín‐Lahoz et al 41 showed that different self‐report and behavioral impulsivity measures reflect distinct brain structural correlates. Namely, the impulsivity traits appeared to be associated with lower gray matter volume in the dorsolateral prefrontal cortices. In a recent study conducted in patients who underwent bilateral DBS of the subthalamic nucleus, Ricciardi et al 42 showed a positive correlation between the oscillatory activity in the α band and the impulsivity traits (BIS‐11 score) in patients with PD, irrespective of the presence and severity of active ICDs. The authors proposed that this spectral feature may represent a neural biomarker associated with impulsive behavior.
In conclusion, the results of the present study support the view that impulsivity as a personality trait may characterize patients with PD even in the absence of ICDs. Moreover, our meta‐analysis corroborates findings of clinical studies reporting higher levels of impulsivity in PD patients with ICDs compared with patients without ICDs. Although the present results broaden the knowledge on the personality profiles of patients with PD, 35 , 43 they are currently not exhaustive. Thus, the complex relationship between impulsivity traits and ICDs in PD warrants further investigation.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
F.G.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
G.G.: 1C, 2A, 2B, 2C, 3A
C.N.: 1C, 2A, 2B, 2C, 3A
P.P.: 1A, 3B
M.P.V.: 1A, 3B
M.C.: 1A, 1B, 1C, 2C, 3A, 3B
Disclosures
Ethical Compliance Statement: The authors confirm that the approval of an institutional review board was not required for this work. The authors confirm that patient consent was not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: The authors declare that there are no additional disclosures to report.
Supporting information
Supplementary Material S1. Details on the data extraction, formulas, reference list of studies included in the quantitative analyses, and funnel plots for risk of publication bias.
Figure S1. Barratt Impulsiveness Scale–11 subscale (attentional, motor, and nonplanning impulsivity) scores: patients with Parkinson's disease versus healthy controls.
Figure S2. Barratt Impulsiveness Scale–11 subscale (attentional, motor, and non‐planning impulsivity) scores: patients with Parkinson's disease with versus without impulse control disorders.
Acknowledgments
This study was part of a project supported by a grant by “Ente Cassa di Risparmio di Firenze.” We are grateful to Professor G. Koch, Dr. S. Picazio, Professor D.O. Claassen, and Dr. M. Aumann who kindly provided us additional information on their studies.
Relevant disclosures and conflicts of interest are listed at the end of this article.
REFERENCES
- 1. DeYoung CG. Impulsivity as a personality trait. In: Vohs KD, Baumeister RF, eds. Handbook of self‐regulation: Research, theory, and applications. New York, NY: Guilford Press; 2011:485–502. [Google Scholar]
- 2. Dalley JW, Robbins TW. Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci 2017;18:158–171. [DOI] [PubMed] [Google Scholar]
- 3. Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 2013;108:44–79. [DOI] [PubMed] [Google Scholar]
- 4. Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry 2001;158:1783–1793. [DOI] [PubMed] [Google Scholar]
- 5. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross‐sectional study of 3090 patients. Arch Neurol 2010;67:589–595. [DOI] [PubMed] [Google Scholar]
- 6. Voon V, Mehta AR, Hallett M. Impulse control disorders in Parkinson's disease: recent advances. Curr Opin Neurol 2011;24:324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weintraub D, Mamikonyan E. Impulse control disorders in Parkinson's disease. Am J Psychiatry 2019;176:5–11. [DOI] [PubMed] [Google Scholar]
- 8. Kelly MJ, Baig F, Hu MT, Okai D. Spectrum of impulse control behaviours in Parkinson's disease: pathophysiology and management. J Neurol Neurosurg Psychiatry 2020;91:703–711. [DOI] [PubMed] [Google Scholar]
- 9. Isaias IU, Siri C, Cilia R, de Gaspari D, Pezzoli G, Antonini A. The relationship between impulsivity and impulse control disorders in Parkinson's disease. Mov Disord 2008;23:411–415. [DOI] [PubMed] [Google Scholar]
