Abstract
Objective
Inflammatory biomarkers are novel tools to assess the prognosis of different cardiovascular diseases. We evaluated the impact of the monocyte-to-lymphocyte ratio (MLR) on clinical outcomes in patients with coronary heart disease (CHD).
Methods
We systematically screened English-language articles in PubMed, Scopus, and Web of Science to 31 August 2022. Relevant articles reporting the MLR and its association with clinical outcomes (major adverse cardiovascular events (MACE), coronary artery disease (CAD) severity, mortality, cardiac rupture, subclinical CAD, acute coronary syndrome (ACS) prediction, thin-cap fibroatheroma, no-reflow phenomenon, MLR-related differences in percutaneous coronary intervention, heart failure hospitalization, and depression) in patients with CHD were collected for further analysis.
Results
Nineteen articles were selected. The mean MLR was 0.34. A higher MLR was significantly associated with an increased risk of MACE among patients with CHD. The MLR was an independent predictor of MACE in patients with ACS. No significant association was found for CAD severity. A complementary analysis was not performed because of few studies focusing on the other predefined endpoints.
Conclusions
The MLR is a simple and widely available tool to predict MACE in patients with CHD. This biomarker can be utilized in emergency settings to prioritize high-risk patients and optimize therapeutic interventions.
Keywords: Monocyte-to-lymphocyte ratio, coronary disease, coronary artery disease, acute coronary syndrome, systematic review, meta-analysis
Introduction
Coronary artery disease (CAD) is one of the major causes of morbidity and mortality worldwide. 1 This disorder is the leading cause of death in middle- and high-income nations. 2 CAD is reportedly the foremost cause of disability-adjusted life years and mortality around the globe, with a higher burden among individuals residing in low- and middle-income countries. Previous studies showed that 129 million disability-adjusted life years and 7 million deaths occurred each year in these nations.3–6 Although the term CAD might be used interchangeably with the term coronary heart disease (CHD), the latter is the result of CAD. 7 The most severe and common CAD subcategory is acute coronary syndrome (ACS), defined as the presence of unstable angina, ST segment elevation myocardial infarction (STEMI), and non-ST segment elevation myocardial infarction (NSTEMI).7,8
Atherosclerosis of the coronary arteries is the underlying mechanism of CAD, and the interplay between atherosclerosis and inflammation has been investigated in the literature. 9 The inflammatory response plays a significant role in both the initiation and progression of atherosclerotic coronary plaques.10,11 The main circulating inflammatory cells are neutrophils, monocytes, lymphocytes, and platelets. Previous studies have shown that increased numbers of neutrophils and monocytes and decreased numbers of lymphocytes promote atherosclerosis.12–14 Additionally, monocytes play more significant roles in atherosclerotic disease than neutrophils.15,16 Monocytes transmigrate to cardiac tissue, differentiate into macrophages, and activate inflammatory cytokine secretion. Monocytes are thus pivotal cells in atherosclerosis, and assessment of these inflammatory cells might be helpful for prognostic evaluation. Several inflammatory markers have been introduced for different cardiovascular diseases.17–23 One of these recently introduced markers is the monocyte-to-lymphocyte ratio (MLR), defined as the monocyte count divided by the lymphocyte count. This ratio has been investigated in several cardiovascular diseases.24–27 However, the role of the MLR in patients with CHD remains controversial.
In this systematic review and meta-analysis, we evaluated all relevant records regarding the impact of the MLR on clinical outcomes among patients with CHD.
Materials and methods
Protocol registration
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used in the current study. 28 Moreover, we registered this systematic review and meta-analysis in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022357282).
Inclusion and exclusion criteria
The literature was screened for any relevant studies published in English (cross-sectional studies, case-control studies, cohort studies, and randomized clinical trials) that assessed the potential effect of the MLR on clinical outcomes of patients with CHD. The following study designs were excluded: meeting abstracts, editorials, case reports, case series, animal studies, and any records without complete desired data.
Literature review strategy
The medical databases of PubMed (title, abstract), Scopus, and Web of Science (title, abstract, and keywords) were screened up to 31 August 2022 using the following keyword search strategy: (“monocyte* to lymphocyte* ratio” OR “monocyte *-lymphocyte*” OR “monocyte *-lymphocyte* ratio” OR “monocyte * to lymphocyte*” OR “monocyte *-to-lymphocyte* ratio” OR “monocyte *-to lymphocyte* ratio” OR “monocyte * to-lymphocyte* ratio” OR “monocyte */lymphocyte* ratio” OR “monocyte */lymphocyte*”) AND (“cardiovascular disease*” OR “myocardial infarction*” OR “cardiac infarction” OR “coronary artery disease*” OR “acute coronary syndrome*” OR “unstable angina” OR “ST segment elevation myocardial infarction*” OR “ST-segment elevation myocardial infarction*” OR “non ST segment elevation myocardial infarction*” OR “non ST-segment elevation myocardial infarction*” OR “stable angina” OR “stable cardiac disease*” OR “stable heart disease*”).
Study selection process
In the aforementioned databases, the titles and abstracts were screened by two authors independently. If relevant records were found, the full texts were collected for further assessment. In cases of duplication, only a single record was considered, and any disagreement was resolved by consensus. Figure 1 shows the flow diagram of the current study.
Figure 1.
Flow diagram of current review.
Data collection
The following items were collected from each record: first author’s name and year of publication, study design, total sample size and subgroups (if applicable), frequency of male patients (percentage), age (mean ± standard deviation (SD) or median (interquartile range (IQR)), as reported), follow-up duration (if applicable), MLR (mean ± SD, median (IQR), cut-off, or tertiles, as reported), and clinical outcomes (major adverse cardiovascular events (MACE), severity of CAD, all-cause mortality, cardiac rupture (CR), subclinical CAD, ACS prediction, thin-cap fibroatheroma (TCFA), no-reflow phenomenon, MLR-related differences in percutaneous coronary intervention (PCI), heart failure (HF) hospitalization, and depression, as reported).
Risk of bias assessment
The quality of cross-sectional studies was evaluated using the Appraisal Tool for Cross-Sectional Studies (AXIS). 29 The National Institutes of Health Quality Assessment Tool was used for case-control studies. 30 The Joanna Briggs Institute critical appraisal checklists for cohort studies and randomized clinical trials were utilized to evaluate the quality of cohort studies and randomized clinical trials, respectively.31,32
Statistical analysis
The pooled effect size is presented as mean, hazard ratio (HR), and odds ratio (OR) with their specific 95% confidence interval (CI), as appropriate. The method established by Wan et al. 33 was used to convert median (IQR) to mean ± SD. Cochran’s Q statistic, I2, and tau squared (τ2) were utilized for heterogeneity evaluation. Random-effects or fixed-effects models were used if I2 ≥ 25% or I2 < 25%, respectively. Forest plots were created to show the mean MLR, and the HR of MACE and OR of CAD severity were assessed according to the multivariate adjusted models reported in each study, as appropriate. Publication bias was examined using funnel plots as well as Begg’s and Egger’s tests. Data entry was performed with an Excel datasheet, and all analyses were performed using Comprehensive Meta-Analysis (CMA) Version 2.0 software.
