Abstract
The pharmacokinetics of moxifloxacin were investigated in six studies after oral administration of 50, 100, 200, 400, 600, and 800 mg. Eight healthy male volunteers were included in each study. With doses of up to 200 mg the study was performed as a double-blind, randomized group comparison (n = 6 verum and n = 2 matched placebo); with the higher doses the study was conducted with a double-blind, randomized, crossover design. Safety and tolerability were assessed by evaluation of vital signs, electrocardiograms, electroencephalograms, clinical chemistry parameters, results of urinalysis, and adverse events. The drug was well tolerated. The concentrations of moxifloxacin in plasma, urine, and saliva were determined by a validated high-pressure liquid chromatography assay with fluorescence detection. In addition, plasma and urine samples were analyzed by a bioassay. A good correlation between both methods was seen, indicating an absence of major active metabolites. The mean maximum concentrations of moxifloxacin in plasma (Cmax) ranged from 0.29 mg/liter (50-mg dose) to 4.73 mg/liter (800-mg dose) and were reached 0.5 to 4 h following drug administration. After reaching the Cmax, plasma moxifloxacin concentrations declined in a biphasic manner. Within 4 to 5 h they fell to about 30 to 55% of the Cmax, and thereafter a terminal half-life of 11 to 14 h accounted for the major part of the area under the concentration-time curve (AUC). During the absorption phase concentrations in saliva were even higher than those in plasma, whereas in the terminal phase a constant ratio of the concentration in saliva/concentration in plasma of between 0.5 and 1 was observed, indicating a correlation between unbound concentrations in plasma and levels in saliva (protein binding level, approximately 48%). AUC and Cmax increased proportionally to the dose over the whole range of doses investigated. Urinary excretion amounted to approximately 20% of the dose. Data on renal clearance (40 to 51 ml/min/1.73 m2) indicated partial tubular reabsorption of the drug. The pharmacokinetic parameters derived from compartmental and noncompartmental analyses were in good agreement. The kinetics could be described best by fitting the data to a two-compartment body model.
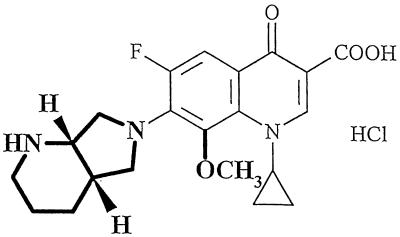
Moxifloxacin hydrochloride (Bayer name, BAY 12-8039), 1-cyclopropyl-7-(2,8-diazabicyclo [4.3.0] nonane)-6-fluoro-8-methoxy-1,4-di-hydro-4-oxo-3-quinoline carboxylic acid hydrochloride, is a new enantiomerically pure 8-methoxy quinolone that was first synthesized by Petersen and colleagues (2, 7) and that has potent antimicrobial activity against both gram-negative and gram-positive bacteria and anaerobes. Figure 1 presents the chemical structure of the compound. In vitro, moxifloxacin is highly effective against a wide spectrum of respiratory tract pathogens, showing enhanced activity against gram-positive microorganisms compared to those of other antibiotics. It has excellent efficacy against pneumococcal strains resistant to beta-lactam and macrolide antibiotics and good activity against atypical respiratory tract pathogens (2, 8). The MICs at which 90% of isolates are inhibited for clinical isolates of penicillin-resistant Streptococcus pneumoniae were below 0.125 mg/liter (8).
FIG. 1.
Chemical structure of moxifloxacin (substituents differing from those for ciprofloxacin are represented in boldface type).
In vivo, moxifloxacin is effective in mouse models of typical and atypical respiratory tract infections and in guinea pigs infected with Mycoplasma pneumoniae (6, 8). Moxifloxacin is rapidly bactericidal and effectively penetrates extravascular tissues, including lung tissue. Unlike the multihalogenated quinolones, moxifloxacin shows no phototoxic potential (11).
This paper describes the single-dose pharmacokinetics following oral administration of ascending doses of 50 to 800 mg as part of the clinical evaluation of this drug.
(Parts of the paper were presented at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy New Orleans, La., 15 to 18 September 1996 [6a] and at the 8th ECCMID, Laussanne, Switzerland 25 to 28 May 1997 [10a].)
MATERIALS AND METHODS
Study design.