- 10. Voon V, Reynolds B, Brezing C, et al. Impulsive choice and response in dopamine agonist‐related impulse control behaviors. Psychopharmacology (Berl) 2010;207:645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poletti M, Bonuccelli U. Impulse control disorders in Parkinson's disease: the role of personality and cognitive status. J Neurol 2012;259:2269–2277. [DOI] [PubMed] [Google Scholar]
- 12. Voon V, Thomsen T, Miyasaki JM, et al. Factors associated with dopaminergic drug‐related pathological gambling in Parkinson disease. Arch Neurol 2007;64:212–216. [DOI] [PubMed] [Google Scholar]
- 13. Voon V, Sohr M, Lang AE, et al. Impulse control disorders in parkinson disease: a multicenter case‐control study. Ann Neurol 2011;69:986–996. [DOI] [PubMed] [Google Scholar]
- 14. Aumann MA, Stark AJ, Hughes SB, et al. Self‐reported rates of impulsivity in Parkinson's disease. Ann Clin Transl Neurol 2020;7:437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen TX, Lin CR, Aumann MA, Yan Y, Amokrane N, Desai NA, et al. Impulsivity trait profiles in patients with cerebellar ataxia and Parkinson disease. Neurology 2022;99:e176–e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aiello M, Eleopra R, Foroni F, Rinaldo S, Rumiati RI. Weight gain after STN‐DBS: the role of reward sensitivity and impulsivity. Cortex 2017;92:150–161. [DOI] [PubMed] [Google Scholar]
- 17. Hammes J, Theis H, Giehl K, et al. Dopamine metabolism of the nucleus accumbens and fronto‐striatal connectivity modulate impulse control. Brain 2019;142:733–743. [DOI] [PubMed] [Google Scholar]
- 18. Marín‐Lahoz J, Pagonabarraga J, Martinez‐Horta S, et al. Parkinson's disease: impulsivity does not cause impulse control disorders but boosts their severity. Front Psych 2018;9:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JY, Jeon B, Koh SB, et al. Behavioural and trait changes in parkinsonian patients with impulse control disorder after switching from dopamine agonist to levodopa therapy: results of REIN‐PD trial. J Neurol Neurosurg Psychiatry 2019;90:30–37. [DOI] [PubMed] [Google Scholar]
- 20. Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt impulsiveness scale: an update and review. Pers Individ Differ 2009;47:385–395. [Google Scholar]
- 21. Caspar EA, Cleeremans A. “Free will”: are we all equal? A dynamical perspective of the conscious intention to move. Neurosci Conscious 2015;2015:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giovannelli F, Mastrolorenzo B, Rossi A, et al. Relationship between impulsivity traits and awareness of motor intention. Eur J Neurosci 2016;44:2455–2459. [DOI] [PubMed] [Google Scholar]
- 23. Giovannelli F, Menichetti C, Kiferle L, et al. Impulsivity traits and awareness of motor intention in Parkinson's disease: a proof‐of‐concept study. Neurol Sci 2022;43:335–430. [DOI] [PubMed] [Google Scholar]
- 24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 1995;6:768–774. [DOI] [PubMed] [Google Scholar]
- 26. Maggi G, Altieri M, Ilardi CR, Santangelo G. Validation of a short Italian version of the Barratt impulsiveness scale (BIS‐15) in non‐clinical subjects: psychometric properties and normative data. Neurol Sci 2022;43:4719–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Antonini A, Siri C, Santangelo G, et al. Impulsivity and compulsivity in drug‐naïve patients with Parkinson's disease. Mov Disord 2011;26:464–468. [DOI] [PubMed] [Google Scholar]
- 28. Poletti M, Frosini D, Pagni C, Claudio L, Paolo del D, Roberto C, Bonuccelli U. Alexithymia is associated with impulsivity in newly‐diagnosed, drug‐naïve patients with Parkinson's disease: an affective risk factor for the development of impulse‐control disorders? J Neuropsychiatry Clin Neurosci 2012;24:E36–E37. [DOI] [PubMed] [Google Scholar]
- 29. van der Vegt JP, Hulme OJ, Zittel S, Madsen KH, Weiss MM, Buhmann C, et al. Attenuated neural response to gamble outcomes in drug‐naive patients with Parkinson's disease. Brain 2013;136:1192–1203. [DOI] [PubMed] [Google Scholar]
- 30. Canesi M, Rusconi ML, Isaias IU, Pezzoli G. Artistic productivity and creative thinking in Parkinson's disease. Eur J Neurol 2012;19:468–472. [DOI] [PubMed] [Google Scholar]