Results
After full screening of the aforementioned databases and removal of duplicate and non-relevant records from a total of 203 studies, 19 articles that reported the effects of the MLR on CHD were finally selected (Figure 1).34–52 With the exception of one article, 39 all reported the participants’ ages (mean ± SD or median (IQR)). The mean age of all participants was 62.07 ± 11.19 years, and 64.09% of the population was male. One study was a cohort study, and another was a case-control study.38,47 In all articles, the MLR was calculated as the monocyte count divided by the lymphocyte count.34–52 We used a random-effects model because I2 ≥ 25%. A summary of all included studies is shown in Table 1. The details of the risk-of-bias assessment based on the study designs are provided in the supplementary appendix (Tables S1, S2, and S3). Three articles did not report the MLR values (either as mean ± SD or median (IQR)).36,39,47 Figure 2 shows a forest plot of the mean MLR in patients with CHD (0.34; 95% CI, 0.30–0.38). The heterogeneity indices were as follows: Q = 3444.436, I2 = 99.56%, τ2 = 0.006, and P < 0.001. In terms of publication bias, the P-values of Begg’s and Egger’s tests were 0.172 and 0.015, respectively (the funnel plot is shown in Figure S1).
Table 1.
Summary of included studies reporting MLR and clinical outcomes.
| Reference | Design | Sample size | Male (%) | Age (years) | Follow-up duration | MLR | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Li et al. 2022 34 | Cross-sectional | Total | 1701 | 1305 (76.72) | Mean ± SD: 60 ± 10 | Median (IQR): 30 (30–36) months | Mean ± SD: 0.2 ± 0.07Median (IQR): 0.2 (0.16–0.26) MLR cut-off: 0.24 | MACE |
| Negative MACE | 1594 | 1227 (76.98) | Mean ± SD: 59 ± 10 | Mean ± SD: 0.2 ± 0.08Median (IQR): 0.2 (0.15–0.26) | ||||
| Positive MACE | 107 | 78 (72.90) | Mean ± SD: 64 ± 12 | Mean ± SD: 0.25 ± 0.09Median (IQR): 0.25 (0.19–0.32) | ||||
| Dai et al. 2022 35 | Cross-sectional | Total | 180 | 109 (60.56) | Mean ± SD: 70.52 ± 10.75 | NA | Mean ± SD: 0.64 ± 0.6MLR cut-off: 0.61 | Cardiac rupture |
| Negative cardiac rupture | 90 | 55 (61.11) | Mean ± SD: 71.03 ± 11.42 | Mean ± SD: 0.41 ± 0.24Median (IQR): 0.41 (0.25–0.58) | ||||
| Positive cardiac rupture | 90 | 54 (60.00) | Mean ± SD: 70.01 ± 10.09 | Mean ± SD: 0.88 ± 0.76Median (IQR): 0.75 (0.44–1.45) | ||||
| Song et al. 2021 36 | Cross-sectional | Total | 3461 | 2383 (68.85) | Mean ± SD: 63.24 ± 10.6 | Mean ± SD: 37.59 ± 22.24 months | MLR cut-off: 0.34 | MACE |
| Si et al. 2021 37 | Cross-sectional | Total | 335 | 204 (60.90) | Mean ± SD: 63.33 ± 6.7Median (IQR): 63 (59–68) | NA | Mean ± SD: 0.25 ± 0.11Median (IQR): 0.25 (0.18–0.33) MLR cut-off: 0.142 | Subclinical CAD |
| Shumilah et al. 2021 38 | Case-control | Total | 100 | 60 (60.00) | Mean ± SD: 55.5 ± 15 | NA | Mean ± SD: 0.61 ± 0.29MLR cut-off: 0.375 | ACS presence |
| Gao et al. 2021 39 | Cross-sectional | Total | 1558 | 1166 (74.84) | NR | Median: 1142 days | MLR cut-off: 0.33 and 0.43 | MACE |
| Dai et al. 2022 40 | Cross-sectional | Total | 304 | 215 (70.72) | Mean ± SD: 65.09 ± 12.46 | NA | Mean ± SD: 0.47 ± 0.39MLR cut-off: 0.64 | Cardiac rupture |
| Negative cardiac rupture | 228 | 167 (73.25) | Mean ± SD: 63.03 ± 12.65 | Mean ± SD: 0.36 ± 0.21Median (IQR): 0.34 (0.23–0.52) | ||||
| Positive cardiac rupture | 76 | 48 (63.16) | Mean ± SD: 71.3 ± 9.58 | Mean ± SD: 0.81 ± 0.58Median (IQR): 0.76 (0.45–1.22) | ||||
| Candemir et al. 2021 41 | Cross-sectional | Total | 669 | 395 (59.04) | Mean ± SD: 62.21 ± 10.86 | NA | Mean ± SD: 0.28 ± 0.13 | Severity of CAD |
| Zhang et al. 2020 42 | Cross-sectional | Total | 72 | 56 (77.78) | Mean ± SD: 55.65 ± 12.08 | NA | Mean ± SD: 0.21 ± 0.1MLR cut-off: 0.25 | TCFA |
| Tanriverdi et al. 2020 43 | Cross-sectional | Total | 421 | 215 (51.07) | Mean ± SD: 56.3 ± 10.2 | NA | Mean ± SD: 0.22 ± 0.06 | Severity of CAD |
| Chen et al. 2020 44 | Cross-sectional | Total | 1009 | 608 (60.26) | Mean ± SD: 56.22 ± 9.45 | 90 days | Mean ± SD: 0.64 ± 0.37 | All-cause mortalityNo-reflow phenomenon |
| Horváth et al. 2019 45 | Cross-sectional | Total | 21 | 18 (85.71) | Mean ± SD: 62.9 ± 10.6 | NA | Mean ± SD: 0.33 ± 0.18 | MLR-related difference in PCI |
| Chen et al. 2019 46 | Cross-sectional | Total | 963 | 758 (78.71) | Mean ± SD: 60.77 ± 11.34 | Median (IQR): 22 (12–35) months | Mean ± SD: 0.2 ± 0.14Median (IQR): 0.28 (0.21–0.40) MLR tertiles: T1: <0.23T2: 0.23–0.35T3: >0.35MLR cut-off: 0.43 and 0.31 | MACE |
| Fan et al. 2018 47 | Retrospective cohort | Total | 678 | 435 (64.16) | Mean ± SD: 62.81 ± 15.07 | Median (range): 26 (1–30) months | MLR cut-off: 0.36 | MACE |
| Ji et al. 2017 48 | Cross-sectional | Total | 381 | 211 (55.38) | Mean ± SD: 62.79 ± 9.52 | NA | Mean ± SD: 0.23 ± 0.09Median (IQR): 0.23 (0.17–0.30) MLR cut-off: 0.18 and 0.25 | CAD detectionSeverity of CAD |
| Gijsberts et al. 2017 49 | Cross-sectional | Total | 1754 | 478 (27.25) | Mean ± SD: 63.97 ± 10.76 | 484 days | Mean ± SD: 0.33 ± 0.14Median (IQR): 0.32 (0.24–0.43) | HF hospitalization |
| Fan et al. 2017 50 | Cross-sectional | Total | 133 | 96 (72.18) | Mean ± SD: 62 ± 12 | NA | Mean ± SD: 0.28 ± 0.38MLR cut-off: 0.25 | TCFA |
| Serfőző et al. 2016 51 | Cross-sectional | Total | 23 | 18 (78.26) | Mean ± SD: 62.9 ± 10.6 | NA | Mean ± SD: 0.32 ± 0.14 | Depression |
| Gijsberts et al. 2016 52 | Cross-sectional | Total | 1015 | 742 (73.10) | Mean ± SD: 65.11 ± 11.32 | 805 days | Mean ± SD: 0.33 ± 0.14 | MACEAll-cause death |
| Survived | 950 | 693 (72.95) | Mean ± SD: 64.6 ± 11.2 | Mean ± SD: 0.33 ± 0.13Median (IQR): 0.32 (0.25–0.43) | ||||
| Death | 65 | 49 (75.38) | Mean ± SD: 72.7 ± 10.4 | Mean ± SD: 0.47 ± 0.25Median (IQR): 0.44 (0.32–0.65) | ||||
ACS: acute coronary syndrome, CAD: coronary artery disease, HF: heart failure, IQR: interquartile range, MACE: major adverse cardiovascular events, MLR: monocyte-to-lymphocyte ratio, NA: not applicable, NR: not reported, PCI: percutaneous coronary intervention, SD: standard deviation, T: tertile, TCFA: thin-cap fibrous atheroma
Figure 2.