The pharmacokinetics, safety, and tolerability of oral moxifloxacin were investigated in healthy male volunteers which gave their written informed consent prior to admission to the study. The study was conducted in accordance with the principles of the Declaration of Helsinki. The protocol was approved by the local ethics committee.
Doses ranging from 50 to 800 mg were covered in six escalation stages (50, 100, 200, 400, 600, and 800 mg). A total of 45 healthy male volunteers participated in the program (Table 1). With doses of up to 200 mg the study was performed as a double-blind, randomized group comparison (n = 6 verum and n = 2 matching placebo); for the other doses a double-blind, randomized, crossover design (n = 7) was applied. The washout phase between two treatments was at least 1 week. The drug was administered in the morning, after an overnight fast. Eating was not allowed for 4 h after drug administration, but water was allowed ad libitum. The state of health of the volunteers was determined by physical examination and a laboratory test panel that allowed evaluation of interfering diseases (e.g., renal or hepatic impairment or hematological disorder). The healthy subjects were not allowed to take any medication from 2 weeks prior to the study to the end of the study.
TABLE 1.
Demographic data for the study populationa
| Characteristic | Mean | SD | Range |
|---|---|---|---|
| Age (yr) | 33.6 | 5.24 | 23–45 |
| Wt (kg) | 81.47 | 9.03 | 59–100 |
| Ht (cm) | 182.2 | 7.13 | 168–196 |
| Broca index | 0.99 | 0.10 | 0.72–1.18 |
The study population consisted of 45 Caucasian subjects.
Specimen sampling.
Blood samples of 3 ml were collected in heparinized (ammonium heparin) containers at predosing and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, and 48 h after administration of the drug. From the 200-mg-dose study on sampling was extended to 72 h postdosing, and in the 800-mg-dose study an additional sample was drawn at 96 h to fully describe the terminal phase of the plasma concentration-time curves. Maximal blood loss was approximately 300 ml (45 ml for pharmacokinetic study sampling). The separated plasma was transferred to polypropylene tubes and was stored at −20°C prior to analysis.
For the first three stages of dose escalation (50 to 200 mg), saliva samples were collected in parallel with the blood sampling schedule. The subjects were asked to chew a cotton wool swab for approximately 1 min. The samples were extracted in three stages. After initial centrifugation (1,900 × g, 5 min) an extraction with 0.5 ml of acetonitrile and phosphate buffer (pH 4) at 0.1 mol/liter followed by centrifugation was applied twice. The samples were transferred to polypropylene tubes and were stored at −20°C prior to analysis. To determine the volumes of saliva, known amounts of ofloxacin as an internal standard were added after the last extraction step, and a weight control of the cotton wool swabs was performed (10).
Urine samples were collected from 0 to 4.0, 4.1 to 8.0, 8.1 to 12.0, and 12.1 to 24.0 h after drug administration. From the second day on collection was done at intervals of 24 h up to the last time of plasma collection. The total volume of each sampling interval was recorded. Samples of 2 ml were stored at −20°C prior to analysis.
Plasma protein binding.
The level of plasma protein binding of moxifloxacin was determined ex vivo over a concentration range of 0.05 to 4.7 mg/liter by a membrane filtration method. Two pooled plasma samples from two subjects each were deproteinated by ultrafiltration with a Centricon device (Centricon; Amicon). The concentration of unbound drug in the ultrafiltrate was measured. To validate the results of the experiment, heparinized plasma samples spiked with known concentrations of moxifloxacin hydrochloride were analyzed together with the study samples, and the amounts recovered in the ultrafiltrate and the residue were determined. Additional extraction of the used filtration devices did not reveal any binding of the drug to the filter material. The content of unbound drug (fu) was calculated as ratio of the concentration in the ultrafiltrate versus the concentration in plasma ×100.
Bioanalytics.
All drug assays were performed by Bayer AG in the laboratories of the Institute of Clinical Pharmacology, Wuppertal, Germany. A validated high-pressure liquid chromatography (HPLC) method with fluorescence detection (10) was used to determine the concentrations of moxifloxacin in plasma, urine, and saliva after the separation of moxifloxacin from its metabolites, M1 (sulfo compound) and M2 (glucuronide). The validity of the concentration results was verified by assaying quality control samples produced from blank plasma, urine, and saliva spiked with known concentrations of moxifloxacin in the range of 0.01 to 1.0 mg/liter. The precision for the quality control samples was between 3.1, 0.8, and 3.3% and 3.9, 3.5, and 8.6% for the three types of samples, respectively. The accuracy ranged between 95.2, 102.0, and 102.9% and 100.3, 102.2, and 106.6% for the three types of samples, respectively. The limit of quantification of the method was 0.0025 mg/liter, independent of the matrix. Additionally, concentrations in plasma and urine were determined by the agar diffusion plate method with Bacillus subtilis as the test strain (10). The limit of quantification was 0.063 mg/liter.