- 31. Fonoff FC, Fonoff ET, Barbosa ER, et al. Correlation between impulsivity and executive function in patients with Parkinson disease experiencing depression and anxiety symptoms. J Geriatr Psychiatry Neurol 2015;28:49–56. [DOI] [PubMed] [Google Scholar]
- 32. de Chazeron I, Durif F, Lambert C, et al. A case‐control study investigating food addiction in Parkinson patients. Sci Rep 2021;11:10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Izzo VA, Donati MA, Torre E, Ramat S, Primi C. Impulse control disorders in Parkinson's disease versus in healthy controls: a different predictive model. J Neuropsychol 2020;14:318–332. [DOI] [PubMed] [Google Scholar]
- 34. Bayard S, Joly E, Ghisletta P, et al. A multidimensional approach to impulsivity in Parkinson's disease: measurement and structural invariance of the UPPS impulsive behaviour scale. Psychol Med 2016;46:2931–2941. [DOI] [PubMed] [Google Scholar]
- 35. Meira B, Lhommée E, Schmitt E, Klinger H, Bichon A, Pélissier P, et al. Early Parkinson's disease phenotypes tailored by personality, behavior, and motor symptoms. J Parkinsons Dis 2022;12:1665–1676. [DOI] [PubMed] [Google Scholar]
- 36. Dawson A, Ortelli P, Carter A, et al. Motivational and myopic mechanisms underlying dopamine medication‐induced impulsive‐compulsive behaviors in Parkinson's disease. Front Behav Neurosci 2023;16:949406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olley J, Blaszczynski A, Lewis S. Dopaminergic medication in Parkinson's disease and problem gambling. J Gambl Stud 2015;31:1085–1106. [DOI] [PubMed] [Google Scholar]
- 38. Hlavatá P, Linhartová P, Šumec R, et al. Behavioral and neuroanatomical account of impulsivity in Parkinson's disease. Front Neurol 2020;10:1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marques A, Roquet D, Matar E, Taylor NL, Pereira B, O'Callaghan C, Lewis SJG. Limbic hypoconnectivity in idiopathic REM sleep behaviour disorder with impulse control disorders. J Neurol 2021;268:3371–3380. [DOI] [PubMed] [Google Scholar]
- 40. Strickland JC, Johnson MW. Rejecting impulsivity as a psychological construct: a theoretical, empirical, and sociocultural argument. Psychol Rev 2021;128:336–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marín‐Lahoz J, Martínez‐Horta S, Sampedro F, Pagonabarraga J, Horta‐Barba A, Bejr‐Kasem H, et al. Measuring impulsivity in Parkinson's disease: a correlational and structural neuroimaging study using different tests. Eur J Neurol 2020;27:1478–1486. [DOI] [PubMed] [Google Scholar]
- 42. Ricciardi L, Fischer P, Mostofi A, et al. Neurophysiological correlates of trait impulsivity in Parkinson's disease. Mov Disord 2021;36:2126–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Santangelo G, Garramone F, Baiano C, D'Iorio A, Piscopo F, Raimo S, Vitale C. Personality and Parkinson's disease: a meta‐analysis. Parkinsonism Relat Disord 2018;49:67–74. [DOI] [PubMed] [Google Scholar]
- 44. Koh J, Kaneoke Y, Donishi T, Ishida T, Sakata M, Hiwatani Y, et al. Increased large‐scale inter‐network connectivity in relation to impulsivity in Parkinson's disease. Sci Rep 2020;10:11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pickering JS, Leroi I, McBride J, Poliakoff E. Continuous force measurements reveal no inhibitory control deficits in Parkinson's disease. Exp Brain Res 2020;238:1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kubera KM, Schmitgen MM, Nagel S, Hess K, Herweh C, Hirjak D, et al. A search for cortical correlates of trait impulsivity in Parkinson's disease. Behav Brain Res 2019;369:111911. [DOI] [PubMed] [Google Scholar]
- 47. Girard R, Obeso I, Thobois S, Park SA, Vidal T, Favre E, et al. Wait and you shall see: sexual delay discounting in hypersexual Parkinson's disease. Brain 2019;142:146–162. [DOI] [PubMed] [Google Scholar]
- 48. Picazio S, Ponzo V, Caltagirone C, Brusa L, Koch G. Dysfunctional inhibitory control in Parkinson's disease patients with levodopa‐induced dyskinesias. J Neurol 2018;265:2088–2096. [DOI] [PubMed] [Google Scholar]
- 49. Duprez J, Houvenaghel JF, Argaud S, Naudet F, Robert G, Drapier D, et al. Impulsive oculomotor action selection in Parkinson's disease. Neuropsychologia 2017;95:250–258. [DOI] [PubMed] [Google Scholar]