Forest plot for mean MLR based on studies that reported this marker.
MLR and MACE
Six studies reported MACE as the clinical endpoint among patients with CHD.34,36,39,46,47,52 MACE was defined as the following in each study: Li et al. 34 : composite of all-cause death, non-fatal ischemic stroke, and non-fatal myocardial infarction (MI); Song et al. 36 : combination of cardiovascular death, recurrent MI, stroke, and target vessel reconstruction; Gao et al. 39 : all-cause mortality, cardiac mortality (death due to HF, MI, fatal arrhythmia, or other heart-related conditions), and readmission for severe HF; Chen et al. 46 : in-hospital MACE (cardiac arrest, cardiac death, cardiogenic shock or syncope, CR, malignant arrhythmia, and acute congestive HF) and long-term MACE (cardiac arrest, malignant arrhythmia, MI, unstable angina, cardiogenic shock or syncope, cardiac death, readmission for coronary revascularization, or HF decompensation); Fan et al. 47 : composite of all-cause death, stroke, non-fatal MI, cardiac death, and target vessel or target lesion revascularization; and Gijsberts et al. 52 : cardiovascular death, stroke, non-fatal MI, and unplanned coronary revascularization. Among all 9376 patients, MACE occurred in 1150 (12.26%). The mean MLR in three articles (n = 3679; male patients, 76.24%) that reported this endpoint was 0.24 (95% CI, 0.17–0.32) (data not shown).34,46,52
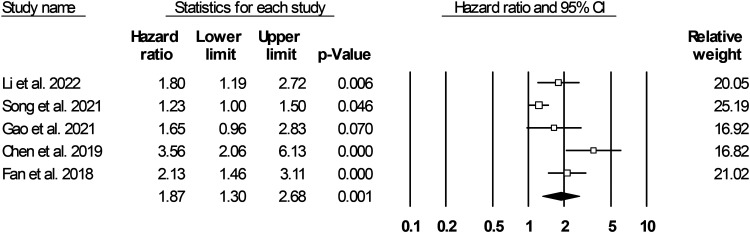
Five articles reported the HR of the MACE incidence among patients with CHD (including both CAD and ACS).34,36,39,46,47 Further analysis revealed that a high MLR was associated with a 1.87-times increased risk of MACE (95% CI, 1.30–2.68; P = 0.001) (Figure 3, heterogeneity indices: Q = 17.338, I2 = 76.93%, τ2 = 0.123, P = 0.002, Begg’s test P-value = 0.231, Egger’s test P-value = 0.033) (funnel plot: Figure S2).
Figure 3.
Forest plot for hazard ratio of MACE in coronary heart disease (coronary artery disease and acute coronary syndrome) based on MLR.
For a more uniform description of MACE, we selected studies that reported at least death (either cardiovascular or all-cause mortality), MI, and stroke. This complementary analysis revealed a similar outcome in that a high MLR was associated with a significantly increased risk of MACE among patients with CHD (HR = 1.62, 95% CI = 1.12–2.34, P = 0.010) (Figure 4). The heterogeneity indices were low (Q = 7.613, I2 = 73.73%, τ2 = 0.077, P = 0.022), and neither the funnel plot (Figure S3) nor Begg’s (P = 0.500) or Egger’s test (P = 0.106) was in favor of publication bias.
Figure 4.
Forest plot for hazard ratio of MACE (uniform definition) in coronary heart disease (coronary artery disease and acute coronary syndrome) based on MLR.
As shown in Figure 5, a higher MLR was significantly associated with an increased occurrence of MACE among patients with ACS (HR = 2.14, 95% CI = 1.59–2.87, P < 0.001). Heterogeneity was low among the included studies (Q = 4.920, I2 = 39.02%, τ2 = 0.035, P = 0.178). The funnel plot showed no publication bias among the studies (Figure S4, Begg’s test P-value = 0.500, Egger’s test P-value = 0.335).
Figure 5.
Forest plot for hazard ratio of MACE in acute coronary syndrome based on MLR.
MLR and severity of CAD
Three studies involving 1471 patients assessed the effect of the MLR on CAD severity.41,43,48 Figure 6 shows the forest plot of the MLR odds on CAD severity. Although the MLR was associated with the severity of CAD, this relationship was not statistically significant (OR = 1.25, 95% CI = 0.95–1.65, P = 0.115). The heterogeneity data were as follows: Q = 27.577, I2 = 92.74%, τ2 = 0.046, P < 0.001. The funnel plot, as shown in Figure S5, as well as Begg’s test (P = 0.500) and Egger’s test (P = 0.101), were in favor of no publication bias.
Figure 6.
Forest plot for MLR odds ratio of coronary artery disease severity.