To assess the comparability of the bioassay and the HPLC method and to detect the activity of potential microbially active metabolites relevant in humans, the concentrations determined by both methods were correlated.
Pharmacokinetic evaluation.
Compartmental (5) and noncompartmental (4) pharmacokinetic parameters were calculated from the concentration data. For the determination of noncompartmental parameters the Kincalc (version 2.3) software package was used, compartmental parameters were estimated with TopFit (version 2.0) software (5).
Maximum concentrations in plasma (Cmaxs) and times to Cmax (Tmax) were taken directly from the plasma concentration-time profiles. Terminal half-lives (t1/2) were obtained by linear regression analysis of the last datum points after logarithmic transformation of the data. Areas under the curves extrapolated to infinity (AUC) were calculated by using the log-linear trapezoidal rule. Cmax,norm and AUCnorm were calculated by normalizing Cmax and AUC, respectively, to the dose (in milligrams per kilogram). The apparent oral volume of distribution during the terminal phase (V/F) and the apparent oral clearance of the drug (CL/F) were calculated as [(dose/AUC)] · [t1/2/ln2] and (dose/AUC), respectively. Amounts excreted into urine (Aeur) were based on drug concentrations in urine and the volumes of urine collected in the postdosing interval. Renal clearance was calculated as Aeur/AUC. For all variables descriptive statistics including mean values, SDs, and minimum, median, and maximum values were applied.
Compartmental parameters were calculated after fitting the data to a two-compartment body model.
RESULTS
Study population.
The study population consisted of 45 healthy male Caucasian volunteers (39 of whom received the study drug) whose data were valid for safety evaluation. Details of their demographic characteristics are presented in Table 1.
Safety and tolerability.
The study drug was well tolerated by all volunteers. No clinically relevant changes in physical findings or vital signs were detected. There were no serious adverse events, dropouts, or deaths in this study.
A total of 20 adverse events were reported: 8 among subjects receiving active treatment and 12 among subjects receiving placebo. Only one (headache in a subject receiving placebo) was judged to be moderate; all the others were judged to be mild. Overall, three episodes of headache were reported in the active treatment group and four episodes of headache were reported in the placebo group. Flu syndrome occurred more often in the placebo group (n = 4) than in the verum group (n = 2). Asthenia was reported more often in the active treatment group (n = 2) than in the placebo group (n = 1). The digestive system was involved once in the active treatment group (50 mg SD, loose stool) and once in the placebo treatment group (flatulence). Acne was observed in one subject during active and placebo treatments. The only other adverse events reported in subjects receiving active treatment were herpes simplex labialis and an ear disorder. Voice alteration and an increased intensity of cough were reported for one subject each treated with placebo. In conclusion, no influence of the dose on the frequency or quality of adverse events was observed.
The only laboratory parameter that slightly exceeded the upper range of normal was an alkaline phosphatase level which was already elevated prior to drug administration. It did not increase further during treatment. No other laboratory parameter exceeded the upper limit of normal.
There were no clinically relevant changes in electrocardiogram or electroencephalogram parameters or pathological findings on physical examination.
Plasma protein binding.
An ex vivo assay indicated that 48% ± 2.5% of the total concentration was bound to plasma proteins. The degree of protein binding was consistent across the range of concentrations in plasma tested (0.05 to 4.7 mg/liter). The findings were in agreement with data from in vitro studies (3).
Bioanalytics.