- 50. Sharp ME, Foerde K, Daw ND, Shohamy D. Dopamine selectively remediates 'model‐based' reward learning: a computational approach. Brain 2016;139:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grogan J, Bogacz R, Tsivos D, Whone A, Coulthard E. Dopamine and consolidation of episodic memory: Timing is everything. J Cogn Neurosci 2015;27:2035–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herz DM, Haagensen BN, Christensen MS, Madsen KH, Rowe JB, Løkkegaard A, Siebner HR. The acute brain response to levodopa heralds dyskinesias in Parkinson disease. Ann Neurol 2014;75:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nombela C, Rittman T, Robbins TW, Rowe JB. Multiple modes of impulsivity in Parkinson's disease. PLoS One 2014;9:e85747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Piray P, Zeighami Y, Bahrami F, Eissa AM, Hewedi DH, Moustafa AA. Impulse control disorders in Parkinson's disease are associated with dysfunction in stimulus valuation but not action valuation. J Neurosci 2014;34:7814–7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schomaker J, Berendse HW, Foncke EM, van der Werf YD, van den Heuvel OA, Theeuwes J, Meeter M. Novelty processing and memory formation in Parkinson's disease. Neuropsychologia 2014;62:124–136. [DOI] [PubMed] [Google Scholar]
- 56. Florin E, Müller D, Pfeifer J, Barbe MT, Fink GR, Timmermann L. Subthalamic stimulation modulates self‐estimation of patients with Parkinson's disease and induces risk‐seeking behaviour. Brain 2013;136:3271–3281. [DOI] [PubMed] [Google Scholar]
- 57. Leroi I, Barraclough M, McKie S, Hinvest N, Evans J, Elliott R, McDonald K. Dopaminergic influences on executive function and impulsive behaviour in impulse control disorders in Parkinson's disease. J Neuropsychol 2013;7:306–325. [DOI] [PubMed] [Google Scholar]
- 58. Rustamov N, Rodriguez‐Raecke R, Timm L, Agrawal D, Dressler D, Schrader C, et al. Absence of congruency sequence effects reveals neurocognitive inflexibility in Parkinson's disease. Neuropsychologia 2013;51:2976–2987. [DOI] [PubMed] [Google Scholar]
- 59. Cools R, Miyakawa A, Sheridan M, D'Esposito M. Enhanced frontal function in Parkinson's disease. Brain 2010;133:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Balconi M, Angioletti L, Siri C, Meucci N, Pezzoli G. Gambling behavior in Parkinson's Disease: Impulsivity, reward mechanism and cortical brain oscillations. Psychiatry Res 2018a;270:974–980. [DOI] [PubMed] [Google Scholar]
- 61. Balconi M, Siri C, Meucci N, Pezzoli G, Angioletti L. Personality traits and cortical activity affect gambling behavior in parkinson's disease. J Parkinsons Dis 2018b;8:341–352. [DOI] [PubMed] [Google Scholar]
- 62. Ruitenberg MFL, Wu T, Averbeck BB, Chou KL, Koppelmans V, Seidler RD. Impulsivity in Parkinson's disease is associated with alterations in affective and sensorimotor striatal networks. Front Neurol 2018;9:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pettorruso M, Martinotti G, Fasano A, Loria G, Di Nicola M, De Risio L, et al. Anhedonia in Parkinson's disease patients with and without pathological gambling: a case‐control study. Psychiatry Res 2014;215:448–452. [DOI] [PubMed] [Google Scholar]
- 64. Bentivoglio AR, Baldonero E, Ricciardi L, De Nigris F, Daniele A. Neuropsychological features of patients with Parkinson's disease and impulse control disorders. Neurol Sci 2013;34:1207–1213. [DOI] [PubMed] [Google Scholar]
- 65. Ray NJ, Miyasaki JM, Zurowski M, Ko JH, Cho SS, Pellecchia G, et al. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson's patients with medication‐induced pathological gambling: a [11C] FLB‐457 and PET study. Neurobiol Dis 2012;48:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1. Details on the data extraction, formulas, reference list of studies included in the quantitative analyses, and funnel plots for risk of publication bias.
Figure S1. Barratt Impulsiveness Scale–11 subscale (attentional, motor, and nonplanning impulsivity) scores: patients with Parkinson's disease versus healthy controls.
Figure S2. Barratt Impulsiveness Scale–11 subscale (attentional, motor, and non‐planning impulsivity) scores: patients with Parkinson's disease with versus without impulse control disorders.