MLR and mortality
Three studies investigated the association of the MLR with death in patients with CHD.36,44,52 Song et al. 36 enrolled 3461 patients with CAD and categorized them into two groups based on the reported MLR cut-off point (MLR < 0.34, n = 2338 and MLR ≥ 0.34, n = 1123). CAD was defined as ≥1 coronary artery stenosis (≥50%) confirmed through coronary angiography with ≥1 implemented stent. After follow-up for 37.59 ± 22.24 months, the rates of both all-cause and cardiac death were significantly higher in patients with an MLR of ≥0.34 than in those with an MLR of <0.34 (6.8% vs. 2.3%, P < 0.001 and 4.3% vs. 1.4%, P < 0.001, respectively). The multivariate adjusted hazard models indicated that a high MLR was associated with a 1.766-times higher (95% CI = 1.336–3.650, P = 0.001) and 2.379-times higher (95% CI = 1.611–3.511, P < 0.001) rate of all-cause and cardiovascular mortality, respectively. 36 In another study, 1009 patients with STEMI were followed for 90 days, and 83 (8.23%) deaths were observed. Further analysis revealed that a high MLR significantly increased the 90-day mortality risk in both the univariate and multivariate models (HR = 1.423, 95% CI = 1.183–1.702, P < 0.001 and HR = 1.313, 95% CI = 1.150–1.586, P = 0.012, respectively). 44 Gijsberts et al. 52 followed 1015 patients with suspected CAD for 805 days and observed 65 (6.40%) death events. The MLR was significantly associated with all-cause and cardiovascular mortality (HR = 1.35, 95% CI = 1.14–1.59, P < 0.001 and HR = 1.42, 95% CI = 1.11–1.81, P = 0.005, respectively). 52
MLR and CR
Dai et al. 35 investigated the predictive role of the MLR on CR among patients with acute MI. CR was defined as signs and symptoms of pericardial tamponade, electromechanical separation, large pericardial effusion, and pericardial puncture with production of non-coagulable fluids. After propensity score matching, 180 patients were selected and divided into two groups of equal numbers. The MLR differed significantly between patients with and without CR (0.75 (0.44–1.45) vs. 0.41 (0.25–0.58), respectively; P < 0.001). The authors defined 0.61 as the optimal cut-off point for CR prediction. The complementary analysis indicated that the MLR was an independent risk factor for CR (univariate OR = 6.64, 95% CI = 2.69–16.43, P < 0.001 and multivariate OR = 3.57, 95% CI = 1.28–9.97, P = 0.015). 35
Another study involved 76 patients who had MI with concurrent CR and 228 patients who had MI without CR. 40 The MLR was higher in patients with than without CR (0.76 (0.45–1.22) vs. 0.34 (0.23–0.52), P < 0.001). An MLR of 0.64 was defined as the optimal cut-off point for prediction of CR. A high MLR was found to increase the likelihood of CR in patients with MI (univariate OR = 9.81, 95% CI = 4.11–23.35 and multivariate OR = 5.99, 95% CI = 2.09–17.16, P = 0.001). 40
MLR and subclinical CAD
Si et al. 37 investigated the ability of the MLR to predict subclinical CAD in 335 patients with CAD and 362 controls. Subclinical CAD was defined as a coronary artery calcium score of ≥100 Agatston units and/or coronary stenosis of ≥50%. The total MLR (median (IQR)) in the study population was 0.23 (0.17–0.31), and patients with CAD had significantly higher MLR values than controls (0.25 (0.18–0.33) vs. 0.21 (0.16–0.29), P < 0.001). Further analysis revealed that an MLR of 0.142 was the optimal cut-off point for predicting subclinical CAD. Moreover, the multivariable adjusted regression model indicated that the MLR was an independent predictor of subclinical CAD (OR = 1.693, 95% CI = 1.032–2.775, P = 0.037). 37
MLR and ACS prediction
A case-control study of 100 patients with ACS and 100 controls with no significant differences in age or sex was performed to investigate the ability of the MLR to predict the incidence of ACS. The mean MLR was significantly different between the groups (ACS group: 0.61 ± 0.29 vs. control group: 0.23 ± 0.10, P < 0.001). The optimal MLR cut-off was determined to be 0.375. However, the complementary analysis failed to prove any independency of the MLR to predict ACS (OR = 4.12, P = 0.597). 38
MLR and TCFA
Two studies assessed the impact of the MLR on TCFA in patients with CHD.42,50 Zhang et al. 42 enrolled 72 patients with ACS and divided them into lower and higher MLR groups according to the median value (0.21). The median (IQR) of the MLR was 0.26 (0.23–0.36) and 0.15 (0.11–0.18) in the high and low groups, respectively (P < 0.001). Fibrous cap thickness was defined as the thickness of the fibrous layer covering a lipid plaque, with a criterion of <65 µm, and a lipid core involving more than two quadrants. It was measured by optical coherence tomography. Lower thickness was found among patients with higher MLR values (112.37 ± 60.24 vs. 153.49 ± 73.29 µm, P = 0.013). The authors defined 0.25 as the optimal cut-off for the presence of TCFA, and further analyses were in favor of MLR independency for predicting TCFA in patients with ACS (multivariate OR = 3.316, 95% CI = 1.448–7.593, P = 0.005). 42
Another study of 133 patients with stable angina (age, 62 ± 12 years; male patients, 72.18%) indicated that the MLR was higher among those with TCFA (0.36 ± 0.12 vs. 0.22 ± 0.5, P < 0.001). 50 The authors defined TCFA as a necrotic core of >10% in at least three consecutive frames with no evidence of overlying fibrous tissue in the presence of a ≥40% plaque burden. The optimal cut-off value was proposed as 0.25, and they suggested that the MLR could independently identify TCFA among patients with stable angina (OR = 2.61, 95% CI = 1.13–6.04, P = 0.025). 50
MLR and no-reflow phenomenon
Chen et al. 44 investigated the association of the MLR with the no-reflow phenomenon in patients with STEMI. In total, 1009 patients underwent PCI. A no-reflow event was defined as no effective myocardial tissue perfusion (thrombolysis in MI grade of <3) after coronary recanalization with no evidence of spasm, residual stenosis, or dissection. The mean MLR in the no-reflow group (n = 262) was higher than that in the normal flow group (n = 747) (0.56 ± 0.48 vs. 0.43 ± 0.33, P < 0.001). Logistic regression analysis showed independency of the MLR for predicting the no-reflow phenomenon (univariable OR = 1.432, 95% CI = 1.221–1.632, P < 0.001 and multivariable OR = 1.335, 95% CI = 1.176–1.558, P = 0.018). 44