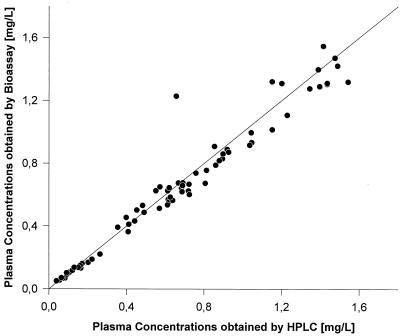
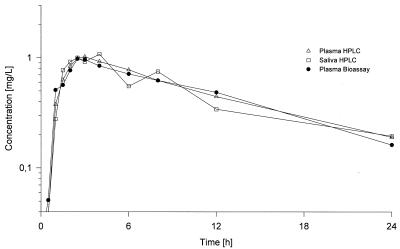
A bioassay (with a sensitive B. subtilis test strain) for urine and plasma and an HPLC assay (reverse-phase chromatography with fluorescence detection) for plasma, saliva, and urine were developed to assess the pharmacokinetics of moxifloxacin in different biological matrices (10). The results of the correlation are illustrated in Fig. 2. The correlation was excellent, with a slope of 1.05 and an almost negligible intercept of −0.16 (r2 = 0.993). Figure 3 indicates that the plasma concentration-versus-time curves obtained by both methods are comparable. The pharmacokinetic parameters derived from these experiments yielded almost identical values.
FIG. 2.
Correlation between concentrations obtained by HPLC and bioassay.
FIG. 3.
Time courses of the moxifloxacin concentration in plasma (HPLC and bioassay data) and saliva (HPLC data) following oral administration of a single 200-mg dose.
Concentration-time profiles for plasma and saliva.
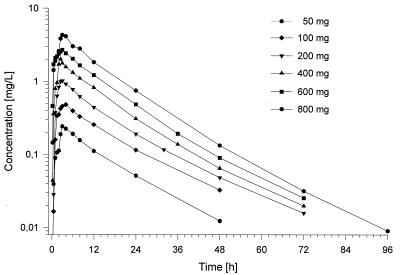
The plasma concentration-versus-time curves obtained by HPLC are presented in Fig. 4. They followed very similar patterns over the dose range of 50 to 800 mg, and no significant dose dependency was detectable. Following administration of the dose, Cmaxs were reached in about 0.5 to 4 h. Cmaxs ranged from 0.22 to 0.42 mg/liter for the lowest dose to 4.0 to 5.6 mg/liter for the 800-mg dose. Within 3 to 10 h the concentrations decreased to about 50% of the Cmax, and then the concentrations declined, with t1/2s of about 9 to 17 h. A comparison of the profiles with data derived from the bioassay resulted in almost identical curves, as shown in Fig. 3.
FIG. 4.
Time courses of the moxifloxacin concentration in plasma following the administration of single ascending doses ranging from 50 to 800 mg (HPLC data).
Pharmacokinetics in saliva were investigated over the dose range of 50 to 200 mg. Following administration of the drug, maximum concentrations in saliva were reached about 1 to 4 hours after drug administration. The peak concentrations ranged from 0.14 to 0.52 mg/liter after the ingestion of a 50-mg dose, 0.41 to 1.80 mg/liter after the ingestion of a 100-mg dose, and 1.12 to 2.83 mg/liter following the ingestion of a 200-mg dose; these concentrations are generally higher than those in plasma. Overall, the patterns of the saliva concentration-versus-time profiles, although biased by a higher variability, were similar to the corresponding curves for plasma (Fig. 3).
Pharmacokinetics.
The results of the noncompartmental pharmacokinetic analysis derived from concentrations in plasma and urine are summarized in Table 2, and those calculated from concentrations in saliva are tabulated in Table 3. AUC data were comparable for plasma (mean AUCnorm, approximately 5.3 to 6.5 kg · h/liter) and saliva (mean AUCnorm, approximately, 4.7 to 5.6 kg · h/liter); they indicate that a high level of systemic exposure to drug is achieved in humans. Tmaxs were almost identical for saliva and plasma. The mean apparent volume of distribution during the terminal phase amounted to approximately 2.5 to 3.6 liters/kg for plasma and 3.0 to 3.7 liters/kg for saliva (noncompartmental analysis), a value on the order of magnitude typical for fluoroquinolones (1, 13). Statistical analysis of the noncompartmental parameters AUCnorm (P = 0.18) and Cmax,norm (P = 0.76) confirmed that pharmacokinetics are independent of the dose. The data are supported by the urinary recovery of the substance, which also indicated the linearity of the pharmacokinetics.
TABLE 2.