MLR differences in PCI
Horváth et al. 45 investigated the MLR alterations before PCI, immediately after PCI, and 1 day after PCI. Twenty-three patients with stable CHD (age, 62.9 ± 10.6 years) were selected. The mean MLR before the PCI procedure was 0.33 ± 0.18. The MLR was also assessed immediately after completion of PCI (0.33 ± 0.17), and no significant difference was found between these two time points. However, the mean MLR was significantly higher 1 day after PCI (0.42 ± 0.20) than before PCI (P < 0.01). The authors proposed that stenting might be associated with coronary wall injury, leading to an increased monocyte count. 45
MLR and HF hospitalization
The association of the MLR with HF admission among patients with CAD was also investigated. 49 Among 1754 patients who underwent coronary angiography for unstable angina, MI, or stable CAD (defined as chest pain or dyspnea upon exertion and >50% stenosis in at least one coronary artery), the MLR was calculated before the procedure and showed a median (IQR) of 0.32 (0.24–0.43). Recruited patients were followed for a mean of 1.3 years, and 46 HF admissions were reported. The authors defined an MLR value in the upper quartile (0.43) as the cut-off and divided the patients into high and low groups accordingly. Multivariate adjusted Cox regression analysis showed that a high MLR was significantly associated with an increased risk of HF hospitalization (HR = 2.1, 95% CI = 1.1–4.1, P = 0.039). 49
MLR and depression
The association of the MLR with depression in patients with CHD was examined by Serfőző et al. 51 They enrolled 23 patients (age, 62.9 ± 10.6 years) with stable CAD, defined as >50% stenosis of at least two major coronary arteries, and assessed depression using the Beck Depression Inventory. The mean MLR in the study population was 0.32 ± 0.14. The multivariate correlation analysis was in favor of a significant correlation between the MLR and the depression questionnaire responses (r = 0.624, P = 0.004). The authors suggested that the MLR was associated with depressive symptoms among patients with CAD. 51
MLR cut-off points
Twelve articles reported specific MLR cut-off values for their endpoints in patients with CHD.34–40,42,46–48,50 In terms of MACE, five studies indicated different cut-off points of 0.24, 0.34, 0.33, 0.43, 0.31, and 0.36.34,36,39,46,47 Two different cut-off points (0.61 and 0.64) were reported to predict CR.35,40 Si et al. 37 and Shumilah et al. 38 defined cut-off points of 0.142 and 0.375 for prediction of subclinical CAD and the presence of ACS, respectively. Two studies revealed a cut-off point of 0.25 for detection of TCFA.42,50 The best cut-off for prediction of CAD severity was also 0.25. Moreover, the cut-off value to determine CAD was 0.18. 48 Detailed information regarding each cut-off value according to each study endpoint is shown in Table 2.
Table 2.
Summary of MLR cut-off characteristics.
| Reference | Outcome | MLR cut-off | Sensitivity (%) | Specificity (%) | Area under curve | 95% confidence interval | P-value |
|---|---|---|---|---|---|---|---|
| Li et al. 2022 34 | MACE | 0.24 | 59.1 | NR | NR | NR | NR |
| Dai et al. 2022 35 | Cardiac rupture | 0.61 | 63 | 80 | 0.74 | 0.65–0.83 | NR |
| Song et al. 2021 36 | MACE | 0.34 | NR | NR | NR | NR | NR |
| Si et al. 2021 37 | Subclinical CAD | 0.142 | 90.1 | 18.2 | 0.582 | 0.540–0.624 | <0.001 |
| Shumilah et al. 2021 38 | ACS presence | 0.375 | 79 | 91 | 0.896 | NR | <0.001 |
| Gao et al. 2021 39 | MACE 1 | 0.33 | 52.5 | 66.1 | 0.588 | 0.508–0.668 | NR |
| MACE 2 | 0.43 | 44.4 | 81.3 | 0.627 | 0.521–0.734 | NR | |
| Dai et al. 2022 40 | Cardiac rupture | 0.64 | 62.2 | 86.4 | 0.811 | 0.734–0.889 | <0.001 |
| Zhang et al. 2020 42 | TCFA | 0.25 | 60 | 85.1 | 0.741 | 0.619–0.864 | 0.001 |
| Chen et al. 2019 46 | In-hospital MACE | 0.43 | 63.2 | 80.8 | 0.728 | 0.604–0.852 | 0.001 |
| Long-term MACE | 0.31 | 57.4 | 61.5 | 0.609 | 0.556–0.661 | <0.001 | |
| Fan et al. 2018 47 | MACE | 0.36 | 54.74 | 73.57 | 0.683 | 0.647–0.718 | 0.022 |
| Ji et al. 2017 48 | CAD detection | 0.18 | 69.03 | 64.81 | 0.727 | 0.683–0.771 | NR |
| Severity of CAD | 0.25 | 60.26 | 78.49 | 0.761 | 0.702–0.820 | <0.001 | |
| Fan et al. 2017 50 | TCFA | 0.25 | 73.7 | 61.8 | 0.759 | 0.676–0.842 | <0.001 |
ACS: acute coronary syndrome, CAD: coronary artery disease, MACE: major adverse cardiovascular events, MLR: monocyte-to-lymphocyte ratio, NR: not reported, TCFA: thin-cap fibrous atheroma.
MLR tertiles
One article reported specific MLR tertiles. Chen et al. 46 selected 963 patients with NSTEMI to assess MACE according to MLR tertiles. The median (IQR) MLR was 0.28 (0.21–0.40), and the authors categorized the patients into low, intermediate, and high groups based on the MLR tertiles (T1: <0.23 (n = 321), T2: 0.23–0.35 (n = 322), and T3: >0.35 (n = 320). They found that the MLR differed significantly between the groups (P < 0.001). Twenty-seven patients developed MACE during admission (acute HF: n = 10, cardiogenic shock: n = 10, malignant arrhythmia: n = 3, cardiac arrest: n = 2, and CR: n = 2). The rate of in-hospital MACE was remarkably higher among patients in the highest tertile than in the other tertiles (4.7% vs. 2.2% vs. 1.6%, P = 0.016). After a median follow-up of 22 months, 176 long-term MACE occurred with a similar trend (high group: 27.2%, intermediate group: 16.2%, and low group: 13.3%; P< 0.001). 46
Discussion
The main aim of the current study was to assess the potential effects of the MLR on patients with CHD. The mean MLR in all studies that reported this ratio was 0.34 (95% CI = 0.30–0.38). We also found that the MLR affected the prognosis in that a higher MLR was associated with a 1.87-times higher risk of MACE (95% CI = 1.30–2.68, P = 0.001) in patients with CHD, and this association remained significant even after selection of studies with quite similar definitions of MACE. This impact was more robust for ACS (HR = 2.14, 95% CI = 1.59–2.87, P < 0.001). This widely available tool is easily accessible in healthcare settings and can be used to evaluate the prognosis of patients with CHD.
Atherosclerotic plaque formation is a multifactorial process involving intimal inflammation, fibrosis, calcification, and angiogenesis. Inflammation is a major player in this regard, influencing the entire process from the development of atherosclerosis to its final life-threatening outcome (i.e., plaque instability and rupture). 53 The major inflammatory cytokines, including C-reactive protein, tumor necrosis factor-α, and interleukin-1, are reportedly elevated in patients with atherosclerotic heart disease.54,55 Two main subgroups of interplaying inflammatory cells in patients with CHD are monocytes and lymphocytes. The former has been reported to be the main component of the innate immune system. 56 Circulating monocytes and resident vascular macrophages are the first leukocytes to be recruited to an atheromatous plaque. 57 In response to various inflammatory cytokines, these cells transmigrate from the circulation to the involved tissue and subsequently differentiate into macrophages, foam cells, and dendritic cells and activate the secretion of matrix metalloproteinases, reactive oxidative substances, and several proinflammatory cytokines. These factors are responsible for atheromatous plaque formation as well as plaque degradation and rupture.15,16 Furthermore, monocytes are capable of producing higher cytokine levels than neutrophils, and both monocytes and monocyte-derived macrophages contribute more to atherosclerotic plaque initiation and progression.16,58 Interestingly, MI itself can induce the bone marrow to release hematopoietic stem cells and increase the monocyte frequency and availability. 59
Lymphocytes are major cells responsible for inflammatory response regulation. These cells also play important roles in atherosclerosis development. 60 Under conditions of stress-induced increased cortisol and catecholamine secretion, as in patients with CHD, lymphocyte apoptosis increases, resulting in lymphocytopenia.61,62 Low lymphocyte counts have been related to poorer outcomes in patients with CAD and ACS.63,64 Moreover, lymphopenia is an index of impaired coronary microcirculation and is considered a pathogenic mechanism for CAD.65,66 Thus, the composite of high monocytes and low lymphocytes into a single inflammatory index (the MLR) could provide more prognostic information than each parameter alone. 46
The MLR is frequently used to assess the prognosis of non-cardiovascular diseases including tuberculosis, lung and laryngeal squamous cell cancer, and rheumatoid arthritis.67–70 This inflammatory marker has been less frequently investigated in cardiac disorders. Zhai et al. 71 investigated the prognostic utility of the MLR in 5512 patients admitted to cardiac intensive care units. Their final analysis showed that higher MLR values were associated with increased in-hospital mortality (MLR Q4 vs. MLR Q1: OR = 1.87, 95% CI = 1.38–2.56, P < 0.001). They also found that patients within the last MLR quartile had remarkably longer intensive care unit and hospital stays (median (IQR)) than patients within the first quartile (Q4 vs. Q1: 2.8 (1.7–5.4) vs. 2.1 (1.2–3.7) days, P < 0.001 and 8.3 (4.8–11.1) vs. 5.3 (3.1–9.3) days, P < 0.001, respectively). 71
Although it was not possible to perform a meta-analysis on CR because of the few available records, the enrolled articles suggested a detrimental effect of the MLR on this life-threatening outcome.35,40 Monocyte activation and accumulation results in initiation of considerable inflammatory responses, leading to infiltration of inflammatory cells to the infarcted area. This process can disrupt and dissolve the cardiac cell membrane and fibrin cytoskeleton. 72 Additionally, the combination of tumor necrosis factor-α and cell surface receptors aggravates the inflammatory cascade in myocardial cells, causing cardiac collagen fiber degradation and fragmentation and elevating the risk of CR. 73
The enrolled articles indicated that the MLR increases the risk of plaque rupture.42,50 A potential mechanism is the presence of higher monocyte-derived macrophages in the plaque cap. 74 Lymphocytopenia may also be involved as suggested by the fact that lower lymphocyte counts have been associated with growth of atherosclerotic plaques, development of a lipid core, and finally plaque rupture. 75
Data regarding an association between the MLR and psychiatric issues in patients with CAD are very limited. 51 However, the association of inflammatory cytokines released by activated monocytes in the context of CHD increases the likelihood of depressive symptoms by interfering with neurotransmitter functions.76,77
Hematologic indices are widely available in most centers and routinely ordered for patients with CHD. Thus, calculation of the MLR can be rapidly performed at the bedside and used to simply evaluate the prognosis of CHD before use of further expensive and time-consuming risk assessment tools, which might be unavailable in some nations with restricted healthcare resources.
This systematic review and meta-analysis is the first to investigate the potential prognostic impact of the MLR on patients with CHD. We utilized a comprehensive search strategy for collection of all relevant articles with no time restriction. However, the current study is not free from limitations. We only screened records published in English; no non-English studies were evaluated. Because of the presence of few studies on some predefined variables (including CR, subclinical CAD, ACS prediction, TCFA, no-reflow phenomenon, MLR-related differences in PCI, HF hospitalization, and depression), performing a meta-analysis on these variables was not feasible. A meta-analysis of all-cause mortality was also not performed because of inconsistent CIs in the published articles. Additionally, we were unable to define the optimal MLR cut-off point for each endpoint because the studies were heterogeneous and had different sample sizes. Although we assessed the HR of the MLR on MACE, this variable was defined differently among the studies, and dichotomous MLR values were quite variable based on the proposed MLR cut-off values. However, we selected the studies with very similar MACE definitions and performed a further downstream analysis to cover this limitation. Finally, because the main focus of the current review was the MLR, we did not screen studies that investigated the inverse ratio (lymphocyte-to-monocyte ratio).
In conclusion, this study indicates that the MLR is a practical inflammatory marker that can be used in the emergency setting to appropriately categorize high-risk patients with CHD, especially in developing countries with limited healthcare resources. However, complementary studies are imperative to define the optimal MLR cut-off value with which to assess the prognosis of different clinical outcomes.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605231204469 for Prognostic impact of monocyte-to-lymphocyte ratio in coronary heart disease: a systematic review and meta-analysis by Mehrbod Vakhshoori, Sepehr Nemati, Sadeq Sabouhi, Mehrnaz Shakarami, Behzad Yavari, Sayed Ali Emami, Niloofar Bondariyan and Davood Shafie in Journal of International Medical Research
Footnotes
ORCID iD: Mehrbod Vakhshoori https://orcid.org/0000-0002-1380-4791
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available because of confidentiality issues but are available from the corresponding author on reasonable request.
Authors’ contributions
Study concept and design: M.V., N.B., S.S., D.S., S.N., M.S., B.Y., SA.E.
Acquisition of data: N.B, M.V., S.S., SA.E.
Analysis and interpretation of data: M.V., N.B., D.S.
Drafting of the manuscript: N.B., M.V., S.S, SA.E., S.N., B.Y., M.S., D.S.
Critical revision of the manuscript for valuable intellectual content: M.V., N.B., S.S., SA.E., S.N., B.Y., M.S., D.S.
Statistical analysis: M.V.
Administrative, technical, and material support: M.V., D.S., S.S., S.N.
Supervision: D.S., M.V.