Noncompartmental pharmacokinetic parameters of moxifloxacin following administration of single ascending doses ranging from 50 to 800 mga
| Dose (mg [no. of subjects]) | AUC (mg · h/liter) | AUCnorm (kg · h/liter) | Cmax (mg/liter) | Cmax,norm (kg/liter) | Tmax (h)b |
|---|---|---|---|---|---|
| 50 (6) | 3.88/1.13 (3.30–4.42) | 6.53/1.18 (5.34–8.10) | 0.29/1.25 (0.22–0.42) | 0.48/1.30 (0.35–0.78) | 1.75 (0.50–4.00) |
| 100 (6) | 8.51/1.21 (8.06–11.3) | 6.18/1.18 (5.08–7.80) | 0.59/1.21 (0.46–0.79) | 0.43/1.23 (0.31–0.54) | 2.00 (1.00–4.00) |
| 200 (6) | 15.4/1.20 (12.1–20.8) | 6.13/1.13 (5.44–7.57) | 1.16/1.35 (0.72–1.54) | 0.46/1.27 (0.32–0.57) | 2.50 (1.00–3.00) |
| 400 (7) | 26.9/1.18 (20.7–33.2) | 5.33/1.18 (4.14–6.39) | 2.50/1.31 (1.62–3.64) | 0.50/1.25 (0.32–0.65) | 1.50 (0.50–2.57) |
| 600 (7) | 39.9/1.11 (34.1–45.6) | 5.45/1.24 (3.75–6.72) | 3.19/1.19 (2.41–3.99) | 0.44/1.29 (0.31–0.63) | 2.50 (0.50–3.00) |
| 800 (7) | 59.9/1.24 (42.2–78.1) | 6.39/1.22 (4.64–7.76) | 4.73/1.16 (3.98–5.61) | 0.51/1.16 (0.44–0.68) | 3.00 (2.00–4.00) |
| Mean residence time (h) | t1/2 (h) | V/F (liters/kg)c | CL/F (liters/h) | CLR (liters/h)d | Aeur (%) |
|---|---|---|---|---|---|
| 16.2/1.17 (12.5–19.9) | 11.4/1.11 (9.3–12.4) | 2.52/1.15 (2.17–3.22) | 12.9/1.13 (11.3–15.2) | 2.77/1.22 (0.06–3.38) | 20.4/1.15 (17.2–23.7) |
| 17.8/1.13 (15.7–22.2) | 12.2/1.11 (11.0–14.6) | 2.85/1.13 (2.32–3.11) | 11.8/1.21 (8.9–15.3) | 2.38/1.25 (1.72–3.26) | 18.9/1.34 (10.7–24.6) |
| 17.0/1.11 (14.8–19.9) | 14.0/1.14 (12.2–16.7) | 3.30/1.14 (2.77–3.87) | 13.0/1.20 (9.6–16.6) | 2.64/1.25 (2.01–3.52) | 19.8/1.16 (16.3–24.0) |
| 14.9/1.14 (11.8–17.9) | 13.1/1.06 (12.2–14.6) | 3.55/1.23 (2.76–5.10) | 14.9/1.18 (12.1–19.3) | 3.03/1.17 (2.45–3.75) | 20.1/1.20 (15.7–24.7) |
| 14.6/1.18 (12.0–19.0) | 12.5/1.14 (10.3–15.0) | 3.30/1.12 (2.90–3.95) | 15.0/1.11 (13.2–17.6) | 2.67/1.10 (2.35–3.08) | 17.5/1.16 (14.4–21.8) |
| 14.7/1.13 (12.0–17.3) | 12.3/1.13 (9.3–14.3) | 2.77/1.19 (2.23–3.41) | 13.4/1.24 (10.3–19.0) | 2.50/1.28 (1.98–4.14) | 18.7/1.25 (13.3–24.7) |
Values are geometric mean/SD (range) unless indicated otherwise.
Values are median (range).
V/F, volume of distribution during the terminal phase.
CLR, renal clearance.
TABLE 3.