Consent for publication
Not applicable.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethical approval and consent to participate
The requirement for ethics approval was waived because of the nature of this study (review).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO): The top 10 causes of death [Available from: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 3.Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol 2013; 168: 934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran AE, Oliver JT, Mirzaie M, et al. Assessing the global burden of ischemic heart disease: part 1: methods for a systematic review of the global epidemiology of ischemic heart disease in 1990 and 2010. Glob Heart 2012; 7: 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forouzanfar MH, Moran AE, Flaxman AD, et al. Assessing the global burden of ischemic heart disease: part 2: analytic methods and estimates of the global epidemiology of ischemic heart disease in 2010. Glob Heart 2012; 7: 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vedanthan R, Seligman B, Fuster V. Global perspective on acute coronary syndrome: a burden on the young and poor. Circ Res 2014; 114: 1959–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchis-Gomar F, Perez-Quilis C, Leischik R, et al. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 2016; 4: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox KA. Acute coronary syndromes: presentation—clinical spectrum and management. Heart 2000; 84: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauersachs R, Zeymer U, Brière JB, et al. Burden of coronary artery disease and peripheral artery disease: a literature review. Cardiovasc Ther 2019; 2019: 8295054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012; 32: 2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med 2015; 278: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto E, Sugiyama S, Hirata Y, et al. Prognostic significance of circulating leukocyte subtype counts in patients with coronary artery disease. Atherosclerosis 2016; 255: 210–216. [DOI] [PubMed] [Google Scholar]
- 13.Pinheiro Machado G, Araujo GN, Carpes CK, et al. Elevated neutrophil-to-lymphocyte ratio can predict procedural adverse events in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron Artery Dis 2019; 30: 20–25. [DOI] [PubMed] [Google Scholar]
- 14.Her AY, Cho KI, Singh GB, et al. Plaque characteristics and inflammatory markers for the prediction of major cardiovascular events in patients with ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging 2017; 33: 1445–1454. [DOI] [PubMed] [Google Scholar]
- 15.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010; 7: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol 2011; 31: 1506–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidarpour M, Bashiri S, Vakhshoori M, et al. The association between platelet-to-lymphocyte ratio with mortality among patients suffering from acute decompensated heart failure. BMC Cardiovasc Disord 2021; 21: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delcea C, Buzea CA, Vijan A, et al. Comparative role of hematological indices for the assessment of in-hospital outcome of heart failure patients. Scand Cardiovasc J 2021; 55: 227–236. [DOI] [PubMed] [Google Scholar]
- 19.Majmundar M, Kansara T, Park H, et al. Absolute lymphocyte count as a predictor of mortality and readmission in heart failure hospitalization. Int J Cardiol Heart Vasc 2022; 39: 100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koosha P, Roohafza H, Sarrafzadegan N, et al. High sensitivity C-reactive protein predictive value for cardiovascular disease: a nested case control from Isfahan cohort study (ICS). Glob Heart 2020; 15: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva N, Bettencourt P, Guimarães J. The lymphocyte-to-monocyte ratio: an added value for death prediction in heart failure. Nutr Metab Cardiovasc Dis 2015; 25: 1033–1040. [DOI] [PubMed] [Google Scholar]
- 22.Heidarpour M, Sourani Z, Vakhshoori M, et al. Prognostic utility of shock index and modified shock index on long-term mortality in acute decompensated heart failure; Persian Registry of cardioVascular diseasE/Heart Failure (PROVE/HF) study. Acta Cardiol 2022; 78: 217–226. [DOI] [PubMed] [Google Scholar]
- 23.Bondariyan N, Vakhshoori M, Sadeghpour N, et al. Prognostic value of shock index, modified shock index, and age-adjusted derivatives in prediction of in-hospital mortality in patients with acute decompensated heart failure: Persian Registry of Cardiovascular Disease/Heart Failure Study. Anatol J Cardiol 2022; 26: 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Wang R, Bai L, et al. The leukocyte subtype counts and ratios can effectively predict the risk of arterial stiffness assessed by cardio-ankle vascular index: a retrospective study. Front Cardiovasc Med 2021; 8: 671885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbanowicz T, Olasińska-Wiśniewska A, Michalak M, et al. The prognostic significance of neutrophil to lymphocyte ratio (NLR), monocyte to lymphocyte ratio (MLR) and platelet to lymphocyte ratio (PLR) on long-term survival in off-pump coronary artery bypass grafting (OPCAB) procedures. Biology (Basel) 2021; 11: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirna M, Schmutzler L, Topf A, et al. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Sci Rep 2021; 11: 18101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boos CJ, Toon LT, Almahdi H. The relationship between ambulatory arterial stiffness, inflammation, blood pressure dipping and cardiovascular outcomes. BMC Cardiovasc Disord 2021; 21: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. JAMA 2015; 313: 1657–1665. [DOI] [PubMed] [Google Scholar]
- 29.Downes MJ, Brennan ML, Williams HC, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016; 6: e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Study Quality Assessment Tools [Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 31.The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews. Checklist for Cohort Studies
- 32.The Joanna Briggs Institute Critical Appraisal tools for use in JBI Systematic Reviews. Checklist for Randomized Controlled Trials [Available from: https://jbi.global/sites/default/files/2019-05/JBI_RCTs_Appraisal_tool2017_0.pdf.
- 33.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Ma X, Shao Q, et al. Prognostic impact of multiple lymphocyte-based inflammatory indices in acute coronary syndrome patients. Front Cardiovasc Med 2022; 9: 811790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai K, Li Z, Luo Y. The predictive value of the monocyte-to-lymphocyte ratio and monocyte-to-haematocrit ratio for cardiac rupture patients with acute myocardial infarction: a propensity score matching analysis [corrigendum]. Risk Manag Healthc Policy 2022; 15: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song FH, Zheng YY, Tang JN, et al. A correlation between monocyte to lymphocyte ratio and long-term prognosis in patients with coronary artery disease after PCI. Clin Appl Thromb Hemost 2021; 27: 1076029621999717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Si Y, Fan W, Han C, et al. Atherogenic index of plasma, triglyceride-glucose index and monocyte-to-lymphocyte ratio for predicting subclinical coronary artery disease. Am J Med Sci 2021; 362: 285–290. [DOI] [PubMed] [Google Scholar]
- 38.Shumilah AM, Othman AM, Al-Madhagi AK. Accuracy of neutrophil to lymphocyte and monocyte to lymphocyte ratios as new inflammatory markers in acute coronary syndrome. BMC Cardiovasc Disord 2021; 21: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao X, Liu Y, Tian Y, et al. Prognostic value of peripheral blood inflammatory cell subsets in patients with acute coronary syndrome undergoing percutaneous coronary intervention. J Int Med Res 2021; 49: 3000605211010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai K, Li Z, Luo Y, et al. Neutrophil percentage‐to‐albumin ratio and monocyte‐to‐lymphocyte ratio as predictors of free‐wall rupture in patients with acute myocardial infarction. J Clin Lab Anal 2022; 36: e24136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Candemir M, Kiziltunç E, Nurkoç S, et al. Relationship between systemic immune-inflammation index (SII) and the severity of stable coronary artery disease. Angiology 2021; 72: 575–581. [DOI] [PubMed] [Google Scholar]