Comparison of compartmental (plasma) and noncompartmental (plasma, saliva) pharmacokinetic parameters of moxifloxacina
| Compartment, dose (mg), and model | AUC (mg · h/liter) | AUCnorm (kg · h/liter) | Cmax (mg/liter) | Cmax,norm (kg/liter) | Tmax (h) | t1/2 (h) | Mean residence time (h) | CL/F (ml/min) | CLR (ml/min)b | Aeur (%) | V/F (liters)c |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma, 200 | |||||||||||
| Noncompartmental model | 15.4/1.20 | 6.13/1.13 | 1.16/1.35 | 0.46/1.27 | 1.96/1.69 | 14.0/1.14 | 17.0/1.11 | 217/1.20 | 44/1.25 | 19.8/1.16 | 264/1.14 |
| Two-compartmental model | 15.3/1.19 | 6.11/1.12 | 1.15/1.38 | 0.46/1.30 | 1.66/1.74 | 11.8/1.25 | 16.2/1.16 | 218/1.19 | —d | — | 222/1.15 |
| Saliva | |||||||||||
| 200 | 14.0/1.29 | 5.60/1.27 | 1.62/1.44 | 0.65/1.40 | 1.89/1.62 | 12.4/1.29 | 14.8/1.14 | 238/1.29 | — | — | 254/1.34 |
| 100 | 8.27/1.54 | 6.01/1.49 | 0.84/1.74 | 0.61/1.77 | 1.98/1.78 | 12.0/1.19 | 18.3/1.12 | 202/1.54 | — | — | 216/1.57 |
| 50 | 2.81/1.40 | 4.72/1.37 | 0.31/1.55 | 0.53/1.56 | 1.45/1.57 | 12.0/1.29 | 16.5/1.31 | 297/1.40 | — | — | 308/1.36 |
Data were obtained after the administration of a 200-mg single dose and, for saliva, after the administration of single 50- and 100-mg doses. Data are geometric mean/SD
CLR, renal clearance.
V/F, volume of distribution during the terminal phase.
—, not applicable.
Data on elimination are summarized in Table 2. The geometric mean CL/F amounted to approximately 12 to 15 liters/h (200 to 250 ml/min) for plasma and was comparable for saliva (Table 3). Moxifloxacin is eliminated from plasma and saliva, with mean t1/2s of approximately 11 to 14 h (plasma) and 12 h (saliva) accounting for the major part (>90%; compartmental analysis) of the AUC.
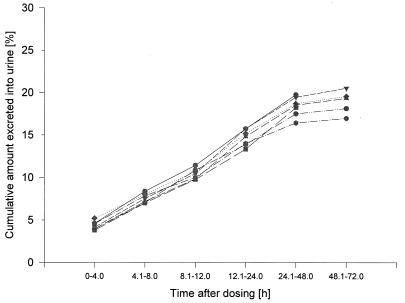
Figure 5 gives an overview of the cumulative amounts of unchanged drug excreted into urine. High-dose-dependent concentrations in urine ranging between 1.4 to 137.6 mg/liter were found in the first 24 h following administration of the drug, with high interindividual variability. The level of urinary excretion of moxifloxacin amounted to 18 to 20% of the dose. The statistical tests with renal excretion data revealed no differences between the dosages with regard to Aeur (P = 0.55). The data on renal excretion (individual renal clearances of approximately 2.4 to 3.0 liters/h [40 to 50 ml/min]) indicate partial tubular reabsorption of the drug (fu, approximately 52%).
FIG. 5.
Urinary excretion of moxifloxacin (percentage of dose) following administration of single ascending doses ranging from 50 to 800 mg. —•—, 50 mg, HPLC; —■—, 100 mg, HPLC; —▾—, 200 mg, HPLC; …⧫…, 400 mg, HPLC; — —, 600 mg, HPLC; --—•—--, 800 mg, HPLC.
Compartmental modeling.
The plasma concentration-versus-time profiles for the 200-mg dose (HPLC data) were modeled by compartmental methods (7). Table 3 presents a comparison of the results obtained by compartmental analysis with the data from noncompartmental analysis. They could be described best by a two-compartment body model. The compartmental and noncompartmental data were in good agreement.
DISCUSSION
In the present study moxifloxacin was safe and well tolerated when it was administered as oral single doses ranging from 50 to 800 mg. No clinically relevant changes in vital signs, electrocardiograms, electroencephalograms, or results of physical examinations were seen. Only a few adverse events were reported, and, with the exception of one adverse event, were all mild in intensity; the exception (moderate headache) occurred after the administration of placebo. The elevation of the alkaline phosphatase level in one volunteer was a constant finding starting before treatment with active drug and placebo and is therefore of no clinical relevance. No other clinically relevant changes in the laboratory values were found.
In this study the basic pharmacokinetic properties of moxifloxacin in human volunteers were determined. Application of both HPLC and bioassay helped us to better understand the relevance of the pharmacokinetic parameters. While the bioassay produces results based on the total antimicrobial activity of the sample, HPLC differentiates between the different structures of the compounds produced during metabolic transformation of the drug in vivo. In this study the bioassay and HPLC data showed an excellent correlation, indicating that there seem to be no significant concentrations of metabolites with noticeable antimicrobial activity present in plasma and urine over the time interval after single-dose administration investigated. Thus, in contrast to the results of studies of other quinolones published earlier (1, 13), it was proven that the concentrations measured by bioassay and HPLC are adequate for assessment of the pharmacokinetics of moxifloxacin in humans with respect to pharmacological (antimicrobial) activity. However, HPLC was superior because of its higher sensitivity and better specificity with respect to metabolites.
The pharmacokinetics of moxifloxacin were linear in the range of 50 to 800 mg following the administration of a single dose in all matrices investigated. The plasma concentration-time curves followed very similar patterns for all doses, and no significant dose dependency was detectable. High peak concentrations were reached 1 to 3 h following administration, indicating a fast, slightly variable rate of absorption; thus, concentrations far above the MIC (more than 10 times) for the susceptible microorganisms (e.g., at which 90% of Streptococcus pneumoniae strains are inhibited, ≤0.125 mg/liter [8]) were reached.
High concentrations of antimicrobial agents at the target of infection are mandatory. Besides a high level of exposure to the drug (high AUC) during the dosing interval, the rapid distribution of a drug into extravascular spaces is a key requirement. Data for saliva prove that the initial distribution of the drug is apparently a very fast process which cannot be detected from the plasma concentration-time profiles alone, since it may be hidden by slower processes, like absorption of the drug. They provide strong evidence that moxifloxacin, like other quinolones (1, 13), behaves in this way. Thus, kinetics in plasma and saliva together may be an excellent means of obtaining a first impression of the distribution of the drug when other data (intravenous infusion data) are not yet available. They are very useful for enhancing our understanding of the pharmacokinetics of antimicrobial agents, if possible pitfalls, for example, the presence of drug residue in the oral cavity, are avoided (9, 15).
The high mean apparent volume of distribution during the terminal phase, which is in the order of magnitude typical for fluoroquinolones (1, 13), also provides evidence of the good distribution of the drug.
Data on the elimination of moxifloxacin from plasma prove that high drug concentrations could be maintained in plasma and saliva over 24 h following ingestion of the dose. Therefore, taking into account the good safety and tolerability features seen in this study, in combination with the sensitivity of the drug against gram-positive microorganisms (2, 6, 7, 8, 12), a once-daily dosing regimen (e.g., 400 mg once daily) seems to be possible from a pharmacokinetic point of view (e.g., the MIC at which 90% of S. pneumoniae strains are inhibited was exceeded for more than 24 h following the administration of a 400-mg dose).
Taking into account the data on renal and apparent nonrenal clearances as well as the results of the HPLC and bioassay correlation experiment, further conclusions can be drawn. Besides renal excretion, biliary excretion and metabolism seem to be the elimination pathways. The metabolites that are formed seem to have no major antimicrobial activity. Determination of the metabolic fate of moxifloxacin in vivo will provide new insights and allow us to have a better understanding of the pharmacokinetic characteristics of the drug; corresponding investigations are ongoing.
The high-dose-dependent concentrations in urine, ranging between 1.4 and 137.6 mg/liter, found during the first 24 h following administration of the dose indicate that a high level of antimicrobial activity can be maintained against microorganisms in the urinary tract. Consequently, the drug should also be useful even at doses as low as 50 or 100 mg for treating bacterial infections in the urinary tract.
In conclusion, the favorable pharmacokinetics of moxifloxacin (good absorption and distribution, concentrations above the MICs for relevant microorganisms in plasma, saliva, and urine, and a long terminal half-life controlling the level of exposure to the drug) combined with the excellent safety profile allow a once-daily oral dosing regimen, assuming that AUC/MIC is the relevant parameter for predicting the clinical outcome.