- 42.Zhang TY, Zhao Q, Liu ZS, et al. Relationship between monocyte/lymphocyte ratio and non-culprit plaque vulnerability in patients with acute coronary syndrome: an optical coherence tomography study. Medicine (Baltimore) 2020; 99: e21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanriverdi Z, Gungoren F, Tascanov MB, et al. Comparing the diagnostic value of the C-reactive protein to albumin ratio with other inflammatory markers in patients with stable angina pectoris. Angiology 2020; 71: 360–365. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Meng Y, Shao M, et al. Prognostic value of pre-infarction angina combined with mean platelet volume to lymphocyte count ratio for no-reflow and short-term mortality in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. Med Sci Monit 2020; 26: e919300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horváth T, Serfőző G, Györkei Á, et al. Neutrophil count as the centerpiece in the joined association networks of inflammatory and cell damage markers, and neuroendocrine stress markers in patients with stable angina pectoris following stenting. PLoS One 2019; 14: e0215209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen H, Li M, Liu L, et al. Monocyte/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients with non-ST-elevation myocardial infarction. Medicine (Baltimore) 2019; 98: e16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan Z, Li Y, Ji H, et al. Prognostic utility of the combination of monocyte-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with NSTEMI after primary percutaneous coronary intervention: a retrospective cohort study. BMJ Open 2018; 8: e023459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji H, Li Y, Fan Z, et al. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: a syntax score assessment. BMC Cardiovasc Disord 2017; 17: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gijsberts CM, Ellenbroek GH, Maarten J, et al. Effect of monocyte-to-lymphocyte ratio on heart failure characteristics and hospitalizations in a coronary angiography cohort. Am J Cardiol 2017; 120: 911–916. [DOI] [PubMed] [Google Scholar]
- 50.Fan Z, Ji H, Li Y, et al. Relationship between monocyte-to-lymphocyte ratio and coronary plaque vulnerability in patients with stable angina. Biomark Med 2017; 11: 979–990. [DOI] [PubMed] [Google Scholar]
- 51.Serfőző G, Horváth T, Földesi I, et al. The monocyte-to-lymphocyte ratio correlates with psycho-neuro-inflammatory factors in patients with stable coronary artery disease. Neuroimmunomodulation 2016; 23: 67–74. [DOI] [PubMed] [Google Scholar]
- 52.Gijsberts CM, Ellenbroek GH, Ten Berg MJ, et al. Routinely analyzed leukocyte characteristics improve prediction of mortality after coronary angiography. Eur J Prev Cardiol 2016; 23: 1211–1220. [DOI] [PubMed] [Google Scholar]
- 53.Raggi P, Genest J, Giles JT, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018; 276: 98–108. [DOI] [PubMed] [Google Scholar]
- 54.Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009; 54: 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montecucco F, Liberale L, Bonaventura A, et al. The role of inflammation in cardiovascular outcome. Curr Atheroscler Rep 2017; 19: 11. [DOI] [PubMed] [Google Scholar]
- 56.Santos-Gallego CG, Picatoste B, Badimón JJ. Pathophysiology of acute coronary syndrome. Curr Atheroscler Rep 2014; 16: 401. [DOI] [PubMed] [Google Scholar]
- 57.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011; 473: 317–325. [DOI] [PubMed] [Google Scholar]
- 58.Ramsay SC, Maggs JA, Ketheesan N, et al. Relative uptake of technetium 99m stannous colloid by neutrophils and monocytes is altered by gram-negative infection. Nucl Med Biol 2005; 32: 101–107. [DOI] [PubMed] [Google Scholar]
- 59.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature 2012; 487: 325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Núñez J, Miñana G, Bodí V, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem 2011; 18: 3226–3233. [DOI] [PubMed] [Google Scholar]
- 61.Petru CD, Noboru W, Mitsuaki I. Apoptosis of peripheral blood lymphocytes is induced by catecholamines. Jpn Heart J 2000; 41: 385–398. [DOI] [PubMed] [Google Scholar]
- 62.Krüger K, Agnischock S, Lechtermann A, et al. Intensive resistance exercise induces lymphocyte apoptosis via cortisol and glucocorticoid receptor-dependent pathways. J Appl Physiol (1985) 2011; 110: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 63.Ommen SR, Gibbons RJ, Hodge DO, et al. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol 1997; 79: 812–814. [DOI] [PubMed] [Google Scholar]
- 64.Núñez J, Núñez E, Bodí V, et al. Low lymphocyte count in acute phase of ST-segment elevation myocardial infarction predicts long-term recurrent myocardial infarction. Coron Artery Dis 2010; 21: 1–7. [DOI] [PubMed] [Google Scholar]
- 65.Camici PG, D'Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015; 12: 48–62. [DOI] [PubMed] [Google Scholar]
- 66.Lee MJ, Park SD, Kwon SW, et al. Relation between neutrophil-to-lymphocyte ratio and index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2016; 118: 1323–1328. [DOI] [PubMed] [Google Scholar]
- 67.Yuan C, Li N, Mao X, et al. Elevated pretreatment neutrophil/white blood cell ratio and monocyte/lymphocyte ratio predict poor survival in patients with curatively resected non‐small cell lung cancer: results from a large cohort. Thorac Cancer 2017; 8: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L, Zeng H, Yang J, et al. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer 2018; 18: 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du J, Chen S, Shi J, et al. The association between the lymphocyte-monocyte ratio and disease activity in rheumatoid arthritis. Clin Rheumatol 2017; 36: 2689–2695. [DOI] [PubMed] [Google Scholar]
- 70.Naranbhai V, Kim S, Fletcher H, et al. The association between the ratio of monocytes: lymphocytes at age 3 months and risk of tuberculosis (TB) in the first two years of life. BMC Med 2014; 12: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhai G, Liu Y, Wang J, et al. Association of monocyte-lymphocyte ratio with in-hospital mortality in cardiac intensive care unit patients. Int Immunopharmacol 2021; 96: 107736. [DOI] [PubMed] [Google Scholar]
- 72.Solomon SD, Pfeffer MA. Renin-angiotensin system and cardiac rupture after myocardial infarction. Circulation 2002; 106: 2167–2169. [DOI] [PubMed] [Google Scholar]
- 73.Askari AT, Brennan ML, Zhou X, et al. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med 2003; 197: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghattas A, Griffiths HR, Devitt A, et al. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol 2013; 62: 1541–1551. [DOI] [PubMed] [Google Scholar]
- 75.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005; 45: 1638–1643. [DOI] [PubMed] [Google Scholar]
- 76.Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry 2000; 157: 683–694. [DOI] [PubMed] [Google Scholar]
- 77.Miller AH, Haroon E, Raison CL, et al. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 2013; 30: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605231204469 for Prognostic impact of monocyte-to-lymphocyte ratio in coronary heart disease: a systematic review and meta-analysis by Mehrbod Vakhshoori, Sepehr Nemati, Sadeq Sabouhi, Mehrnaz Shakarami, Behzad Yavari, Sayed Ali Emami, Niloofar Bondariyan and Davood Shafie in Journal of International Medical Research
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because of confidentiality issues but are available from the corresponding author on reasonable request.