REFERENCES
- 1.Borner K, Höffken G, Lode H, Koeppe P, Prinzing C, Glätzel P, Wiley R, Olschewski P, Sievers B. Pharmacokinetics of ciprofloxacin after oral and intravenous administration. Eur J Clin Microbiol. 1986;5:179–186. doi: 10.1007/BF02013983. [DOI] [PubMed] [Google Scholar]
- 2.Dalhoff A, Petersen U, Endermann R. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone. Chemotherapy (Basel) 1996;42:410–425. doi: 10.1159/000239474. [DOI] [PubMed] [Google Scholar]
- 3.Domdey-Bette, A. (Bayer AG). 1996. Private communication.
- 4.Gibaldi M, Perrier D. Pharmacokinetics. New York, N.Y: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 5.Heinzel G, Woloszczak R, Thomann P. Topfit 2.0, pharmacokinetic and pharmacodynamic data analysis system for the PC. Stuttgart, Germany: Gustav Fischer; 1993. [Google Scholar]
- 6.Jacobs E, Dalhoff A, Brunner H. Program and abstract of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Efficacy of BAY 12-8039 in Mycoplasma pneumoniae infected guinea pigs, abstr. F17; p. 102. [Google Scholar]
- 6a.Kubitza D, Stass H H, Wingender W, Kuhlmann J. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. BAY 12-8039 (I), a new 8-methoxy-quinolone: safety (S), tolerability (T) and steady state pharmacokinetics (PK) in healthy male volunteers, abstr. F25; p. 104. [Google Scholar]
- 7.Petersen U, Dalhoff A, Endermann R. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Synthesis and in vitro activity of BAY 12-8039, a new 8-methoxy-quinolone, abstr. F1; p. 100. [Google Scholar]
- 8.Rouse M S, Piper K E, Patel R, Wilson W R, Steckelberg J M. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro and in vivo activity of BAY 12-8039 or trovafloxacin against penicillin-resistant Streptococcus pneumoniae experimental pneumonia in immunocompetent mice, abstr. B45; p. 29. [Google Scholar]
- 9.Smith K, Weber A, Pandher R, Williams-Warren J, Cohen M L, Ramsey B. Utilization of salivary concentrations of ciprofloxacin in subjects with cystic fibrosis. Infection. 1997;25:106/38–108/40. doi: 10.1007/BF02113587. [DOI] [PubMed] [Google Scholar]
- 10.Stass H H, Dalhoff A. Determination of BAY 12-8039, a new 8-methoxy-quinolone, in human body fluids by HPLC with fluorescence detection using on column focusing. J Chromatogr. 1997;702:163–174. doi: 10.1016/s0378-4347(97)00371-x. [DOI] [PubMed] [Google Scholar]
- 10a.Stass H, Kubitza D, Schüly V, Wingendes W. Program and abstracts of the 8th European Congress of Microbiology and Infectious Diseases, Lausanne, Switzerland. 1997. Pharmacokinetics, safety, and tolerability of 800 mg BAY 12-8039 administered orally as a single dose. [Google Scholar]
- 11.Vohr H W, Wasinska-Kempa G, Ahr H J. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Studies on the phototoxic potential of BAY 12-8039, abstr. B45; p. 29. [Google Scholar]
- 12.Waterbury K, Wang J J, Barbiero M, Federici J, Ohlin A C, Huguenel E D. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Efficacy of BAY 12-8039, a potent new quinolone, in mouse models of typical and atypical respiratory infection, abstr. F18; p. 101. [Google Scholar]
- 13.Wingender W, Graefe K-H, Gau W, Förster D, Beermann D, Schacht P. Pharamcokinetics of ciprofloxacin after oral and intravenous administration in healthy volunteers. Eur J Clin Microbiol. 1984;3:355–359. doi: 10.1007/BF01977494. [DOI] [PubMed] [Google Scholar]
- 14.Zeiler H J, Petersen U, Gau W, Ploschke H J. Antibacterial activity of the metabolites of ciprofloxacin and its significance in the bioassay. Drug Res. 1987;37:131–134. [PubMed] [Google Scholar]
- 15.Zhai S, Wei X, Parker B, Kunze K, Vestal R E. Do saliva concentrations predict plasma concentrations of enoxacin, ciprofloxacin and theophylline. Clin Pharmacol Ther. 1995;57:191. doi: 10.1097/00007691-199612000-00007. . (Abstract PII-110.) [DOI] [PubMed] [Google Scholar]